Abstract
EPS formed by lactobacilli in situ during sourdough fermentation may replace hydrocolloids currently used as texturizing, antistaling, or prebiotic additives in bread production. In this study, a screening of >100 strains of cereal-associated and intestinal lactic acid bacteria was performed for the production of exopolysaccharides (EPS) from sucrose. Fifteen strains produced fructan, and four strains produced glucan. It was remarkable that formation of glucan and fructan was most frequently found in intestinal isolates and strains of the species Lactobacillus reuteri, Lactobacillus pontis, and Lactobacillus frumenti from type II sourdoughs. By the use of PCR primers derived from conserved amino acid sequences of bacterial levansucrase genes, it was shown that 6 of the 15 fructan-producing lactobacilli and none of 20 glucan producers or EPS-negative strains carried a levansucrase gene. In sourdough fermentations, it was determined whether those strains producing EPS in MRS medium modified as described by Stolz et al. (37) and containing 100 g of sucrose liter−1 as the sole source of carbon also produce the same EPS from sucrose during sourdough fermentation in the presence of 12% sucrose. For all six EPS-producing strains evaluated in sourdough fermentations, in situ production of EPS at levels ranging from 0.5 to 2 g/kg of flour was demonstrated. Production of EPS from sucrose is a metabolic activity that is widespread among sourdough lactic acid bacteria. Thus, the use of these organisms in bread production may allow the replacement of additives.
Sourdough has traditionally been used as a leavening agent in bread production. Sourdough fermentations, as well as baking agents based on sourdoughs, have retained their importance in contemporary baking technology because of the improved aroma, texture, and shelf life of sourdough breads (7, 35, 40). The production of a wide variety of traditionally prepared baked goods continues to rely exclusively on the use of sourdough as a leavening agent. In most industrial applications, sourdough or dried sourdough preparations are added to bread doughs which also contain baker's yeast as a leavening agent (13, 14, 45). Knowledge of the metabolic activities and corresponding genes of sourdough lactic acid bacteria that are responsible for their positive influence on bread quality is a prerequisite for the deliberate choice of starter cultures for specific applications. The predominant microorganisms isolated from traditional sourdoughs (type I doughs) sustained by continuous propagation are yeasts and lactic acid bacteria, mainly Lactobacillus sanfranciscensis and Lactobacillus pontis. In industrial sourdoughs prepared by using elevated temperatures and/or longer fermentation times (type II sourdough), as well as in cereal fermentations in tropical climates, thermophilic, acid-tolerant lactobacilli, such as L. pontis, Lactobacillus panis, Lactobacillus reuteri, Lactobacillus amylovorus, and Lactobacillus frumenti, are predominant (1, 31, 45). The microbiotae of type II sourdoughs are highly similar to those species of lactobacilli most frequently found in the digestive tracts of humans and animals.
Exopolysaccharides (EPS) from lactic acid bacteria are used to improve the textural properties of fermented foods. Two classes of EPS from lactic acid bacteria can be distinguished: extracellularly synthesized homopolysaccharides and heteropolysaccharides with (ir)regularly repeating units that are synthesized from intracellular sugar nucleotide precursors. Studies of the application of EPS-forming starter cultures have primarily focused on heteropolysaccharides from lactobacilli in dairy fermentations. Heteroexopolysaccharides are produced in small amounts, usually below 0.5 g liter−1 (9, 22).
The production from sucrose of glucan and levan by L. reuteri LB121 and the production of a levan-type fructan by L. sanfranciscensis LTH2590 have been described by van Geel-Schutten et al. (42) and Korakli et al. (17), respectively. Leuconostoc citreum was reported to produce inulin based on a biochemical characterization of the inulosucrase, as well as a structural characterization of the inulin formed (32). Bacterial glycansucrases generally have two activities, an invertase activity, splitting sucrose into fructose and glucose, and a fructosyltransferase activity responsible for the formation of polymers (28). Because of the poor specificity of fructansucrases and glucansucrases for the linkages formed from sucrose, the formation of oligosaccharides is also observed (10, 28, 44). Two fructosyltransferases and a glucosyltransferase responsible for the production of fructans and glucan by L. reuteri LB121 were recently characterized on the biochemical and/or genetic level (20, 43, 44).
The fructan from L. sanfranciscensis has recently been found to positively affect dough rheology and bread texture (4, 17). EPS-forming sourdough lactic acid bacteria thus have the potential to replace xanthan and guar gums currently used as additives to improve dough rheology and bread texture. Furthermore, fructose-oligosaccharides and inulin are increasingly used as prebiotic additives in baked goods and other foods. They are not digested by pancreatic enzymes and thus are available for metabolism by intestinal microorganisms, mainly Bifidobacteria. Prebiotics are defined as nondigestible food ingredients that affect the host beneficially by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon and thus improving host health (12).
To determine the potential of lactobacilli to improve the textural and nutritional qualities of bread based on EPS production during sourdough fermentation, we screened cereal-associated lactobacilli for the production of EPS from sucrose and investigated EPS formation on the physiological and genetic levels. EPS formation in vitro was compared to the formation of EPS during sourdough fermentation.
MATERIALS AND METHODS
Strains, media, and growth conditions.
One hundred eleven strains of the genera Lactobacillus and Weissella that were previously isolated from sourdough fermentations, sour-wort fermentation, and spoiled beer were screened for EPS formation. This selection comprised strains of Lactobacillus alimentarius (2 strains), Lactobacillus amylolyticus (1 strain) L. amylovorus (2 strains), Lactobacillus brevis (5 strains), Lactobacillus buchneri (1 strain), Lactobacillus farciminis (3 strains), Lactobacillus fructivorans, (1 strain), L. frumenti (7 strains), Lactobacillus fermentum (2 strains), Lactobacillus mindensis (3 strains), L. panis (2 strains), Lactobacillus pentosus (3 strains), Lactobacillus plantarum (6 strains), L. pontis (9 strains), Lactobacillus rhamnosus (1 strain), L. reuteri (5 strains), Lactobacillus sakei (1 strain), L. sanfranciscensis (30 strains), Lactobacillus suebicus (2 strains), Lactobacillus viridescens (1 strain), Weissella confusa (3 strains), and 21 Lactobacillus strains that were not classified to the species level. The strains were incubated in MRS medium modified as described by Stolz et al. (37) and containing 100 g of sucrose liter−1 as the sole source of carbon (mMRS-sucrose) or 10 g of maltose liter−1, 5 g of glucose liter−1, and 5 g of fructose liter−1 as sole carbon sources (mMRS-maltose). The incubation temperature was 30°C unless otherwise indicated. The medium pH was adjusted to 6.2 before it was autoclaved (121°C; 20 min), and sucrose was autoclaved separately from the other medium components. Solid media contained 15 g of agar liter−1. Prior to each experiment, the strains were inoculated on plates and the absence of contaminants was verified by the observation of a uniform colony morphology of the inoculum used for the experiments.
