Abstract
Sodium salicylate and ammonium sulfate were applied to leaf surfaces along with suspensions of the biological control agents Pseudomonas syringae Cit7(pNAH7), which catabolizes salicylate, and Cit7, which does not catabolize salicylate, to determine whether enhanced biological control of bacterial speck of tomato could be achieved. Foliar amendment with salicylate alone significantly enhanced the population size and the efficacy of Cit7(pNAH7), but not of Cit7, on tomato leaves. Application of ammonium sulfate alone did not result in enhanced population size or biological control efficacy of either Cit7(pNAH7) or Cit7; however, when foliar amendments with both sodium salicylate and ammonium sulfate were applied, a trend toward further increases in population size and biological control efficacy of Cit7(pNAH7) was observed. This study demonstrates the potential of using a selective carbon source to improve the efficacy of a bacterial biological control agent in the control of a bacterial plant disease and supports previous conclusions that the growth of P. syringae in the phyllosphere is primarily carbon limited and secondarily nitrogen limited.
Effective colonization and high population size of introduced bacterial biological control agents on plant surfaces have been considered to be important factors in the successful control of plant diseases (4, 21, 24, 25). Hence, many strategies have been employed to enhance colonization and population size of bacterial biological control agents in order to improve biological control efficacy, including attempts to use nutritional amendments to favor the growth and activity of those biological control agents (2, 8, 11, 23, 27). Chitin applied as an amendment along with chitinolytic Bacillus cereus strain 304, an antagonist of early leaf spot of peanut, increased the population size of this bacterium on peanut leaves, resulting in significant disease control (18). Amino acids were used as an amendment to enhance the population size of an antagonistic nonpathogenic bacterial strain on apple fruits, resulting in improved biological control of blue mold on ripe apples (15). Methionine increased the population size of the biological control agent Pseudomonas putida AP-1 in soil, and the suppression of Fusarium wilt on tomato was greater when AP-1 was used with methionine than when AP-1 was used alone (35).
The ability to selectively increase the population size and biological control efficacy of a target biological control agent through nutritional amendment is based on the observation that availability of nutrients is a limiting factor for growth of microbial populations in various plant habitats (1, 12, 14, 31-35). An understanding of the nutritional composition of these environments, therefore, is important when using nutrient amendments to enhance the population size of introduced bacteria. Leaf surfaces are a nutrient-limited environment, and it has been reported that bacteria in the phyllosphere are primarily carbon limited and secondarily nitrogen limited (31-34). Exogenous application of the carbon source salicylate increased the population size of a salicylate-catabolizing P. putida strain in the phyllosphere of beans (33). Endogenous provision of mannityl opines by mannityl opine-producing transgenic tobacco also increased the population size of Pseudomonas syringae Cit7(pYDH208) in the phyllosphere due to its ability to catabolize mannityl opines as a source of carbon and nitrogen (34). It was hypothesized, therefore, that amendment with selective carbon sources could be employed to increase the population size and efficacy of a biological control agent in the phyllosphere, if the primary mechanism of the biological control agent was population size dependent.
In this study, the goal was to use carbon source amendment to selectively increase the phyllosphere population size of the biological control agent P. syringae Cit7 (30) and to determine whether this resulted in increased efficacy against bacterial speck of tomato. To achieve this selectivity, and because of the similarity in nutrient utilization profiles between P. syringae Cit7 and the pathogen P. syringae pv. tomato, the biological control agent was provided with a novel catabolic activity. The salicylate catabolism plasmid pNAH7 (10, 36) was mobilized into the biological control agent Cit7 to confer upon it the selective use of the carbon source salicylate. However, since bacteria in the phyllosphere are believed to be primarily carbon limited and secondarily nitrogen limited (31-34), only a certain increase in bacterial population size could be achieved by provision of carbon alone. Therefore, the nonselective nitrogen source ammonium sulfate was applied in conjunction with the salicylate to achieve a maximal increase in population size and, hopefully, biological control efficacy, without losing the selectivity provided by the carbon source.
Bacterial strains.
