Abstract
A gene encoding a new thermostable d-stereospecific alanine amidase from the thermophile Brevibacillus borstelensis BCS-1 was cloned and sequenced. The molecular mass of the purified enzyme was estimated to be 199 kDa after gel filtration chromatography and about 30 kDa on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, indicating that the enzyme could be composed of a hexamer with identical subunits. The purified enzyme exhibited strong amidase activity towards d-amino acid-containing aromatic, aliphatic, and branched amino acid amides yet exhibited no enzyme activity towards l-amino acid amides, d-amino acid-containing peptides, and NH2-terminally protected amino acid amides. The optimum temperature and pH for the enzyme activity were 85°C and 9.0, respectively. The enzyme remained stable within a broad pH range from 7.0 to 10.0. The enzyme was inhibited by dithiothreitol, 2-mercaptoethanol, and EDTA yet was strongly activated by Co2+ and Mn2+. The kcat/Km for d-alaninamide was measured as 544.4 ± 5.5 mM−1 min−1 at 50°C with 1 mM Co2+.
d-Amino acids occur in bacterial cell wall peptidoglycan (28), mammalian cells (11), higher plants (25), and active peptides (5, 13, 14, 24) and are important materials for various pharmaceuticals, herbicides, and food additives (1). Unlike l-amino acids, almost all d-amino acids are obtained by using enzymatic methods; otherwise it is difficult to obtain a high state of optical purity and productivity (1, 22). Peptides incorporating d-amino acids exhibit stronger antimicrobial properties than peptides with l-isomers because d-isomers appear to be more stable against proteolytic digestion than l-isomers (12). These facts have already been verified by various studies on the fate of d-amino acids in peptides and proteins (21, 23).
Enzymatic biotransformations in which optically pure d-amino acids are produced from dl-amino acid racemic mixtures by d-amino acid-specific enzymes have been determined to be most feasible for the production of d-amino acids with a high optical purity and yield (1). To apply this system, many microbial d-amino acid-specific enzymes have already been screened and subjected to direct enzyme methods (22).
Although the synthesis of bioactive peptides incorporating d-amino acids instead of their l-counterparts could lead to metabolically stable and long-acting products, this has been hampered because of the need to use expensive processes that suffer from low stereoselectivity, low temperature stability, and the production of undesired by-products due to the use of an undesirable biocatalyst (1). Accordingly, thermolabile enzymes have been considered inappropriate for the harsh reaction conditions required in industrial processes. However, d-amino acid-specific enzymes have recently attracted much attention in regard to the synthesis of useful bioactive d-peptides and enantioselective synthesis of d-amino acids from dl-amino acid racemic mixtures (16, 18, 19, 22). Among these enzymes, d-aminoacylase (9, 29), d-aminopeptidase (2), and d-amino acid amidase (15, 22) are the most notable as potential enzyme catalysts. However, all of these enzymes are thermolabile and there have been no previous reports on the production of thermostable d-amino acid-specific enzymes from thermophiles. It is well known that the stability of the biocatalyst is the most important factor determining the productivity in enzymatic biotransformation. Generally, thermophilic bacteria are known to produce thermostable enzymes with a high stability relative to heat, organic solvents, pH, and chemical denaturants.
Previously, we isolated and characterized a thermostable d-stereospecific amino acid amidase named d-stereospecific methionine amidase from Brevibacillus borstelensis BCS-1 (4) and investigated its industrial applications. The present study presents the molecular cloning, sequencing, and expression of a gene encoding a new thermostable d-alanine amidase (BDA) from B. borstelensis BCS-1. In addition, the enzymatic characterization and chiral resolution of d-phenylalanine from dl-phenylalaninamide are described.
MATERIALS AND METHODS
Materials.
Restriction enzymes, T4 DNA ligase, DNA polymerase, and other DNA-modifying enzymes were purchased from Invitrogen Life Technologies, New England Biolabs, Takara, and Promega and used as recommended by the manufacturers. d-Amino acid amides, l-amino acid amides, d-amino acid esters, and d-alanine-p-nitroanilide (d-AlaPNA) were all purchased from Bachem (Bubendorf, Switzerland). d-Dipeptides and oligopeptides were purchased from Sigma (St. Louis, Mo.). Aliphatic amides were purchased from Wako Pure Chemicals (Osaka, Japan). o-Phthaldialdehyde (OPA) and N-acetyl-l-cysteine (NAC) were purchased from Sigma. Resource Q, phenyl-Superose, Mono Q HR 5/5, Mono S, and Superdex 200 HR 10/30 columns were all purchased from Amersham Pharmacia Biotech (Uppsala, Sweden).
Bacterial strains and plasmids.
Escherichia coli DH5α [F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1] and BL21 [F− ompT hsdSB (rB− mB+) gal dcm] were used as the hosts. The plasmids pUC118 BamHI/BAP and pHCE IIB (Bohan Biomedicals Co., Seoul, Korea) were used as the DNA cloning and an expression vectors, respectively. DNA manipulations were carried out by standard procedures (20).
Preparation of genomic library and screening.
