Abstract
Cercosporin is a non-host-selective, perylenequinone toxin produced by many phytopathogenic Cercospora species. The involvement of Ca2+/calmodulin (CaM) signaling in cercosporin biosynthesis was investigated by using pharmacological inhibitors. The results suggest that maintaining endogenous Ca2+ homeostasis is required for cercosporin biosynthesis in Cercospora nicotianae. The addition of excess Ca2+ to the medium slightly increased fungal growth but resulted in a reduction in cercosporin production. The addition of Ca2+ chelators [EGTA and 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid] also reduced cercosporin production. Ca2+ channel blockers exhibited a strong inhibition of cercosporin production only at higher concentrations (>2 mM). Cercosporin production was reduced greatly by Ca2+ ionophores (A23187 and ionomycin) and internal Ca2+ blocker [3,4,5-trimethoxybenzoic acid 8-(diethylamino)octyl ester]. Phospholipase C inhibitors (lithium, U73122, and neomycin) led to a concentration-dependent inhibition of cercosporin biosynthesis. Furthermore, the addition of CaM inhibitors (compound 48/80, trifluoperazine, W-7, and chlorpromazine) also markedly reduced cercosporin production. In contrast to W-7, W-5, with less specificity for CaM, led to only minor inhibition of cercosporin production. The inhibitory effects of Ca2+/CaM inhibitors were partially or completely reversed by the addition of external Ca2+. As assessed with Fluo-3/AM (a fluorescent Ca2+ indicator), the Ca2+ content in the cytoplasm decreased significantly when fungal cultures were grown in a medium containing Ca2+/CaM antagonists, confirming the specificity of those Ca2+/CaM antagonists in C. nicotianae. Taken together, the results suggest that Ca2+/CaM signal transduction may play a pivotal role in cercosporin biosynthesis in C. nicotianae.
Many phytopathogenic fungi of the genus Cercospora produce a light-activated perylenequinone toxin, cercosporin (10). Cercosporin and other structurally diverse compounds are classified as photosensitizing compounds, as they can absorb and transfer light energy to oxygen, producing reactive oxygen species. Reactive oxygen is highly toxic to plant cells because it causes membrane peroxidation (8). Cercosporin has been shown to generate both singlet oxygen (1O2) and superoxide (O2−) in vitro; however, the primary toxicity of cercosporin is dependent on the formation of singlet oxygen (9). With this unique weapon, Cercospora species are among the most successful fungal phytopathogens, and the production of cercosporin has been considered important for fungal pathogenesis (40).
The biosynthesis of cercosporin is highly affected by many environmental factors, including nutrient conditions, temperature, and light, and its production is highly variable among species (17). Light not only is required for cercosporin activity but also is a primary regulator for cercosporin toxin biosynthesis. The production of cercosporin toxin can be detected at 2 days after culture transfer (17). Brief exposure of Cercospora cultures to light is sufficient to induce cercosporin production in fungi grown in the dark. Cercosporin is red and is not soluble in water; thus, it is easily visible as red crystals in the culture medium, allowing an easy means for toxin identification.
The biosynthesis of cercosporin through the polyketide pathway was proposed several decades ago (29). However, its detailed biosynthetic pathway and regulation have been investigated in few studies, leaving a large gap in the understanding of the pathogenic role of this important phytotoxin. Recently, it was found that flanking DNA from a rescued plasmid showed amino acid homology to polyketide synthase sequences from several fungi, confirming the notion that cercosporin is synthesized via the polyketide pathway (K.-R. Chung et al., unpublished data). Another gene related to cercosporin production is that for cercosporin facilitator protein, which shows homology to the family of membrane facilitators responsible for toxin pumping and resistance in both bacterial and fungal cells (5). Cercosporin facilitator protein presumably functions in cercosporin secretion through the membrane rather than in biosynthesis (41, 42).
Cytosolic Ca2+ plays a crucial role in cell signaling and can regulate a wide range of physiological functions and cell development in diverse organisms (3). The Ca2+ concentration in cells is highly regulated by the simultaneous interplay of multiple counteracting processes (4). In general, Ca2+ signaling in cells is initiated by a response to environmental cues through membrane receptors, causing a conformational change in GTP binding protein (G protein). G protein then activates phospholipase C, which is functional in the hydrolysis of inositol-1,4-bisphosphate (PIP2), to form two secondary messages, diacylglycerol and inositol-1,4,5-triphosphate (IP3) (39). The role of IP3 is to stimulate the release of Ca2+ from intracellular stores in the endoplasmic reticulum or vacuoles (2, 4). In many fungi, Ca2+/calmodulin (CaM) has been demonstrated to be involved in various aspects of fungal development, including conidium and appressorium formation, hyphal extension and branching, mycelial dimorphism, photomorphogenesis, and fungal pathogenicity (14, 19, 22, 27, 28, 30, 34, 35, 45). The Ca2+/CaM signaling system also mediates zoospore germination and encystment in oomycetes (11, 16) and is apparently involved in aflatoxin biosynthesis in Aspergillus parasiticus (33) and in melanin biosynthesis in Colletotrichum gloeosporioides (19). Little is known about the involvement of Ca2+ signaling in the biosynthesis of secondary metabolites in fungi. In preliminary experiments, neomycin, which interferes with internal Ca2+ release by inhibiting phospholipase C activity (13, 31), was found to abolish completely cercosporin production in Cercospora nicotianae, suggesting the involvement of Ca2+ in cercosporin biosynthesis.
