Abstract
Purpose
To determine the efficiency of baculoviruses (BVs) to transfer recombinant genes in vivo into murine ocular tissues.
Methods
Recombinant (r)BVs carrying fluorescent protein (FP) cDNA under the control of cytomegalovirus (CMV) immediate early promoter were constructed. Initially, cultured HEK293 and ARPE19 cells were infected with these rBVs and analyzed for efficiency and stability of transgene expression. The rBV-CMV green (G)FP was also injected into the intravitreal and subretinal space of mouse eye. Mice were periodically analyzed to determine the efficiency and stability of expression by histologic examination under fluorescence microscopy. The effect of rBV-CMV-GFP on the physiology of the retina was analyzed by electroretinography.
Results
cDNAs encoding fluorescent proteins were efficiently transduced in HEK293 and ARPE19 cells in vitro. GFP expression in vivo was observed exclusively in retinal pigment epithelial (RPE) cells after subretinal injections. Intravitreal injections of rBV resulted in GFP expression in the corneal endothelium, lens, RPE, and retina. GFP expression was observed for up to 14 days after injection. The infiltration of macrophages, observed 2 days after injection in the area of GFP transduction, had dissipated by day 8 after injection. No alteration in ERG responses was observed 6 weeks after injection of rBV-CMV-GFP.
Conclusions
BV efficiently transduces cultured RPE cells and many cell types in vivo in the eye, including endothelial, epithelial, and neuronal cells. BV may be a useful vector for transferring genes in cultured cells and in vivo into ocular tissue.
The investigation of novel vectors for gene delivery in vivo is an important part of the process of advancing human gene therapy and developing simple gene transfer methods to study gene regulation and function in animal models. Vector-mediated gene transfer could serve as an alternative approach to the time-consuming generation of transgenic mice. Furthermore, gene transfer has great potential for application to the eye, because vectors can be administrated directly to the affected tissues. Moreover, the eye exhibits a high degree of immune privilege that limits the immune response against the recombinant vectors.
The ideal vector for gene therapy should have low toxicity and high transduction efficiency, confer stable expression of the transgene, have the capacity to carry large inserts, and be safe to handle in a laboratory setting. Multiple viral and nonviral vectors have been investigated for gene therapy (for review see Refs. 1,2). Recombinant adeno-associated viruses (AAVs), retroviruses, adenoviruses (AVs), and herpes virus are the most extensively studied viral vectors. Some of these vectors are capable of transferring genes into the retina (rAAV, rAV, lentivirus, and herpes virus).3–11 rAAV is the most promising of these viral vectors, due to its apparent stability, efficiency, and safety. The major limitation of rAAV is the size of the transgene that can be packaged in the virus (≤4 kb). This excludes larger gene cassettes that may contain either multiple genes or large promoters.
One vector that has not been previously investigated in ocular tissue is baculovirus (BV). The double-stranded DNA BV has been investigated as a candidate vector for gene therapy in other organ systems. BV does not cause human diseases and has been extensively studied for its potential utility in insect pest control and, more recently, to overexpress recombinant proteins in vitro.12 Volkman and Goldsmith were the first to report the entry of BV in vertebrate cells (nonpermissive cells), by identifying nucleocapsids in cytoplasmic vacuoles of infected mammalian cell, but not in the nucleus.13 BV enters nondividing mammalian cells by endocytosis and loses its envelope when it passes from the endosome to the cytoplasm. Finally, the nucleocapsid is transported to the nucleus through the nucleopore.14 In addition, recombinant (r)BV has the capability not only of entering but also of transducing hepatocytes15 and a variety of mammalian cells from different tissues in vitro.14,16–28
BVs do not replicate in vertebrate cells and constitute appropriate vectors to deliver genes in mammals. The prerequisite for rBV-mediated expression in mammalian cells was the insertion of a mammalian promoter. Despite the ability to transduce mammalian cells in vitro, only limited success has been reported in more complex systems. BV-mediated gene transfer into perfused human liver tissue has been achieved ex vivo,29 into rabbit carotid artery through collar-mediated delivery,30 and into astrocytes in the striatum in vivo.26 This limited success in transferring genes in several tissues is most likely due to viral inactivation by the complement system.22 Blocking antibodies against C-component 5 have been shown to prevent the inactivation of BVs in human serum. The anterior chamber, the subretinal space, and to a lesser extent, the vitreous cavity are immune-privileged sites.31–33 Antigens in these areas are not subject to the complement pathway. This trait makes intraocular tissues an attractive target for gene transfer through rBV. Currently, there are no reports showing rBV-mediated gene transfer into ocular tissue.
In this study, we tested the capability of rBV to transduce RPE cells in vitro. We then investigated whether rBV can mediate the transfer of exogenous genes in vivo into mouse eyes.