The thermophile B. borstelensis BCS-1 was cultured in Luria-Bertani (LB) broth medium in a reciprocal shaking incubator (180 rpm) for 24 h at 55°C. The genomic DNA was isolated from the harvested cells by the Saito-Miura method (26) and partially digested with Sau3AI. The resulting fragments of 2 to 10 kb in size were isolated from a 0.8% agarose gel by using a miniprep DNA purification system (Promega). The size-fractionated DNAs were ligated into BamHI-cleaved and dephosphorylated plasmid pUC118 by using T4 DNA ligase. E. coli DH5α was transformed with the recombinant plasmid DNA by using the variation method of Cohen et al. (10), and ampicillin-resistant transformants were selected. Visualization of the BDA activity expressed in the transformants was done on a 5.0 mM d-AlaPNA plate containing 0.1 M Tris-HCl (pH 8.0) and 2% (wt/vol) agar. The detection of yellow color (color of p-nitroanilide formed by the action of amidase) around a colony indicated a positive clone.
Construction of thermostable BDA expression plasmid pBDA.
The open reading frame (ORF) of the cloned BDA gene was amplified from the recombinant plasmid pDAP by PCR (27). An upstream primer (5′-AAAGTTTATATTAGTGCA-3′) was designed to create a blunt-end ligation with pHCE IIB after treatment with NcoI and E. coli DNA polymerase I, while a downstream primer (5′-GGGGATCCTTAAGTTAAA-3′) (the restriction site is underlined) containing a BamHI site was designed from the terminal sequences of the proenzyme. Amplification of the DNA fragment by PCR produced a single product of approximately 0.8 kb in length. This PCR product was then digested with BamHI, and the resulting fragment was ligated onto the same restriction site of pHCE IIB. The resulting plasmid, pBDA, was transformed into E. coli BL21.
Expression and purification of recombinant BDA gene.
E. coli BL21 cells transformed with pBDA were cultured for 28 h at 37°C in 200 ml of LB medium containing 100 μg of ampicillin per ml. The cells were harvested by centrifugation, suspended in buffer A (0.1 M Tris-HCl buffer [pH 8.0] containing 0.3 mM phenylmethylsulfonyl fluoride [PMSF]), and passed through a French press twice at 12,000 lb/in2. The cell debris was removed by centrifugation at 10,000 × g for 20 min, and then the cell lysate was incubated for 30 min at 55°C. The denatured E. coli proteins were removed by centrifugation at 15,000 × g for 20 min, and the crude enzyme solution was dialyzed against buffer A. The dialyzed enzyme solution was loaded onto a Resource Q column (16-mm inner diameter [i.d.] by 30 mm; Amersham Pharmacia Biotech,) equilibrated with buffer A. The proteins were eluted with 200 ml of 0.1 M Tris-HCl (pH 8.0) by using a linear gradient of 0.0 to 1.0 M NaCl. The active fractions (20 ml) were loaded onto a phenyl-Superose column (16 [i.d.] by 300 mm; Amersham Biosciences) equilibrated with buffer B (0.1 M Tris-HCl [pH 8.0] containing 0.3 mM PMSF and 0.5 M ammonium sulfate). The enzyme was then eluted by using a linear descending gradient of 0.5 to 0 M ammonium sulfate. The active fractions (5 ml) were dialyzed and loaded onto a Mono Q HR 5/5 column (5 [i.d.] by 50 mm; Amersham Biosciences). The proteins were eluted by using a linear gradient of 0.0 to 1.0 M NaCl, and the active fractions were dialyzed against buffer A. The dialyzed enzyme solution (2 ml) was loaded onto a Mono S HR 5/5 column (5 [i.d.] by 50 mm; Amersham Biosciences), and the active fractions were eluted by using a linear gradient of 0.0 to 1.0 M NaCl and concentrated with an Amicon PM-10 ultrafiltration membrane.
BDA assay.
The enzyme activity was assayed at 55°C by measuring the production of d-amino acids liberated from the dl-amino acid amides. The assay mixture (total volume, 1 ml) contained 0.1 M Tris-HCl (pH 8.0), 5.0 mM dl-amino acid amides, and 0.5 ml of the enzyme solution. The reaction was carried out at 55°C and terminated after 20 min by boiling for 10 min.
For quantitative determination, the d-amino acids liberated from the dl-amino acid amides were assayed by reversed-phase high-pressure liquid chromatography (HPLC) on a Rexchrome S5-100-ODS column (4.6 mm by 25 cm; Regis Chemical Co.) and monitored with a fluorescence detector (excitation wavelength of 342 nm and emission wavelength of 452 nm). The reactant was analyzed with a 50 mM sodium acetate buffer (pH 6.8)-methanol linear gradient HPLC system after derivatization with OPA together with NAC (30). One unit of enzyme activity was defined as the amount of enzyme that liberated 1 μmol of d-amino acids from the d-amino acid amides and esters per min at 55°C.
Enantiomer selectivity assay.
