Abstract
The mode of action of reutericyclin was determined with fluorescent dyes that probed the permeability of the cytoplasmic membrane by large molecules, protons, and potassium. A comparison of reutericyclin activity with those of nisin, nigericin, and valinomycin demonstrated that reutericyclin does not form pores but selectively dissipates the transmembrane proton potential.
Reutericyclin is a tetramic acid derivative produced by sourdough isolates of Lactobacillus reuteri. It is inhibitory for a broad range of gram-positive bacteria at concentrations ranging from 0.1 to 1 mg liter−1 (2, 4). Yeasts, fungi, and gram-negative bacteria are resistant to reutericyclin, and the permeability barrier of gram-negative bacteria mediates resistance to reutericyclin (2). Reutericyclin is produced at active concentrations during sourdough fermentation and has been suggested to contribute to the stable persistence of L. reuteri in industrial sourdough fermentation (3). It was the aim of this study to elucidate the mode of action of reutericyclin. Because reutericyclin is an amphiphilic molecule consisting of a hydrophilic, negatively charged head group with two hydrophobic side chains, the experiments were based on the hypothesis that the membrane is the cellular target.
Bacterial strains and growth conditions.
Lactobacillus plantarum TMW 1.460 and Lactobacillus sanfranciscensis LTH2581 were grown at 30°C in MRS medium (mMRS) modified according to Stolz et al. (8). The cultures were subcultured twice with a 1% inoculum for 24 and 16 h prior to each experiment.
Chemicals.
Reutericyclin was purified from cultures of L. reuteri LTH2584 as previously described (3). The concentration of the stock solution in propanol-water (4:1, vol/vol) was determined by high-performance liquid chromatography (3) with synthetic reutericyclin (EMC, Tübingen, Germany) used as an external standard. Stock solutions of nisin, valinomycin, and nigericin (Sigma, Deisenhofen, Germany) were prepared by dissolving the compounds to concentrations of 0.5 mg ml−1, 100 μM, and 100 μM, respectively, in 50% ethanol. Propidium iodide (PI), 5 (and 6-)-carboxyfluorescein diacetate succinimidyl ester (cFDASE), and 3,3-dipropylthiacarbocyanine [DiSC3(5)] were purchased from Molecular Probes (Eugene, Oreg.).
Determination of the MICs.
The MICs of reutericyclin and nisin for L. sanfranciscensis and L. plantarum were determined by critical-dilution assays (2) with mMRS with the pH values adjusted to 6.2 and 5.0. The MIC of reutericyclin or nisin in mMRS at pH 6.2 and 5.0 for either strain ranged from 0.1 to 1 mg liter−1.
Evaluation of pore-forming activity of reutericyclin.
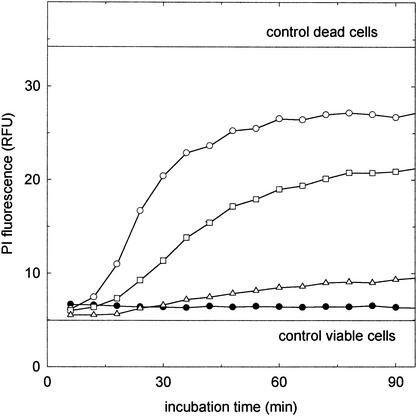
The pore-forming activity of reutericyclin was assayed by determination of the permeability of the cells to PI. Nisin served as the control. The cells were washed twice with 50 mM sodium phosphate buffer (pH 6.5) and resuspended in the same buffer containing 13 μM PI. Reutericyclin (final concentrations of 8, 17, and 33 mg liter−1) and nisin (final concentrations of 2.5, 5, and 10 mg liter−1) were added to aliquots of this suspension, and the PI fluorescence was determined at intervals over a period of 3 h at 30°C. During incubation of L. plantarum with nisin and PI, a dose-dependent increase in PI fluorescence was observed (Fig. 1), in accordance with the pore-forming activity of this antibiotic (5).The cells were not found to be permeable to PI when reutericyclin was added at levels by far in excess of the MIC. Corresponding results were obtained with L. sanfranciscensis (data not shown).
FIG. 1.