Methods
Preparation of Recombinant BV for In Vivo Injection
To replace the BV polyhedrin promoter with the cytomegalovirus (CMV) promoter, a CMV promoter fragment (BglII-XhoI) from the pcDNA3.1 vector (Invitrogen, Carlsbad, CA) was cloned into pFastBac1 (Life Technologies, Inc., Rockville, MD) between the sites SnaBI and BamHI (vector pFastCMV). The coding region for the enhanced green fluorescent protein (GFP) was then transferred as a fragment EcoRI-NotI from pEGFP1 (Clontech Laboratories, Palo Alto, CA) into pFastCMV opened at the EcoRI and NotI sites. Similarly, the coding sequence for either the blue fluorescent protein (BFP) or the red fluorescent protein (RFP) was transferred as a fragment EcoRI-NotI from pEBFP-N1 (Clontech Laboratories) or pDsRed1-N1 (Clontech Laboratories), respectively, into pFastCMV, opened at the EcoRI and NotI sites. The expression cassette was then transferred into the BV shuttle vector by transposition. SF9 insect cells were transfected with the recombinant bacmid using cationic liposome–mediated transfection (CellFECTIN reagent; Life Technologies, Inc.). After amplification of the rBV for 3 days in SF9 cells, cell debris was removed by centrifugation at 1000g for 15 minutes, and the rBV-CMV-GFP was then concentrated from the cell culture supernatant by centrifugation at 80,000g for 1 hour at 4°C. The resultant pellet containing the rBV was resuspended in PBS (1.5 mM KH2PO4, 12 mM NaH2PO4, 2.7 mM KCl, and 139 mM NaCl [pH 7.4]) and used for in vitro and in vivo experiments. The rBV was further purified by the 25% to 60% linear sucrose gradient centrifugation method (96,000g for 3 hours).34 A white band containing the rBV formed at approximately 47% sucrose, was collected and diluted approximately 10 times in PBS. The rBV was collected by centrifugation at 80,000g for 75 minutes, resuspended in 200 μl PBS, and then used for the experiments. The rBV titer was determined using a rapid titer kit (BacPak BV; Clontech).
Cell Culture and Infection
ARPE19 and HEK293 cells were obtained from the American Type Culture Collection (Rockville, MD). The cells were cultured in DMEM-F12 supplemented with 10% fetal bovine serum (FBS). During infection with rBV, the cells were maintained in DMEM-F12 supplemented with 1% FBS. After 2 hours, the supernatant was removed and replaced with DMEM-F12 supplemented with 10% FBS and 10 mM sodium butyrate.35 Twenty-four hours after infection, the cells were collected and analyzed for the expression of fluorescent proteins. To analyze the stability of GFP expression in vitro, HEK293 cells were maintained in culture for 8 months. To limit the effect of the dilution factor, the cells were passaged only once a week. Every 8 days, the cells were trypsinized and diluted three times with the fresh medium. Expression of GFP was monitored under a fluorescence microscope once a week at the cells’ passage.
Cell Preparation for Electron Microscopy
ARPE19 cells were cultured in a 12-well plate for 20 hours. BVs were added to the cells (multiplicity of infection [MOI] of ~100) and incubated for 1 or 2 hours at 37°C. The cells were then washed twice with PBS and trypsinized for 5 minutes. After two washes with PBS, the cells were fixed for 30 minutes at 4°C in 1% final glutaraldehyde in PBS. After two more washes with PBS, the cells were resuspended in molten 3% phosphate-buffered, low-gelling-temperature agarose solution and then fixed with 1% OsO4 in 1.0 M phosphate buffer (pH 7.3) for 30 to 45 minutes at room temperature. After complete dehydration in a graded ethanol series, the specimens were embedded in Epon 812. Ultrathin transmission electron microscopy sections were then cut and stained with saturated aqueous uranyl acetate and lead citrate.
Injection of Recombinant BVs in Mice
C57Bl/6J mice (Jackson Laboratory, Bar Harbor, ME) were housed in the Department of Comparative Medicine at the University of Washington and treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Anesthesia was achieved by intraperitoneal injection with 15 μl/g body weight of 6 mg/ml ketamine and 0.44 mg/ml xylazine diluted in PBS. The rBV solution or PBS control (1 μl), was injected under microscopic visualization into the subretinal space or in the vitreous with a blunt 33-gauge needle through a 30-gauge starter, opening just behind the ciliary ruff with the tip angled toward the posterior pole. Caution was taken to avoid the lens material. The blunt needle was advanced into the vitreous cavity for intravitreal injections and was pushed forward across the vitreous to the subretinal space for subretinal injections. The animals recovered from anesthesia on a warming blanket. Approximately 50 mice, about 8 weeks old, were used for both the subretinal and intravitreal injections.
Histologic Analysis
Enucleated eyes or eye cups were fixed in 4% paraformaldehyde-PBS for 6 hours and infiltrated with 20% sucrose in 100 mM sodium phosphate (pH 7.4) at 4°C. The eyes were then embedded in 33% optimal cutting temperature (OCT) compound (Miles; Elkhart, IN) diluted with 20% sucrose in the same buffer.36 The tissues were frozen and cut at 12 μm and the sections analyzed under a fluorescence microscope.