The enantiomers of the amino acids were clearly separated after derivatization with OPA and NAC, as described above. The mobile phase consisted of 50 mM sodium acetate (pH 6.8) and methanol (90:10, vol/vol). If ee (percent) is the enantiomer excess, i.e., [(d − l)/(d + l)] × 100, then the enantiomeric ratio E can be determined as ln [1 − c(1 + ee)]/ln [1 − c(1 − ee)], where c represents the extent of the conversion and eep is the enantiomeric excess of the product fraction (8).
The enantioselectivity of the purified enzyme in dl-amino acid amides was analyzed by HPLC (Youngin, Seoul, Korea). The reaction mixture contained 0.1 M Tris-HCl (pH 8.0), 5.0 mM dl-amino acid amides, and 0.03 U of the enzyme. The reaction mixture was incubated at 55°C with shaking, and 100-μl aliquots were withdrawn and diluted 10-fold with a 20 mM HCl solution. After inactivation of the enzyme, the supernatant of the reaction mixture was subjected to HPLC.
The production of d-phenylalanine from dl-phenylalaninamide by the enzymatic conversion reaction was analyzed by HPLC or chiral thin-layer chromatography (TLC) (Merck, Darmstadt, Germany), and the TLC was developed with methanol-H2O-acetonitrile (5:5:20 [vol/vol/vol]). The plate was sprayed with a 2% (wt/vol) ninhydrin solution (in absolute ethanol), and the separated d-phenylalanine spots were visualized by drying in an oven on a control of authentic dl-phenylalanine.
Determination of molecular mass.
The molecular mass of the native enzyme was determined by gel permeation chromatography. A small amount of the purified enzyme solution (100 μl) was applied to a Superdex 200 preparative-grade column (HR 10/30; Pharmacia) and eluted with 0.1 M Tris-HCl (pH 8.0) containing 0.1 M KCl at a flow rate of 0.7 ml/min. Only a single protein peak was observed, and the corresponding fractions contained the BDA activity. To determine the molecular mass of the BDA, the column was calibrated with bovine thyroglobulin (Mr, 669,000), catalase (Mr, 232,000), aldolase (Mr, 158,000), bovine serum albumin (Mr, 67,000), chymotrypsinogen A (Mr, 25,000), and RNase A (Mr, 13,700) as reference proteins (gel filtration calibration kit; Pharmacia). The subunit molecular mass was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under denaturing conditions as described by Laemmli (17), using reference proteins (LMW electrophoresis calibration kit; Pharmacia).
Protein determination.
The protein concentrations were determined by the method of Bradford (6), using bovine serum albumin as the standard.
Effect of metal ions and inhibitors.
The enzyme solution was preincubated with a 1.0 mM concentration of metal ions and various concentrations of enzyme inhibitors for 20 min in 0.1 M Tris-HCl (pH 8.0). Twenty microliters of the incubation mixture was withdrawn, and the enzyme activity was determined as described above. The inhibitor concentrations were as follows: EDTA, 1.0 and 100 mM; 2-mercaptoethanol, 1.0 and 10.0 mM; dithiothreitol (DTT), 1.0 and 10.0 mM; and PMSF, 0.1 and 10.0 mM.
Determination of kinetic constants, pH, temperature optima, and thermostability.
The enzyme activity versus the pH was determined over a pH range of 5.5 to 11.0. A reaction buffer containing 50.0 mM 2-(N-morpholino)ethanesulfonic acid (MES) (pH 5.5 to 6.5), bis-Tris (pH 6.5 to 7.5), Tris-HCl (pH 7.5 to 9.0), and 3-(cyclohexylamino)-1-propanesulfonic acid (CAPS) (pH 9.0 to 11.0) was used for the enzyme assay. The optimum temperature of the BDA reaction was determined in pH 8.0 buffers within a range of 40 to 100°C. The thermostability was determined after heat treatment of 0.1 ml of the enzyme solution (containing 1.0 mg of protein per ml) for 20 min at various temperatures between 30 and 100°C. The apparent Km values were determined at various concentrations of d-amino acid amides (1.0 to 12.0 mM), while kcat and Km were determined from the Michaelis-Menten equation. For all experiments to characterize the enzyme, preincubated enzyme solution with 1 mM Co2+ was used.
Kinetic resolution of dl-phenylalaninamide.
The kinetic resolution of dl-phenylalaninamide was performed at 50°C in a 50-ml reactor with agitation (300 rpm) and nitrogen flushing. The reaction mixture consisted of 0.2 M dl-phenylalaninamide (pH 8.0) and 70 mg of a cell extract of recombinant BDA. The pH of the reaction mixture was not controlled during the reaction.
Nucleotide sequence accession number.
The nucleotide sequence of the thermostable BDA was deposited in the GenBank nucleotide sequence database under accession no. AF441121.
RESULTS
Cloning and nucleotide sequencing of the BDA gene.