PI fluorescence of L. plantarum during incubation with nisin and reutericyclin. Control lines represent the fluorescence of viable and heat-killed (80°C, 10 min) cells. Inhibitors were added to obtain the following concentrations: 10 (○), 5 (□), and 2.5 (▵) mg of nisin liter−1 and 33 mg of reutericyclin liter−1 (•). The results are representative for two independent experiments. Corresponding results were obtained with L. sanfranciscensis (data not shown). RFU, relative fluorescence units.
Dissipation of transmembrane proton potential (ΔpH).
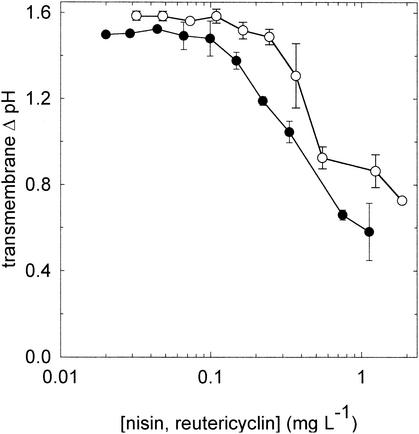
The effects of reutericyclin and nisin on the transmembrane proton potential (measured as the transmembrane pH difference [ΔpH]) of L. plantarum and L. sanfranciscensis were determined by labeling the cells with cFDASE and calibrating each batch of labeled cells as previously described (1). For experiments with L. sanfranciscensis, maltose and fructose were used in place of glucose as energy sources. cFDASE-labeled cells were resuspended in 50 mM citrate buffer (pH 5.0) containing 10 mM glucose or maltose and fructose and 0.4 mM MgSO4 and 0.3 mM MnSO4. Nisin and reutericyclin were added at concentrations ranging from 0 to 2 mg liter−1. The internal pH was determined after 15 min of incubation at 30°C. If they were added at the MIC, nisin and reutericyclin dissipated the ΔpH in cells of L. plantarum (Fig. 2) and L. sanfranciscensis (data not shown). Comparable results were obtained if the experiment was carried out at a buffer pH of 6.5 (data not shown).
FIG. 2.
Decrease of transmembrane ΔpH of L. plantarum upon incubation with reutericyclin (○) or nisin (•). The buffer pH was 5.0, and the internal pH of L. plantarum in the absence of inhibitors was 6.6 ± 0.3. The results indicate the means ± standard deviations of three independent experiments. Corresponding results were obtained with L. sanfranciscensis (data not shown).
Dissipation of transmembrane potassium potential (Δψ).
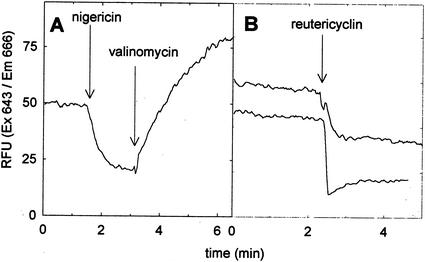
The transmembrane potassium potential was determined with the fluorescent probe DiSC3(5). Fluorescence quenching of DiSC3(5) is observed if the dye becomes concentrated in the cells as a response to a Δψ (9). Cells were washed twice with potassium phosphate buffer (50 mM, pH 6.5) containing 10 mM glucose as the energy source and then were resuspended to an optical density (at 578 nm) of 0.5. DiSC3(5) was added to a final dye concentration of 5 μM, and the cells were incubated for 10 min to equilibrate the internal and external dye concentrations. Fluorescent traces were measured in a fluorescence spectrophotometer (model LB50, Perkin-Elmer, Überlingen, Germany) at the excitation and emission wavelengths of 643 and 666 nm, respectively. The temperature was maintained at 30°C during measurements. Reutericyclin was added during the measurements, and the proton ionophore nigericin and the potassium ionophore valinomycin served as the controls. The addition of nigericin to cells of L. plantarum resulted in decreased DiSC3(5) fluorescence, corresponding to an increased Δψ as a response to the nigericin-mediated dissipation of the transmembrane ΔpH (Fig. 3A). The subsequent addition of valinomycin dissipated the transmembrane Δψ, resulting in the release of the dye from the cells and increased fluorescence. The addition of reutericyclin at levels of 2 and 8 mg liter−1 increased the Δψ to a level comparable to that observed after the addition of nigericin (Fig. 3B). The addition of nigericin after the addition of 8 mg of reutericyclin liter−1 induced no further changes in the fluorescence intensity, and the addition of valinomycin after the addition of reutericyclin increased the fluorescence intensity to those levels observed in the presence of nigericin and valinomycin (data not shown).
