Abstract
This study was initiated to understand whether differential biological control efficacy of Enterobacter cloacae on various plant species is due to differences in the ability of E. cloacae to inactivate the stimulatory activity of seed exudates to Pythium ultimum sporangium germination. In biological control assays, E. cloacae was effective in controlling Pythium damping-off when placed on the seeds of carrot, cotton, cucumber, lettuce, radish, tomato, and wheat but failed to protect corn and pea from damping-off. Seeds from plants such as corn and pea had high rates of exudation, whereas cotton and cucumber seeds had much lower rates of exudation. Patterns of seed exudation and the release of P. ultimum sporangium germination stimulants varied among the plants tested. Seed exudates of plants such as carrot, corn, lettuce, pea, radish, and wheat were generally more stimulatory to P. ultimum than were the exudates of cotton, cucumber, sunflower, and tomato. However, this was not directly related to the ability of E. cloacae to inactivate the stimulatory activity of the exudate and reduce P. ultimum sporangium germination. In the spermosphere, E. cloacae readily reduced the stimulatory activity of seed exudates from all plant species except corn and pea. Our data have shown that the inability of E. cloacae to protect corn and pea seeds from Pythium damping-off is directly related to its ability to inactivate the stimulatory activity of seed exudates. On all other plants tested, E. cloacae was effective in suppressing damping-off and inactivating the stimulatory activity of seed exudates.
Pythium ultimum is a widespread and important oomycete plant pathogen causing seed decay, pre- and postemergence damping-off, and root rot in many plant species (8). The sporangium is a major survival structure in soil, even though oospores are produced (29, 30). This is especially true for asexual strains of P. ultimum (10). Sporangia are exogenously dormant propagules that will germinate in response to stimulants from the seeds of host plants (14, 15, 17, 19, 28). This response occurs within the first few hours of seed germination, resulting in seed infection within the first 24 h after sowing (13). The molecules in seed exudates eliciting these rapid responses are largely unknown (14) but are believed to consist of long-chain unsaturated fatty acids (28).
Enterobacter cloacae is a common plant-associated rhizobacterium effective in controlling Pythium diseases (3, 5-7, 9, 12, 13, 16, 22, 23, 31, 33, 34). Other biological control agents such as Pseudomonas and Bacillus species suppress Pythium diseases largely through the biosynthesis of antibiotics or other Pythium-inhibitory substances (2, 32, 34). However, no antibiotic production or parasitism has been found in E. cloacae, even though E. cloacae attaches to the hyphae of P. ultimum-colonizing seed surfaces (16). The control of Pythium damping-off by E. cloacae can be attributed to its ability to reduce or eliminate responses of sporangia to germinating seeds by metabolizing long-chain fatty acids, mainly linoleic acid, released from the seed during germination (28, 33, 34).
In earlier studies, we demonstrated that E. cloacae protected cucumber, cotton, and ryegrass from damping-off but was ineffective in protecting the seeds of snap bean, lima bean, soybean, corn, and pea (16). Results indicated that this response was regulated by the exudation patterns of seed exudate carbohydrates, since E. cloacae was effective only on those plants whose exudates contained low levels of carbohydrates. Additional experiments in which different mono-, di-, and trisaccharides were added with E. cloacae to the spermosphere of cucumber revealed that some sugars (e.g., d-galactose, d-glucose, sucrose, and β-methyl-d-glucoside) significantly reduced the efficacy of biological control of Pythium damping-off, whereas other sugars (e.g., 3-O-methyl-d-glucose, d-trehalose, l-glucose, and l-sorbose) did not (16). In culture, d-galactose, d-glucose, sucrose, and β-methyl-d-glucoside were capable of supporting abundant growth of E. cloacae, whereas 3-O-methyl-d-glucose, d-trehalose, l-glucose, and l-sorbose supported no growth of E. cloacae.
We also observed that none of the sugars tested increased the colonization or infection of seeds by P. ultimum, suggesting that the differential responses observed were due to direct effects on E. cloacae. Because of the importance of seed exudates in controlling the spermosphere environment, we have hypothesized that seed exudates may affect the ability of E. cloacae to inactivate sporangium germination stimulants and thus affect disease control. We therefore initiated this study to understand whether differential efficacy of E. cloacae on different plant species could be explained by differences in the ability of E. cloacae to inactivate the stimulatory activity of seed exudates to P. ultimum sporangium germination and thus protect plants from damping-off. The objectives of our study were as follows: (i) to determine the exudation patterns and levels of P. ultimum sporangium germination stimulation in the seed exudates of different plant species, (ii) to determine the ability of E. cloacae to inactivate the stimulatory activity of seed exudates from different plant species in vitro and in plant bioassays, and (iii) to evaluate the biological control efficacy of E. cloacae when placed on seeds of different plant species.
MATERIALS AND METHODS
Collection of seed exudate in vitro.
