Abstract
Naxos disease is a recessively inherited condition with arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) and a cutaneous phenotype, characterised by peculiar woolly hair and palmoplantar keratoderma. The disease was first described in families originating from the Greek island of Naxos. Moreover, affected families have been identified in other Aegean islands, Turkey, Israel and Saudi Arabia. A syndrome with the same cutaneous phenotype and predominantly left ventricular involvement has been described in families from India and Ecuador (Carvajal syndrome). Woolly hair appears from birth, palmoplantar keratoderma develop during the first year of life and cardiomyopathy is clinically manifested by adolescence with 100% penetrance. Patients present with syncope, sustained ventricular tachycardia or sudden death. Symptoms of right heart failure appear during the end stages of the disease. In the Carvajal variant the cardiomyopathy is clinically manifested during childhood leading more frequently to heart failure. Mutations in the genes encoding the desmosomal proteins plakoglobin and desmoplakin have been identified as the cause of Naxos disease. Defects in the linking sites of these proteins can interrupt the contiguous chain of cell adhesion, particularly under conditions of increased mechanical stress or stretch, leading to cell death, progressive loss of myocardium and fibro-fatty replacement. Implantation of an automatic cardioverter defibrillator is indicated for prevention of sudden cardiac death. Antiarrhythmic drugs are used for preventing recurrences of episodes of sustained ventricular tachycardia and classical pharmacological treatment for congestive heart failure, while heart transplantation is considered at the end stages.
Alternative names of the disease
Naxos syndrome
Associated diseases
Arrhythmogenic right ventricular dysplasia
Arrhythmogenic right ventricular cardiomyopathy
Carvajal syndrome
Woolly hair
Palmoplantar keratoderma
Definition
Naxos disease is a recessively inherited stereotype association of arrhythmogenic cardiomyopathy with a cutaneous phenotype, characterised by peculiar woolly hair and palmoplantar keratoderma [1]. Clinical and histological studies that compared Naxos disease with arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) showed that the heart disorder was identical in both diseases [2-4]. Since 1995, according to the classification of World Health Organisation, Naxos disease has been considered as the recessive form of ARVD/C [5].
Epidemiology
The disease was first described by Protonotarios et al in families originating from the Greek island of Naxos [1]. Apart from Naxos, affected families have been detected in other Greek Aegean islands, Turkey, Israel and Saudi Arabia [6-9]. The prevalence of the disease in the Greek islands may be as high as 1:1000. A variety of Naxos disease, reported as Carvajal syndrome [6], has been described in families from India and Ecuador [10,11]. It clinically presents at younger age with predominantly left ventricular involvement leading to early heart failure and exhibits a clinical phenotype similar to that of dilated cardiomyopathy [11,12].
Clinical description
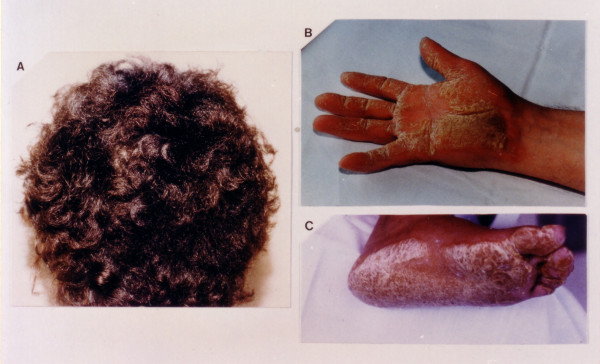
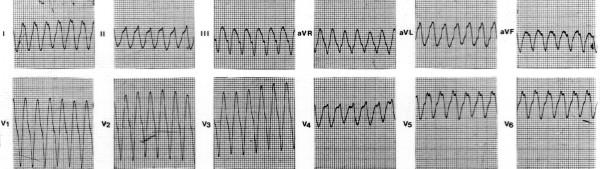
Woolly hair appears from birth, whereas palmoplantar keratoderma develop during the first year of life when infants start to use their hands and feet (Figure 1) [13]. The cardiomyopathy clinically manifests by adolescence and shows 100% penetrance [14]. The symptomatic presentation is usually with syncope and/or sustained ventricular tachycardia of left bundle branch block configuration (Figure 2). Sudden death may be the first manifestation of the disease. One third of patients become symptomatic before the thirtieth year of life. In some cases, a few clinical findings of an early heart disease can be detected during childhood.
