Abstract
A post-proline endopeptidase (PepO2) was detected in cell extracts from a genomic library of Lactobacillus helveticus CNRZ32 by using the synthetic substrate N-acetyl-β-casein-(f203-209)-ρ-nitroanilide in a coupled reaction with aminopeptidase N. Isolates with activity for this substrate contained plasmids with visually indistinguishable restriction profiles. Nucleotide sequence analysis revealed a 1,947-bp open reading frame, designated pepO2, encoding a putative 71.4-kDa protein. Analysis of the predicted peptide sequence revealed that L. helveticus PepO2 contained the zinc-dependent metalloprotease motif HEXXH and exhibited levels of amino acid sequence similarity of 72, 61, 59, and 53% to L. helveticus PepO, Lactococcus lactis PepO2, L. lactis PepO, and Lactobacillus rhamnosus PepO, respectively. Northern hybridization results indicated that the transcript containing pepO2 was monocistronic. Despite the high degrees of amino acid similarity to PepO proteins from other lactic acid bacteria, the specificity of the L. helveticus PepO2 for post-proline bonds distinguishes it from other PepO-type endopeptidases characterized to date. The specificity for post-proline bonds also suggests that this enzyme may play a central role in the hydrolysis of casein-derived bitter peptides, such as β-casein(f193-209).
The proteolytic systems of dairy lactic acid bacteria (LAB) have received extensive research attention due to their importance in the physiology of these organisms and in cheese flavor development. LAB are fastidious microorganisms with multiple amino acid auxotrophies (18). During growth in milk, LAB rely on their proteolytic systems to obtain essential amino acids from caseins (CNs), the most abundant proteins in milk (9, 17). Additionally, proteolytic enzymes from LAB produce flavor compounds and precursors that are essential for cheese flavor development (9, 23).
The proteolytic systems of LAB can be functionally divided into three components: (i) cell envelope-associated proteinases which hydrolyze CNs to oligopeptides; (ii) peptide transport systems, of which the oligopeptide transport system is the most important in milk and cheese; and (iii) numerous intracellular peptidases (9, 17). The intracellular peptidases of LAB include both endopeptidases and aminopeptidases. Endopeptidases, due to their ability to hydrolyze peptide bonds within a peptide, are of particular interest in targeting peptides for rapid hydrolysis. In Lactococcus lactis, the best-characterized LAB, the endopeptidases that have been identified include PepO, PepO2, PepF1, and PepF2. All of these enzymes are metalloproteases, and PepO, PepF1, and PepF2 are encoded in operons (9, 17). The physiological roles of these endopeptidases remain unclear; however, PepF appears to be important for protein turnover during nitrogen starvation (24). To date, one metalloendopeptidase, designated PepO (8), and a thiol-dependent endopeptidase, designated PepE (13), have been characterized from Lactobacillus helveticus.
The ability of L. helveticus CNRZ32 to accelerate cheese ripening and reduce bitterness when it is used as an adjunct culture is well documented (2, 3, 21). While numerous enzymes of the proteolytic system of L. helveticus have been identified (9), our understanding of the specific enzymes responsible for this strain's ability to reduce the bitterness in cheese is incomplete. The peptide β-CN(f193-209), which is produced by the activity of chymosin on β-CN, has been implicated in the development of bitterness in cheese (4, 20). The purpose of this study and another study (10) was to identify and characterize an L. helveticus endopeptidase(s) involved in the hydrolysis of β-CN(f193-209). Additionally, the accumulation of αs1-CN(f1-9) has been associated with bitterness (4, 5, 15); therefore, the hydrolysis of this peptide was also examined.
MATERIALS AND METHODS
Bacterial strains, plasmid, and media.
L. helveticus CNRZ32 (16) and its derivatives were grown in MRS broth (Difco Laboratories, Detroit, Mich.) (12) at 37°C. L. lactis LM0230 was obtained from L. L. McKay (University of Minnesota, St. Paul) and was propagated at 30°C in M17-glucose broth (Difco Laboratories) (30). Escherichia coli DH5α (Gibco-BRL Life Technologies Inc., Gaithersburg, Md.) and derivatives of this strain were grown in Luria-Bertani broth (27) at 37°C with aeration. Agar plates were prepared by adding 1.5% (wt/vol) granulated agar (Difco Laboratories) to liquid media. Erythromycin (Sigma Chemical Co., St. Louis, Mo.) was added to liquid media or agar plates at a concentration of 500 μg/ml to select for pJDC9 (7) in E. coli.
Screening of L. helveticus CNRZ32 genomic library.