Seed exudates were collected from 10 plant species (Table 1) by methods similar to those described previously (17, 19). Twelve grams of seed was used for all seed exudate preparations. Seeds with no cracks or other visible deformations were surface disinfected for 5 min (10% sodium hypochlorite) or 20 min (0.5% sodium hypochlorite) in solutions of sodium hypochlorite containing 1 or 2 drops of Tween 20 (polyoxyethylene sorbitan monolaurate; Sigma Chemical Co., St. Louis, Mo.) per 100 ml as a wetting agent. Seeds were then rinsed three times with sterile water. Surface- disinfested seeds were added to 100 ml of sterile distilled water and kept at 25°C on a rotary shaker at 120 rpm. After incubation, the seeds were removed, and the solution was filtered sequentially through two layers of Whatman no. 1 filter paper (0.8- and 0.2-μm-pore-size filters), evaporated under vacuum at 37°C to a volume of 2 ml, vacuum dried, weighed, and stored under argon gas at −20°C. There were three replicate collections per experiment, and the experiment was repeated three times.
TABLE 1.
Plant species and cultivars tested in this study
| Plant species | Common name | Cultivar | Mean seed wt (mg) | Exudate wt (mg/g of seed)a |
|---|---|---|---|---|
| Daucus carota L. | Carrot | Scarlet Nantes | 0.08 | 9.6 A |
| Zea mays L. | Corn | Hybridb | 7.59 | 9.6 A |
| Gossypium hirsutum L. | Cotton | Deltapine 50 | 4.50 | 7.6 AB |
| Cucumis sativus L. | Cucumber | Marketmore | 1.19 | 3.7 C |
| Lactuca sativa L. | Lettuce | Green Grand Rapids | 0.05 | 8.7 A |
| Pisum sativum L. | Pea | Sugar Ann | 8.92 | 6.1 B |
| Raphanus sativum L. | Radish | Specialty | 0.62 | 7.3 AB |
| Helianthus annuus L. | Sunflower | 891-F1 | 6.83 | 6.4 B |
| Lycopersicon esculentum Mill. | Tomato | New Yorker Special 313A | 0.13 | 2.8 C |
| Triticum aestivum L. | Wheat | Batavia | 1.69 | 3.4 C |
Exudate collected after 6 h of incubation. Means followed by the same letter are not significantly (P = 0.05) different according to Tukey's method of pairwise comparisons.
Cultivar is a hybrid of Yellow Super Sweet crossed with Northern Xtra-Sweet.
Production of P. ultimum sporangia.
Sporangia of P. ultimum were used to assess the stimulatory activity of seed exudates and for inoculum in bioassays. P. ultimum strain P4 was routinely grown on potato dextrose agar (PDA) prior to use in sporangium germination or biocontrol experiments. For production of P. ultimum sporangia, a mycelial disk from a 4- to 10-day-old PDA culture (24°C) was transferred to a defined mineral salts medium containing soy lecithin (SM+L). SM+L was used because sporangia produced on this medium respond to stimulants in a manner that mimicked sporangia produced on plant tissue (17, 19). After a 5-day incubation at 27°C, 5-mm-diameter mycelial disks were excised, placed in sterile petri dishes, and leached for two consecutive 10-min intervals in a buffer (pH 5.8) containing 10 mM Ca(NO3)2 · 4H2O, 4 mM MgSO4 · 7H2O, and 5 mM KNO3 (1), followed by a final 3-h interval in darkness (17, 19). Buffer was replaced after each leaching period. After the final leaching period, leachate was removed, and the disks were rinsed twice with sterile water and kept at 24°C in darkness for 1 day. Sporangia were used immediately in germination assays and biocontrol experiments.
Germination of sporangia in seed exudate.
Sporangia were tested in in vitro germination assays as described previously (17, 19) to determine the stimulatory activity of seed exudates. Ten microliters of seed exudate, adjusted to concentrations ranging from 1 to 20 mg/ml in 10 mM ammonium acetate buffer (pH 5.5), was placed directly on each of three agar disks positioned on a microscope slide. After a 4-h incubation at 24°C, disks were stained with 0.03% acid fuchsin in 85% lactic acid and observed under the microscope (at a magnification of ×40). The percentage of sporangia that germinated was determined from random observations across the sporangial disk. Sporangia were considered germinated if a developing germ tube was visible. There were three replicate disks, and the experiment was repeated three times.
Inactivation of seed exudate stimulation of sporangium germination by E. cloacae.
These experiments were designed to determine whether E. cloacae could eliminate the stimulatory activity of exudates released from seeds of different plant species. Wells of a 96-well microtiter plate were filled with 50 μl of seed exudate at various concentrations. Exudates were collected after 2 or 6 h of incubation of seeds (2-h or 6-h exudates, respectively). Fifty microliters of a bacterial culture grown overnight in Trypticase soy broth (TSB) was added to each well. After the cells were washed with ammonium acetate buffer, the final concentration of bacteria was adjusted to 5 × 106 CFU/ml in 10 mM ammonium acetate (pH 6.0). After 0, 3, and 6 h, the solution in each well was removed and passed through a 0.2-μm-pore-size microtube filter. The cell-free filtrates were stored at −20°C under argon gas and then used in sporangium germination assays as described above. There were four replicate wells per treatment, and experiments were repeated twice.
Assays to assess biological control and sporangium germination in the spermosphere.