A B. borstelensis BCS-1 total DNA library was constructed with pUC118 in E. coli DH5α, and the transformants were screened for BDA activity based on the development of a yellow color, as described in Materials and Methods. Among approximately 20,000 clones, one clone developed a yellow color, indicating the functional expression of the BDA gene, and a 2.2-kb insert was sequenced. The plasmid (5.3 kb) with the 2.2-kb insert was designated pDAP and used for further characterization. Subcloning of a 792-bp BamHI fragment of pDAP in pHCE IIB resulted in plasmid pBDA, which still expressed d-AlaPNA-hydrolyzing activity. One complete 792-bp ORF (ATG initiation codon at nucleotide 948 and TAA termination codon at nucleotide 1742) was identified in the sequence, and this ORF could encode a polypeptide of 264 amino acids with a deduced molecular mass of 29,045 Da.
Sequence comparison and phylogenetic analysis.
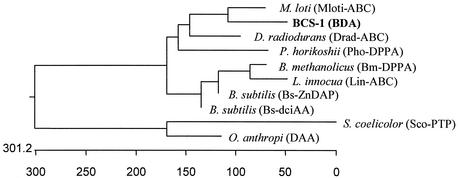
Alignment by using the GenBank database and the BLAST program showed that the deduced amino acid sequence exhibited a strong similarity to that of ABC transport proteins from various organisms and showed the highest similarity with dipeptide ABC transporter from Mesorhizobium loti (44% identical over 265 amino acids). It showed low or no sequence similarity with other d-amino acid-specific enzymes, except for the d-aminopeptidase DppA from Bacillus subtilis (7), with which it showed a relatively low sequence similarity. The linear alignment of the BDA from B. borstelensis BCS-1 and related proteins from various organisms exhibited several homologous regions (Fig. 1). A consensus maximum-parsimony tree was established from the amino acid sequences of the ABC transport proteins and d-amino acid-specific enzymes from various microorganisms (Fig. 2).
FIG. 1.
Multiple sequence alignment of B. borstelensis BCS-1 BDA and other related proteins. Highly conserved residues are in black (100% identity), and less strongly conserved residues are in gray (90% identity). Proteins: BDA, d-stereospecific alanine amidase from B. borstelensis BCS-1 (GenBank accession number AF441121); Bs-ZnDAP, Zn-dependent d-aminopeptidase DppA from B. subtilis (GenBank accession number 15825746); Bs-dciAA, dipeptide transport system from B. subtilis (GenBank accession number CAA40002); Bm-DPPA, dipeptide transport protein from Bacillus methanolicus (GenBank accession number AAB39857); Lin-ABC, dipeptide ABC transporter from Listeria innocua (GenBank accession number NP469546); Mloti-ABC, dipeptide ABC transporter from M. loti (GenBank accession number BAB52911); Drad-ABC, putative dipeptide ABC transporter from Deinococcus radiodurans (GenBank accession number NP295566); Sco-PTP, putative transport-associated protein from Streptomyces coelicolor A3 (GenBank accession number CAA22734); Pho-DPPA, hypothetical dipeptide transport protein dppA from Pyrococcus horikoshii (GenBank accession number BAA30694).
FIG. 2.
Phenogram of consensus maximum-parsimony tree of BDA and related proteins obtained by using the PHYLIP package. Protein DAA is a d-stereospecific amino acid amidase from O. anthropi (GenBank accession number BAA94704), and others are the same as in Fig. 1.
Overproduction and purification of BDA in E. coli BL21.
The amplified BDA products (792 bp in length) were ligated into pHCE IIB after treatment with NcoI, followed by Klenow and BamHI. The constructed recombinant expression vector was designated pBDA and transformed into E. coli BL21, and thereafter the BDA gene was expressed under the control of the high-level constitutive expression (HCE) promoter. When E. coli BL21 cells harboring pBDA were cultured in LB broth at 37°C without any inducer, the transformant exhibited potent BDA activity (23.5 U/mg), and its enzyme activity increased about 160-fold compared with that of the wild-type B. borstelensis BCS-1. The molecular mass of the expressed protein matched the value (29,045 Da) calculated from the amino acid sequence deduced from the gene sequence. The BDA from B. borstelensis BCS-1 remained in the soluble fraction after incubation at 55°C for 30 min. The BDA protein was purified to greater than 90% purity by using ion-exchange and hydrophobic interaction chromatographies (Table 1).
TABLE 1.
Summary of purification of BDA
| Step | Total protein (mg · ml−1) | Total activity (U · ml−1) | Sp act (U · mg−1) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Crude | 288.0 | 5,652.0 | 19.6 | 100.0 | 1.0 |
| Heat treatment | 166.7 | 4,295.8 | 25.8 | 76.0 | 1.3 |
| Resouce Q | 44.7 | 3,734.3 | 83.5 | 66.1 | 4.3 |
| Phenyl Superose | 13.3 | 1,433.8 | 108.1 | 25.4 | 5.5 |
Characteristics of BDA from E. coli BL21.