In this study, a pharmacological approach with a wide range of inhibitors specifically involved in Ca2+/CaM regulation was used to examine the role of Ca2+/CaM signaling in cercosporin biosynthesis. The results provide further understanding of the biosynthesis and regulation of cercosporin toxin.
MATERIALS AND METHODS
Fungal isolate and culture conditions.
C. nicotianae ATCC 18366 was used throughout the experiments. The fungal culture was maintained routinely on malt medium at 28°C as described previously (17). For cercosporin production, fungal mycelium (<0.5 mm) was transferred to potato dextrose agar (PDA; Difco, Detroit, Mich.) plates and incubated under constant fluorescent light (20 microeinsteins m−2 s−1) for 7 days at room temperature. Fungal growth measured as colony diameter (millimeters) was measured at day 7 prior to cercosporin extraction. PDA (containing approximately 150 mg of calcium liter−1) has been demonstrated to be the best medium for cercosporin production (17). PDA (pH 5.6) was prepared fresh, and each plate contained 4 ml (15 by 60 mm) in order to obtain rapid and optimal cercosporin production. For testing of EGTA, the pH of PDA was adjusted to 7.5 but tended to change to 6.3 by the end of incubation (7 days).
Purification and quantification of cercosporin toxin.
Cercosporin was purified and assayed as described previously (17, 18) with modifications. Briefly, five agar plugs (6-mm diameter) cut from mycelial cultures were extracted with 5 N KOH for 16 h, and the absorbance of the solution was measured with a spectrophotometer at a wavelength of 480 nm by using a model Genesys 5 spectrophotometer (Spectronic Instruments, Rochester, N.Y.). The cercosporin concentration was calculated by using a molar extinction coefficient of 23,300 (46) and was reported as nanomoles per agar plug.
Preparation of chemicals.
All chemicals were purchased from Sigma (St. Louis, Mo.) unless otherwise indicated. Chemicals were dissolved in water or in appropriate solvents to make stock solutions. All aqueous solutions were sterilized by filtration. EGTA, a Ca2+ chelator, was dissolved in distilled water, and the solution pH was adjusted to 7.5 with 10 N NaOH. 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) was dissolved in water to make a 100 mM stock solution. Compound A23187 (calcimycin) (Calbiochem, La Jolla, Calif.), a Ca2+ ionophore, was dissolved in ethanol to make a 10 mM stock solution. Ionomycin, another Ca2+ ionophore, was dissolved in dimethyl sulfoxide (DMSO) to make a 1 mM stock solution. Nifedipine, verapamil, nicardipine, and amiloride (Ca2+ channel blockers) were dissolved in DMSO to make 100 mM stock solutions. Lanthanum (La3+), gadolinium (Gd3+), and LiCl were dissolved in water and added from 100 mM stock solutions. 3,4,5-Trimethoxybenzoic acid 8-(diethylamino)octyl ester (TMB-8) (Calbiochem), an antagonist of intracellular Ca2+ release, was dissolved in DMSO to make a 58 mM stock solution. Neomycin was dissolved in water to make a 0.5 M stock solution. U73122 (Calbiochem), a phospholipase C inhibitor, was dissolved in DMSO to yield a 10 mM stock solution.
To examine the role of CaM in cercosporin production, the following CaM antagonists were used. Compound 48/80, with an unknown molecular weight (condensation product of N-methyl-p-methoxy-phenethylamine with formaldehyde), was dissolved in water to make a final concentration of 10 mg ml−1. Trifluoperazine (TFP; phenothiazine) was dissolved in water to make a 100 mM stock solution. Compound W-7 [N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide] (naphthalenesulfonamide) was dissolved in DMSO to make a 100 mM stock solution. Compound W-5 [N-(6-aminohexyl)-1-naphthalenesulfonamide] was dissolved in water to make a 5 mM stock solution. Chlorpromazine was dissolved in water to make a 100 mM stock solution.
All chemicals were added to solid PDA in 60- by 15-mm petri dishes. The final concentrations of solvents added to the medium were adjusted to less than 1% and had no discernible effects on fungal growth or cercosporin production. All treatments were performed at least three times with five replicates each.