Glass cylinders (2.2 cm high; 2.2-cm diameter) were placed on sheets of moist blotter paper positioned on a Plexiglas plate. The paper was marked so that the centers of the cylinders were 4 cm apart. Washed sand (particles ranging in size from 0.5 to 1 mm) was placed in each cylinder at a depth of 6 or 9 mm for plants with small seeds (carrot, cucumber, lettuce, radish, and tomato) and large seeds (corn, cotton, pea, sunflower, and wheat), respectively, followed by an agar disk containing P. ultimum sporangia. The agar disk was then covered with 3 mm of sand. One surface-disinfested seed of corn, cotton, cucumber, pea, sunflower and wheat, 5 seeds (ca. 1 g) of radish, 10 seeds (ca. 1 g) of tomato, or 20 seeds (ca. 1 g) of carrot and lettuce were placed in each cylinder. One milliliter of bacterial suspension prepared as described above was poured over the seeds. Seeds were covered with a 3 or 6 mm depth of additional sand for the large or small seeds, respectively. One milliliter of bacterial suspension was then poured over the sand. Control seeds were drenched with sterile distilled water. Each glass plate supporting the filter paper and cylinders was placed in a clear plastic box, and the entire assembly was incubated at 25°C with 16 h of light. Cylinders were watered daily with sterile water.
For sporangium germination assays, 10 replicate disks were removed after 6, 24, and 48 h of incubation, and the percentage of germinated sporangia was determined as described above. For biological control assays, cylinders were incubated for 10 days, and healthy seedling stands were determined. There were 10 replicate cylinders per treatment. Biocontrol efficacy was calculated using the following formula: efficacy = [1 − (nontrt/noninoc − trt/inoc)/(nontrt/noninoc − nontrt/inoc)] × 100, where nontrt/noninoc is the number of sporangia that were not treated with Pythium and not inoculated with E. cloacae that germinated, trt/inoc is the number of sporangia that were treated with E. cloacae and inoculated with P. ultimum that germinated, and nontrt/inoc is the number of sporangia that were not treated with E. cloacae but were inoculated with P. ultimum that germinated. Sporangium germination experiments and biocontrol experiments were each repeated three times.
Statistical analysis.
Data from nearly all experiments were analyzed using analysis of variance. Means were separated by the least significant difference test or Tukey's method for pairwise comparisons. When necessary, sporangium germination data were normalized by performing arcsin transformations. In dose-response experiments with seed exudates, the concentrations that induce 50% sporangium germination (EC50s) of exudates from different plant species were estimated by probit analyses. All experiments were conducted at least three times. Where no significant experiment effects were observed, data from each of the three experiments were pooled and reanalyzed.
RESULTS
Seed exudation in vitro.
Levels of exudates released from germinating seeds varied greatly in the different species (Table 1). Seeds of carrot, corn, cotton, lettuce, and radish released higher levels of solids during the first 6 h of seed imbibition than plant species such as cucumber, tomato, and wheat. Exudate levels ranged from 2.8 to 9.6 mg/seed. For four representative plants (cotton, corn, cucumber, and pea), exudation dynamics over the first 24 h of seed germination were determined in detail (data not shown). Exudation from seeds of all four tested plant species was evident as early as 30 min after the start of imbibition. In cucumber, exudation was low (<4 mg/g of seeds) even after up to 24 h of incubation. Exudation from seeds of corn, cotton, and peas increased with increasing incubation period up to 12 h of imbibition. The amount of exudates did not increase significantly beyond 12 h of imbibition.
Stimulatory activity of seed exudates to P. ultimum sporangia.
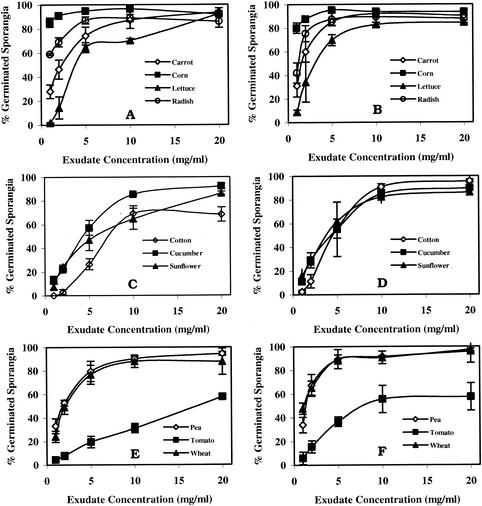
The higher the exudate concentrations to which the sporangia were exposed, the greater the percentage of germinated sporangia (Fig. 1). However, the stimulatory activity of the exudates varied significantly in the plants tested. At the highest concentration (20 mg/ml), the percentage of germinated sporangia was greater than 85% in response to the 2-h exudates for all plant species except for cotton and tomato. With 6-h exudates, all species but tomato induced greater than 85% germinated sporangia. Corn, radish, wheat, and pea seed exudates were the most stimulatory to P. ultimum sporangium germination across all concentrations, whereas sunflower, cucumber, cotton, and tomato seed exudates were the least stimulatory on the basis of EC50s (Table 2). Greater than 80% sporangium germination was induced by corn seed exudates in as little as 30 min of imbibition when added at concentrations as low as 2 mg/ml (data not shown).