Figure 1.
Cutaneous phenotype of Naxos disease: woolly hair (A), palmar (B) and plantar (C) keratoses.
Figure 2.
Spontaneous sustained ventricular tachycardia originating from the right ventricular posterior wall, showing left bundle branch block configuration and superior axis.
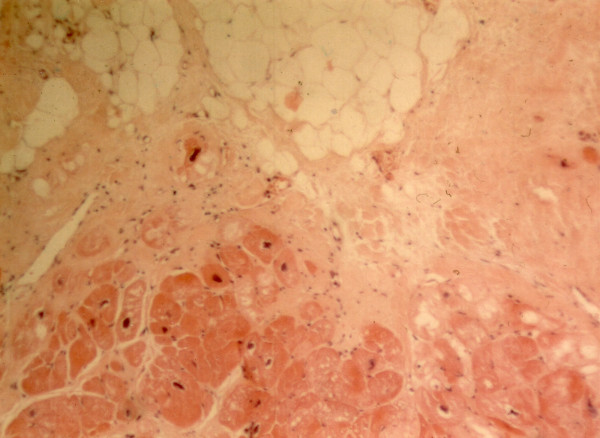
All patients exhibit repolarisation and/or depolarisation abnormalities on resting electrocardiogram and structural/functional abnormalities of the right ventricle on two-dimensional echocardiography leading to the diagnosis of ARVC according to established criteria [15]. Cardiac histology reveals the characteristic loss of right ventricular myocardium, mainly in the subepicardial and mediomural layers with fibro-fatty replacement (Figure 3) [6,14].
Figure 3.
Haematoxylin-eosin stained section from the right ventricular free wall of a patient with Naxos disease (surgical sample). There is extensive myocyte loss with fibrofatty replacement (magnification × 100).
During a mean follow-up period of 10 years, more than 50% of patients develop progressive heart disease involving the right or both ventricles [14]. The progression appears to be stepwise, associated in some cases with an arrhythmic storm or sudden death [16]. Symptoms of right heart failure appear in the final stages when the right or both ventricles are severely affected. In Carvajal syndrome the heart disease is clinically manifested earlier during childhood as dilated cardiomyopathy [10,11]. Fifty percent of patients developed heart failure and most of them died during adolescence. Cardiac pathology of a single case revealed (on gross examination) aneurysms of the outflow tract, apex and posterior wall of the right ventricle at the "triangle of dysplasia", and involvement of the left ventricle. Histology showed areas of extensive myocardial loss and replacement fibrosis, particularly in subepicardial layers, that is very similar to ARVD/C pathology although without the fatty component of replacement process [17].
Naxos ARVD/C is a rather progressive heart disease with adverse prognosis, especially in the young. The annual disease-related and sudden death mortality have been estimated at 3% and 2.3% respectively [14]. In a long-term study of an unselected population of patients with Naxos disease it was shown that risk factors for sudden death included history of syncope, the appearance of symptoms and severely progressed disease to the right ventricle before the age of 35 years, and the involvement of the left ventricle [14].
Aetiology/Genetics
A two base-pair deletion in the plakoglobin (cell adhesion protein) gene (Pk2157del2TG), which maps to17q21, has been identified as the cause of Naxos disease and provided evidence that the pathogenesis of ARVD/C might be related to a defect in myocardial mechanical coupling [13,18]. This mutation was identified in 13 families from Greece and in one family from Turkey [6]. The prevalence of heterozygous carriers is up to 5% of the Naxos population. Apart from a small minority who show woolly hair, as well as a few electrocardiographic or echocardiographic abnormalities not fulfilling the criteria for ARVD/C, heterozygotes dysplay normal phenotype [14]. In the Naxos disease variety described in families from Ecuador and Israel (Arab families), two different mutations of the desmoplakin gene (Dsp7901del1G and DspG2375R), affecting the C-terminal of the protein, have been found as causative genes [19,20].