Screening for EPS synthesis.
In a first screening, all strains were incubated for 60 h at 30°C in 200 μl of mMRS-sucrose. After incubation, the cells were removed by centrifugation (1,500 × g; 10 min), and 100 μl of the supernatants was analyzed by gel permeation chromatography (GPC) coupled to a refractive-index detector to detect high-molecular-weight EPS. Culture supernatant (20 μl) was injected on a Superdex 200 GPC column (Amersham Pharmacia Biotech, Uppsala, Sweden), and the samples were eluted with 50 mM sodium phosphate, pH 6.9, at a flow rate of 0.6 ml min−1. High-molecular-weight and low-molecular-weight (LMW) gel filtration calibration kits (Amersham Pharmacia Biotech) were used for calibration of the GPC column. The GPC column employed separates polymers with relative molecular weights (Mr) ranging from 1 × 104 to 5 × 106.
In a second step, EPS-positive strains were inoculated into 600 μl of mMRS-sucrose, and after fermentation, the supernatants were precipitated by adding 2 volumes of ethanol and storing them at −20°C for at least 1 h. After centrifugation (10 min; 1,200 × g), the precipitates were dried under vacuum, redissolved in 300 μl of demineralized water, and analyzed by GPC as described above. The first screening and the second screening were each carried out in two representative experiments (a total of four replicates for EPS-positive strains) with consistent results.
Monosaccharide composition of EPS.
To analyze the monosaccharide composition of the EPS, positive strains were inoculated on 10 ml of mMRS-sucrose. Strains of L. sanfranciscensis were incubated at 30°C, and strains of L. reuteri, L. frumenti, and L. pontis were incubated at 37°C. After 60 h, the culture supernatants were precipitated with ethanol and redissolved in demineralized water as described above. A preparative GPC run was performed with a 200-μl injection volume at a flow rate of 0.6 ml min−1, and the polymer peak fractions were collected in an elution volume ranging from 7 to 10 ml, corresponding to an Mr above 106. The polymer fractions containing high-molecular-weight EPS were dried in a vacuum and dissolved in 800 μl of demineralized water. To hydrolyze the EPS, 15% (vol/vol) perchloric acid (70%) was added, and the samples were heated to 80°C for 1 h. To precipitate perchlorate, 250 μl of 5 M KOH was added, precipitated potassium perchlorate was removed by centrifugation (4°C; 12,000 × g; 5 min), and the supernatant was used for the analysis of monosaccharides in the hydrolysate. The monosaccharide compositions were analyzed by high-performance liquid chromatography (HPLC) using a Polyspher CH PB column (Merck, Darmstadt, Germany) and refractive-index detection; the injection volume was 20 μl. The mobile phase was deionized H2O at a flow rate of 0.4 ml min−1, and the column temperature was maintained at 80°C. For peak identification, an external standard containing arabinose, fructose, glucose, and xylose was used. Determination of the monosaccharide composition was done in two independent experiments with consistent results.
Preparation of doughs.
Sourdough fermentations were carried out essentially as described by Korakli et al. (18). For the fermentation of doughs, five strains forming fructan (L. sanfranciscensis LTH2590; L. frumenti TMW1.103, TMW1.660, and TMW1.669; and L. pontis TMW1.675), one strain forming glucan (L. reuteri TMW1.106), and one EPS-negative control (L. sanfranciscensis LTH 2581) were used. The doughs were prepared with 100 g of wheat flour (ash content, 510 to 630 mg/100 g), 12 g of sucrose, and 100 g of sterilized tap water. For the inoculation of the doughs, cells from 10 ml of overnight cultures of each strain in mMRS-sucrose were harvested by centrifugation, resuspended in 5 ml of physiological salt solution, and added to the doughs. Aseptically fermented control doughs were prepared without inoculum, and 20 ppm chloramphenicol and 10 ppm erythromycin were added to inhibit microbial growth. The control dough and the doughs with L. sanfranciscensis were incubated for 24 h at 30°C; all other doughs were incubated at 37°C.
Determination of pH and cell counts in sourdoughs.
Cell counts were determined on mMRS-maltose agar. Appropriate dilutions were plated using a spiral plater (IUL, Königswinter, Germany), and the plates were incubated at 30°C for 48 h under a controlled atmosphere (76% N2, 20% CO2, and 4% O2). The pH values of the dough were determined with a glass electrode.
EPS isolation and purification from doughs.
The isolation from doughs of water-soluble polysaccharides (WS-PS) originating from the flour and microbial EPS from doughs was performed essentially as described previously (18). Two parts water were added to one part dough (wt/wt). Solids were removed by centrifugation (8,000 × g; 10 min), and the resulting supernatant was precipitated with ethanol, dialyzed against demineralized H2O (Mr cutoff, ca. 104), and lyophilized. Hydrolyses of the dried polysaccharides were carried out by incubating the samples for 2 h in 1 M H2SO4 at 80°C, and monosaccharide compositions were determined using HPLC as described above.
Construction of primers targeting levansucrase genes.
Primers to amplify fructansucrase genes were constructed to target the conserved amino acid sequences of the levansucrases of lactic acid bacteria. The amino acid sequences DVWDSWP and DEV(IL)ER are conserved in levansucrases from Bacillus subtilis, Bacillus stearothermophilus, Bacillus amyloliquefaciens, Streptococcus mutans, and Streptococcus salivarius (EMBL [Heidelberg, Germany; www.embl.heidelberg.de] accession numbers P05655, P94468, P21130, P11701, and Q55242, respectively) and in levan- and fructansucrases of L. reuteri strain 121 (43, 44). The primer sequences were 5′-GA(CT) GTI TGG GA(CT) (AT)(GC)I TGG C-3′ (LevV; forward primer) and 5′-TCI T(CT)(CT) TC(AG) TCI (GC)(AT)I (AG)(AC)C AT-3′ (LevR; backward primer), where I stands for inosine. The letters in parentheses represent wobble positions.
DNA isolation and PCR conditions
DNA was prepared according to the method of Lewington et al. (25) from cells of an overnight culture in 50 ml of mMRS-maltose. To check the yield and quality of the DNA preparations, a randomly amplified polymorphic DNA-PCR was carried out with M13 universal primers (5′-GTT TTC CCA GTC ACG AC-3′) as described previously (29). PCRs with the LevV and LevR primers were carried out in 50-μl volumes using 0.5 μl of chromosomal DNA as a template and 7.25 U of Taq polymerase. Oligonucleotides, Taq polymerase, and reaction buffer for use in PCRs were obtained from Amersham Pharmacia, Uppsala, Sweden. Amplification products were separated on 1% agarose gels, stained with ethidium bromide, and visualized by UV transillumination.