FIG. 1.
Germination of sporangia of P. ultimum in response to 2-h seed exudates (A, C, and E) and 6-h seed exudates (B, D, and F) from different plant species. Seeds with high (A and B), moderate (C and D), and low (E and F) levels of exudation were used (grouped according to the level of solid exudation at 2 h). Means ± standard deviations (error bars) are shown.
TABLE 2.
Stimulatory activity of seed exudate from various plant species to the germination of P. ultimum sporangia
| Plant species | 2-h exudate
|
6-h exudate
|
||
|---|---|---|---|---|
| EC50 (mg/ml)a | Goodness of fit (P value) | EC50 (mg/ml) | Goodness of fit (P value) | |
| Corn | 0.01 | 0.126 | 0.06 | 0.429 |
| Radish | 0.41 | 0.140 | 0.44 | 0.001 |
| Pea | 1.81 | 0.384 | 1.47 | 0.048 |
| Carrot | 2.19 | 0.099 | 1.59 | 0.029 |
| Wheat | 2.21 | 0.090 | 1.10 | 0.410 |
| Lettuce | 3.55 | 0.003 | 2.46 | 0.001 |
| Cucumber | 3.90 | 0.248 | 3.93 | 0.358 |
| Sunflower | 5.39 | 0.178 | 3.43 | 0.094 |
| Cotton | 9.21 | 0.001 | 4.41 | 0.234 |
| Tomato | 16.89 | 0.748 | 10.43 | 0.133 |
Dose-response regressions from the probit analysis were significant at P < 0.001 for exudates from all of the plant species tested.
In vitro degradation of seed exudate by E. cloacae.
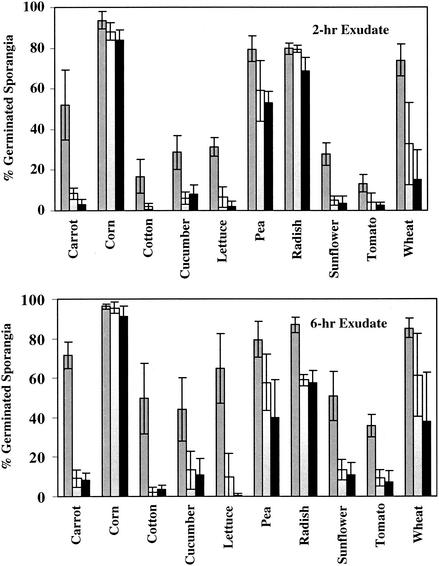
E. cloacae grown for 3 or 6 h in the presence of seed exudates (collected at 2 or 6 h of imbibition) reduced the stimulatory activity to P. ultimum sporangia (Fig. 2). E. cloacae failed to reduce the stimulatory activity of 2-h exudates of corn, pea, and radish after a 3-h incubation time. Significant reductions in the stimulatory activity of seed exudates collected from all other species were observed. By 6 h of incubation, significant reductions in the stimulatory activity of seed exudates compared to that of the noninoculated control were observed with all plant species except corn. Despite this, high levels of stimulatory activity remained in exudates from corn, pea, and radish.
FIG. 2.
In vitro germination of sporangia of P. ultimum in 2-h and 6-h seed exudates of various plant species following incubation with E. cloacae for 0 (  ), 3 (□), and 6 (▪) h. Means ± standard deviations (error bars) are shown.
), 3 (□), and 6 (▪) h. Means ± standard deviations (error bars) are shown.
Similar trends were observed among 6-h exudates. After 3 h of E. cloacae incubation, there were no significant reductions in the stimulatory activity of corn, pea, or wheat seed exudates compared to that of the noninoculated control. However, after 6 h of E. cloacae incubation, only corn seed exudates retained levels of stimulatory activity that did not differ from that of noninoculated exudates. Even after 6 h of incubation, high levels of stimulatory activity remained in the exudates from corn, pea, radish, and wheat. In carrot, cotton, cucumber, lettuce, sunflower, and tomato seed exudates, sporangium germination was reduced to less than 15% following a 3-h incubation with E. cloacae. When the culture filtrate of E. cloacae was used as a control, sporangium germination was not stimulated (data not shown).
Reduction of sporangium germination by E. cloacae in the spermospheres of different plant species.