A previously constructed genomic library of L. helveticus CNRZ32 in E. coli DH5α (25) was screened for endopeptidase activity by using an amino-terminal blocked chromogenic substrate, N-acetyl-β-CN(f203-209)-ρ-nitroanilide [N-acetyl-β-CN(f203-209)-ρNA] (SynPep Co., Dublin, Calif.); this substrate is based on the C-terminal amino acid sequence of Bos taurus β-CN. Pooled cultures (10 isolates/pool) were grown overnight in Luria-Bertani broth containing erythromycin. Cells were pelleted by centrifugation at 13,000 × g for 1 min at room temperature, washed, and suspended in 10 mM bis(2-hydroxyethyl)imino-Tris (pH 6.5; Sigma). Cell extracts (CFEs) were obtained from E. coli cultures by vortexing samples with glass beads alternating with cooling on ice (1 min each); this procedure was repeated twice, and the cell debris was removed by centrifugation for 1 min at 13,000 × g. CFEs obtained from mid-log-phase cultures of L. helveticus CNRZ32 and E. coli DH5α(pJDC9) were used as positive and negative controls, respectively. The presence of endopeptidase activity was determined by adding 100 μl of CFE to 395 μl of 10 mM Bis-Tris (pH 6.5) containing 1 mM N-acetyl-β-CN(f203-209)-ρNA. The appearance of an intense yellow color (resulting from release of ρNA) within 15 min was considered an indication of endopeptidase activity. In the coupled reaction, 20 μl of CFE from E. coli DH5α containing aminopeptidase N activity (pJDC9::pepN) was used. All assays were performed in duplicate.
Plasmid isolation and cloning.
Plasmid isolation from E. coli was performed as described by Sambrook et al. (27). Restriction enzymes and T4 DNA ligase were purchased from Gibco-BRL and were used as recommended by the manufacturer. Electroporation of E. coli was performed by using a Gene Pulser (Bio-Rad Laboratories, Richmond, Calif.) as recommended by the manufacturer.
DNA sequencing and sequence analysis.
All primers were synthesized by GIBCO-BRL Custom Primers (Grand Island, N.Y.). PCR and DNA sequencing reactions were performed with a Perkin-Elmer model 480 thermal cycler (Perkin-Elmer Corp., Norwalk, Conn.). DNA sequencing reactions were performed by using a Prism Ready Reaction DyeDeoxy terminator cycle sequencing kit (Applied Biosystems, Inc., Foster City, Calif.). DNA templates were purified with a Qiagen Inc. (Hilden, Germany) PCR purification kit. Sequencing was initially performed with primers M13 and M13R (GIBCO-BRL). As the known sequenced progressed, new primers were designed accordingly. Additional primers were designed by using the Affinity program supplied by Ransom Hill Bioscience, Inc. (Ramona, Calif.). DNA sequences were determined by the Nucleic Acid and Protein Facility of the University of Wisconsin-Madison Biotechnology Center with an ABI model 370/3 automated sequencer. Sequences were analyzed by using the GCG sequence analysis package (Genetics Computer Group, Inc., Madison, Wis.). Protein homology searches were performed by using the BLAST network service (1). All reported DNA sequence data were confirmed by sequencing both DNA strands from at least two independent PCR products.
mRNA analysis.
Transcription of the pepO2 gene was investigated by using an 810-bp internal pepO2 fragment (nucleotides 607 to 1416) that was amplified and the PCR product end labeled with digoxigenin (Genius system; Boehringer Mannheim GmbH, Mannheim, Germany) for Northern hybridization. The primers used for probe amplification were YC-2290 (5′GATGCGATTGCACTCG) and YC-2000 (5′GATAGCGGCAGGGAAG). Total RNA was isolated by using an RNeasy kit (Qiagen). RNA molecular weight markers, solutions, and reagents used for Northern hybridization and chemiluminescent detection were purchased from Boehringer Mannheim. Northern hybridization was performed by using the procedure recommended by the manufacturer. Mapping of the 5′ end of the pepO2 transcript was accomplished by using a kit for 5′ end rapid amplification of cDNA (5′RACE) (version 2.0; GIBCO-BRL). The gene-specific primers used for 5′RACE were YC-2340 (5′GTTTTCGGTTTGCTTTTG), YC-2600 (5′CGGCATCTCTTTTGGC), and YC-2840 (5′GGACGATCGGCAGGG). First-strand cDNA synthesis was performed with primer YC-2340. Nested amplification of first-strand cDNA was carried out with primer YC-2600 and the anchor primer supplied with the 5′RACE kit. Sequencing reactions were conducted with primer YC-2840 by using the nested amplification product as the template.
Synthesis of peptide substrates.