Ca2+ imaging.
Fluo-3/AM (Biotium, Hayward, Calif.; hereafter referred to as Fluo-3) was used as a Ca2+-specific probe to assess the cytoplasmic calcium in C. nicotianae (7). C. nicotianae was grown on PDA plates with and without Ca2+/CaM antagonists under light for 5 to 7 days. Small pieces of fungal hyphae were transferred to liquid potato dextrose broth (PDB) (pH 4.2) and ground with a disposable grinder. Fluo-3 was prepared from a 1 mM stock solution in DMSO and added to acidic PDB to a final concentration of 150 μM. Pluronic F-127 (Biotium), a nonionic polyol, was added to a final concentration of 2% to facilitate dye penetration. The cultures were incubated at 21°C with gentle shaking for 24 h for dye loading. Images of calcium green fluorescence were observed under a Nikon microscope by using a 450- to 490-nm excitation filter and a 520-nm barrier filter.
Statistical analysis.
The significance of treatments was determined by analysis of variance, and treatment means were separated by the Waller-Duncan k ratio t test (P ≤ 0.01).
RESULTS
Effects of external Ca2+ and Ca2+ chelators on cercosporin production.
Two salts of Ca2+ and Ca2+ chelators were added to PDA to determine the effect of external Ca2+ on cercosporin biosynthesis. The addition of Ca2+ promoted fungal growth slightly but resulted in decreased cercosporin production (Table 1). The inhibitory effect was more clear when Ca(NO3)2 · 4H2O was added to PDA, whereas inhibition was lower with CaCl2. The depletion of external Ca2+ by EGTA or BAPTA reduced fungal growth and cercosporin production (Table 1). The addition of 100 mM CaCl2 to PDA containing EGTA restored fungal growth and cercosporin production. However, the addition of 200 mM CaCl2 restored fungal growth but not cercosporin production. The results indicated that external Ca2+ interfered with cercosporin biosynthesis in C. nicotianae.
TABLE 1.
Effects of Ca2+ and EGTA on fungal growth and cercosporin toxin production in C. nicotianaea
| Treatment | Concn (mM) | Mean colony diam (mm) ± SEM | Cercosporin (nmol per plug), mean ± SEM |
|---|---|---|---|
| None | 13.4 ± 2.5 abc | 130.9 ± 27.4 a | |
| CaCl2 | 10 | 13.3 ± 2.7 abc | 118.2 ± 28.6 ab |
| 50 | 15.1 ± 2.2 ab | 120.4 ± 8.5 ab | |
| 100 | 15.8 ± 3.1 a | 124.8 ± 25.1 ab | |
| 200 | 15.7 ± 3.3 a | 116.7 ± 6.7 ab | |
| Ca(NO3)2 · 4H2O | 10 | 13.0 ± 2.1 abc | 116.2 ± 8.8 ab |
| 50 | 14.3 ± 3.0 ab | 94.8 ± 12.6 bcd | |
| 100 | 15.3 ± 2.4 ab | 88.9 ± 16.9 bcd | |
| 200 | 14.2 ± 2.6 ab | 76.2 ± 27.6 cde | |
| EGTA (pH 7.5) | 1 | 13.9 ± 3.0 abc | 143.0 ± 30.1 a |
| 2 | 9.6 ± 2.0 de | 129.6 ± 30.9 abc | |
| 3 | 10.4 ± 0.8 cde | 84.8 ± 31.6 cde | |
| EGTA + CaCl2 | 3 + 100 | 13.4 ± 1.8 abc | 112.3 ± 29.4 ab |
| 3 + 200 | 15.5 ± 1.4 abc | 42.8 ± 1.8 e | |
| BAPTA | 0.1 | 13.8 ± 1.6 ab | 126.0 ± 10 a |
| 1 | 8.2 ± 0.3 e | 68.0 ± 2.5 de |
Fungal mycelium of C. nicotianae was grown on PDA containing calcium salts, EGTA, or BAPTA, as indicated, and incubated for 7 days. Cercosporin toxin was extracted by using 5 N KOH and quantified by using a spectrophotometer at a wavelength of 480 nm (16). The data are the mean of at least three different experiments with five replicates of each treatment and standard error of the mean (SEM). Mean separation was done with the Waller-Duncan k ratio t test (P ≤ 0.01). Means followed by the same letter were not significantly different.
Effects of Ca2+ channel blockers on cercosporin production.