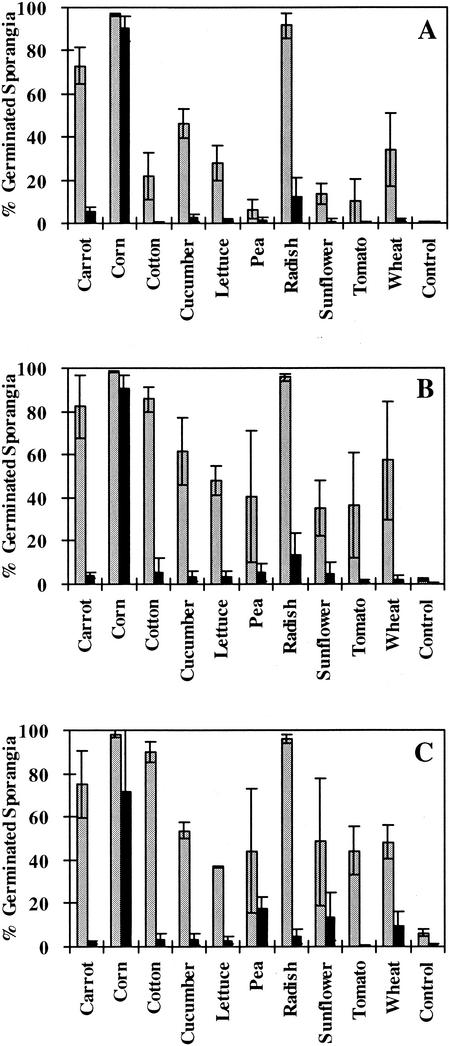
When tested on seeds sown in plant bioassays, E. cloacae was able to reduce the germination of P. ultimum sporangia (Fig. 3). By 6 h after sowing, germination of P. ultimum sporangia in the spermospheres of corn, pea, and tomato treated with E. cloacae did not differ significantly from the levels of germination in the spermosphere of nontreated seeds. However, germination of P. ultimum sporangia in the spermosphere of tomato was significantly reduced by 24 and 48 h after sowing. In the spermospheres of carrot, cotton, cucumber, lettuce, pea, radish, sunflower, tomato, and wheat, germination of P. ultimum was reduced to less than 15% when seeds were treated with E. cloacae. In the absence of seeds, little or no sporangium germination was observed.
FIG. 3.
Germination in soil of P. ultimum sporangia in response to seeds of various plant species treated with E. cloacae. Seeds treated with E. cloacae (∼5 × 106 cells/seed) (▪) and seeds that were not treated with E. cloacae (  ) are shown. Sporangia were removed from soil adjacent to seeds after 6 (A), 24 (B), or 48 (C) h.
) are shown. Sporangia were removed from soil adjacent to seeds after 6 (A), 24 (B), or 48 (C) h.
Biological control efficacy of E. cloacae on seeds of various plant species.
P. ultimum caused preemergence damping-off in all plant species tested with the exception of radish, where both preemergence and postemergence damping-off symptoms were observed (Table 3). Sunflower seedlings were not severely infected by P. ultimum, and there was a significant number of healthy seedlings (55.6%) among the plants tested. In the absence of Pythium inoculum, the percentages of healthy seedlings varied, ranging from 68.1 for lettuce to 100.0 for corn. Furthermore, seeds treated with E. cloacae but in the absence of Pythium gave rise to healthy seedling stands that varied from 55.6% for wheat to 100.0% for corn. Biocontrol efficacy of E. cloacae was calculated to account for this variation in the healthy seedling stands in the different plant species. E. cloacae was most effective in protecting seedlings from Pythium damping-off when placed on seeds of carrot, cotton, cucumber, lettuce, radish, sunflower, tomato, and wheat. Efficacy ranged from 62.1 to 81.5%. E. cloacae was significantly less effective when placed on the seeds of corn and pea, for which the biocontrol efficacy was 20.0 and 31.4%, respectively.
TABLE 3.
Biological control of Pythium damping-off on various plant species by E. cloacae
| Plant species | Healthy seedlings (%)a
|
Efficacyd | |||
|---|---|---|---|---|---|
| Noninoculated
|
Inoculatedb
|
||||
| Nontreated | E. cloacaec | Nontreated | E. cloacae | ||
| Carrot | 70.7 B | 67.6 AB | 5.7 B | 49.0 BC | 66.7 AB |
| Corn | 100.0 A | 88.9 AB | 38.9 A | 51.1 BC | 20.0 C |
| Cotton | 88.9 AB | 77.8 AB | 0.0 B | 69.4 AB | 78.1 A |
| Cucumber | 80.6 AB | 83.3 AB | 0.0 B | 50.0 BC | 62.1 AB |
| Lettuce | 68.1 B | 76.8 AB | 12.1 AB | 55.6 B | 77.7 A |
| Pea | 97.2 A | 100.0 A | 0.0 B | 30.6 C | 31.4 BC |
| Radish | 92.2 AB | 86.7 AB | 8.3 AB | 70.6 AB | 74.2 AB |
| Sunflower | 94.4 AB | 80.6 AB | 55.6 A | 83.3 A | 71.4 AB |
| Tomato | 85.8 AB | 78.9 AB | 0.3 B | 58.3 BC | 67.9 AB |
| Wheat | 75.0 B | 55.6 B | 0.0 B | 61.1 B | 81.5 A |
Healthy seedling stands were determined 7 days after sowing.
Inoculated by placing a disk containing P. ultimum sporangia adjacent to the seed (see Materials and Methods).
Coated onto the seed at the rate of 5.6 × 106 cells/seed.
Efficacy = [1 − (nontrt/noninoc − trt/inoc)/(nontrt/noninoc − nontrt/inoc)] × 100, Numbers in each column followed by the same letter are not significantly different (P = 0.05) according to Tukey's method for pairwise comparisons. where nontrt/noninoc is the number of sporangia that were not treated with Pythium and not inoculated with E. cloacae that germinated, trt/inoc is the number of sporangia that were treated with E. cloacae and inoculated with P. ultimum that germinated, and nontrt/inoc is the number of sporangia that were not treated with E. cloacae but were inoculated with P. ultimum that germinated.