The peptides αS1-CN(f1-9) and β-CN(f193-209) were synthesized at the Utah State University Biotechnology Center. The synthesized peptides were subsequently purified by collection of appropriate fractions after preparatory reverse-phase high-performance liquid chromatography (RP-HPLC). The peptides were analyzed by mass spectrometry, and the identities were confirmed by Edman degradation with an Applied Biosystems model 477B protein sequencer. The peptides were lyophilized and stored at −80°C. Stock solutions were prepared in sterile double-distilled water (ddH2O) and also stored at −80°C.
Peptide hydrolysis reactions.
Peptide hydrolysis reactions were performed essentially as described in the accompanying paper (10). A 10-μl aliquot of CFE (0.95 to 1.05 mg of protein per ml) was diluted in 500 μl (total reaction volume) of 0.1 M Bis-Tris buffer (pH 6.5). The reactions were initiated by adding the substrate, and the reaction mixtures were incubated at 37°C for a minimum of 30 min. The initial substrate concentration in the reaction samples was 0.2 μg/μl for both β-CN(f193-209) and αs1-CN(f1-9). Reactions were stopped by immediately freezing preparations at −20°C.
Peptide separation and identification.
Samples were injected into a 20-μl loop by using a Gilson model 231 sample injector equipped with a model 401 dilutor module containing a ddH2O-acetonitrile wash solution (1:1; Gilson Medical Electronics, Paris, France). The peptides were separated by using a Phenomenex Columbus C18 column (250 by 2 mm; 5μ; 100 Å; Phenomenex Columbus, Torrance, Calif.) preceded by a Brownlee RP-18 precolumn. The mobile phase flow rate and gradient were controlled with a Hitachi L-6200A pump (Hitachi Instruments, San Jose, Calif.). Mobile phases were continuously degassed by slow helium sparging. Peptides were detected with a Hitachi L-4500A diode array detector in the low-absorbance mode. Data was collected by using the Hitachi Chromatography Data Station software with a wavelength range of 200 to 300 nm, a 4-nm spectral bandwidth, and a 3,200-ms spectral interval.
Mobile phase A consisted of ddH2O-MeCN (99:1) with 0.1% trifluoroacetic acid, and mobile phase B consisted of ddH2O-MeCN (20:80) with 0.05% trifluoroacetic acid. Separation and elution of αS1-CN(f1-9) hydrolysis samples were accomplished with the following gradient: 1 to 16% mobile phase B from 0 to 20 min at a rate of 0.25 ml/min, 90% mobile phase B from 20 to 22 min at a rate of 0.25 ml/min, and 90 to 1% mobile phase B from 22 to 25 min at a rate of 0.25 ml/min. Separation and elution of β-CN(f193-209) hydrolysis samples were accomplished with the following gradient: 4 to 60% mobile phase B from 0 to 40 min at a rate of 0.25 ml/min, 60 to 98% mobile phase B from 40 to 41 min at a rate of 0.25 ml/min, 98% mobile phase B from 41 to 45 min at a rate of 0.25 to 0.50 ml/min, and 98 to 4% mobile phase B from 45 to 47 min at a rate of 0.50 to 0.25 ml/min. The pump back pressure was ∼1400 lb/in2 at zero time and remained below 1,600 lb/in2 for the duration of the gradients. Samples being separated for fraction collection were monitored in real time. Fractions were collected manually, taking into account a predetermined time for the peptide to travel from the detector flow cell to the capture point.
The masses of RP-HPLC-separated peptide fractions were determined by using a triple quadrupole mass spectrometer (Micromass Quattro II) with electrospray ionization sources at the Utah State University Biotechnology Center. To identify the hydrolysis products, the masses were compared to calculated molecular masses of peptides and/or amino acids derived from β-CN(f193-209) and αs1-CN(f1-9).
Nucleotide sequence accession number.
The nucleotide sequence of pepO2 has been deposited in the GenBank database under accession no. AF321529.
RESULTS
Screening of the genomic library.
Before the L. helveticus genomic library was screened, a number of preliminary tests were conducted. CFEs of L. helveticus CNRZ32 and E. coli DH5α were examined for endopeptidase activities capable of hydrolyzing acetyl-β-CN(f203-209)-ρNA. CFE of the L. helveticus CNRZ32 wild-type strain resulted in an intense yellow color (A410, >0.30) within 15 min, while E. coli DH5α CFEs resulted in only a very light yellow color (A410, <0.025) after 10 h. To determine if any of the previously identified L. helveticus proteolytic enzymes were required for hydrolysis of β-CN(f203-209), CFEs prepared from several peptidase mutants were examined for the ability to hydrolyze acetyl-β-CN(f203-209)-ρNA (Table 1). Aminopeptidase N (PepN) was found to be required for the release of ρNA from acetyl-β-CN(f203-209)-ρNA. However, no hydrolysis of acetyl-β-CN(f203-209)-ρNA was observed when we used CFEs prepared from E. coli DH5α expressing L. helveticus PepN (strain JLS242) (Christensen, unpublished data). Together, these results indicate that PepN is required, but is not sufficient, to release ρNA from acetyl-β-CN(f203-209)-ρNA. Therefore, the genomic library screening analysis was performed by using a coupled enzyme reaction with PepN.