Immunocytochemical Analysis of Inflammatory Response Induced by rBV
Sections were prepared from mouse eyes as described earlier and used for immunostaining. To reduce nonspecific labeling, retinal sections were incubated for 30 minutes in ICC buffer (PBS, 0.1% Triton X-100 [pH 7.3]; BD PharMingen, San Diego, CA), containing 1.5% normal goat serum. Rat biotinylated anti-CD11b (Mac-1 α-chain), anti-CD4 (L3T4), or anti-CD8a (Ly2) antibodies (BD PharMingen) were diluted in ICC buffer (1:500). Sections were incubated in primary antibody for 12 hours at 4°C, washed repeatedly in ICC buffer (three times for 20 minutes each and one time for 60 minutes), incubated for 30 minutes in Cy3-streptavidin diluted 1:1000 in ICC buffer at room temperature, washed in ICC buffer (three times for 20 minutes each and one time for 60 minutes), coverslipped with 50 μl 2% 1,4-diazabicyclo-2,2,2-octane (DABCO) in 90% glycerol to retard photobleaching, and analyzed under a fluorescence microscope. Negative control samples were prepared without primary antibody. Two to four 5-μm sections from four animals were analyzed under a fluorescence microscope and counted at the highest magnification (lens: ×40), at or away from the site of injection.
Fluorescence Microscopy
Images were collected with an episcopic fluorescence microscope (Nikon Corp., Melville, NY) that was equipped with a mercury lamp and a triple-excitation filter for red, green, and blue excitation (4′,6-diamidino-2-phenylindole [DAPI], FITC, and rhodamine; Nikon). When needed, fluorochromes were visualized with a detection filter for red or green fluorescence. Digital images were captured using a digital camera (Diagnostic Instruments; Sterling Heights, MI).
Mouse Electroretinograms
Mice were dark adapted for more than 2 hours and anesthetized as described above. The pupils were dilated with 1% tropicamide. Electroretinograms (ERGs) were recorded 6 weeks after injection from four eyes using a universal testing and analysis electrophysiologic system (UTAS E-3000; LKC Technologies Inc., Gaithersburg, MD). A contact lens electrode was placed on the eye with a drop of methylcellulose. A ground electrode was placed in the ear. The animals were placed in a Ganzfeld chamber and stimulated with flash intensities of −3.3 to −1.4 log candelas (cd)/sec · m2. Averages of five responses at every intensity were calculated for each four eyes injected with PBS or rBV-CMV-GFP.
Results
Efficient Gene Transfer into Mammalian Cells by Recombinant BVs
The rBV was constructed with GFP under the control of the CMV promoter (rBV-CMV-GFP). After infection with rBV-CMV-GFP, fluorescence was detected in SF9, HEK293, and ARPE19 cells (Fig. 1A). A high multiplicity of infection was necessary to observe fluorescence in 100% of ARPE19 and HEK293 cells (MOI > 100). Sodium butyrate (10 mM), an inhibitor of histone deacetylase,35 increased the expression of GFP in ARPE19 and HEK293 cells. GFP expression was observed as soon as 6 hours after infection. GFP expression in transduced HEK293 cells was stable for at least 8 months in vitro without any selective pressure. However, the ratio of cells expressing GFP compared with nontransduced cells decreased with time because of the dilution of originally transduced cells by dividing native HEK293 cells. In the coinfection experiment with multiple viruses, HEK293 cells were simultaneously infected with rBV encoding a green, blue, or red fluorescent protein. Some cells showed coexpression of two or three fluorescent proteins (Fig. 1B).
Figure 1.

Transduction of mammalian cells with rBV-CMV-GFP. (A) Expression of GFP in ARPE19 cells transduced with rBV-CMV-GFP. (B) Expression of fluorescent proteins in HEK293 cells transduced by three rBVs (rBV-CMV-BFP, rBV-CMV-GFP, and rBV-CMV-RFP). (C–E) Detection of BVs in ARPE19 cells by transmission electron microscopy. (C) Virus particle engulfed by membrane processes at the cell surface; (D) virus particle inside vesicles in the cytoplasm; (E) BVs near the plasma membrane and inside a cytoplasmic vesicle close to the nucleus membrane. (C′–E′) Higher magnifications of (C–E), respectively. Magnification, (C) × 9080; (D) ×5290; (E) ×6670; (C′) ×70,400; (D′) ×50,300; (E′) × 101,000.
Cultures of ARPE19 infected with rBV-CMV-GFP were also analyzed by electron microscopy. BVs were observed at the entry into ARPE19 cell (Figs. 1C, 1E) and inside endosomal vesicles in the cytoplasm (Figs. 1D, 1E).
Subretinal Injections of rBV into the Mouse Retina
We used rBV carrying GFP under the control of the CMV promoter, rather than a cell type–specific promoter, because a goal of this study was to analyze which cell types are transducible by BVs. GFP expression was detected in RPE cells 3 days after subretinal injections of rBV-CMV-GFP (~106–107 infection units) into mouse eyes by fluorescence microscopy (Figs. 2A, 2C). No background fluorescence was observed in the control-injected mice (Figs. 2B, 2D). GFP expression in RPE cells ranged from local areas around the injection site (in ~50% of the 60 analyzed eyes) to widespread distribution across the entire retina (in ~25% of the eyes; Fig. 2A). In approximately 25% of the eyes, GFP expression was very low to undetectable. GFP expression was observed as soon as 20 hours after injection and persisted up to more than 2 weeks after subretinal injections. The peak of GFP expression was observed between days 1 and 4 after injection. RPE cells expressing GFP were detected in more than 50% of the eyes 2 weeks after injections. No GFP expression was observed 2 months after injection.