Sequencing of amplification products.
PCR products were purified using the QIAquick PCR purification kit (Qiagen, Hilden, Germany) according to the instructions of the supplier and sequenced by SequiServe (Vaterstetten, Germany). Nucleotide and amino acid sequence analysis was carried out using the DNASis for Windows software (Hitachi Software Engineering Co., Yokohama, Japan).
Isolation of mRNA, RT-RCR, and amplification of a levansucrase gene internal fragment from cDNA.
Total RNA was isolated from cultures of L. sanfranciscensis LTH2590 and TMW 1.53 grown to the exponential growth phase (optical density at 578 nm, 0.2) in mMRS-sucrose and mMRS-maltose; 0.6 ml was taken from each culture and resuspended in 1.2 ml of stop buffer (RNA Protect bacterial reagent; Qiagen). The isolation of RNA was performed using the RNeasy Plant Mini Kit (Qiagen). In the RNA preparations, DNA was digested by incubation with RQ1 RNase-free DNase (Promega, Mannheim, Germany). Reverse transcription (RT)-PCR was performed using 100 U of Moloney murine leukemia virus reverse transcriptase and was primed with 20 μg of hexameric random primers ml−1 (rRNase H minus and random primers from Promega). From the cDNA library, an internal fragment of the levansucrase gene was amplified using Taq polymerase and the primers Lev3V (5′-CAA GGG TTA TCA ACT AG −3′) and LevshortR (5′-CCG CAT TAC CGG AAT GTG −3′) matching internal sequences of the 800-bp LevV-LevR levansucrase gene fragment of L. sanfranciscensis. PCR with the Lev3V and LevshortR primers was also carried out with DNase-digested RNA preparations to ensure complete hydrolysis of chromosomal DNA in RNA preparations.
RESULTS
Screening of sourdough lactobacilli for EPS production.
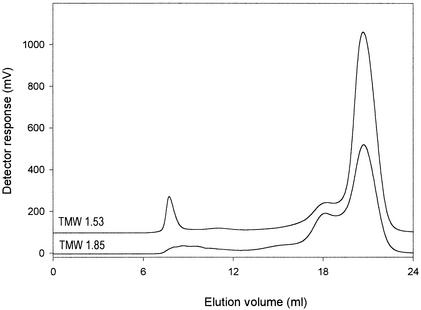
A total of 111 strains of the genera Lactobacillus and Weissella were cultivated on medium containing excessive amounts of sucrose and screened by GPC for EPS production. This selection comprised mainly strains isolated from sourdough fermentations, sour-wort fermentations, and spoiled beer. Additionally, intestinal isolates of L. reuteri were used. Examples of chromatograms from culture supernatants of EPS-positive and EPS-negative strains are shown in Fig. 1. A peak corresponding to high-molecular-weight EPS was observed at a 7.2-ml elution volume, corresponding to an Mr above 106. EPS with molecular weights of 105 to 106 were not observed in any of the strains, and LMW EPS is not detectable by this method because the medium used contains polysaccharides or other compounds eluting in the Mr range of 104 to 105.
FIG. 1.
GPC chromatograms from culture supernatants of L. sanfranciscensis TMW 1.53 (EPS positive) and L. pontis TMW 1.85 (EPS negative). A peak corresponding to high-molecular-weight EPS is observed at a 7.2-ml elution volume. The medium used is essentially free of compounds with Mrs above 105, but LMW EPS is not detectable because it is covered by an LMW peak from the medium.
Twenty-two of the 111 strains were found to produce high-molecular-weight EPS. Table 1 lists the EPS-forming strains, their origins, and the types of EPS formed. The EPS was called fructan or glucan if fructose or glucose, respectively, accounted for >95% of the sugars liberated by hydrolysis. Three strains were found to produce small amounts of polysaccharides with unknown compositions. Because of the small amounts of polysaccharides formed by these strains and/or incomplete hydrolysis of the polysaccharides, the analysis of the respective hydrolysates as performed in this study did not allow an unambiguous identification of the monosaccharides. Production of fructan and glucan was detected in 15 and 4 strains, respectively. The fructan-forming species were L. sanfranciscensis, L. frumenti, L. pontis, L. reuteri, L. panis, and W. confusa. The glucan-forming species were L. reuteri, W. confusa, and Lactobacillus sp. strain TMW1.624. Based on the correlation of peak areas of EPS in GPC chromatograms, as well as those of monosaccharides in HPLC chromatograms, to the EPS concentrations of culture supernatants, it can be estimated that the amounts of glucan and fructan formed by these strains during growth in mMRS-sucrose range from 2 to 20 g liter−1, as previously described for L. sanfranciscensis LTH2590 (18).
TABLE 1.
EPS-forming species and their origins
| Organism and TMW no. | EPS formeda | Origin (reference) | PCR product with Lev primer |
|---|---|---|---|
| L. sanfranciscensis 1.392c | Fructan | Strain D1 (LTH2590); sourdough (1) | 800 bp |
| L. sanfranciscensis 1.53 | Fructan | DSM 20451, sourdough (16) | 800 and 700 bp |
| L. sanfranciscensis 1.54c | Fructan | LTH1729, sourdough (2) | No |
| L. sanfranciscensis 1.896 | Fructan | Sourdough | 800 bp |
| L. sanfranciscensis 1.953 | Fructan | Sourdough | 800 bp |
| L. sanfranciscensis 1.1149 | Unknownd | Sourdough | No |
| L. frumenti 1.103 | Fructan | Sourdough | 800 bp |
| L. frumenti 1.660 | Fructan | Sourdough (29) | No |
| L. frumenti 1.665 | Fructan | Sourdough (29) | No |
| L. frumenti 1.666 | Fructan | DSM 13145T; sourdough (29, 30, 31) | No |
| L. frumenti 1.669 | Fructan | Sourdough (29) | 800 bp |
| L. pontis 1.1115 | Fructan | Sourdough | No |
| L. pontis 1.675 | Fructan | Sourdough (29) | No |
| L. pontis 1.682 | Unknown | Sourdough (29) | No |
| L. panis 1.649 | Fructan | DSMZ 6036; sourdough (47) | No |
| L. reuteri 1.693 | Fructanb | DSMZ 20016; intestinal isolate | No |
| L. reuteri 1.977 | Glucan | Duck colon (21) | No |
| L. reuteri 1.106 | Glucan | Sourdough | No |
| L. reuteri 1.109 | Unknown | Sourdough | No |
| Lactobacillus sp. strain 1.624 | Glucan | Sourdough | No |
| W. confusa 1.617 | Glucan | Sourdough | No |
| W. confusa 1.934 | Fructan | Sourdough | No |
EPS formation was determined in four independent experiments, and the monomer compositions of EPS were determined in two independent experiments.