TABLE 1.
Ability of L. helveticus CNRZ32 and peptidase-deficient derivatives of this strain to hydrolyze N-acetyl-β-CN(f203-209)-ρNA
| Strain | Relevant features | Activitya | Reference |
|---|---|---|---|
| CNRZ32 | Wild type | + | 16 |
| JLS232 | pepO derivative of CNRZ32 | + | 8 |
| JLS233 | pepE derivative of CNRZ32 | + | Unpublished data |
| JLS251 | prtH derivative of CNRZ32 | + | 26 |
| JLS242 | pepN derivative of CNRZ32 | − | 10 |
| JLS241 | pepC derivative of CNRZ32 | + | 10 |
| JLS243 | pepX derivative of CNRZ32 | + | 10 |
Enzyme activity was determined with 1.0 mM substrate at 37°C for 30 min. A reaction was considered positive if the A410 was more than 0.025.
A genomic library of L. helveticus CNRZ32 in E. coli DH5α was screened for endopeptidase activities with acetyl-β-CN(f203-209)-ρNA. Of the 1,880 isolates screened, 2 had activity in a coupled reaction with PepN. The restriction endonuclease profiles of these two isolates were visually indistinguishable (data not shown). One plasmid, designated pSUW99, was selected and used for further analysis.
Sequencing of the endopeptidase clone.
Restriction mapping of pSUW99 revealed a 6.0-kb insert. Two 3.0-kb SstI fragments and two PstI fragments (2.0 and 4.0 kb) were obtained when the insert was digested with restriction endonucleases SstI and PstI, respectively. Subclones containing individual SstI fragments or PstI fragments in pJDC9 were examined for endopeptidase activity with acetyl-β-CN(f203-209)-ρNA. Activity was detected only in strains containing one of the 3.0-kb SstI fragments, suggesting that the gene was present on this SstI fragment and contained a PstI site.
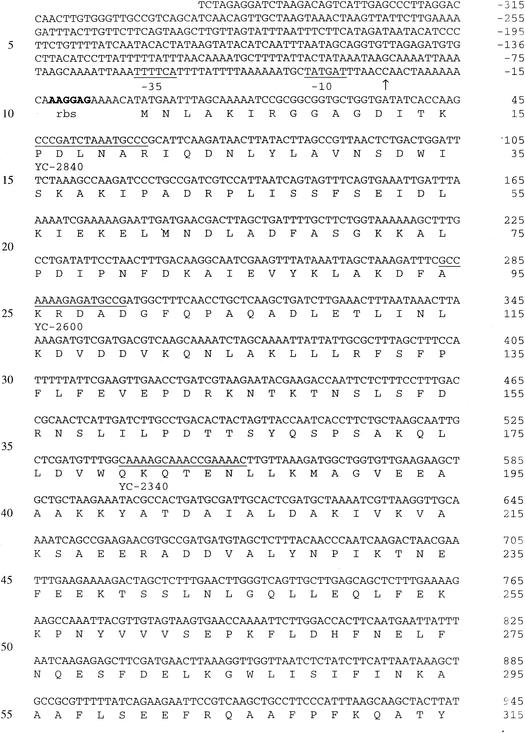
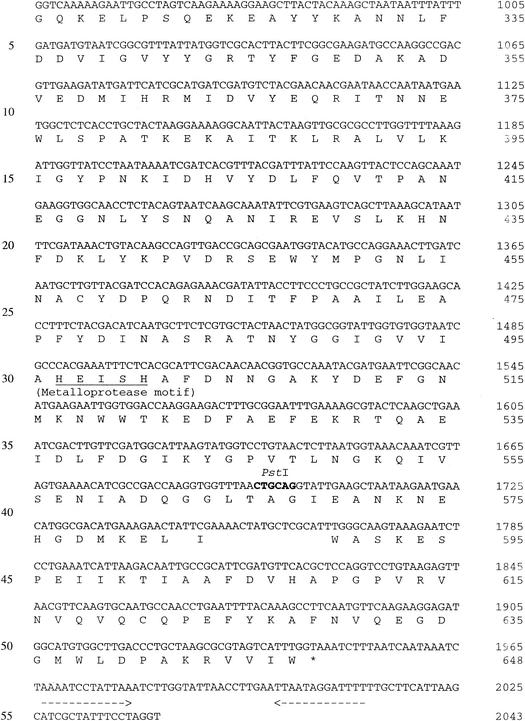
The complete nucleotide sequence of the 3.0-kb SstI fragment encoding endopeptidase activity was determined, and a 1,947-bp open reading frame (ORF) was identified (Fig. 1). This ORF could encode a 649-amino-acid polypeptide with a deduced molecular mass of 71.4 kDa. Protein sequence homology searches with current BLAST databases revealed high levels of similarity between the deduced amino acid sequence and the amino acid sequences of other LAB PepO-type endopeptidases (6, 14, 22, 32; GenBank accession no. AF179267). This protein exhibited 56% identity and 72% similarity to L. helveticus CNRZ32 endopeptidase PepO (8); therefore, the gene was designated pepO2. L. helveticus PepO2 exhibited 42% identity and 59% similarity to L. lactis PepO (22, 32), 41% identity and 61% similarity to L. lactis PepO2, 38% identity and 57% similarity to Streptococcus thermophilus PepO (6), and 36% identity and 53% similarity to Lactobacillus rhamnosus PepO (11). Significant levels of similarity to mammalian metallopeptidases, including endothelin-converting enzyme (45% similarity) and enkephalinase (neutral endopeptidase; 43% similarity), were also observed. The sequence motif His-Glu-Xxx-Xxx-His, which is characteristic of many zinc-dependent