DISCUSSION
Previous studies have shown that E. cloacae can effectively protect plants such as cotton (13, 33, 34), cucumber (6, 16), table beets (31), radish (16), lettuce (12), and bent grass (18) from Pythium damping-off when applied to the seeds. However, E. cloacae is much less effective when applied to seeds of corn (16), pea (6, 16), soybean, snap bean, and lima bean (16). Our study has confirmed that E. cloacae is less effective in protecting plants such as corn and pea from Pythium damping-off than in protecting cotton, cucumber, lettuce, and radish. We have further documented the efficacy of E. cloacae in controlling Pythium damping-off when placed on seeds of carrot, sunflower, tomato, and wheat.
The success of E. cloacae as a biological control organism is directly related to its ability to rapidly interfere with the early responses of Pythium propagules to germinating seeds (13, 16, 33, 34). Based on studies with cotton, unsaturated fatty acids such as linoleic and oleic acids are largely responsible for these rapid responses of Pythium propagules to germinating seeds (28). The metabolism of these fatty acid stimulants by E. cloacae early in seed germination will prevent seed infections and protect plants from damping-off (33, 34). Similar responses involving different molecules have been observed in other systems with seed-applied microorganisms (3, 4, 21).
Because of the key role played by seed exudates in Pythium pathogenesis and the important interaction of E. cloacae with seed exudate components in determining the success or failure of biological control, we hypothesized that the different biological control efficacies of E. cloacae on seeds of different plant species would be related to the differential ability of E. cloacae to rapidly inactivate the stimulatory activity of exudates to P. ultimum sporangia through the metabolism of fatty acids.
In examining the inactivation of exudate stimulants by E. cloacae, we observed that E. cloacae could rapidly (within 6 h) inactivate exudates collected in vitro from seeds of all plants except corn, with reduced inactivation on pea and radish. Also, when studied in plant bioassays, E. cloacae was ineffective in inactivating sporangia germination stimulation only from corn and pea at all time periods studied up to 48 h. These are the same seeds on which E. cloacae was ineffective in biocontrol assays. This association between lack of efficacy on corn and pea and the inability to inactivate seed exudate stimulants of P. ultimum sporangium germination in the spermosphere point to the importance of exudate interactions in the biological control activity of E. cloacae.
We did, however, observe some discrepancies between in vitro inactivation, in vivo inactivation, and biocontrol efficacy, particularly with radish, sunflower, and wheat. For example, E. cloacae was slow to inactivate radish exudates in vitro but inactivated them very rapidly in the spermosphere. The reasons for the discrepancy between in vitro inactivation of radish seed exudates by E. cloacae and the lack of inactivation when tested in the spermosphere is not known but likely involves the way in which the in vitro exudate was collected. The responses of P. ultimum sporangia to exudate collected in vitro over a 2- or 6-h period represent responses to cumulative levels of exudation for those time periods. At either 2 or 6 h, E. cloacae was introduced at a given population level and stage of development. However, in plant bioassays, E. cloacae, P. ultimum sporangia, and the seed were introduced at the time that the seed were sown. Therefore, sporangium germination and the impact of E. cloacae on sporangium germination are the results of concurrent germination and exudate inactivation as stimulants are released from the seed and not cumulative exudation, which was evaluated in in vitro experiments. It is possible, therefore, that in in vitro studies, because of the accumulation of exudate molecules and the possibly higher imbibition rates than those of plant bioassays, different compounds were released from the seeds or the timing of their release was not the same as what would have occurred in the spermosphere. Compounds that interfere with the ability of E. cloacae to inactivate stimulatory molecules that appeared in vitro but not in the spermosphere could explain this observation.
We would predict that if the biological control activity of E. cloacae were related simply to its ability to indiscriminately utilize seed exudate molecules for growth, seeds releasing the highest levels of exudate early in the germination process or those seeds with the highest levels of stimulatory activity early in germination would support less biological control activity than those plants whose seeds release lower levels of exudate or lower levels of stimulatory activity. Within this narrow time frame (0 to 6 h), E. cloacae could not consume or inactivate sufficient levels of exudate stimulants to reduce their availability to P. ultimum sporangia. Our results do not support this prediction. First, we demonstrated that seeds of different plant species vary not only in the amount of exudate released early in the imbibition process but also in the stimulatory activity of those exudates to P. ultimum sporangia. Within the first few hours of seed germination, seeds such as corn and pea (plants on which E. cloacae was ineffective in suppressing Pythium damping-off) released higher total amounts of exudate than seeds such as cotton or cucumber (plants on which E. cloacae was effective). However, other seeds on which E. cloacae was highly effective (i.e., lettuce, radish, and wheat) also released high levels of total exudate or high levels of stimulatory activity early in seed germination. Additionally, pea seeds released significantly lower levels of total exudates than seeds of corn. From the above observations, we have reasoned that the exudates of different plant species either contain levels of nonfatty acid stimulants, some of which E. cloacae cannot metabolize, or they contain compounds that prevent the metabolism of known fatty acid stimulants.