The amount of fructan produced by L. reuteri TMW 1.693 was significantly lower than those of the other fructan-forming strains.
Fructan formation by strains TMW 1.392 (LTH2590) and TMW1.54 (LTH1729) was previously described (8, 17).
Unknown composition of EPS because of the small amounts of polysaccharides formed and/or incomplete hydrolysis of the polysaccharides.
PCR screening for a levansucrase gene and sequence of the amplification product.
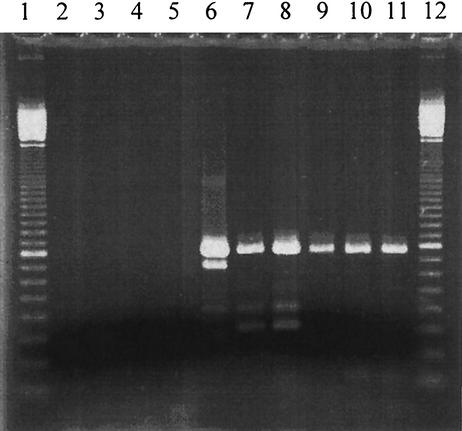
To determine the presence of levansucrase genes, DNA was isolated from the 15 fructan-forming strains, 4 glucan-forming strains, and 16 EPS-negative organisms. Primers were constructed targeting conservative regions of known bacterial levansucrases, and a PCR was carried out. Figure 2 shows the amplification products which could be detected for 6 of the 15 fructan-forming strains. One major PCR product was observed in 6 of the 15 fructan-forming strains. The size of 800 bp corresponds to the length of the amplicon calculated from known sequences of bacterial levansucrases, 700 to 900 bp. No PCR product was detected if DNA from fructan-negative strains was used as a template (Fig. 2 and data not shown).
FIG. 2.
Agarose gel with PCR products obtained with LevV-LevR primers and DNAs from EPS-positive and EPS-negative lactobacilli as templates. Lanes 1 and 12, 100-bp standards; lanes 2 to 5, PCR with non-fructan-forming strains; lanes 6 to 11, fructan-forming lactobacilli (L. sanfranciscensis TMW1.53, TMW1.953, TMW 1.896, and LTH2590 and L. frumenti TMW1.669 and TMW1.103, respectively).
Comparison of the sequences of LevV-LevR PCR products with those of other bacterial levansucrases.
The amplification products from L. frumenti TMW 1.103 and TMW 1.669 and from L. sanfranciscensis TMW 1.53, LTH2590, TMW 1.896, and TMW 1.953 were purified and sequenced. Based on searches using the EMBL protein and nucleotide databases, the amplicons were identified as partial sequences of levansucrase genes. The deduced amino acid sequences of genes amplified from L. sanfranciscensis TMW 1.53 and L. frumenti TMW 1.103 were compared to known sequences of L. reuteri, S. mutans, and S. salivarius (Fig. 3). The partial sequences of L. sanfranciscensis LTH2590, TMW 1.896, and TMW 1.953 were identical to that of L. sanfranciscensis TMW 1.53, and the partial sequence of L. frumenti TMW1.669 was identical to that of L. frumenti TMW 1.103 (data not shown). By comparison of the amino acid sequences of the levansucrase fragments from L. frumenti and L. sanfranciscensis with those of the corresponding segments from S. salivarius and S. mutans and the levansucrase of L. reuteri, identities of 48 and 88%, respectively, were observed (Fig. 3). Comparison of the sequences of the levansucrases of L. sanfranciscensis and L. frumenti showed only 78% identity. The corresponding partial sequences of levansucrases from B. amyloliquefaciens and B. stearothermophilus are only 15.8% identical to those from Lactobacillus and Streptococcus (data not shown).
FIG. 3.
(A) Multiple alignment of the partial levansucrase sequences of L. sanfranciscensis TMW 1.53 (SEQ53.AMI) and L. frumenti TMW1.103 (SEQ103.AMI) with the partial levansucrase sequences from S. mutans (SMUTANS.AMI), and S. salivarius (SSALIV.AMI) and two fructansucrases from L. reuteri (LEVREU.AMI [43] and INULO.AMI [44]). (B) Genealogical tree based on the alignments in panel A. The partial sequences of L. sanfranciscensis LTH2590, TMW 1.896, and TMW 1.953 were identical to that of L. sanfranciscensis TMW 1.53, and the partial sequence of L. frumenti TMW1.669 was identical to that of L. frumenti TMW 1.103 (data not shown).
Using DNA from L. sanfranciscensis TMW1.53 as a template for the Lev PCR, an additional minor band with lower molecular weight was detected. Sequencing of this PCR product and comparison with the EMBL nucleotide database did not reveal significant homologies to levansucrase genes or any other documented DNA sequences.
Expression of the levansucrase gene in L. sanfranciscensis.
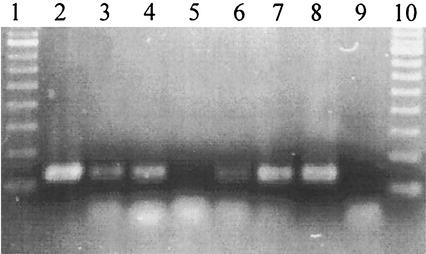
To determine whether the putative levansucrase genes encompassing the 800-bp LevV-LevR PCR product are expressed in L. sanfranciscensis, PCR targeting an internal sequence of the LevV-LevR PCR product was carried out using the cDNAs of L. sanfranciscensis LTH2590 and TMW 1.53. RNA was isolated from cultures growing in the presence or absence of sucrose. Figure 4 shows the amplification products obtained from chromosomal DNA from either strain and from cDNA libraries of either strain growing on mMRS-maltose or mMRS-sucrose. The PCR products had the expected size of 120 bp. Expression of the putative levansucrase gene was observed in either strain growing on maltose or sucrose as a carbon source.
FIG. 4.