metalloproteases, was also identified in PepO2 between residues 497 and 501 (Fig. 1). The start codon of the ORF is preceded by a putative ribosome binding site (AAGGAG; nucleotides −8 to −13) and by putative promoter −10 (TATGAT; nucleotides −32 to −37) and −35 (TTTTCA; nucleotides −56 to −61) sequences (28). An inverted repeat (nucleotides 1967 to 1979 and 2000 to 2012) was observed in the 3′ noncoding region and may function as a rho-independent transcriptional terminator with a ΔG at 25°C of −21 kcal (31). No signal sequence was detected by using a hydrophilicity plot constructed as described by Kyte and Doolittle (19).
FIG. 1.
Nucleotide sequence of pepO2 from L. helveticus CNRZ32 and deduced amino acid sequence. A putative Shine-Dalgarno sequence is indicated by boldface type and labeled rbs. The −10 and −35 regions and the zinc metalloprotease motif are underlined and labeled. The two horizontal arrows indicate the putative transcriptional terminator. The 5′ end of the pepO2 mRNA is indicated by a vertical arrow. Relevant restriction endonuclease sites are indicated by boldface type and labeled. Primers used in 5′RACE are underlined and labeled with their designations.
mRNA analysis.
Northern hybridization performed with total RNA from an exponential culture of L. helveticus CNRZ32 resulted in detection of a transcript that was 2.1 kb long (data not shown). This size corresponds to the size of the pepO2 ORF and indicates that pepO2 is monocistronic. The transcriptional start site for the pepO2 promoter was mapped 26 bp upstream of the pepO2 start codon by 5′RACE (data not shown).
Substrate specificity of PepO2.
To determine if the PepO2 substrate specificity is similar to that of previously described endopeptidases from L. helveticus CNRZ32, the ability of CFE from E. coli DH5α expressing PepO2 to hydrolyze N-benzoyl-Phe-Val-Arg-ρNA, N-benzoyl-Pro-Phe-Arg-ρNA, and N-benzoyl-Val-Gly-Arg-ρNA was examined. These substrates were utilized previously to identify and differentiate PepO and PepE in a genomic library of L. helveticus constructed in E. coli DH5α (8, 13). Derivatives of E. coli DH5α expressing PepO hydrolyzed N-benzoyl-Pro-Phe-Arg-ρNA and N-benzoyl-Val-Gly-Arg-ρNA, while derivatives of E. coli DH5α expressing PepE hydrolyzed N-benzoyl-Phe-Val-Arg-ρNA and N-benzoyl-Pro-Phe-Arg-ρNA. Hydrolysis of these substrates by PepO2, with or without PepN, was not observed (data not shown). Additionally, hydrolysis of acetyl-β-CN(f203-209)-ρNA by CFEs of E. coli DH5α expressing L. helveticus PepO or PepE in coupled assays with PepN was not observed. These results indicated that PepO2 substrate specificity is distinct from PepO and PepE substrate specificity. CFEs from E. coli DH5α expressing L. helveticus PepC or PepX were also examined in a coupled reaction with PepO2 (in place of PepN); the results indicated that only the combined activity of PepN and PepO2 was capable of releasing ρNA from acetyl-β-CN(f 203-209)-ρNA.