Although Pythium germination stimulants other than fatty acids may be present in seed exudates (28), other evidence suggests that it is likely that other types of compounds are present in seed exudates that prevent the metabolism of fatty acids. For example, corn and pea seeds release high levels of simple sugars early in seed germination (16, 24, 25). During the first 24 h of seed germination, pea and corn seeds release nearly 3,000 and 1,800 times, respectively the amount of carbohydrates found in cucumber seed exudates (24). Cucumber exudate consists largely of glucose and fructose, whereas high levels of stachyose are found in pea exudate. Seeds such as radish, cucumber, and sunflower release very low levels of sugars during the initial stages of seed germination (24). Simple sugars such as these are utilized as carbon and energy sources by E. cloacae during growth and development in the spermosphere (11, 16, 26, 27). Whereas sugars can be used as carbon and energy sources, they can also repress β-oxidation and thus prevent the metabolism of exudate fatty acids (20). As a result, if sugars are present in seed exudates in high levels, it is likely that E. cloacae predominantly metabolizes simple sugars so that remaining fatty acids would induce the sporangium germination (S. T. Windstam and E. B. Nelson, unpublished data). If sugar concentrations are low, fatty acid metabolism could occur, giving rise to the suppression of P. ultimum sporangium germination and subsequent disease control. This would also explain our earlier observations in which seeds were not protected by E. cloacae if high levels of sugars that support E. cloacae growth (e.g., d-galactose, d-glucose, sucrose, and β-methyl-d-glucoside) were present in the spermosphere (16).
Our data have shown that on seeds such as corn and pea, the inability of E. cloacae to protect seeds from Pythium damping-off is related to its ability to inactivate the stimulatory activity of seed exudates. On all other plants tested, E. cloacae was effective in suppressing damping-off and also in inactivating the stimulatory activity of seed exudates. Since seed exudate inactivation occurs largely through the metabolism of unsaturated fatty acids (33), the presence of high exudate sugar concentrations could be at least partially responsible for the inability of E. cloacae to metabolize fatty acids. Research is currently under way to test this hypothesis.
REFERENCES
- 1.Chen, D. W., and G. A. Zentmyer. 1970. Production of sporangia by Phytophthora cinnamomi in axenic culture. Mycologia 62:397-402. [Google Scholar]
- 2.Elad, Y., and R. Baker. 1985. The role of competition for iron and carbon in suppression of chlamydospore germination of Fusarium spp. by Pseudomonas spp. Phytopathology 75:1053-1059. [Google Scholar]
- 3.Elad, Y., and I. Chet. 1987. Possible role of competition for nutrients in biocontrol of Pythium damping-off by bacteria. Phytopathology 77:190-195. [Google Scholar]
- 4.Ellis, R. J., T. M. Timms-Wilson, J. E. Beringer, D. Rhodes, A. Renwick, L. Stevenson, and M. J. Bailey. 1999. Ecological basis for biocontrol of damping-off disease by Pseudomonas fluorescens 54/96. J. Appl. Microbiol. 87:454-463. [DOI] [PubMed] [Google Scholar]
- 5.Fravel, D. R., R. D. Lumsden, and D. P. Roberts. 1990. In situ visualization of the biocontrol rhizobacterium Enterobacter cloacae with bioluminescence. Plant Soil 125:233-238. [Google Scholar]
- 6.Hadar, Y., G. E. Harman, A. G. Taylor, and J. M. Norton. 1983. Effects of pregermination of pea and cucumber seeds and of seed treatment with Enterobacter cloacae on rots caused by Pythium spp. Phytopathology 73:1322-1325. [Google Scholar]
- 7.Harman, G. E., and Y. Hadar. 1983. Biological control of Pythium species. Seed Sci. Technol. 11:893-906. [Google Scholar]
- 8.Hendrix, F. F., Jr., and W. A. Campbell. 1973. Pythiums as plant pathogens. Annu. Rev. Phytopathol. 11:77-98. [Google Scholar]
- 9.Howell, C. R., R. C. Beier, and R. D. Stipanovic. 1988. Production of ammonia by Enterobacter cloacae and its possible role in the biological control of Pythium preemergence damping-off by the bacterium. Phytopathology 78:1075-1078. [Google Scholar]
- 10.Kageyama, K., and T. Ui. 1982. Survival structure of Pythium spp. in the soils of bean fields. Ann. Phytopathol. Soc. Jpn. 48:308-313. [Google Scholar]
- 11.Lin, E. C. C. 1987. Dissimilatory pathways for sugars, polyols, and carboxylates, p. 244-284. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 1. American Society for Microbiology, Washington, D.C.