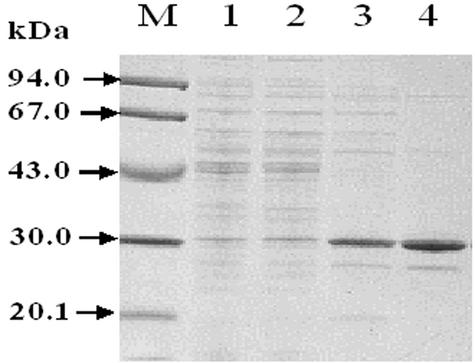
The BDA gene was cloned and expressed, and, based on its enzyme characteristics, the protein was found to be a new BDA. In addition, since B. borstelensis BCS-1 is a thermophilic bacterium, it also exhibited thermostability, as expected. A crude extract of recombinant E. coli was treated at 55°C for 30 min to denature most of the E. coli proteins. The supernatant was then subjected to purification by chromatography on Resource Q, phenyl-Superose, and Mono Q columns. Table 1 shows a summary of the purification of the recombinant BDA. Approximately 22 mg of thermostable BDA was purified from 1 liter of culture. As shown in Fig. 3, the molecular mass of the enzyme was estimated to be 30 kDa by SDS-PAGE. This value was comparable to the theoretical value (29,045 Da) calculated from the predicted amino acid sequence of the BDA.
FIG. 3.
SDS-PAGE of BDA from B. borstelensis BCS-1 at different stages of purification. The proteins were analyzed by SDS-12% PAGE. Lane M, molecular mass markers; lane 1, crude extract of E. coli BL21; lane 2, soluble fraction of the crude extract after heat treatment for 30 min at 55°C; lane 3, after Resource Q column chromatography; lane 4, after phenyl-Superose column chromatography.
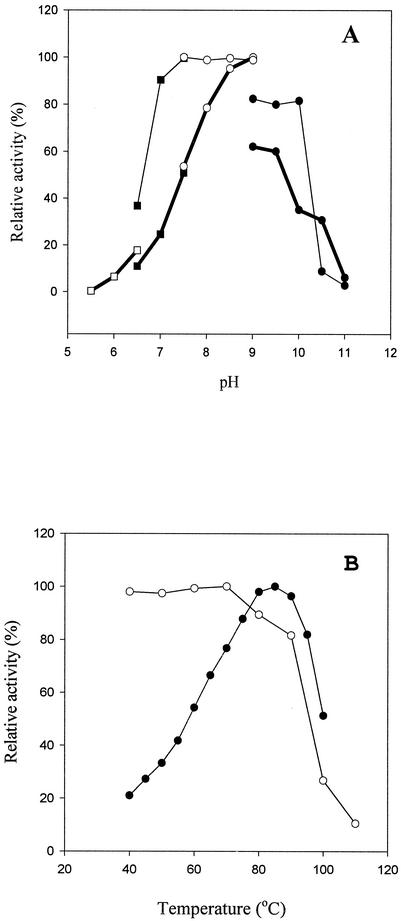
The purified enzyme remained stable between pH 7.0 and 10.0 at 55°C during 30 min of incubation and exhibited about 50% of its original activity at pH 10.0. The pH optimum was determined to be 9.0 (Tris-HCl buffer) under the same buffer conditions (Fig. 4A). When the BDA was tested at various temperatures, it exhibited maximum activity at 85°C (Fig. 4B) and thermostability at 70°C. None of these characteristics has been detected in any other microbial d-amino acid-specific enzymes. The enzyme retained approximately 80% of its activity when it was incubated at 90°C for 30 min and about 10% of its original activity after being heated for 30 min at 100°C. The enzyme was inhibited by DTT, EDTA, and mercaptoethanol. When the reactivation of the EDTA-inactivated enzyme was carried out with several divalent metal ions, Co2+ and Mn2+ reactivated the enzyme activity most strongly. When Co2+ was added to the reaction mixture at a final concentration of 1.0 mM, the specific activity increased about sevenfold compared with the control samples (data not shown). To investigate the substrate preference, the purified enzyme was used to hydrolyze d-amino acid amides, esters, and l-amino acid amides, and then the enzyme activity was assayed. The enzyme was highly active towards d-alaninamide, d-leucinamide, d-norleucinamide, d-norvalinamide, d-phenylalaninamide, d-phenylglycinamide, d-tyrosinamide, d-valinamide, d-lysinamide, and d-tryptophanamide, whereas most l-amino acid-containing amides, N-terminally protected amides, and esters were not hydrolyzed by the enzyme. Besides these l-amino acid amides, aliphatic amino acid amides, such as acetamide, n-butylamide, propionamide, and benzamide, were also determined to be inert substrates (data not shown). The stereoselectivity of the purified enzyme was examined by studying the hydrolysis of dl-phenylalaninamide. The enzyme exhibited the optical purity of d-phenylalanine, while the EEp (enantiomeric excess of the product) [(d − l)/(d + l) × 100] and E (enantiomeric ratio) [(kcat/Km)d/(kcat/Km)l] were 99.0 and 592, respectively. The kcat and Km for d-alaninamide were determined to be 7,946 ± 217 min−1 and 14.5 ± 0.2 mM, respectively, and the catalytic efficiency (kcat/Km) of the enzyme for d-alaninamide was determined to be 544.4 ± 5.5 min−1 mM−1 in the presence of Co2+.
FIG. 4.