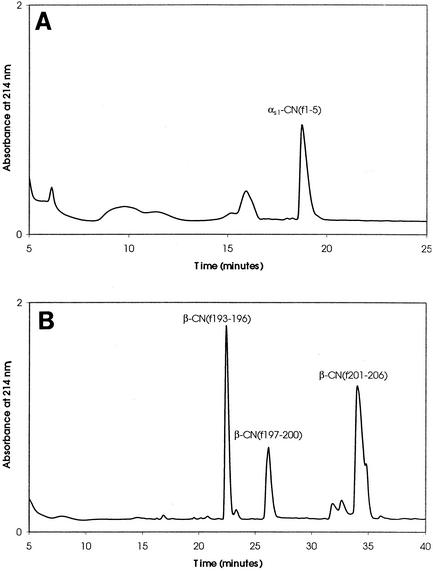
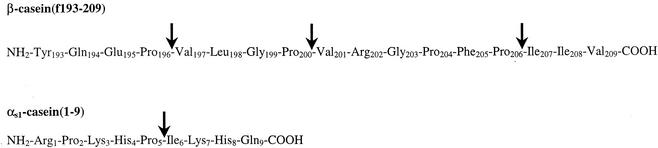
To examine hydrolysis of the model CN-derived bitter peptides β-CN(f193-209) and αs1-CN(f1-9) by PepO2, RP-HPLC was performed to separate and collect peptide hydrolysis products. No significant hydrolysis of either substrate was detected with CFEs from E. coli DH5α(pJDC9). However, significant hydrolysis of both β-CN(f193-209) and αs1-CN(f1-9) was detected with CFEs from E. coli DH5α(pSUW99) (Fig. 2). The predominant peptide fractions were collected and analyzed. PepO2 was found to hydrolyze β-CN(f193-209) at the Pro-196-Val-197, Pro-200-Val-201, and Pro-206-Ile-207 bonds. Hydrolysis of αs1-CN(f1-9) was observed at the Pro-5-Ile-6 bond (Fig. 3).
FIG. 2.
Chromatograms of peptides resulting from hydrolysis of αs1-CN(f1-9) (A) and β-CN(f193-209) (B) by E. coli DH5α(pSUW99). Hydrolysis reactions were conducted for 30 min. Major accumulated hydrolysis products are indicated.
FIG. 3.
Specificity of PepO2 for the substrates αs1-CN(f1-9) and β-CN(f193-209). The arrows indicate the bonds that were hydrolyzed by CFEs of E. coli DH5α(pSUW99) expressing PepO2 from L. helveticus, as determined by mass spectrometry and from the calculated molecular masses of peptides derived from both substrates.
DISCUSSION
Bitterness in cheese is believed to be the result of accumulation of low-molecular-weight hydrophobic peptides, such as αs1-CN(f1-9) and β-CN(f193-209) (4, 5, 20). In another study, hydrolysis of αs1-CN(f1-9) and β-CN(f193-209) by the L. helveticus CNRZ32 wild-type strain and several peptidase-deficient mutants was investigated (10). The results of that study indicated that L. helveticus contains a previously undetected endopeptidase capable of hydrolyzing β-CN(f193-209) under conditions simulating the conditions in ripening cheese. In this study, the endopeptidase was identified by screening a genomic library of L. helveticus for the ability to hydrolyze the chromogenic substrate N-acetyl-β-CN(f203-209)-ρNA. The gene identified was determined to code for a protein with the highest level of identity (55%) to PepO from L. helveticus CNRZ32, a previously described metal-dependent endopeptidase (8). Additionally, this endopeptidase exhibits 42% identity to PepO from L. lactis (22, 32), 41% identity to PepO2 from L. lactis, and 36% identity to PepO from L. rhamnosus (11). Unlike the lactococcal PepO proteins, the L. helveticus PepO proteins include cysteine residues and are translated from a monocistronic message. The presence of duplicated PepO proteins in both lactococci and lactobacilli suggests that these enzymes may have important physiological functions. However, inactivation of L. helveticus PepO did not result in any observable change in the ability to grow in milk or amino acid-containing defined media (8). Several attempts to construct a PepO2 deletion mutant derivative of L. helveticus CNRZ32 via two-step gene replacement were unsuccessful (data not shown), suggesting that this enzyme is required for viability. Additional research is required to establish the physiological roles of L. helveticus PepO and PepO2.