Agarose gel with PCR products obtained with Lev3V-LevshortR primers using chromosomal DNA or cDNA libraries from EPS-positive L. sanfranciscensis strains as templates. Lev3V-LevshortR PCR ensured that RNA preparations used as templates for the RT-PCR were free of DNA. The strains were grown on mMRS-maltose or mMRS-sucrose. Lanes 1 and 10, molecular weight standards; lanes 5 and 9, negative control (no template); lanes 2 to 4, LTH2590 chromosomal DNA (lane 2) and cDNAs from maltose-grown cells (lane 3) and sucrose-grown cells (lane 4); lanes 6 to 8, TMW 1.53 chromosomal DNA (lane 6) and cDNAs from maltose-grown cells (lane 7) and sucrose-grown cells (lane 8).
In situ EPS formation in sourdough.
Sourdough fermentations were carried out using five strains producing fructan and one strain producing glucan in order to compare EPS formation in sourdough with the results obtained in vitro. Sourdough fermentation and analysis of the dough for the presence of EPS were carried out as described previously (18). Dough fermented with the EPS-negative strain L. sanfranciscensis LTH2581 and dough in which microbial growth and metabolism were inhibited by antibiotics served as negative controls. Table 2 shows the monosaccharide compositions of WS-PS extracted from the sourdoughs. Small amounts of fructose were found in WS-PS from control doughs fermented with EPS-negative L. sanfranciscensis or aseptic fermented doughs. More than 10-fold-higher fructose levels in WS-PS were found in all doughs fermented with fructan-producing strains, indicating fructan formation by these strains during sourdough fermentation. Dough fermented with L. sanfranciscensis LTH 2581 or fructan-forming strains contained <1 mmol of glucose kg−1 in WS-PS. In the control dough with antibiotics, a higher glucose content was observed in WS-PS because in that dough amylases were not inhibited by acidification, resulting in the generation of WS-PS from insoluble starch during fermentation. A significantly higher glucose content was observed in dough fermented with the glucan-forming strain L. reuteri TMW1.106, indicating glucan formation from sucrose by this strain. The amounts of high-molecular-weight fructan and/or glucan can be estimated to range between 0.5 (L. frumenti TMW 1.103) and 2 (L. sanfranciscensis LTH 2590) g/kg flour. An increase in the arabinose and xylose levels in WS-PS indicated a solubilization of arabinoxylans during fermentation in all doughs. The content of arabinoxylans in WS-PS was not appreciably affected by the fermentation of EPS-positive or EPS-negative lactobacilli compared to the aseptic control dough.
TABLE 2.
Characterization of wheat sourdoughs fermented with EPS-positive and EPS-negative lactobacilli and monosaccharide compositions of WS-PS extracted from these doughsa
| Strain used for sourdough fermentation | t (h) | pH | Cell count (log CFU g−1) | Composition (mmol kg−1)
|
|||
|---|---|---|---|---|---|---|---|
| Glucose | Xylose | Arabinose | Fructose | ||||
| All doughs | 0 | 6.07 ± 0.03 | 6.80 ± 0.80b | 0.70 ± 0.04 | 4.85 ± 0.05 | 4.78 ± 0.10 | 0.10 ± 0.01 |
| Control dough (with antibiotics) | 24 | 6.05 ± 0.16 | 3.98 ± 0.28 | 2.87 ± 0.33 | 15.97 ± 0.72 | 12.90 ± 1.03 | 0.13 ± 0.02 |
| L. sanfranciscensis LTH2581 | 24 | 3.49 ± 0.23 | 9.11 ± 0.21 | 0.37 ± 0.20 | 15.80 ± 0.55 | 11.05 ± 0.67 | 0.18 ± 0.09 |
| L. sanfranciscensis LTH2590 | 24 | 3.79 ± 0.07 | 8.86 ± 0.18 | 0.44 ± 0.23 | 12.37 ± 0.05 | 10.04 ± 0.21 | 6.08 ± 1.00c |
| L. frumenti TMW 1.103 | 24 | 3.42 ± 0.14 | 9.57 ± 0.09 | 0.81 ± 0.30 | 12.70 ± 0.08 | 12.05 ± 1.24 | 1.11 ± 0.30c |
| L. frumenti TMW 1.660 | 24 | 3.47 ± 0.04 | 8.63 ± 0.37 | 0.76 ± 0.51 | 14.49 ± 1.11 | 13.60 ± 0.46 | 3.28 ± 0.00c |
| L. pontis TMW 1.675 | 24 | 3.40 ± 0.07 | 9.10 ± 0.16 | 0.64 ± 0.33 | 13.04 ± 0.23 | 12.06 ± 1.19 | 1.39 ± 0.09c |
| L. frumenti TMW 1.669 | 24 | 3.44 ± 0.04 | 9.36 ± 0.13 | 1.49 ± 0.50 | 13.87 ± 1.18 | 12.84 ± 0.30 | 2.33 ± 0.11c |
| L. reuteri TMW 1.106 | 24 | 3.52 ± 0.06 | 9.38 ± 0.23 | 3.58 ± 0.89c | 13.12 ± 1.05 | 12.95 ± 0.74 | 0.57 ± 0.03 |
Data are means ± standard deviations of two independent experiments. The aseptic control dough and sourdoughs inoculated with L. sanfranciscensis LTH2581 and LTH2590 were incubated at 30°C; other doughs were incubated at 37°C.
The cell count in control dough at zero hour was 2.50 ± 0.50.
The amount of high-molecular-weight fructan and/or glucan can be estimated by comparison of fructose or glucose levels in WS-PS from control doughs (EPS-negative strain and aseptic control) to levels of fructose or glucose in WS-PS from doughs fermented with EPS-positive strains: LTH 2590, 2 g of fructan/kg of flour; TMW 1.103, 0.3 g of fructan/kg of flour; TMW 1.660, 1.0 g of fructan/kg of flour; TMW 1.675, TMW 1.669, and TMW 1.106, 0.4, 0.7, and 1.0 g of EPS/kg of flour, respectively.
DISCUSSION
The microflora of traditionally prepared sourdoughs typically consists of two to five strains in a single dough, rarely more. As ∼20% of the strains screened were found to produce EPS from sucrose, it is likely that any given sourdough contains EPS-producing lactobacilli. The frequencies of fructan- or glucan-positive strains were highest in the phylogenetically closely related species L. reuteri, L. frumenti, L. panis, and L. pontis originating from type II sourdoughs or the intestinal tract. Van Geel-Schutten et al. (42) identified two strains of L. reuteri among 182 lactobacilli of various origins as potent producers of EPS. Outside of the genus Lactobacillus, glucan and fructan formation have been found in Leuconostoc spp. and oral streptococci. Strains of species predominating in type II sourdough fermentations are frequently found in the intestinal tracts of mammals and birds (21, 24, 38, 46). In particular, virtually all species of lactobacilli detected in pig intestines are also recognized as organisms predominating in type II sourdough fermentations (1, 24, 36, 45), although evidence for the occurrence of L. pontis and L. panis in pig intestines is based on culture-independent techniques only. Remarkably, the frequency of EPS-forming strains of lactobacilli is highest in intestinal isolates and species typical of intestinal microbiotae. It is tempting to speculate that glucan and fructan formation are physiological properties with relevance for the growth and survival of lactobacilli in intestinal environments.