Figure 2.

GFP expression in mouse eye after subretinal injections of rBV-CMV-GFP. A section of mouse retina 3 days after subretinal injections with 1 μl rBV-CMV-GFP (A, C) or 1 μl rBV-CMV (B, D). (A, B) Fluorescence images. (C, D) Fluorescent view overlaid with bright-field view. GFP expression was observed across the mouse retina (A). There was no background fluorescence observed in mouse retina injected with the control rBV (B). OS, outer segment.
Subretinal injections with rBV stocks prepared by ultracentrifugation or by further purification on a sucrose gradient resulted in similar efficiency of GFP expression. The gradient step removed few contaminants from the cell culture supernatant as observed by SDS-PAGE analysis of rBV (data not shown). However, filtration of the rBV preparations on a 0.22-μm filter decreased the viral titer but also decreased the infiltration of macrophages after injection into the eye without affecting the transduction efficiency.
Intravitreal Injections of rBV into the Mouse Eye
Intravitreal injections of rBV-CMV-GFP (106–107 infection units) were performed to investigate transduction to intraocular cell layers other than the RPE. As shown in Figure 3, GFP expression was observed in multiple cell types. Fluorescence was observed in the corneal endothelium (Figs. 3B, 3D), the retinal inner nuclear layer, the ganglion cell layer (Figs. 3A, 3C), and in the RPE cell layer (Figs. 3A, 3B). Occasionally, we also observed GFP expression in the lens epithelium (Fig. 3B) and in photoreceptor cells (Fig. 3A). The retinal GFP expression was sporadically observed across all layers to the RPE and localized in one area of the retina. Fluorescence was also observed in cells, possibly Müller cells, extending from the inner nuclear layer to the ganglion cell layer (Fig. 3C).
Figure 3.

GFP expression in mouse eye after intravitreal injections of rBV-CMV-GFP. Mice were injected in the vitreous with 1 μl rBV-CMV-GFP. Fluorescence detected with an FITC filter was observed in the retina (A–C), cornea (B, D), and lens (B). INL, inner nuclear layer; ONL, outer nuclear layer; GCL, ganglion cell layer; lens, lens subcapsular anterior epithelium; cornea, posterior corneal endothelium.
Analysis of Inflammatory Response Induced by rBV
An analysis of the inflammation induced by rBV-CMV-GFP was performed at different times after injection. The sections were first analyzed for GFP expression and then used for immunocytochemistry with antibodies recognizing macrophages, granulocytes, dendritic cells, and natural killer cells (anti-CD11b); cytotoxic T cells (anti-CD8a); or T-helper cells (anti-CD4). Two days after injection into the subretinal space (time of optimal GFP expression), an infiltration of macrophages was observed in the subretinal space. There were also some macrophages across the retina and in the area where GFP expression was detected (data not shown). After intravitreal injection, macrophage infiltration was observed in the anterior chamber close to the corneal endothelium, where GFP expression was found. Macrophages were also detected in the vitreous and concentrated in areas of GFP expression (data not shown). No CD4+ or CD8+ cells were observed 2 days after injection.
Three days after injection into the subretinal space, many CD11b+ cells (60 ± 30 cells; number of positive cells in a 5-μm section/high-power field [HPF, 40×]) were found at the site of inoculation (Fig. 4A). A few CD4+ cells were also detected (10 ± 5 cells/HPF) at day 3 after injection (Fig. 4B). At more distal areas of the retina, CD11b+ (12 ± 4 cells/HPF) and CD4+ (3 ± 1 cells/HPF) were scattered, even though GFP expression was still observed (data not shown). No positive cells were observed in the retina of the animals injected with PBS or in the noninjected contralateral eye.
Figure 4.