The substrate specificity of PepO2 was assessed with chromogenic peptide substrates and two CN-derived peptides. The inability of PepO2 to hydrolyze the chromogenic substrates used to identify PepO and PepE from L. helveticus suggested that PepO2 has distinct substrate specificity. This suggestion was supported by the ability of PepO2 to hydrolyze acetyl-β-CN(f203-209)-ρNA in conjunction with PepN, while no hydrolysis by either PepE or PepO was observed under the same conditions. The bonds hydrolyzed in αs1-CN(f1-9) and β-CN(f193-209) by PepO2 were either Pro-Val or Pro-Ile bonds, indicating that PepO2 is a post-proline endopeptidase. Hydrolysis of the β-CN(f193-209) Pro-204-Phe-205 and αs1-CN(f1-9) Pro-2-Lys-3 bonds was not observed, suggesting that PepO2 may have a preference for small uncharged amino acids on the carboxy side of the scissile bond. The hydrolysis of peptide bonds involving Pro is likely to be important in the hydrolysis of CN-derived peptides as Pro constitutes 16.7% of β-CN and 8.5% of αs1-CN amino acid residues (29). Additionally, CN-derived bitter peptides have been observed to contain relatively large amounts of Pro, and it has been proposed that the spatial structure resulting from the presence of Pro in a peptide is directly related to bitterness (20). Therefore, the specificity of PepO2 for bonds containing Pro suggests that this enzyme may have a central role in the demonstrated ability of L. helveticus CNRZ32 to reduce bitterness in cheese.
In future studies we will examine if strains overexpressing PepO2 can reduce bitterness and increase flavor development in bacterial ripened cheeses (i.e., Cheddar and Gouda). Additionally, the possible interaction between PepO2 and other components of the L. helveticus CNRZ32 proteolytic system, such as PepN, will be assessed by using combinations of strains overexpressing peptidases.
Acknowledgments
We thank the Utah State University Biotechnology Center for peptide synthesis and Marie Strickland for technical assistance.
This project was funded by Dairy Management, Inc. through the Wisconsin Center for Dairy Research and the College of Agricultural and Life Sciences at the University of Wisconsin-Madison.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Meyers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Bartels, H. J., M. E. Johnson, and N. F. Olson. 1987. Accelerated ripening of Gouda cheese. 1. Effect of heat-shocked thermophilic lactobacilli and streptococci on proteolysis and flavor development. Milchwissenschaft 42:83-88. [Google Scholar]
- 3.Bartels, H. J., M. E. Johnson, and N. F. Olson. 1987. Accelerated ripening of Gouda cheese. 1. Effect of freeze-shocked Lactobacillus helveticus on proteolysis and flavor development. Milchwissenschaft 42:139-144.
- 4.Broadbent, J. R., M. Barnes, C. Brennand, M. Strickland, K. Houck, M. E. Johnson, and J. L. Steele. 2002. Contribution of Lactococcus lactis cell envelope proteinase specificity to peptide accumulation and bitterness in reduced-fat Cheddar cheese. Appl. Environ. Microbiol. 68:1778-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broadbent, J. R., M. Strickland, B. C. Weimer, M. E. Johnson, and J. L. Steele. 1998. Peptide accumulation and bitterness in Cheddar cheese made using single-strain Lactococcus lactis starters with distinct proteinase specificities. J. Dairy Sci. 81:327-337. [Google Scholar]
- 6.Chavagnat, F., J. Meyer, and M. G. Casey. 2000. Purification, characterization, cloning and sequencing of the gene encoding oligopeptidase PepO from Streptococcus thermophilus A. FEMS Microbiol. Lett. 191:79-85. [DOI] [PubMed] [Google Scholar]
- 7.Chen, J.-D., and D. A. Morrison. 1988. Construction and properties of a new insertion vector, pJDC9, that is protected by transcriptional terminators and useful for cloning of DNA from Streptococcus pneumoniae. Gene 64:155-164. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Y.-S., and J. L. Steele. 1998. Genetic characterization and physiological role of endopeptidase O from Lactobacillus helveticus CNRZ32. Appl. Environ. Microbiol. 64:3411-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen, J. E., E. G. Dudley, J. A. Pederson, and J. L. Steele. 1999. Peptidases and amino acid catabolism in lactic acid bacteria. Antonie Leeuwenhoek 76:217-246. [PubMed] [Google Scholar]
- 10.Christensen, J. E., J. R. Broadbent, and J. L. Steele. 2003. Hydrolysis of casein derived peptides αS1-CN(f1-9) and β-CN(f193-209) by Lactobacillus helveticus peptidase deletion mutants indicates the presence of a previously undetected endopeptidase. Appl. Environ. Microbiol. 69:1283-1286. [DOI] [PMC free article] [PubMed]