P. syringae strain Cit7, resistant to rifampin (100 μg/ml), was provided by S. E. Lindow (University of California, Berkeley). The pathogen P. syringae pv. tomato strain PT12 was provided by D. A. Cooksey (University of California, Riverside). P. putida strain PpG7 Leu− was provided by S. F. Colbert (University of California, Berkeley) and is a stable leucine auxotroph of PpG7 which is sensitive to rifampin. PpG7 Leu− carries the plasmid pNAH7, which confers the ability to catabolize salicylate (10, 36). P. syringae Cit7(pNAH7) was generated in this study by transferring the plasmid pNAH7 to Cit7 through biparental mating with PpG7 Leu− by methods described previously (6). Growth of Cit7(pNAH7) on King's medium B (17) was typical of P. syringae Cit7 rather than P. putida, and the identity of the transconjugant was further confirmed by using the Sherlock GC-FAME system (MIDI, Newark, Del.).
Foliar carbon and nitrogen amendments and experimental design.
Sodium salicylate and ammonium sulfate were used as carbon and nitrogen sources, respectively, to investigate their influence on population size and efficacy of inoculated biological control agents P. syringae Cit7 and Cit7(pNAH7) under both growth chamber and greenhouse conditions. Bacteria were cultured on King's medium B or minimal medium A (20) amended with sodium salicylate (10 mM), washed from the plates, and suspended in sterile potassium phosphate buffer (PPB;10 mM, pH 7.0). Bacterial suspensions were spray inoculated onto upper and lower leaf surfaces of 5-week-old tomato plants (cv. Agriset 761; Agrisales, Inc., Plant City, Fla.) at a concentration of 106 or 107 CFU per ml. Sodium salicylate and ammonium sulfate were added to the bacterial suspensions immediately prior to inoculation onto tomato leaves. In accordance with published studies using salicylate (5, 33), both sodium salicylate and ammonium sulfate were applied at a concentration of 0.6 g/liter. However, 0.6-g/liter salicylate was occasionally phytotoxic to 5-week-old tomato plants; hence, a concentration of 0.3 g/liter was used in all subsequent experiments.
Tomato plants were grown in pots containing Promix (Premier Peat Ltd., Riviere-du-Loup, Quebec, Canada) and incubated in a growth chamber under conditions of 25°C, 100% relative humidity, and 12-h photoperiod or in the greenhouse under ambient conditions suitable for bacterial speck development (16, 30). A randomized complete block design was used in each experiment. Each experiment consisted of 12 treatments and four or five replications for each treatment with one plant in each replicate. All experiments were performed at least twice.
Effect of salicylate and ammonium amendment on bacterial population sizes.
Tomato leaflets were sampled at the time of inoculation and at 24-h intervals up to 4 or 5 days after inoculation. Fifteen or 20 leaflets were collected at each time from each treatment in the growth chamber or greenhouse experiment, respectively, 3 or 4 leaflets per plant. Leaves were collected from the top, middle, and bottom parts of individual tomato seedlings at each sampling time. Assessment of bacterial population sizes in the greenhouse was conducted with the same tomato plants used for disease control assays. For enumeration of bacterial population sizes, individual leaves were placed in 20 ml of PPB in sterile Whirl-Pak bags (Macalaster Bicknell Co., New Haven, Conn.). The bags were sonicated in an ultrasonic bath (FS28; Fisher Scientific) for 7 min to dislodge bacteria from leaf surfaces and shaken briefly to suspend the bacterial cells. Leaf washings were diluted and plated with a spiral plater (Spiral Biotech, Inc.) onto tryptic soy agar amended with rifampin (100 μg/ml) and cycloheximide (100 μg/ml), the latter to suppress fungal growth. The plates were incubated at 28°C for 30 h, and bacterial colonies were counted with a laser colony counter (Spiral Biotech, Inc.). The mean log10-transformed population size was estimated from the 15 or 20 individual leaves. Population sizes were compared statistically through analysis of variance and Fisher's protected-least-significant-difference test with Proc GLM of the Statistical Analysis System (SAS Institute Inc., Cary, N.C.).