Effects of temperature and pH on BDA activity of B. borstelensis BCS-1. (A) Optimum pH (thick lines) and stability (thin lines); (B) optimum temperature and thermostability. Symbols: □, MES; ▪, bis-Tris; ○, Tris-HCl; •, CAPS buffer. The maximum relative activity is indicated as 100%. Each experiment was performed in duplicate.
Kinetic resolution of dl-phenylalaninamide.
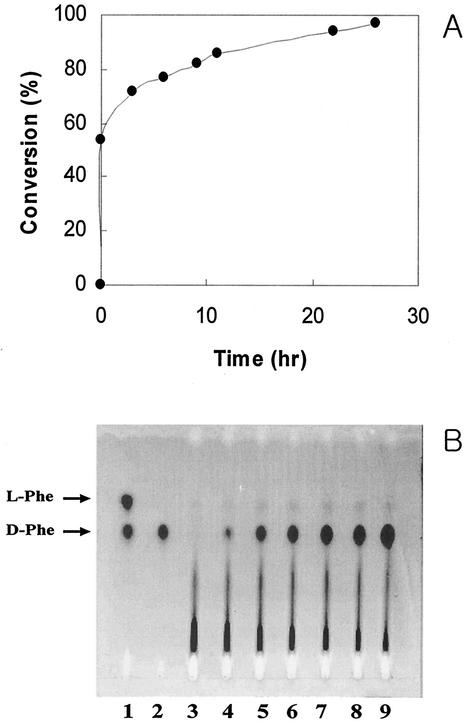
To study the enantioselective synthesis of d-amino acids, the kinetic resolution of dl-phenylalaninamide by the thermostable BDA was confirmed under high-temperature reaction conditions. As shown in Fig. 5, the BDA from B. borstelensis BCS-1 exhibited only BDA activity even though the reaction exceeded several hours. After 11 h, the conversion yield of d-phenylalanine from dl-phenylalaninamide reached 86%, while 97% of the substrate was converted after 26 h and only l-phenylalaninamide remained in the reaction mixture. In these experiments, only d-phenylalanine was produced, as detected by HPLC (Fig. 5A) and chiral TLC (Fig. 5B), and its optical purity was more than 99.0% enantiomeric excess.
FIG. 5.
Chiral resolution of dl-phenylalanine. Direct enantiomeric resolution of d-phenylalanine from dl-phenylalaninamide was achieved by HPLC (A) and TLC (B). (A) Determination of the dl-amino acids by HPLC was monitored with a fluorescence detector (excitation at 342 nm and emission at 452 nm). The reactant was resolve with a 50 mM sodium acetate (pH 6.8)-methanol linear gradient system after derivatization with OPA. (B) dl-Isomers were developed with methanol-H2O-acetonitrile (5:5:20 [vol/vol/vol]). The amino acid spots were detected with ninhydrin (0.2% in ethanol). Reactant samples were withdrawn at various times. Lanes 1 and 2, authentic dl-Phe and d-Phe, respectively; lane 3, after 0 min; lane 4, after 5 min; lane 5, after 3 h; lane 6, after 6 h; lane 7, after 9 h; lane 8, after 12 h; lane 9, after 24 h.
DISCUSSION
In the present study the gene encoding the thermostable BDA from B. borstelensis BCS-1 was cloned and its nucleotide sequence was determined. The purification and characterization of the recombinant BDA and kinetic studies were also performed. Thus, this is the first report on the gene cloning of a new thermostable BDA from the thermophilic bacterium B. borstelensis BCS-1. The deduced amino acid sequence exhibited no significant similarity with sequences of other d-amino acid-specific enzymes or related proteins yet exhibited relatively high sequence similarities with many bacterial dipeptide ABC transport proteins, and the highest sequence identity (44%) was with the dipeptide ABC transporter from M. loti. The characteristics of the enzyme, such as its molecular mass and subunit structure, were generally similar to those of the d-methionine amidase (4) purified from the parental strain, B. borstelensis BCS-1, except for the optimum pH, thermostability, and substrate preference. In the parent strain, the d-methionine amidase remained stable for 20 min at 65°C yet the recombinant BDA showed a higher temperature stability (Fig. 4B). A comparison of the substrate preferences of the parent d-methionine amidase and the recombinant d-alanine amidase revealed different substrate specificities. As shown in Table 2, the recombinant BDA exhibited the highest amidase activity with d-alaninamide, whereas the parent d-methionine amidase showed the maximum substrate preference with d-methioninamide, although no amidase activity was detected in aliphatic amino acid amides.
TABLE 2.
Substrate preference of purified BDA on d-amino acid amides
| Substrate | Relative activity (%)a |
|---|---|
| d-Alaninamide | 100 |
| d-Leucinamide | 38 |
| d-Norleucinamide | 22 |
| d-Norvalinamide | 19 |
| d-Phenylalaninamide | 17.2 |
| d-Phenylglycinamide | 17 |
| d-Tyrosinamide | 16 |
| d-Valinamide | 15.2 |
| d-Lysinamide | 15 |
| d-Tryptophanamide | 13 |
| d-Glutaminamide | 10 |
| d-Asparaginamide | 7.3 |
| d-Methioninamide | 6.3 |
| d-Prolinamide | 0.4 |
| d-Aspartic acid amide | 0.2 |
| Z-d-alanine amideb | 0.0 |
| Z-d-alanine ester | 0.0 |
| l-Phenylalaninamide | 0.0 |
| l-Leucinamide | 0.0 |
| l-Alaninamide | 0.0 |
| l-Aspartic acid amide | 0.0 |
The activity for d-alaninamide, corresponding 166.7 U/mg, was taken as 100%.