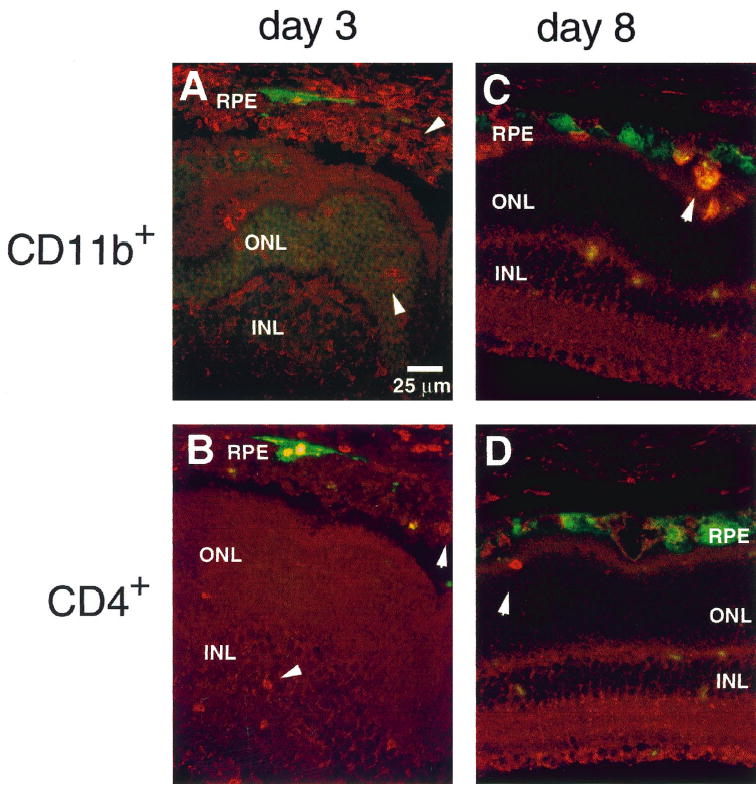
Histologic analysis of inflammation after subretinal injections of rBV-CMV-GFP. Mice were injected with a recombinant rBV-CMV-GFP, and their eyes were analyzed for GFP expression and inflammation 3 (A, B) or 8 (C, D) days after injections. The anti-specific cell markers were detected with streptavidin-Cy3. CD11b was expressed by granulocytes, macrophages, dendritic cells, and natural killer cells and CD4 by T-helper cells. The inflammation was analyzed close to the site of inoculation (A, B). An abundant infiltration of macrophages was detected in the subretinal space at the site of inoculation (A). A few CD4+cells were scattered over the retina and in the subretinal space (B). A few CD11b+ and CD4+ cells were detected 8 days after injection (C, D). See Figure 3 for abbreviations.
Eight days after subretinal injection, the inflammation was almost completely resolved. Very few CD11b+ and CD4+ cells were observed in areas of GFP expression (Figs. 4C, 4D); however, some clusters of CD4+ and macrophages were occasionally observed in the vitreous. CD4+ (30 ± 10 cells/HPF) and CD11b+ (15 ± 5 cells/HPF) cells were occasionally observed in the subretinal space and in the retina when the retina was still detached from the RPE. No CD8+ cells were detected. No inflammation or morphologic changes were observed by histologic examination of the retina 2 months after injection (data not shown).
The humoral response against the rBV-CMV-GFP after sub-retinal injection into mouse eyes, was analyzed using enzyme-linked immunosorbent assays (ELISAs). Anti-BV antibodies were detected at low levels 8 days after injection and had doubled 3 weeks after injection (data not shown).
ERG Analysis of Injected Eyes
ERG responses were recorded from injected eyes to test whether there was a functional change in the retina after rBV-CMV-GFP injection. No significant differences were observed between the ERG recordings obtained from four PBS- and rBV-CMV-GFP–injected eyes 6 weeks after injection (Fig. 5). These results suggest that there is no alteration of retinal function caused by subretinal injection of rBV-CMV-GFP or by GFP expression. There was also no difference between the retinoid content of noninjected and rBV-CMV-GFP–injected eyes, as observed by HPLC analysis at day 4 after injection (Haeseleer, unpublished data, 2000).
Figure 5.
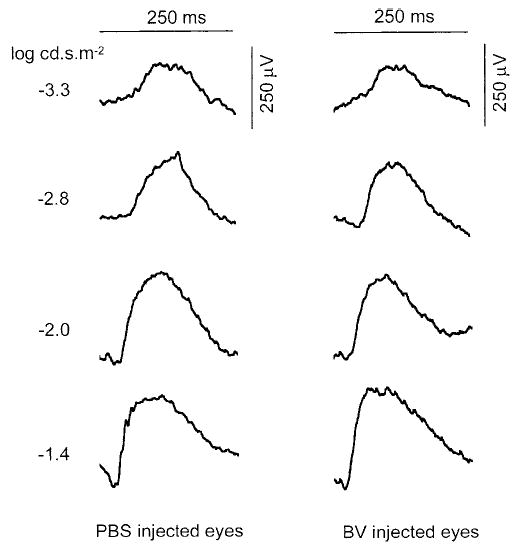
ERG recordings of mice subretinally injected with PBS or rBV-CMV-GFP. ERG responses were recorded 6 weeks after injection with PBS or rBV-CMV-GFP. Each trace is the average of 20 recordings (five responses for each four injected eyes). There were no significant differences between PBS- and rBV-CMV-GFP–injected eyes.
Discussion
Multiple viral vectors have been investigated as a vehicle for transferring genes into the eye, because different viral vectors may be necessary, depending on the type of disease to rescue. For example, depending on the particular condition or animal model, low transgene expression for a long period, strong expression for a short time, or an inducible expression may be needed. The BV, a pathogen for arthropods, is a possible candidate as a safe vehicle to transfer genes in vivo, because it is not capable of replicating in vertebrate cells. Another potential advantage of BV is that it can carry large fragments of recombinant DNA, which would allow the transfer of the gene and its promoter—a requirement for targeting the expression to the native tissue. Its capability of packaging large inserts is also important in cases in which multiple genes must be transferred simultaneously. Furthermore, cells can be coinfected with multiple rBVs.