Glucans and fructans are formed from sucrose by the activity of a single enzyme, i.e., glucan- or fructansucrase (28). Two fructosyltransferases from L. reuteri strain 121 were recently characterized (43, 44). One enzyme was characterized as levansucrase based on homology searches using partial amino acid sequences, as well as the structural characterization of the polymer formed (43). Generally, bacterial levansucrases exhibit fructosyltransferase activities resulting in the formation of the inulin-type fructooligosaccharides kestose and nystose (5, 10). Accordingly, kestose and nystose production were observed in L. reuteri strain 121, in which levansucrase is the only fructosyltransferase which is transcribed and active (43, 44). The second fructosyltransferase gene in L. reuteri 121, a silent gene, exhibits high homology to bacterial levansucrases. The gene product exhibited fructosyltransferase activity upon heterologous expression and was tentatively called inulosucrase based on the formation of kestose and nystose (44). An 800-bp sequence could be amplified in this work from fructan-forming lactobacilli by PCR targeting levansucrases of lactic acid bacteria, and database searches revealed high similarity to known levansucrase genes. These levansucrase genes were present in only 6 of the 15 fructan-forming strains and in none of the fructan-forming L. reuteri strains. The fructan of the Lev PCR-positive strain L. sanfranciscensis LTH2590 (equivalent to TMW 1.392) was previously characterized by enzymatic digestion and reported to be of the levan type (8). An internal fragment of the putative levansucrase gene was amplified from cDNA libraries of L. sanfranciscensis LTH2590 and TMW 1.53, indicating levansucrase expression in these strains (this study). Taken together, these results suggest that a levansucrase is responsible for fructan formation in L. sanfranciscensis strain LTH2590, and they may indicate that the EPS produced by other Lev PCR-positive strains is of the levan type. Because out of 35 strains only fructan-forming lactobacilli were detected by Lev PCR, this PCR is a useful tool for rapid screening of lactobacilli for EPS formation.
L. reuteri 121 contains genes for two fructansucrases and one glucansucrase, exhibits glucansucrase and levansucrase activities, and produces two types of EPS, glucan and levan, depending on environmental conditions (20, 41, 43, 44). L. sanfranciscensis LTH1729 was reported to produce levan (8) but was Lev PCR negative (this study). Furthermore, eight additional strains were described in this study that produce a fructan(s) not characterized on the structural level but that are Lev PCR negative. Therefore, it can be anticipated that a further characterization of genes responsible for glucan and fructan formation in lactic acid bacteria would provide new types of glycosyltransferases.
Korakli et al. (18) have shown by the use of 13C-labeled sucrose the formation of up to 3.6 g of high-molecular-weight fructan per kg of flour by L. sanfranciscensis LTH2590 in wheat and rye sourdoughs. Fructan formation in dough was detected by a higher 13C content of WS-PS in the dough and a 10-fold-higher content of fructose in WS-PS upon fermentation than in control doughs. By the use of the same methodology, it could be demonstrated that those strains producing EPS in mMRS-sucrose also formed EPS during sourdough fermentation in the presence of sucrose. The amounts of high-molecular-weight fructan and/or glucan can be estimated to range between 0.3 and 2 g pf EPS/kg of flour based on the glucose and fructose levels in WS-PS. These amounts are comparable to the fructan levels previously reported (18) and are higher than the levels of heteroexpolysaccharides produced by dairy lactic acid bacteria during growth in milk (10 to 200 mg per liter of milk) (22). Hydrocolloids, such as xanthan or modified cellulose, significantly affect dough rheology and bread texture at levels of 0.1 to 1% of the flour base (6, 34), and levan formed by L. sanfranciscensis LTH2590 was shown to affect the rheological properties of wheat doughs at a level of 0.1% of the flour base (4). Therefore, the amounts of EPS formed during sourdough fermentation can be assumed to be technologically relevant.
Bacterial levansucrases that have been characterized usually have invertase activity in addition to levansucrase activity (15). Because fructose is used as an electron acceptor by heterofermentative lactobacilli from sourdough, resulting in concomitant production of acetate instead of ethanol (37), sourdough fermentation by fructan-forming strains in the presence of sucrose results in higher acetate contents in the dough (18). The acetate formed affects sensorial qualities and improves the shelf life of the bread. During fermentation of wheat doughs with 12% sucrose and fructan-forming L. sanfranciscensis, >50% of the sucrose was metabolized during fermentation (18). Because sourdough preferments are generally included in wheat bread formulas at levels below 30%, 12% sucrose addition at the preferment stage results in sucrose levels at or below 2% of the flour base in the bread dough. The addition of 2% sucrose is commonly used in wheat bread formulas.
EPS from lactic acid bacteria may influence the intestinal flora, because oligofructose and fructans of the levan and inulin types are known to selectively stimulate the growth of bifidobacteria (3, 8, 26). Possible health benefits achieved through stimulation of the growth and metabolism of bifidobacteria by dietary oligofructose or fructans have been proposed (11, 23, 27, 33, 39, 48). In this study, it was shown that two-thirds of EPS-producing strains form fructan. The levan produced by L. sanfranciscensis LTH2590 is metabolized by bifidobacteria (19) and selectively stimulated the growth of bifidobacteria during cultivation of human fecal microflorae in vitro (8). However, wheat and rye flours contain ∼6.6 and 8.5% arabinoxylans, 1.4 and 2% β-glucans, and 1 and 4% fructans, respectively. Therefore, it remains to be established whether fructans produced during sourdough fermentation exert an additional effect on the composition and activity of the intestinal microflora and human health.
In conclusion, it was shown that the production of EPS from sucrose is a metabolic activity that is widespread among sourdough lactic acid bacteria. Lev PCR produced false-negative, but not false-positive, results and thus allows rapid screening of isolates based on fructan formation. EPS-positive strains formed technologically relevant amounts of EPS during sourdough fermentation. These results will allow the deliberate use of EPS-forming lactobacilli in bread production to achieve the replacement of additives currently used in bread production by glucans or fructans formed in situ. The links between type II sourdough and intestinal microflorae on the levels of species composition and EPS production may prove to be helpful for the further development of pre- and probiotic concepts.