Myocardial cells are differentiated bipolar cells mechanically and electrically coupled at intercalated discs [21]. Adherence junctions and desmosomes secure mechanical coupling, while gap junctions are involved in electrical coupling [22]. Plakoglobin (γ-catenin) and desmoplakin are intracellular proteins anchoring desmosomes to desmin intermediate filaments. Moreover, plakoglobin contributes to interlinking adherens junctions with the actin cytoskeleton, showing also signalling roles to the nucleus and to desmosome organisation [23,24]. It is also involved in mechanisms of apoptosis [25]. Defects in linking sites of these proteins can interrupt the contiguous chain of cell adhesion, particularly under conditions of increased mechanical stress or stretch, leading to cell death, progressive loss of myocardium and fibro-fatty replacement [18]. The degree of participation of fat in the repair process may be related to the rate of disease progression or may be mutation-specific [6]. Surviving myocardial fibres within fibro-fatty tissue form zones of slow conduction and provide a substrate for re-entry ventricular arrhythmias [26]. Recent studies on Naxos disease myocardium revealed that remodelling of gap junctions and altered electrical coupling might be an early result of the genetically determined defect in cell adhesion, enhancing the development of a highly arrhythmogenic substrate [27].
Treatment and prevention
The primary goal is the prevention of sudden cardiac death. Implantation of an automatic cardioverter defibrillator is indicated in patients who develop symptoms and/or structural progression, particularly before the age of 35 years [14,28,29]. Antiarrhythmic drugs are indicated for preventing recurrences of episodes of sustained ventricular tachycardia; sotalol and amiodarone, either alone or in combination with classical β-blockers, seem to be the most effective [26,30]. Patients with congestive heart failure are managed with diuretics and angiotensin-converting enzyme (ACE) inhibitors, while heart transplantation is considered at the end stages.
In an attempt to control Naxos disease, systematic genetic screening of the populations at risk has been initiated and is starting to identify the heterozygous carriers of the plakoglobin gene mutation.
A multicentre European study is under way aiming to determine the clinical, pathological and genetic characteristics of ARVD/C, validate the diagnostic criteria and define strategies for disease treatment and prevention of sudden death http://anpat.unipd.it/ARVC/[31]. One of the missions is the study of Naxos disease (participating unit: Yannis Protonotarios Medical Center, Naxos).
Acknowledgments
Acknowledgements
This study was supported by: The European Community Research project for ARVC/D (#QLG1-CT-2000001091)
Contributor Information
Nikos Protonotarios, Email: nprotnaxos@altecnet.gr.
Adalena Tsatsopoulou, Email: adalena@otenet.gr.
References
- Protonotarios N, Tsatsopoulou A, Patsourakos P, Alexopoulos D, Gezerlis P, Simitsis S, Scampardonis G. Cardiac abnormalities in familial palmoplantar keratosis. Br Heart J. 1986;56:321–326. doi: 10.1136/hrt.56.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protonotarios N, Tsatsopoulou A, Scampardonis G. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;319:175. doi: 10.1056/NEJM198807213190312. [DOI] [PubMed] [Google Scholar]
- Fontaine G, Protonotarios N, Tsatsopoulou A, Tsezana R, Fontaliran F, Frank R. Comparisons between Naxos disease and arrhythmogenic right ventricular dysplasia by electrocardiography and biopsy. Circulation. 1994;90:3233. [Google Scholar]