As shown in this study, rBV is a promising vector for gene transfer into the eye. Our in vivo results show that rBV infected multiple cell types with higher transgene expression in epithelial and endothelial cells, such as the corneal endothelium, the lens epithelium, and the RPE. To a lesser extent, multiple neuronal cell types were also transduced by rBV. Specifically, when rBVs were injected into the subretinal space, marked GFP expression was observed only in the RPE cells; when the virus was injected in the vitreous, the GFP expression was spread throughout the eye, including the cornea and lens and across the retina to the RPE. One of the main functions of RPE cells is phagocytosis of the shed photoreceptor discs. When rBVs were injected into the subretinal space, it is possible that they not only infected RPE cells, but that they were also immediately phagocytosed by RPE cells and therefore were not able to reach the neural retina. van Loo et al.14 reported that rBV infects nondividing mammalian cells by endocytosis, followed by an acid-induced fusion event that releases the nucleocapsid into the cytoplasm and ultimately into the nucleus. From both subretinal and intravitreal injections, it appears that RPE cells are the primary cell type transduced by rBV, although, further experiments using cell-type–specific promoters are needed to determine which cell type is the most efficiently transduced by rBV.
The retinal area transduced by rBV varied from approximately a third to almost all the mouse retina. These differences are probably due to the variable volume of the injections reaching the subretinal space. Another factor that may be responsible for the variable success of gene transduction is the inactivation of BVs by the classic pathway of the complement system.22,29 However, the eye, in addition to brain and reproductive organs, possesses immune privilege.31,33 Therefore, it is unlikely that inactivation by the complement system occurs.
We have shown that rBVs induced some inflammation, observed as an influx of macrophages 3 days after injection. However, this inflammation was mostly resolved 8 days after injection, with only a few macrophages and CD4+ cells observed in the area of BV transduction. This limited inflammation response to the vector makes rBV an acceptable candidate for transfer of genes in vivo.
Compared with other viral vectors, the expression of recombinant genes transduced by rBV is detected as soon as 20 hours after injection. The expression is initiated earlier than that induced by transduction with rAVs or lentiviruses, with which it is observed 2 days after injections, or with rAAV, with which it is not detected until 1 to 2 weeks after injection. The expression lasts approximately 2 weeks for rBV-transduced genes compared with at least 3 months for rAAV, AV, and lentivirus.9,37–39 rBVs do not infect layers other than the RPE after subretinal injection. In contrast, rAAV can reach the ganglion cells in anterograde fashion after subretinal injection.37 rAAV is able to transduce the widest range of retinal cell types after subretinal injection (RPE cells, photoreceptor cells, ganglion cells layer, inner nuclear layer) in comparison with the AV, which mostly transduces RPE cells but also photoreceptors and Müller cells, and HIV-based vectors that can efficiently transduce RPE and photoreceptor cells, but seldom transduce bipolar cells.9
BVs transduced a wider range of retinal cell types after intravitreal injection than after subretinal injections, including distant RPE cells, the corneal endothelium, the retinal inner nuclear layer, the ganglion cell layer and, occasionally, lens epithelium cells and photoreceptors. Intravitreal injection of herpes viruses also results in efficient transgene expression in the remote RPE, as well as in ganglion cells and occasionally in the inner nuclear layer.8,40
Few photoreceptors expressed GFP after transduction by rBV. This infrequent expression in photoreceptor cells may be due to the inefficiency of the CMV promoter in these cells, rather than to low transduction by the viruses. The gene expression obtained after transduction by rAAV, AV, and lentivirus, is more efficient in photoreceptor cells when driven by the rhodopsin promoter.6,9 rBV-carrying reporter genes under the control of cell-type–specific promoter, will help in determining which cell type is efficiently transduced by them.
Gene transduction mediated by rBV was observed up to 14 days after injection. This transient transduction is probably due to the nonreplicative, episomal form of the viral double-stranded DNA and the instability of exogenous GFP in the RPE cells. However, a BV carrying the AAV Rep 78 gene and the recombinant cassettes between the AAV-inverted terminal repeats, has been shown to be capable of mediating the transduction of the recombinant gene into the AAV-binding site located on the human chromosome 19q13.3.41 Recently, Hüser et al.42 have shown that the insertion of a decay-accelerating factor into the BV envelope through genetic manipulation, can protect the BV against complement-mediated inactivation. Such approaches could be used to prolong the expression of transduced genes in vivo.
Acknowledgments
The authors thank Preston Van Hooser for help during the project, Hiroshi Ohguro and Tadao Maeda for help with the ERG recordings, Michael W. Kaplan for help with the preliminary experiments, Dan Possin for help with the electron microscopy, and Jing Huang for technical assistance.
Footnotes
Supported by National Institutes of Health Grants EY-08061 (KP) and EY-00347 (DAS); an award from Research to Prevent Blindness, Inc. to the Department of Ophthalmology at the University of Washington; an award from the Alcon Research Institute (KP); the Ruth and Milton Steinbach Fund; the E. K. Bishop Foundation; and a Foundation Fighting Blindness T. Wayne Robertson Career Development Award (DAS). KP is a Research to Prevent Blindness Senior Scientific Investigator.