Acknowledgments
We thank Ernst Böcker GmbH, Minden, Germany, for financial support. Markus Tieking acknowledges the support of a grant from the Fleischmann Fonds, Wissenschaftszentrum Weihenstephan, TU München, Germany.
REFERENCES
- 1.Böcker, G., P. Stolz, and W. P. Hammes. 1995. Neue Erkenntnisse zum Ökosystem Sauerteig und zur Physiologie der sauerteigtypischen Stämme Lactobacillus sanfrancisco und Lactobacillus pontis. Getreide Mehl Brot 49:370-374. [Google Scholar]
- 2.Böcker, G., R. F. Vogel, and W. P. Hammes. 1990. Lactobacillus sanfrancisco als stabiles Element in einem Reinzucht-Sauerteig-Präparat. Getreide Mehl Brot 44:269-274. [Google Scholar]
- 3.Bouhnik, Y. K., K. Vahedi, L. Achour, A. Attar, J. Salfati, P. Pochart, P. Marteau, B. Floure, F. Bornet, and J. C. Rambaud. 1999. Short-chain fructo-oligosaccharide administration dose-dependently increases fecal Bifidobacteria in healthy humans. J. Nutr. 129:113-116. [DOI] [PubMed] [Google Scholar]
- 4.Brandt, M. J. 2001. Mikrobiologische Wechselwirkungen von technologischer Bedeutung in Sauerteigen. Dissertation, Universität Hohenheim, Stuttgart, Germany.
- 5.Crittenden, R. G., and H. W. Doelle. 1993. Structural identification of oligosaccharides produced by Zymomonas mobilis levansucrase. Biotechnol. Lett. 15:1055-1060. [Google Scholar]
- 6.Collar, C., P. Andreu, J. C. Martinez, and E. Armero. 1999. Optimization of hydrocolloid addition to improve wheat bread dough functionality: a response surface methodology study. Food Hydrocolloids 13:467-475. [Google Scholar]
- 7.Corsetti, A., M. Gobbetti, B. de Marco, F. Belestrieri, F. Paoletti, L. Russi, and J. Rossi. 2000. Combined effect of sourdough lactic acid bacteria and additives on bread firmness and staling. J. Agric. Food Chem. 48:3044-3051. [DOI] [PubMed] [Google Scholar]
- 8.Dal Bello, F., J. Walter, C. Hertel, and W. P. Hammes. 2001. In vitro study of prebiotic properties of levan-type exopolysaccharides from lactobacilli and non-digestible carbohydrates using denaturing gradient gel electrophoresis. Syst. Appl. Microbiol. 24:1-6. [DOI] [PubMed] [Google Scholar]
- 9.De Vuijst, L., and B. Degeest. 1999. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol. Rev. 23:153-177. [DOI] [PubMed] [Google Scholar]
- 10.Euzenat, O., A. Guibert, and D. Combes. 1997. Production of fructo-oligosaccharides by levansucrase from Bacillus subtilis C4. Process Biochem. 32:237-243. [Google Scholar]
- 11.Fordaliso, M., N. Kok, J.-P. Desayer, F. Goethals, D. Deboyser, M. Roberfroid, and N. Delzenne. 1995. Dietary oligofructose lowers triglycerides, phospholipids and cholesterol in serum and very low density lipoproteins of rats. Lipids 30:163-167. [DOI] [PubMed] [Google Scholar]
- 12.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 13.Hammes, W. P., and M. G. Gänzle. 1997. Sourdough breads and related products, p. 199-216. In B. J. B. Wood (ed.), Microbiology of fermented food. Chapman and Hall, London, United Kingdom.
- 14.Hammes, W. P., P. Stolz, and M. G. Gänzle. 1996. Metabolism of lactobacilli in traditional sourdoughs. Adv. Food Sci. 18:176-184. [Google Scholar]
- 15.Han, Y. W. 1990. Microbial levan. Adv. Appl. Microbiol. 35:171-194. [DOI] [PubMed] [Google Scholar]
- 16.Kline, L., and T. F. Sugihara. 1971. Microorganisms of the San Francisco sour dough bread process. II. Isolation and characterization of undescribed bacterial species responsible for the souring activity. Appl. Microbiol. 21:459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korakli, M., E. Schwarz, G. Wolf, and W. P. Hammes. 2000. Production of mannitol by Lactobacillus sanfranciscensis. Adv. Food Sci. 22:1-4. [Google Scholar]
- 18.Korakli, M., A. Rossmann, M. Gänzle, and R. Vogel. 2001. Sucrose metabolism and exopolysaccharide production in wheat and rye sourdoughs by Lactobacillus sanfranciscensis. J. Agric. Food Chem. 49:5194-5200. [DOI] [PubMed] [Google Scholar]
- 19.Korakli, M., M. G. Gänzle, and R. F. Vogel. 2002. Metabolism by Bifidobacteria and lactic acid bacteria of polysaccharides from wheat and rye and exopolysaccharides produced by Lactobacillus sanfranciscensis. J. Appl. Microbiol. 92:1-8. [DOI] [PubMed] [Google Scholar]
- 20.Kralj, S., G. H. van Geel-Schutten, H. Rahaoui, R. J. Leer, E. J. Faber, M. H. E. C. van der Maarel, and L. Dijkhuizen. 2002. Molecular characterization of a novel glucosyltransferase from Lactobacillus reuteri strain 121 synthesizing a unique, highly branched glucan with α-(1-4) and α-(1-6) glucosidic bonds. Appl. Environ. Microbiol. 68:4283-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurzak, P., M. A. Ehrmann, and R. F. Vogel. 1998. Diversity of lactic acid bacteria associated with ducks. Syst. Appl. Microbiol. 21:588-592. [DOI] [PubMed] [Google Scholar]
- 22.Laws, A. P., and V. M. Marshall. 2001. The relevance of exopolysaccharides to the rheological properties in milk fermented with ropy strains of lactic acid bacteria. Int. Dairy. J. 11:709-721. [Google Scholar]
- 23.Le Blay, G., C. Michel, H. M. Blottiere, and C. Cherhut. 1999. Prolonged intake of fructose-oligosaccharides induces a short-term elevation of lactic acid-producing bacteria and a persistent increase in cecal butyrate in rats. J. Nutr. 129:2231-2235. [DOI] [PubMed] [Google Scholar]
- 24.Leser, T. D., J. Z. Amenuvor, T. K. Jensen, R. H. Lindecrona, M. Boye, and K. Møller. 2002. Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl. Environ. Microbiol. 68:673-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewington, J., S. D. Greenaway, and B. J. Spillane. 1987. Rapid small scale preparations of bacterial genomic DNA, suitable for cloning and hybridization analysis. Lett. Appl. Microbiol. 5:51-53. [Google Scholar]