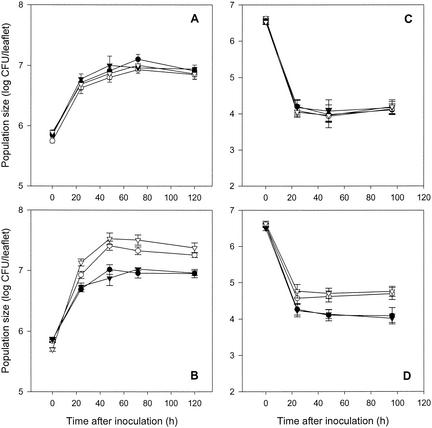
Application of sodium salicylate as a foliar amendment significantly enhanced population size of P. syringae Cit7(pNAH7) on tomato leaves. Under growth chamber conditions, population sizes of Cit7(pNAH7) on leaf surfaces, 24 to 120 h after inoculation, were increased by two- to threefold when salicylate was added to the inoculum (Fig. 1B). In contrast, salicylate amendment did not increase the population size of P. syringae Cit7 on tomato leaves under the same conditions (Fig. 1A). Under greenhouse conditions, population sizes of Cit7(pNAH7) on leaf surfaces, at 24 to 96 h after inoculation, were significantly increased, by two- to fourfold, when sodium salicylate was added to the inoculum (Fig. 1D). Once again, the population size of Cit7 was not increased under the same conditions (Fig. 1C).
FIG. 1.
Influence of foliar sodium salicylate and ammonium sulfate amendments on population size of P. syringae Cit7 and Cit7(pNAH7) on tomato leaves. Sodium salicylate (○) and ammonium sulfate (▾) were applied separately or were used in combination (▿). Application of PPB (•) was used as a control. Error bars, standard errors of the means of population size estimated from 20 or 15 leaflets. (A) P. syringae Cit7 in the growth chamber; (B) P. syringae Cit7(pNAH7) in the growth chamber; (C) P. syringae Cit7 in the greenhouse; (D) P. syringae Cit7(pNAH7) in the greenhouse. Experiments were repeated twice in the greenhouse and three times under growth chamber conditions. One representative experiment is presented for each location.
Amendment of the biological control agent inoculum with ammonium sulfate alone did not increase the population size of either Cit7 or Cit7(pNAH7). However, when ammonium sulfate was used in conjunction with sodium salicylate, further increases in population size of Cit7(pNAH7) were observed under growth chamber conditions (Fig. 1B). These enhancements represented a significant increase of 3- to 6-fold compared with Cit7(pNAH7) applied without nutritional amendment and an increase of 1.2- to 1.6-fold compared with Cit7(pNAH7) amended with salicylate alone, though the latter was not statistically significant. In contrast, no increases in population size of Cit7 were observed when ammonium sulfate was used with sodium salicylate as a foliar amendment (Fig. 1A and C).
Since salicylate was used by Cit7(pNAH7) as a carbon source, the observed increase in population size of Cit7(pNAH7) as a result of salicylate amendment supports the conclusion that carbon source availability is the primary factor limiting growth of P. syringae in the phyllosphere (31-33). Previous studies indicated that application of salicylate to the bean (Phaseolus vulgaris) phyllosphere (33) and to the soil (6, 28) significantly enhanced the population size or favored the survival of salicylate-catabolizing P. putida and Enterobacter agglomerans strains, also suggesting the existence of carbon limitation in these habitats. While application of the nitrogen source ammonium sulfate alone did not increase the population size of either Cit7 or Cit7(pNAH7), application of ammonium sulfate with sodium salicylate further increased the population size of Cit7(pNAH7). These data suggest that, in the phyllosphere of the tomato, P. syringae was primarily carbon limited and secondarily nitrogen limited, which is consistent with observations of P. syringae in the bean phyllosphere (31-33). It was noted that the effect of ammonium sulfate, when applied along with salicylate, on population sizes of Cit7(pNAH7) was more prominent in growth chamber studies than in the greenhouse (Fig. 1B and D). This might indicate that the nutritional compositions of exudates in the phyllosphere of tomato plants under growth chamber and greenhouse conditions were not identical; for example, nitrogen availability in the tomato phyllosphere might have been somewhat lower under growth chamber than under greenhouse conditions.