- 11.Christensson, C., H. Bratt, L. J. Collins, T. Coolbear, R. Holland, M. W. Lubbers, P. W. O'Toole, and J. R. Reid. 2002. Cloning and expression of an oligopeptidase, PepO, with novel specificity from Lactobacillus rhamnosus HN001 (DR20). Appl. Environ. Microbiol. 68:254-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeMan, J., M. Rogosa, and M. E. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 13.Fenster, K. M., K. L. Parkin, and J. L. Steele. 1997. Characterization of a thiol-dependent endopeptidase from Lactobacillus helveticus CNRZ32. J. Bacteriol. 179:2529-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Froeliger, E. H., J. Oetjen, J. P. Bond, and P. Fives-Taylor. 1999. Streptococcus parasanguis pepO encodes an endopeptidase with structure and activity similar to those of enzymes that modulate peptide receptor signaling in eukaryotic cells. Infect. Immun. 67:5206-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaminogawa, S., T. R. Yan, N. Azuma, and K. Yamauchi. 1986. Identification of low molecular weight peptides in Gouda-type cheese and evidence for the formation of these peptides from 23 amino terminal residues of alpha-s1-casein by proteinases of Streptococcus cremoris H61. J. Food Sci. 51:1253-1264. [Google Scholar]
- 16.Khalid, N. M., and E. H. Marth. 1990. Purification and partial characterization of a prolyldipeptidyl aminopeptidase from Lactobacillus helveticus CNRZ32. Appl. Environ. Microbiol. 56:381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunji, E. R. S., I. Mierau, A. Hagting, B. Poolman, and W. N. Konings. 1996. The proteolytic systems of lactic acid bacteria. Antonie Leeuwenhoek 70:187-221. [DOI] [PubMed] [Google Scholar]
- 18.Kok, J., and W. M. De Vos. 1994. The proteolytic system of lactic acid bacteria, p. 169-210. In M. J. Gasson and W. M. de Vos (ed.), Genetics and biotechnology of lactic acid bacteria. Blackie Academic and Professional, Glasgow, United Kingdom.
- 19.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydrophatic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 20.Lemieux, L., and R. E. Simard. 1992. Bitter flavor in dairy products. II. A review of bitter peptides from caseins: their formation, isolation and identification, structure masking and inhibition. Lait 72:335-382. [Google Scholar]
- 21.Madkor, S. A., P. S. Tong, and M. El Soda. 2000. Ripening of cheddar cheese with added attenuated adjunct cultures of lactobacilli. J. Dairy Sci. 83:1684-1691. [DOI] [PubMed] [Google Scholar]
- 22.Mierau, I., P. S. T. Tan, A. J. Haandrikman, J. Kok, K. J. Leenhouts, W. N. Konings, and G. Venema. 1993. Cloning and sequencing of the gene for a lactococcal endopeptidase, an enzyme with sequence similarity to mammalian enkephalinase. J. Bacteriol. 175:2087-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulholland, F. 1997. Proteolytic systems of dairy lactic acid bacteria, p. 299-318. In B. A. Law (ed.), Microbiology and biochemistry of cheese and fermented milk. Blackie Academic and Professional, Glasgow, United Kingdom.
- 24.Nardi, M., P. Renault, and V. Monnet. 1997. Duplication of the pepF gene and shuffling of DNA fragments on the lactose plasmid of Lactococcus lactis. J. Bacteriol. 179:4164-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowakowski, C. M., T. K. Bhowmik, and J. L. Steele. 1993. Cloning of peptidase genes from Lactobacillus helveticus CNRZ32. Appl. Microbiol. Biotechnol. 39:204-210. [Google Scholar]
- 26.Pederson, J. A., G. J. Mileski, B. C. Weimer, and J. L. Steele. 1999. Genetic characterization of a cell envelope-associated proteinase from Lactobacillus helveticus CNRZ32. J. Bacteriol. 181:4592-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 28.Shine, J., and L. Dalgarno. 1974. The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc. Natl. Acad. Sci. USA 71:1342-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swaisgood, H. E. 1982. The chemistry of milk protein, p. 1-59. In P. F. Fox (ed.), Developments in dairy chemistry, vol. 1. Elsevier, London, United Kingdom.
- 30.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tinoco, I. J., P. N. Borer, B. Dengler, M. D. Levine, O. C. Uhlenbeck, and D. M. Crothers. 1973. Improved estimation of secondary structure in ribonucleic acids. Nature (London) New Biol. 246:40-41. [DOI] [PubMed] [Google Scholar]
- 32.Tynkkynen, S., G. Buist, E. Kunji, J. Kok, B. Poolman, G. Venema, and A. Haandrikman. 1993. Genetic and biocemical characterization of the oligopeptide transport system of Lactococcus lactis. J. Bacteriol. 175:7523-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]