- 26.Marx, S. P., S. Winkler, and W. Hartmeier. 2000. Metabolization of β-(2,6)-linked fructose-oligosaccharides by different bifidobacteria. FEMS Microbiol. Lett. 182:163-169. [DOI] [PubMed] [Google Scholar]
- 27.Molis, C., B. Flourie, F. Ouarne, M. F. Gailing, S. Lartigue, A. Guibert, F. Bornet, and J. P. Galmiche. 1996. Digestion, excretion, and energy value of fructooligosaccharides in healthy humans. Am. J. Clin. Nutr. 64:324-328. [DOI] [PubMed] [Google Scholar]
- 28.Monchois, V., R.-M. Willemot, and P. Monsan. 1999. Glucansucrases: mechanism of action and structure-function relationships. FEMS Microbiol. Rev. 23:131-151. [DOI] [PubMed] [Google Scholar]
- 29.Müller, M. R. A., G. Wolfrum, P. Stolz, M. A. Ehrmann, and R. F. Vogel. 2001. Monitoring the growth of lactobacillus species during a rye flour fermentation. Food Microbiol. 18:217-227. [Google Scholar]
- 30.Müller, M. R. A., M. A. Ehrmann, and R. F. Vogel. 2000. Multiplex PCR for the detection of Lactobacillus pontis and two related species in a sourdough fermentation. Appl. Environ. Microbiol. 66:2113-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller, M. R. A., M. A. Ehrmann, and R. F. Vogel. 2000. Lactobacillus frumenti sp. nov., a new lactic acid bacterium isolated from rye-bran fermentations with a long fermentation period. Int. J. Syst. Evol. Microbiol. 50:2127-2133. [DOI] [PubMed] [Google Scholar]
- 32.Oliveras-Illana, V., C. Wacher-Rodarte, S. Le Borgne, and A. López-Munguia. 2002. Characterization of a cell-associated inulosucrase from a novel source: a Leuconostoc citreum strain isolated from Pozol, a fermented corn beverage of Mayan origin. J. Ind. Microbiol. Biotechnol. 28:112-117. [DOI] [PubMed] [Google Scholar]
- 33.Roberfroid, M. B. 1996. Functional effects of food components and the gastrointestinal system: chicory fructooligosaccharides. Nutr. Rev. 54:38S-42S. [DOI] [PubMed]
- 34.Rosell, C. M., J. A: Rojas, and C. Benedito de Barber. 2001. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocolloids 15:75-81. [Google Scholar]
- 35.Rosenquist, H., and A. Hansen. 1998. The antimicrobial effect of organic acids, sour dough and nisin against Bacillus subtilis and B. licheniformis isolated from wheat bread. J. Appl. Microbiol. 85:621-631. [Google Scholar]
- 36.Simpson, J. M., V. J. McCracken, H. R. Gaskins, and R. I. Mackie. 2000. Denaturing gradient gel electrophoresis analysis of 16S ribosomal DNA amplicons to monitor changes in fecal bacterial populations of weaning pigs after introduction of Lactobacillus reuteri strain MM53. Appl. Environ. Microbiol. 66:4705-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stolz, P., G. Böcker, W. P. Hammes, and R. F. Vogel. 1995. Utilization of electron acceptors by lactobacilli isolated from sourdough. I. Lactobacillus sanfrancisco. Z. Lebensm. Unters. Forsch. 201:91-96. [Google Scholar]
- 38.Tannock, G. W., K. Munro, H. J. M. Harmsen, G. W. Welling, J. Smart, and P. K. Gopal. 2000. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl. Environ. Microbiol. 66:2578-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taper, H. S., and M. Roberfroid. 1999. Influence of inulin and oligofructose on breast cancer and tumor growth. J. Nutr. 129:1488S-1491S. [DOI] [PubMed]
- 40.Thiele, C., M. G. Gänzle, and R. F. Vogel. 2002. Contribution of sourdough lactobacilli, yeast, and cereal enzymes to the generation of amino acids in dough relevant for bread flavor. Cereal Chem. 79:45-51. [Google Scholar]
- 41.van Geel-Schutten, G. H., E. J. Faber, E. Smit, K. Bonting, M. R. Smith, B. Ten Brink, J. P. Kamerling, J. F. G. Vliegenthart, and L. Dijkhuizen. 1999. Biochemical and structural characterization of the glucan and fructan exopolysaccharides synthesized by the Lactobacillus reuteri wild-type strain and by mutant strains. Appl. Environ. Microbiol. 65:3008-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Geel-Schutten, G. H., F. Flesch, B. ten Brink, M. R. Smith, and L. Dijkhuizen. 1998. Screening and characterization of Lactobacillus strains producing large amounts of exopolysaccharides; Appl. Microbiol. Biotechnol. 50:697-703. [Google Scholar]
- 43.van Hijum, S., K. Bonting, M. van der Maarel, and L. Dijkhuizen. 2001. Purification of a novel fructosyltransferase from Lactobacillus reuteri strain 121 and characterization of the levan produced. FEMS Microbiol. Lett. 205:323-328. [DOI] [PubMed] [Google Scholar]
- 44.van Hijum, S. A. F. T., G. H. van Geel-Schutten, H. Rahaoui, M. J. E. C. van der Maarel, and L. Dijkhuizen. 2002. Characterization of a novel fructosyltransferase from Lactobacillus reuteri that synthesizes high-molecular-weight inulin and inulin oligosaccharides. Appl. Environ. Microbiol. 68:4390-4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogel, R. F., R. Knorr, M. R. A. Müller, U. Steudel, M. G. Gänzle, and M. A. Ehrmann. 1999. Non-dairy lactic fermentations: the cereal world. Antonie Leeuwenhoek 76:403-411. [PubMed] [Google Scholar]
- 46.Walter, J., C. Hertel, G. W. Tannock, C. M. Lis, K. Munro, and W. P. Hammes. 2001. Detection of Lactobacillus, Peciococcus, Leuconostoc, and Weisella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67:2578-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiese, B. G., W. Strohmar, F. A. Rainey, and H. Diekmann. 1996. Lactobacillus panis sp. nov., from sourdough with a long fermentation period. Int. J. Syst. Bacteriol. 46:449-453. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto, Y., Y. Takahashi, M. Kawano, M. Iizuka, T. Matsumoto, S. Saeki, and H. Yamaguchi. 1999. In vitro digestibility and fermentability of levan and its hypocholesterolomic effects in rats. J. Nutr. Biochem. 10:13-18. [DOI] [PubMed] [Google Scholar]