FIG. 3.
Fluorescent traces of DiSC3(5) in stained cells of L. plantarum (at excitation and emission wavelengths of 643 and 666 nm, respectively). (A) In a control experiment, valinomycin and nigericin were added to a final concentration of 1 μM each at time points indicated by the arrows. (B) Reutericyclin was added to a final concentration of 2 (upper trace) and 8 (lower trace) mg liter−1 at the time points indicated by the arrows. The results are representative for four independent experiments. RFU, relative fluorescence units.
The determination of reutericyclin's effects on the permeability of the cytoplasmic membrane to large molecules, protons, and potassium has shown that reutericyclin at its MIC selectively dissipates the transmembrane ΔpH. Thus, the mode of action of reutericyclin is essentially comparable to that of other weak organic acids that are widely used in food preservation, such as acetic acid and sorbic acid. The much lower MIC of reutericyclin is explained by increased partitioning into the cytoplasmic membrane due to its high hydrophobicity. Comparable to reutericyclin, the hop bitter acids isohumulone, humulone, and humulinic acid act as proton ionophores and at micromolar concentrations inhibit the growth of lactic acid bacteria in but are not inhibitory to yeasts (7). Isohumulone has a pKa of 3.1, and the undissociated acid is 100-fold more active than the corresponding salt (6). Reutericyclin is a weak acid with a pKa between 2 and 3 as determined by isoelectric focusing (M. G. Gänzle and W. P. Hammes, unpublished data). Its activity is increased at low pH (2; present study), suggesting an effect of environmental conditions on its activity comparable to those known for hop bitter acids.
In conclusion, reutericyclin acts as a proton ionophore, selectively dissipating the transmembrane ΔpH in sensitive cells. This information about the mode of action of reutericyclin will allow the assessment of mechanisms of resistance towards this compound and will facilitate the development of food or drug applications.
Acknowledgments
We acknowledge our students Micha Hoffmann, David Walerius, and Clarissa Schwab, who contributed to the work during their laboratory courses.
REFERENCES
- 1.Breeuwer, P., J.-L. Drocourt, F. M. Rombouts, and T. Abee. 1996. A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6-)-carboxyfluorescein succinimidyl ester. Appl. Environ. Microbiol. 62:178-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gänzle, M. G., A. Höltzel, J. Walter, G. Jung, and W. P. Hammes. 2000. Characterization of reutericyclin produced by Lactobacillus reuteri LTH2584. Appl. Environ. Microbiol. 66:4325-4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gänzle, M. G., and R. F. Vogel. 2002.. Contribution of reutericyclin production to the stable persistence of Lactobacillus reuteri in an industrial sourdough fermentation. Int. J. Food Microbiol. 80:31-45. [DOI] [PubMed]
- 4.Höltzel, A., M. G. Gänzle, G. J. Nicholson, W. P. Hammes, and G. Jung. 2000. The first low-molecular-weight antibiotic from lactic acid bacteria: reutericyclin, a new tetramic acid. Angew. Chem. Int. Ed. Engl. 39:2766-2768. [PubMed] [Google Scholar]
- 5.McAuliffe, O., R. P. Ross, and C. Hill. 2001. Lantibiotics: structure, biosynthesis, and mode of action. FEMS Microbiol. Rev. 25:285-308. [DOI] [PubMed] [Google Scholar]
- 6.Simpson, W. J., and A. R. W. Smith. 1992. Factors affecting antibacterial activity of hop compounds and their derivatives. J. Appl. Bacteriol. 72:327-334. [DOI] [PubMed] [Google Scholar]
- 7.Simpson, W. J. 1993. Studies on the sensitivity of lactic acid bacteria to hop bitter acids. J. Inst. Brew. 99:405-411. [Google Scholar]
- 8.Stolz, P., G. Böcker, W. P. Hammes, and R. F. Vogel. 1995. Utilization of electron acceptors by lactobacilli isolated from sourdough. I. Lactobacillus sanfranciscencis. Z. Lebensm. Unters. Forsch. 201:91-96. [Google Scholar]
- 9.Wu, M., and R. E. W. Hancock. 1999. Interaction of the cyclin actimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J. Biol. Chem. 274:29-35. [DOI] [PubMed] [Google Scholar]