References
- 1.Friedmann T. The Development of Human Gene Therapy. Friedmann T, ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999.
- 2.Anderson WF. Human gene therapy. Nature. 1998;392:25–30. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- 3.Ali RR, Sarra GM, Stephens C, de Alwis M, et al. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat Genet. 2000;25:306–310. doi: 10.1038/77068. [DOI] [PubMed] [Google Scholar]
- 4.Bennett J, Maguire AM, Cideciyan AV, et al. Stable transgene expression in rod photoreceptors after recombinant adeno-associated virus-mediated gene transfer to monkey retina. Proc Natl Acad Sci USA. 1999;96:9920–9925. doi: 10.1073/pnas.96.17.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett J, Zeng Y, Bajwa R, Klatt L, Li Y, Maguire AM. Adenovirus-mediated delivery of rhodopsin-promoted bcl-2 results in a delay in photoreceptor cell death in the rd/rd mouse. Gene Ther. 1998;5:1156–1164. doi: 10.1038/sj.gt.3300733. [DOI] [PubMed] [Google Scholar]
- 6.Flannery JG, Zolotukhin S, Vaquero MI, LaVail MM, Muzyczka N, Hauswirth WW. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:6916–6921. doi: 10.1073/pnas.94.13.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauswirth WW, Beaufrere L. Ocular gene therapy: Quo vadis? Invest Ophthalmol Vis Sci. 2000;41:2821–2826. [PubMed] [Google Scholar]
- 8.Liu XY, Brandt CR, Gabelt BT, Bryar PJ, Smith ME, Kaufman PL. Herpes simplex virus mediated gene transfer to primate ocular tissues. Exp Eye Res. 1999;69:385–395. doi: 10.1006/exer.1999.0711. [DOI] [PubMed] [Google Scholar]
- 9.Miyoshi H, Takahashi M, Gage FH, Verma IM. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichel MB, Hudde T, Ali RR, Wiedemann P. Gene transfer in ophthalmology. Ophthalmologe. 1999;96:570–577. doi: 10.1007/s003470050454. [DOI] [PubMed] [Google Scholar]
- 11.Lai CM, Shen WY, Constable I, Rakoczy PE. The use of adenovirus-mediated gene transfer to develop a rat model for photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2000;41:580–584. [PubMed] [Google Scholar]
- 12.Miller L. The Baculoviruses. New York: Plenum Press; 1997.
- 13.Volkman L, Goldsmith P. In vitro survey of Autographa californica nuclear polyhedrosis virus interaction with nontarget vertebrate host cells. Appl Environ Microbiol. 1983;45:1085–1093. doi: 10.1128/aem.45.3.1085-1093.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Loo ND, Fortunati E, Ehlert E, Rabelink M, Grosveld F, Scholte BJ. Baculovirus infection of nondividing mammalian cells: mechanisms of entry and nuclear transport of capsids. J Virol. 2001;75:961–970. doi: 10.1128/JVI.75.2.961-970.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann C, Sandig V, Jennings G, Rudolph M, Schlag P, Strauss M. Efficient gene-transfer into human hepatocytes by baculovirus vectors. Proc Natl Acad Sci USA. 1995;92:10099–10103. doi: 10.1073/pnas.92.22.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barsoum J, Brown R, McKee M, Boyce FM. Efficient transduction of mammalian cells by a recombinant baculovirus having the vesicular stomatitis virus G glycoprotein. Hum Gene Ther. 1997;8:2011–2018. doi: 10.1089/hum.1997.8.17-2011. [DOI] [PubMed] [Google Scholar]
- 17.Boyce FM, Bucher NLR. Baculovirus-mediated gene transfer into mammalian cells. Proc Natl Acad Sci USA. 1996;93:2348–2352. doi: 10.1073/pnas.93.6.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilello JP, Delaney WE, Boyce FM, Isom HC. Transient disruption of intercellular junctions enables baculovirus entry into nondividing hepatocytes. J Virol. 2001;75:9857–9871. doi: 10.1128/JVI.75.20.9857-9871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bucher NLR, Stieglitz-Joseph K, Radner B, Franco E, Boyce FM. Efficient of gene transfer by a baculovirus vector into primary cultures of adult rat hepatocytes depends on their proliferative state (Abstract) Hepatology. 1996;24:38. [Google Scholar]
- 20.Condreay JP, Witherspoon SM, Clay WC, Kost TA. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc Natl Acad Sci USA. 1999;96:127–132. doi: 10.1073/pnas.96.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grotzinger C, Lehnert W, Hofmann C, Schneiders F, Strauss M, Wiedenmann B. Baculovirus-mediated gene transfer into human hepatocytes. Gastroenterology. 1997;112:A1275–A1275. [Google Scholar]