Effect of salicylate and ammonium amendment on biological control efficacy.
Evaluation of disease control efficacy was conducted in the greenhouse. The pathogen was applied 48 h after inoculation of the biological control agents. To prepare the inoculum of the pathogen, P. syringae pv. tomato PT12 was grown on tryptic soy agar plates and bacterial cells were scraped from plates and suspended in PPB. The pathogen suspension (approximately 108 CFU/ml) was spray inoculated onto upper and lower leaf surfaces. To evaluate disease severity, 10 leaflets per plant were collected 7 days after inoculation of the pathogen. Lesions on each leaflet were counted manually, and the area of each leaflet was measured with an image analysis system (AgVision Monochrome system; Decagon Devices, Pullman, Wash.). Disease severity data were subjected to log transformation and expressed as log10 [(number of lesions + 1)/square centimeter] (16, 30). Analysis of variance was performed by using the ANOVA or GLM procedures of the Statistical Analysis System. Means were compared by using Duncan's multiple-range test at P = 0.05. Biological control effectiveness was quantified as the percent reduction in disease severity compared to that for the pathogen-only control (16, 30).
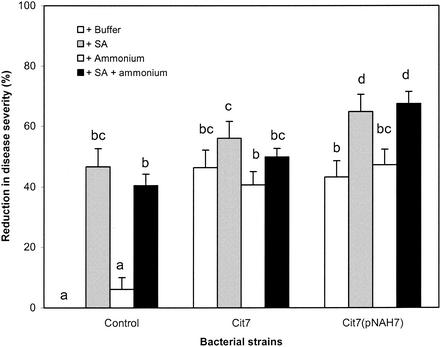
Application of salicylate alone provided moderate but significant control of bacterial speck in the greenhouse (Fig. 2) due to the well-documented induction of systemic acquired resistance (SAR). The combination of Cit7 and salicylate consistently provided disease suppression greater than either Cit7 alone or salicylate alone, though these differences were not statistically significant (Fig. 2). Most importantly, however, applications of the biological control agent P. syringae Cit7(pNAH7) amended with both sodium salicylate and ammonium sulfate provided the greatest disease reduction in three out of four trials (data not shown). This level of disease reduction was significantly higher than that provided by Cit7 plus sodium salicylate and ammonium sulfate or Cit7(pNAH7) alone but was not significantly greater than that provided by Cit7(pNAH7) amended with sodium salicylate alone (Fig. 2). Cit7(pNAH7) amended with sodium salicylate provided a significantly higher degree of disease reduction than Cit7 amended with sodium salicylate. Application of ammonium sulfate alone did not affect disease severity significantly (Fig. 2).
FIG. 2.
Effect of sodium salicylate (SA) and ammonium sulfate amendment on effectiveness of P. syringae Cit7 and Cit7(pNAH7) in reduction of foliar severity of bacterial speck of tomato under greenhouse conditions. Sodium salicylate and ammonium sulfate were applied at concentrations of 0.3 and 0.6 g/liter, respectively. Control plants were treated with buffer or nutritional amendments but no biological control agent. Reduction in disease severity was derived from the mean of four repeated experiments. Same letter indicates no significant difference (P = 0.05).
The use of nutritional amendments has previously been reported to enhance the efficacy of several bacterial and fungal biological control agents of fungal diseases (8, 11, 13-15, 18, 35). There are also reports that certain carbohydrates, such as 2-deoxy-d-ribose, may be useful as nutritional amendments to enhance the growth of bacterial biological control agents which control bacterial pathogens (3, 29). The study presented here, however, is the first to report that application of a nutritional substrate which was selectively utilized by a bacterial biological control agent enhanced the level of control of a disease caused by a bacterial plant pathogen. This study also demonstrated the feasibility of improving disease control efficacy, especially when the pathogen is physiologically similar to the biological control agent, as was the case in this report, by conferring upon the biological control agent a unique catabolic ability.