- Protonotarios N, Tsatsopoulou A. Arrhythmogenic right ventricular cardiomyopathy. Clinical forms of the disease. Hellenic J Cardiol. 1998;39:78–80. [Google Scholar]
- Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- Protonotarios N, Tsatsopoulou A. Naxos disease and Carvajal syndrome: cardiocutaneous disorders that highlight the pathogenesis and broaden the spectrum of arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Path. 2004;13:185–194. doi: 10.1016/j.carpath.2004.03.609. [DOI] [PubMed] [Google Scholar]
- Narin N, Akcakus M, Gunes T, Celiker A, Baykan A, Uzum K, Ferahbas A. Arrhythmogenic right ventricular cardiomyopathy (Naxos disease): report of a Turkish boy. Pacing Clin Electrophysiol. 2003;26:2326–2329. doi: 10.1111/j.1540-8159.2003.00370.x. [DOI] [PubMed] [Google Scholar]
- Djabali K, Martinez-Mir A, Horev L, Christiano AM, Zlotogorski A. Evidence for extensive locus heterogeneity in Naxos disease. J Invest Dermatol. 2002;118:557–560. doi: 10.1046/j.0022-202x.2001.01627.x. [DOI] [PubMed] [Google Scholar]
- Bukhari I, Juma'a N. Naxos disease in Saudi Arabia. J Eur Acad Dermatol Venereol. 2004;18:614–616. doi: 10.1111/j.1468-3083.2004.01010.x. [DOI] [PubMed] [Google Scholar]
- Rao BH, Reddy IS, Chandra KS. Familial occurrence of a rare combination of dilated cardiomyopathy with palmoplantar keratoderma and woolly hair. Indian Heart J. 1996;48:161–162. [PubMed] [Google Scholar]
- Carvajal-Huerta L. pidermolytic palmoplantar keratoderma with woolly hair and dilated cardiomyopathy. J Am Acad Dermatol. 1998;39:E418–421. doi: 10.1016/S0190-9622(98)70317-2. [DOI] [PubMed] [Google Scholar]
- Protonotarios N, Tsatsopoulou A, Fontaine G. Naxos disease: Keratoderma, scalp modifications, and cardiomyopathy. J Am Acad Dermatol. 2001;44:309–310. doi: 10.1067/mjd.2001.110648. [DOI] [PubMed] [Google Scholar]
- Coonar AS, Protonotarios N, Tsatsopoulou A, Needham EW, Houlston RS, Cliff S, Otter MI, Murday VA, Mattu RK, McKenna WJ. Gene for arrhythmogenic right ventricular cardiomyopathy with diffuse nonepidermolytic palmoplantar keratoderma and woolly hair (Naxos disease) maps to 17q21. Circulation. 1998;97:2049–2058. doi: 10.1161/01.cir.97.20.2049. [DOI] [PubMed] [Google Scholar]
- Protonotarios N, Tsatsopoulou A, Anastasakis A, Sevdalis E, McKoy G, Stratos K, Gatzoulis K, Tentolouris K, Spiliopoulou C, Panagiotakos D, McKenna W, Toutouzas P. Genotype-phenotype assessment in autosomal recessive arrhythmogenic right ventricular cardiomyopathy (Naxos disease) caused by a deletion in plakoglobin. J Am Coll Cardiol. 2001;38:1477–1484. doi: 10.1016/S0735-1097(01)01568-6. [DOI] [PubMed] [Google Scholar]
- McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, Camerini F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group on Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71:215–218. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protonotarios N, Tsatsopoulou A, Gatzoulis K. Arrhythmogenic right ventricular cardiomyopathy caused by a deletion in plakoglobin (Naxos disease) Card Electrophysiol Rev. 2002;6:72–80. doi: 10.1023/A:1017943323473. [DOI] [PubMed] [Google Scholar]
- Kaplan SR, Gard JJ, Carvajal-Huerta L, Ruiz-Cabezas JC, Thiene G, Saffitz JE. Structural and molecular pathology of the heart in Carvajal syndrome. Cardiovasc Pathol. 2004;13:26–32. doi: 10.1016/S1054-8807(03)00107-8. [DOI] [PubMed] [Google Scholar]
- McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, Norman M, Baboonian C, Jeffery S, McKenna WJ. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease) Lancet. 2000;355:2119–2124. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- Norgett EE, Hatsell SJ, Carvajal-Huerta L, Cabezas JC, Common J, Purkis PE, Whittock N, Leigh IM, Stevens HP, Kelsell DP. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Molec Genet. 2000;9:2761–2766. doi: 10.1093/hmg/9.18.2761. [DOI] [PubMed] [Google Scholar]