- 22.Hofmann C, Strauss M. Baculovirus-mediated gene transfer in the presence of human serum or blood facilitated by inhibition of the complement system. Gene Ther. 1998;5:531–536. doi: 10.1038/sj.gt.3300607. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann C, Huser A, Lehnert W, Strauss M. Protection of baculovirus-vectors against complement-mediated inactivation by recombinant soluble complement receptor type 1. Biol Chem. 1999;380:393–395. doi: 10.1515/BC.1999.052. [DOI] [PubMed] [Google Scholar]
- 24.Ma L, Tamarina N, Wang Y, et al. Baculovirus-mediated gene transfer into pancreatic islet cells. Diabetes. 2000;49:1986–1991. doi: 10.2337/diabetes.49.12.1986. [DOI] [PubMed] [Google Scholar]
- 25.Merrihew RV, Clay WC, Condreay JP, Witherspoon SM, Dallas WS, Kost TA. Chromosomal integration of transduced recombinant baculovirus DNA in mammalian cells. J Virol. 2001;75:903–909. doi: 10.1128/JVI.75.2.903-909.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkis C, Serguera C, Petres S, et al. Efficient transduction of neural cells in vitro and in vivo by a baculovirus-derived vector. Proc Natl Acad Sci USA. 2000;97:14638–14643. doi: 10.1073/pnas.260472897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoji I, Aizaki H, Tani H, et al. Efficient gene transfer into various mammalian cells, including non-hepatic cells, by baculovirus vectors. J Gen Virol. 1997;78:2657–2664. doi: 10.1099/0022-1317-78-10-2657. [DOI] [PubMed] [Google Scholar]
- 28.Yap CC, Ishii K, Aoki Y, et al. A hybrid baculovirus-T7 RNA polymerase system for recovery of an infectious virus from cDNA. Virology. 1997;231:192–200. doi: 10.1006/viro.1997.8537. [DOI] [PubMed] [Google Scholar]
- 29.Sandig V, Hofmann C, Steinert S, Jennings G, Schlag P, Strauss M. Gene transfer into hepatocytes and human liver tissue by baculovirus vectors. Hum Gene Ther. 1996;7:1937–1945. doi: 10.1089/hum.1996.7.16-1937. [DOI] [PubMed] [Google Scholar]
- 30.Airenne KJ, Hiltunen MO, Turunen MP, et al. Baculovirus-mediated periadventitial gene transfer to rabbit carotid artery. Gene Ther. 2000;7:1499–1504. doi: 10.1038/sj.gt.3301269. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson TA, Griffith TS. A vision of cell death: insights into immune privilege. Immunol Rev. 1997;156:167–184. doi: 10.1111/j.1600-065x.1997.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan HJ, Leibole MA, Tezel T, Ferguson TA. Fas ligand (CD95 ligand) controls angiogenesis beneath the retina. Nat Med. 1999;5:292–297. doi: 10.1038/6509. [DOI] [PubMed] [Google Scholar]
- 33.Niederkorn J. Immune privilege and immune regulation in the eye. Adv Immunol. 1990;48:191–226. doi: 10.1016/s0065-2776(08)60755-5. [DOI] [PubMed] [Google Scholar]
- 34.O’Reilly DR, Miller LK, Luckow VA. Baculovirus expression vectors: a laboratory manual. New York: Oxford University Press; 1994.
- 35.Kruh J. Effects of sodium-butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982;42:65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- 36.Barthel LK, Raymond PA. Improved method for obtaining 3-μm cryosections for immunocytochemistry. J Histochem Cytochem. 1990;38:1383–1388. doi: 10.1177/38.9.2201738. [DOI] [PubMed] [Google Scholar]
- 37.Bennett J, Duan DS, Engelhardt JF, Maguire AM. Real-time, noninvasive in vivo assessment of adeno-associated virus-mediated retinal transduction. Invest Ophthalmol Vis Sci. 1997;38:2857–2863. [PubMed] [Google Scholar]
- 38.Reichel MB, Ali RR, Thrasher AJ, Hunt DM, Bhattacharya SS, Baker D. Immune responses limit adenovirally mediated gene expression in the adult mouse eye. Gene Ther. 1998;5:1038–1046. doi: 10.1038/sj.gt.3300691. [DOI] [PubMed] [Google Scholar]
- 39.Guy J, Qi XP, Muzyczka N, Hauswirth WW. Reporter expression persists 1 year after adeno-associated virus-mediated gene transfer to the optic nerve. Arch Ophthalmol. 1999;117:929–937. doi: 10.1001/archopht.117.7.929. [DOI] [PubMed] [Google Scholar]
- 40.Spencer B, Agarwala S, Miskulin M, Smith M, Brandt CR. Herpes simplex virus-mediated gene delivery to the rodent visual system. Invest Ophthalmol Vis Sci. 2000;41:1392–1401. [PubMed] [Google Scholar]
- 41.Palombo F, Monciotti A, Recchia A, Cortese R, Ciliberto G, La Monica N. Site-specific integration in mammalian cells mediated by a new hybrid baculovirus adeno-associated virus vector. J Virol. 1998;72:5025–5034. doi: 10.1128/jvi.72.6.5025-5034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hüser A, Rudolph M, Hofmann C. Incorporation of decay-accelerating factor into the baculovirus envelope generates complement-resistant gene transfer vectors. Nat Biotechnol. 2001;19:451–455. doi: 10.1038/88122. [DOI] [PubMed] [Google Scholar]