In retrospect, salicylate was not an ideal selective carbon source for these studies since it has effects on host plant physiology. Foliar application of salicylate alone significantly reduced the severity of bacterial speck of tomato (Fig. 2), indicating that salicylate induced SAR in the tomato plant against the pathogen P. syringae pv. tomato. Induction of SAR against a bacterial pathogen has been previously reported for P. syringae pv. tomato (5), P. syringae pv. syringae (19, 26), and Erwinia carotovora subsp. carotovora (22). While application of a selective carbon source that had no indirect effects on the host or pathogen would have been desirable, we can assume that these indirect effects on the host and/or pathogen were equivalent for both Cit7 and Cit7(pNAH7) and, therefore, that any differential effects were due to provision of a carbon source which could be catabolized by Cit7(pNAH7) but not Cit7. The data appear to support this assumption. Salicylate amendment significantly increased the population size and biological control efficacy of Cit7(pNAH7), the bacterial strain that catabolizes salicylate, but not that of the nearly isogenic strain Cit7, which does not catabolize salicylate. Since strains Cit7 and Cit7(pNAH7) are nearly isogenic, apart from the presence of the plasmid conferring salicylate utilization, the increase in population size and biological control efficacy of Cit7(pNAH7) was almost certainly due to the provision of a selective carbon source rather than to other potential effects of salicylate, such as induction of SAR, altered pH of the leaf surface, direct toxicity to the pathogen, or inhibition of antagonistic microorganisms that might have suppressed Cit7 and Cit7(pNAH7).
The mechanism through which an increase in population size of Cit7(pNAH7) resulted in an increase in the level of biological control is uncertain. It has been suggested that Cit7 may induce host defense responses in tomato plants (30). In greenhouse and field experiments, in addition to providing significant control of bacterial speck of tomato (30), Cit7 significantly reduced the severity of bacterial spot of tomato, caused by the pathogen Xanthomonas campestris pv. vesicatoria (M. Wilson et al., unpublished data). P. syringae Cit7 is not known to produce any antibiotics against X. campestris pv. vesicatoria, and preemptive competitive exclusion of epiphytic X. campestris pv. vesicatoria is unlikely, due to different nutrient utilization profiles (9, 21); hence, these factors are not thought to be significant in the control of bacterial spot of tomato, and induced resistance is presumed to be involved (30). There are precedents for population size dependence of induced host responses. Raaijmakers et al. (24) reported that the level of suppression of Fusarium wilt of radish was significantly related to the rhizosphere population density of the bacterial strain WCS358, which suppressed the disease by induction of systemic resistance. So an increase in the population size of Cit7(pNAH7) may have resulted in greater levels of induced resistance in the tomato plants.
In summary, the increase in the population size of Cit7(pNAH7) achieved through sodium salicylate amendment demonstrated the potential of using a selective carbon source to create a catabolic niche for the biological control agent. Selectivity of salicylate was important, not only because this carbon source was unavailable to the pathogen but also because the rarity of the ability to catabolize salicylate (7) might also have limited the consumption by indigenous microorganisms. It is presumed that provision of such a catabolic niche and enhancement of the population size of the biological control agent would only be beneficial in those instances in which the mechanism of action is population size dependent and does not rely on preemptive utilization of carbon sources naturally present in the phyllosphere.
Acknowledgments
We gratefully acknowledge S. E. Lindow, D. A. Cooksey, and S. F. Colbert for providing bacterial strains. We thank J. B. Jones and S. E. Lindow for review of the manuscript prior to submission.
This work was supported by a USDA NRICGP grant (original USDA award no. 95-37303-2043) awarded to M. Wilson.