- Alcalai R, Metzger S, Rosenheck S, Meiner V, Chajek-Shaul T. A recessive mutation in desmoplakin causes arrhythmogenic right ventricular dysplasia, skin disorder, and woolly hair. J Am Coll Cardiol. 2003;42:319–327. doi: 10.1016/S0735-1097(03)00628-4. [DOI] [PubMed] [Google Scholar]
- Perriard JC, Hirschy A, Ehler E. Dilated cardiomyopathy: a disease of the intercalated disc? Trends Cardiovasc Med. 2003;13:30–38. doi: 10.1016/S1050-1738(02)00209-8. [DOI] [PubMed] [Google Scholar]
- Kanno S, Saffitz JE. The role of myocardial gap junctions in electrical conduction and arrhythmogenesis. Cardiovasc Pathol. 2001;10:169–177. doi: 10.1016/S1054-8807(01)00078-3. [DOI] [PubMed] [Google Scholar]
- Zhurinsky J, Shtutman M, Ben-Ze'ev A. Plakoglobin and β-catenin: protein interactions, regulation and biological role. J Cell Sci. 2000;113:3127–3139. doi: 10.1242/jcs.113.18.3127. [DOI] [PubMed] [Google Scholar]
- Lewis JE, Wahl JK, III, Sass KM, Jensen PJ, Johnson KR, Wheelock MJ. Cross-talk between adherens junctions and desmosomes depends on plakoglobin. J Cell Biol. 1997;136:919–934. doi: 10.1083/jcb.136.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancolini C, Sgorbissa A, Schneider C. Proteolytic processing of the adherens junctions components β-catenin and γ-catenin/plakoglobin during apoptosis. Cell Death Differ. 1998;5:1042–1050. doi: 10.1038/sj.cdd.4400443. [DOI] [PubMed] [Google Scholar]
- Fontaine G, Fontaliran F, Hebert JL, Chemla D, Zenati O, Lecarpentier Y, Frank R. Arrhythmogenic right ventricular dysplasia. Ann Rev Med. 1999;50:17–35. doi: 10.1146/annurev.med.50.1.17. [DOI] [PubMed] [Google Scholar]
- Kaplan SR, Gard JJ, Protonotarios N, Tsatsopoulou A, Spiliopoulou C, Anastasakis A, Squarcioni CP, McKenna WJ, Thiene G, Basso C, Brousse N, Fontaine G, Saffitz JE. Remodeling of myocyte gap junctions in arrhythmogenic right ventricular cardiomyopathy due to a deletion in plakoglobin (Naxos disease) Heart Rhythm. 2004;1:3–11. doi: 10.1016/j.hrthm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Gatzoulis K, Protonotarios N, Anastasakis A, Tsatsopoulou A, Vlasseros J, Gialafos J, Toutouzas P. Implantable defibrillator therapy in Naxos disease. Pacing Clin Electrophysiol. 2000;23:1176–1178. doi: 10.1111/j.1540-8159.2000.tb00922.x. [DOI] [PubMed] [Google Scholar]
- Corrado D, Leoni L, Link MS, Della Bella P, Gaita F, Curnis A, Salerno JU, Igidbashian D, Raviele A, Disertori M, Zanotto G, Verlato R, Vergara G, Delise P, Turrini P, Basso C, Naccarella F, Maddalena F, Estes NA, 3rd, Buja G, Thiene G. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003;108:3084–3091. doi: 10.1161/01.CIR.0000103130.33451.D2. [DOI] [PubMed] [Google Scholar]
- Wichter T, Borggrefe M, Haverkamp W, Chen X, Breithardt G. Efficacy of antiarrhythmic drugs in patients with arrhythmogenic right ventricular disease. Results in patients with inducible and noninducible ventricular tachycardia. Circulation. 1992;86:29–37. doi: 10.1161/01.cir.86.1.29. [DOI] [PubMed] [Google Scholar]
- Basso C, Wichter T, Danieli GA, Corrado D, Czarnowska E, Fontaine G, McKenna WJ, Nava A, Protonotarios N, Antoniades L, Wlodarska K, D'Alessi F, Thiene G. Arrhythmogenic right ventricular cardiomyopathy: clinical registry and database, evaluation of therapies, pathology registry, DNA banking. Eur Heart J. 2004;25:531–534. doi: 10.1016/j.ehj.2003.12.025. [DOI] [PubMed] [Google Scholar]