Z, benzyloxycarbonyl.
The d-stereospecific amino acid amidase (DaaA) from Ochrobactrum anthropi is composed of 363 amino acid residues (molecular mass, 40,082 Da) with a monomeric structure, and its deduced amino acid sequence showed homology to the alkaline d-peptidase and dd-peptidase (3). DaaA exhibited maximal activity at 45°C and pH 9.0, while the enzyme activity was completely inactivated in the presence of Zn2+.
In contrast, as shown in Table 2, the recombinant BDA had a molecular mass of 30 kDa, and its most favorable substrate was determined to be d-alaninamide. The optimum temperature and pH of the BDA were 85°C and 9.0, respectively. The BDA activity was strongly inhibited by EDTA, DTT, and 2-mercaptoethanol yet was restored and strongly activated by the addition of Co2+ and Mn2+ after chelation. This suggests that cysteine is an important amino acid residue for the enzyme activity, while conformational changes in the BDA due to sulfhydryl reducing agents resulted in the deactivation of the enzyme.
Cheggour et al. (7) reported that the DppA of B. subtilis exhibited d-aminopeptidase activity towards d-Ala-d-Ala and d-Ala-Gly-Gly and behaved as an octamer composed of identical 30 kDa subunits. They also proposed that the N-terminal conserved region of the amino acid residues (X-D-X-E-X) was involved in the interaction with Zn2+ ions; however, the BDA had no metal ion effect on Zn2+. In particular, DppA showed no amidase activity and was further characterized to be a Zn2+-dependent enzyme, which exhibited a broad pH optimum extending from 9.0 to 11.0.
The characteristics of the BDA, including its sensitivity to EDTA, optimal pH, high temperature optimum, high thermostability, and activity against various substrates containing N-terminal d-amino acids, identify it as a new thermostable enzyme. Studies on the kinetic resolution of dl-phenylalaninamide by the BDA from B. borstelensis BCS-1 clearly demonstrated that this strain is suitable for the enzymatic production of d-phenylalanine from dl-phenylalaninamide. The production yield of d-phenylalanine was very high and efficient. Furthermore, the optical purity of the d-phenylalanine was also satisfactory for an industrial manufacturing process for d-phenylalanine without any other racemase activity. Accordingly, the BDA from B. borstelensis BCS-1 appears to be a new thermostable d-stereospecific amino acid amidase that may be a useful enzyme biocatalyst for the production of various d-amino acids from dl-amino acid amides, esters, and arylamides under high-temperature reaction conditions.
Acknowledgments
We thank Y. Asano (Toyama Prefectural University) for his presentation on synthetic peptide amides.
This work was supported by National Research Laboratory (NRL) program grant NLM0020224 from the Ministry of Science and Technology of Korea.
REFERENCES
- 1.Asano, Y., and T. L. Lübbehüsen. 2000. Enzymes acting on peptides containing d-amino acid. J. Biosci. Bioeng. 89:295-306. [DOI] [PubMed] [Google Scholar]
- 2.Asano, Y., Y. Kato, A. Yamada, and K. Kondo. 1992. Structural similarity of d-aminopeptidase to carboxypeptidase DD and β-lactamase. Biochemistry 31:2316-2328. [DOI] [PubMed] [Google Scholar]
- 3.Baek, D. H., S. J. Kwon, J. S. Park, S. G. Lee, T. A. Mheen, and M. H. Sung. 1999. Discovery of d-stereospecific dipeptidase from thermophilic Bacillus sp. BCS-1 and its application for synthesis of d-amino acid-containing peptide. J. Microbiol. Biotechnol. 9:646-649. [Google Scholar]
- 4.Baek, D. H., S. J. Kwon, S. G. Lee, M. S. Kwak, S. P. Hong, K. H. Yoon, and M. H. Sung. June 2000. A new thermophilic bacterium Brevibacillus borstelensis BCS-1 and a thermostable d-stereospecific amino acid amidase produced therefrom. PCT/KR00/00673. World Intellectual Property Association, Geneva, Switzerland.