REFERENCES
- 1.Andrews, J. H. 1992. Biological control in the phyllosphere. Annu. Rev. Phytopathol. 30:603-635. [DOI] [PubMed]
- 2.Bhatt, D. D., and E. K. Vaughn. 1962. Preliminary investigations on biological control of gray leaf mold (Botrytis cinerea) of strawberries. Plant Dis. Rep. 46:342-345. [Google Scholar]
- 3.Brown, E. W., T. van der Zwet, R. H. Bors, and W. Janisiewicz. 1992. Identification of a carbohydrate which enhances the growth of a bacterial antagonist against Erwinia amylovora. Phytopathology 82:718. [Google Scholar]
- 4.Bull, C. T., D. M. Weller, and L. S. Thomashow. 1991. Relationship between root colonization and suppression of Gaeumannomyces graminis var. tritici by Pseudomonas fluorescens strain 2-79. Phytopathology 81:954-959. [Google Scholar]
- 5.Cokmus, C., and A. H. Sayar. 1991. Effect of salicylic acid on the control of bacterial speck of tomato caused by Pseudomonas syringae pv. tomato. J. Turkish Phytopathol. 20:27-32. [Google Scholar]
- 6.Colbert, S. F., M. Hendson, M. Ferri, and M. N. Schroth. 1993. Enhanced growth and activity of a biological control bacterium genetically engineered to utilize salicylate. Appl. Environ. Microbiol. 59:2071-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colbert, S. F., M. N. Schroth, A. R. Weinhold, and M. Hendson. 1993. Enhancement of population densities of Pseudomonas putida PpG7 in agricultural ecosystems by selected feeding with the carbon source salicylate. Appl. Environ. Microbiol. 59:2064-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, R. F., P. A. Backman, R. Rodriguez-Kabana, and N. Kokalis-Burelle. 1992. Biological control of apple fruit diseases by Chaetomium globosum formulations containing cellulose. Biol. Control 2:118-123. [Google Scholar]
- 9.Dianese, A. C. 1997. Importance of nutritional similarity in pre-emptive biocontrol of bacterial spot. M.S. thesis. Auburn University, Auburn, Ala.
- 10.Dunn, N. W., and I. C. Gunsalus. 1973. Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J. Bacteriol. 114:974-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fokkema, N. J., J. G. den Houter, Y. J. C. Kosterman, and A. L. Nelis. 1979. Manipulation of yeasts on field-grown wheat leaves and their antagonistic effect on Cochliobolus sativus and Septoria nodorum. Trans. Br. Mycol. Soc. 72:19-29. [Google Scholar]
- 12.Fukui, R., M. N. Schroth, M. Hendson, and J. G. Hancock. 1994. Interaction between strains of pseudomonads in sugar beet spermospheres and their relationship to pericarp colonization by Pythium ultimum in soil. Phytopathology 84:1322-1330. [Google Scholar]
- 13.Gullino, M. L., C. Aloi, and A. Garibaldi. 1989. Evaluation of the influence of different temperatures, relative humidities and nutritional supports on the antagonistic activity of Trichoderma spp. against grey mold of grape, p. 231-236. In R. Cavallora (ed.), Influence of environmental factors on the control of grape pests, diseases and weeds. A. A. Balkema Publishers, Brookfield, Vt.
- 14.Janisiewicz, W. J., and B. Bors. 1995. Development of a microbial community of bacterial and yeast antagonists to control wound-invading postharvest pathogens of fruits. Appl. Environ. Microbiol. 61:3261-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janisiewicz, W. J., J. Usall, and B. Bors. 1992. Nutritional enhancement of biological control of blue mold on apples. Phytopathology 82:1364-1370. [Google Scholar]
- 16.Ji, P., and M. Wilson. 2002. Assessment of the importance of similarity in carbon source utilization profiles between the biological control agent and the pathogen in biological control of bacterial speck of tomato. Appl. Environ. Microbiol. 68:4383-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King, E. O., M. K. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44:301-307. [PubMed] [Google Scholar]
- 18.Kokalis-Burelle, N., P. A. Backman, R. Rodriguez-Kabana, and L. D. Ploper. 1992. Potential for biological control of early leafspot of peanut using Bacillus cereus and chitin as foliar amendments. Biol. Control 2:321-328. [Google Scholar]
- 19.Malamy, J., and D. F. Klessig. 1992. Salicylic acid and plant disease resistance. Plant J. 2:643-654. [Google Scholar]
- 20.Miller, J. H. 1972. Experiments in bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 21.Mohamed, S., and I. G. Caunter. 1995. Isolation and characterization of a Pseudomonas fluorescens strain suppressive to Bipolaris maydis. Phytopathol. Z. 143:111-114. [Google Scholar]
- 22.Palva, T. K., M. Hurtig, P. Saindrenan, and E. T. Palva. 1994. Salicylic acid induced resistance to Erwinia carotovora subsp. carotovora in tobacco. Mol. Plant-Microbe Interact. 7:356-363. [Google Scholar]
- 23.Ploper, L. D., P. A. Backman, and R. Rodriguez-Kabana. 1992. Enhanced natural biological control of apple fruit diseases by applications of biopolymers. Biol. Control Tests 7:3.