- 12.Lynch, J. M., R. D. Lumsden, P. T. Atkey, and M. A. Ousley. 1991. Prospects for control of Pythium damping-off of lettuce with Trichoderma, Gliocladium, and Enterobacter spp. Biol. Fertil. Soils 12:95-99. [Google Scholar]
- 13.Nelson, E. B. 1988. Biological control of Pythium seed rot and preemergence damping-off of cotton with Enterobacter cloacae and Erwinia herbicola applied as seed treatments. Plant Dis. 72:140-142. [Google Scholar]
- 14.Nelson, E. B. 1990. Exudate molecules initiating fungal responses to seeds and roots. Plant Soil 129:61-73. [Google Scholar]
- 15.Nelson, E. B. 1987. Rapid germination of sporangia of Pythium species in response to volatiles from germinating seeds. Phytopathology 77:1108-1112. [Google Scholar]
- 16.Nelson, E. B., W. L. Chao, J. M. Norton, G. T. Nash, and G. E. Harman. 1986. Attachment of Enterobacter cloacae to hyphae of Pythium ultimum: possible role in biological control of Pythium pre-emergence damping-off. Phytopathology 76:327-335. [Google Scholar]
- 17.Nelson, E. B., and C. M. Craft. 1989. Comparative germination of culture-produced and plant-produced sporangia of Pythium ultimum in response to soluble seed exudates and exudate components. Phytopathology 79:1009-1013. [Google Scholar]
- 18.Nelson, E. B., and C. M. Craft. 1992. A miniaturized and rapid bioassay for the selection of soil bacteria suppressive to Pythium blight of turfgrasses. Phytopathology 82:206-210. [Google Scholar]
- 19.Nelson, E. B., and J. S. T. Hsu. 1994. Nutritional factors affecting responses of sporangia of Pythium ultimum to germination stimulants. Phytopathology 84:677-683. [Google Scholar]
- 20.Pauli, G., R. Ehring, and P. Overath. 1974. Fatty acid degradation in Escherichia coli: requirement of cyclic adenosine monophosphate and cyclic adenosine monophosphate receptor protein for enzyme synthesis. J. Bacteriol. 117:1178-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulitz, T. C. 1991. Effect of Pseudomonas putida on the stimulation of Pythium ultimum by seed volatiles of pea and soybean. Phytopathology 81:1282-1287. [Google Scholar]
- 22.Roberts, D. P., P. D. Dery, P. K. Hebbar, W. Mao, and R. D. Lumsden. 1997. Biological control of damping-off of cucumber caused by Pythium ultimum with a root-colonization-deficient strain of Escherichia coli. J. Phytopathol. 145:383-388. [Google Scholar]
- 23.Roberts, D. P., P. D. Dery, W. Mao, and P. K. Hebbar. 1997. Use of a colonization-deficient strain of Escherichia coli in strain combinations for enhanced biocontrol of cucumber seedling diseases. J. Phytopathol. 145:461-463. [Google Scholar]
- 24.Roberts, D. P., P. D. Dery, I. Yucel, J. Buyer, M. A. Holtman, and D. Y. Kobayashi. 1999. Role of pfkA and general carbohydrate catabolism in seed colonization by Enterobacter cloacae. Appl. Environ. Microbiol. 65:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts, D. P., P. D. Dery, I. Yucel, and J. S. Buyer. 2000. Importance of pfkA for rapid growth of Enterobacter cloacae during colonization of crop seeds. Appl. Environ. Microbiol. 66:87-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts, D. P., and C. J. Sheets. 1991. Carbohydrate nutrition of Enterobacter cloacae ATCC 39978. Can. J. Microbiol. 37:168-170. [Google Scholar]
- 27.Roberts, D. P., C. J. Sheets, and J. S. Hartung. 1992. Evidence for proliferation of Enterobacter cloacae on carbohydrates in cucumber and pea spermosphere. Can. J. Microbiol. 38:1128-1134. [Google Scholar]
- 28.Ruttledge, T. R., and E. B. Nelson. 1997. Extracted fatty acids from Gossypium hirsutum stimulatory to the seed-rotting fungus Pythium ultimum. Phytochemistry 46:77-82. [Google Scholar]
- 29.Stanghellini, M. E. 1974. Spore germination, growth, and survival of Pythium in soil. Proc. Am. Phytopathol. Soc. 1:211-214. [Google Scholar]
- 30.Stanghellini, M. E., and J. G. Hancock. 1971. The sporangium of Pythium ultimum as a survival structure in soil. Phytopathology 61:157-164. [Google Scholar]
- 31.Taylor, A. G., Y. Hadar, J. M. Norton, A. A. Khan, and G. E. Harman. 1985. Influence of presowing seed treatments of table beets on the susceptibility to damping-off caused by Pythium. J. Am. Soc. Hortic. Sci. 110:516-519. [Google Scholar]
- 32.Trutmann, P., and E. B. Nelson. 1992. Production of non-volatile and volatile inhibitors of Pythium ultimum sporangium germination and mycelial growth by strains of Enterobacter cloacae. Phytopathology 82:1120.
- 33.van Dijk, K., and E. B. Nelson. 2000. Fatty acid competition as a mechanism by which Enterobacter cloacae suppresses Pythium ultimum sporangium germination and damping-off. Appl. Environ. Microbiol. 66:5340-5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Dijk, K., and E. B. Nelson. 1998. Inactivation of seed exudate stimulants of Pythium ultimum sporangium germination by biocontrol strains of Enterobacter cloacae and other seed-associated bacteria. Soil Biol. Biochem. 30:183-192. [Google Scholar]