- 5.Bajusz, S., M. Kovacs, M. Gazdag, L. Bokser, T. Karashima, V. J. Csernus, and T. Janaky. 1988. Highly potent antagonists of luteinizing hormone-releasing hormone free of edematogenic effects. Proc. Natl. Acad. Sci. USA 85:1637-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Cheggour, A., L. Fanuel, C. Duez, B. Joris, F. Bouillenne, B. Devreese, G. Van Driessche, J. Van Beeumen, J. M. Frere, and C. Goffin. 2000. The dppA gene of Bacillus subtilis encodes a new d-aminopeptidase. Mol. Microbiol. 38:504-513. [DOI] [PubMed] [Google Scholar]
- 8.Chen, C. S., Y. Fujimoto, G. Girdaukas, and C. J. Sih. 1982. Quantitative analysis of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 104:7294-7299. [Google Scholar]
- 9.Cheng, H. P., S. H. Wu, and K. T. Wang. 1994. d-Aminoacylase from Alcaligenes faecalis possesses novel activities on d-methionine. Bioorg. Med. Chem. 2:1-5. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, S. N., A. C. Y. Chang, and L. Hsu. 1972. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of E. coli by R-factor DNA. Proc. Natl. Acad. Sci. USA 69:2110-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher, G. H., A. D. Aniello, A. Vetere, L. Padula, G. P. Cusano, and E. H. Man. 1991. Free d-aspartate in normal and Alzheimer brain. Brain Res. Bull. 26:983-985. [DOI] [PubMed] [Google Scholar]
- 12.Friedman, M. 1999. Chemistry, nutrition, and microbiology of d-amino acids. J. Agric. Food Chem. 47:3457-3479. [DOI] [PubMed] [Google Scholar]
- 13.Gill, I., L.-F. R. X. Jorba, and E. N. Vulfson. 1996. Biologically active peptides and enzymatic approaches to their production. Enzyme Microb. Technol. 18:162-183. [DOI] [PubMed] [Google Scholar]
- 14.Janusz, J. M., J. M. Gardlik, P. A. Young, R. V. Burkes, S. J. Stoll, A. F. Estelle, and C. M. Riley. 1990. High potency dipeptide sweeteners. 1. l-Asp-d-phenylglycine esters. J. Med. Chem. 33:1052-1061. [DOI] [PubMed] [Google Scholar]
- 15.Komeda, H., and Y. Asano. 2000. Gene cloning, nucleotide sequencing, and purification and characterization of the d-stereospecific amino-acid amidase from Ochrobactrum anthropi SV3. Eur. J. Biochem. 267:2028-2035. [DOI] [PubMed] [Google Scholar]
- 16.Kuramitsu, H. K., and J. E. Snoke. 1962. The biosynthesis of d-amino acids in Bacillus licheniformis. Biochim. Biophys. Acta 62:114-121. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 18.Makoto, Y., and A. Ozaki. 1998. Industrial biotransformations for the production of d-amino acids. J. Mol. Catal. B 4:1-11. [Google Scholar]
- 19.Makoto, Y. A. Ozaki, and Y. Hashimoto. 1993. Enzymatic production of d-glutamate from l-glutamate by Lactobacillus brevis ATCC 8287. Biosci. Biotech. Biochem. 57:1499-1502. [Google Scholar]
- 20.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Murray, E. D., Jr., and S. Clarke. 1984. Synthetic peptide substrates for the erythrocyte protein carboxyl methyltransferase. J. Biol. Chem. 259:10722-10732. [PubMed] [Google Scholar]
- 22.Ozaki, A., K. Kawasaki, M. Yagasaki, and Y. Hashimoto. 1992. Enzymatic production of d-alanine from dl-alaninamide by novel d-alaninamide specific amide hydrolase. Biosci. Biotechnol. Biochem. 56:1980-1984. [Google Scholar]
- 23.Paquet, A., W. C. Thresher, H. E. Swaisgood, and G. L. Catignani. 1985. Synthesis and digestibility determination of some epimeric tripeptides occurring in dietary proteins. Nutr. Res. 5:891-901. [Google Scholar]
- 24.Pert, C. B., A. Pert, J. K. Chang, and B. T. W. Fong. 1976. d-Ala2-Met-enkephalineamide: a potent, long-lasting synthetic pentapeptide analgesic. Science 194:330-332. [DOI] [PubMed] [Google Scholar]
- 25.Rhobinson, T. 1976. d-Amino acids in higher plants. Life Sci. 19:1097-1102. [DOI] [PubMed] [Google Scholar]
- 26.Saito, H., and K. Miura. 1963. Preparation of transforming deoxynucleic acid by phenol treatment. Biochim. Biophys. Acta 72:619-629. [PubMed] [Google Scholar]
- 27.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed., vol. 2, p. 8.65-8.71. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Schleifer, K. H., and O. Kandler. 1972. Peptidoglycan types of bacterial cell walls and their taxonomical implications. Bacteriol. Rev. 36:407-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang, Y. B., K. M. Hsiao, H. Li, A. Tsugita, and Y. C. Tsai. 1992. Characterization of d-aminoacylase from Alcaligenes denitrificans DA181. Biosci. Biotechnol. Biochem. 56:1392-1395. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida, Y. K., M. Kobayashi, A. Yasuda, T. Fujita, H. Minakata, K. Nomoto, M. Nakamura, and F. Sakiyama. 1997. A novel d-amino acid-containing peptide, fulyal, coexists with fulicin gene-related peptides in Achatina atria. Peptides 18:347-354. [DOI] [PubMed] [Google Scholar]