- 24.Raaijmakers, J. M., M. Leeman, M. M. P. van Oorschot, I. van der Sluis, B. Schippers, and P. A. H. M. Bakker. 1995. Dose-response relationship in biological control of Fusarium wilt of radish by Pseudomonas spp. Phytopathology 85:1075-1081. [Google Scholar]
- 25.Randhawa, P. S., and E. L. Civerolo. 1986. Interaction of Xanthomonas campestris pv. pruni with pruniphage and epiphytic bacteria on detached peach leaves. Phytopathology 76:549-553. [Google Scholar]
- 26.Rasmussen, J. B., R. Hammerschmidt, and M. Zook. 1991. Systemic induction of salicylic acid accumulation in cucumber after inoculation with Pseudomonas syringae pv. syringae. Plant Physiol. 97:1342-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stadler, B., and T. Mueller. 1996. Aphid honeydew and its effect on the phyllosphere microflora of Picea abies (L.) Karst. Oecologia 108:771-776. [DOI] [PubMed] [Google Scholar]
- 28.Utkhede, R., J. Nie, H. Xu, K. Eastwell, and P. Wiersma. 2000. Transformation of biological control agent Enterobacter agglomerans with salicylate utilizing gene and its monitoring in orchard soil. J. Hortic. Sci. Biotechnol. 75:50-54. [Google Scholar]
- 29.van der Zwet, T. 1993. Manipulation of the epiphytic microbial community to promote biological control of Erwinia amylovora on pear and apple. Acta Hortic. 338:351. [Google Scholar]
- 30.Wilson, M., H. L. Campbell, P. Ji, J. B. Jones, and D. A. Cuppels. 2002.. Biological control of bacterial speck of tomato under field conditions at several locations in North America. Phytopathology 92:1284-1292. [DOI] [PubMed]
- 31.Wilson, M., and S. E. Lindow. 1994. Ecological similarity and coexistence of epiphytic ice-nucleating (Ice+) Pseudomonas syringae strains and a non-ice-nucleating (Ice−) biological control agent. Appl. Environ. Microbiol. 60:3128-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson, M., and S. E. Lindow. 1994. Coexistence among epiphytic bacterial populations mediated through nutritional resource partitioning. Appl. Environ. Microbiol. 60:4468-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson, M., and S. E. Lindow. 1995. Enhanced epiphytic coexistence of near-isogenic salicylate-catabolizing and non-salicylate-catabolizing Pseudomonas putida strains after exogenous salicylate application. Appl. Environ. Microbiol. 61:1073-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson, M., M. A. Savka, I. Hwang, S. K. Farrand, and S. E. Lindow. 1995. Altered epiphytic colonization of mannityl opine-producing transgenic tobacco plants by a mannityl opine-catabolizing strain of Pseudomonas syringae. Appl. Environ. Microbiol. 61:2151-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada, M., and M. Ogiso. 1997. Control of soil-borne diseases using antagonistic microorganisms. IV. Study on the available substrates for antagonistic bacterial strains to control Fusarium wilt of tomatoes. Res. Bull. Aichi-Ken Agric. Res. Cent. 29:141-144. (In Japanese.)
- 36.Yen, K. M., and C. M. Serdar. 1988. Genetics of naphthalene catabolism in pseudomonads. Crit. Rev. Microbiol. 15:247-268. [DOI] [PubMed] [Google Scholar]