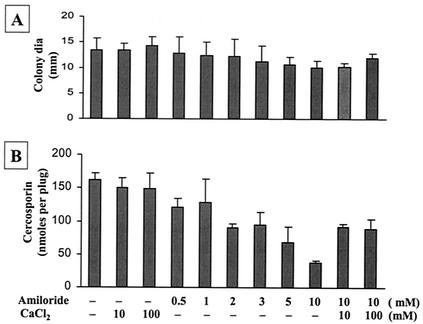
Different types of Ca2+ channel blockers were used to substantiate the roles of external Ca2+ and Ca2+ ion channels in cercosporin biosynthesis. In general, Ca2+ channel blockers, unless tested at high dosages, inhibited cercosporin production only slightly. The inorganic Ca2+ channel blockers gadolinium and lanthanum reduced fungal growth. Lanthanum, but not gadolinium, increased cercosporin production slightly (Table 2). The voltage-dependent Ca2+ channel blockers verapamil and nicardipine decreased fungal growth and cercosporin production. Nifedipine, another voltage-dependent Ca2+ channel blocker, slightly decreased fungal growth but increased cercosporin production at low concentrations (0.1 to 1 mM) (Table 2). At higher concentrations (>2 mM), nifedipine reduced fungal growth and cercosporin production. Amiloride, a T-type-specific (voltage-operated) Ca2+ channel blocker, exhibited mild inhibition of fungal growth but had a negligible effect on cercosporin production in C. nicotianae (Fig. 1). The addition of CaCl2 partially reversed amiloride inhibition of fungal growth and cercosporin production.
TABLE 2.
Effects of Ca2+ channel blockers on fungal growth and cercosporin toxin production in C. nicotianaea
| Treatment | Concn (mMb) | Mean colony diam (mm) ± SEM | Cercosporin (nmol per plug), mean ± SEM |
|---|---|---|---|
| None | 14.1 ± 2.5 a | 154.2 ± 10.6 cde | |
| DMSO | 0.1% | 13.5 ± 2.3 a | 152.1 ± 5.6 de |
| Gadolinium | 1 | 12.4 ± 1.9 abcd | 170.7 ± 32.1 cde |
| 5 | 12.6 ± 2.4 abcd | 156.1 ± 15.5 cde | |
| 10 | 10.9 ± 2.2 bcde | 177.5 ± 12.3 cde | |
| 20 | 8.7 ± 2.3 bcdef | 150.8 ± 27.3 de | |
| Lanthanum | 0.1 | 12.1 ± 1.4 abcde | 177.0 ± 31.5 cde |
| 0.5 | 11.6 ± 1.2 abcde | 185.9 ± 34.2 bcd | |
| 1 | 11.1 ± 1.0 bcde | 204.2 ± 42.6 abc | |
| 2 | 9.7 ± 0.6 def | 204.8 ± 36.7 abc | |
| Verapamil | 0.1 | 13.0 ± 2.8 ab | 139.6 ± 13.9 def |
| 0.5 | 12.3 ± 2.1 abcde | 139.0 ± 36.8 def | |
| 1 | 9.5 ± 0.4 ef | 103.0 ± 20.4 fg | |
| 2 | 7.7 ± 0.7 fg | 56.6 ± 16.0 h | |
| Nicardipine | 0.1 | 12.7 ± 2.2 abc | 172.9 ± 12.7 cde |
| 0.5 | 10.1 ± 1.1 cdef | 134.0 ± 22.3 ef | |
| 1 | 10.4 ± 1.4 bcdef | 71.9 ± 16.4 gh | |
| 2 | 5.2 ± 1.2 g | 48.5 ± 1.2 h | |
| Nifedipine | 0.1 | 12.7 ± 2.4 abc | 170.4 ± 30.0 cde |
| 0.5 | 11.9 ± 0.7 abcde | 230.6 ± 33.9 ab | |
| 1 | 9.5 ± 1.0 def | 234.6 ± 23.2 a | |
| 2 | 7.6 ± 1.1 fg | 49.0 ± 4.4 h |
Fungal mycelium of C. nicotianae was grown on PDA containing various Ca2+ channel blockers, as indicated, and incubated for 7 days. Cercosporin toxin was extracted by using 5 N KOH and quantified by using a spectrophotometer. The data are the mean of at least three different experiments with five replicates of each treatment and standard error of the mean (SEM). Mean separation was done with the Waller-Duncan k ratio t test (P ≤ 0.01). Means followed by the same letter were not significantly different.
Unless otherwise indicated.
FIG. 1.
Effect of the Ca2+ channel blocker amiloride on fungal growth and cercosporin toxin production in C. nicotianae. (A) Effect on fungal growth. dia, diameter. (B) Effect on cercosporin toxin production. The fungus was grown on PDA plates with or without amiloride and CaCl2. Cercosporin toxin was extracted by using 5 N KOH and was quantified by using a spectrophotometer at a wavelength of 480 nm. The data shown are the means and standard errors of at least three different experiments with five replicates of each treatment. CaCl2 reversed amiloride inhibition of cercosporin biosynthesis.
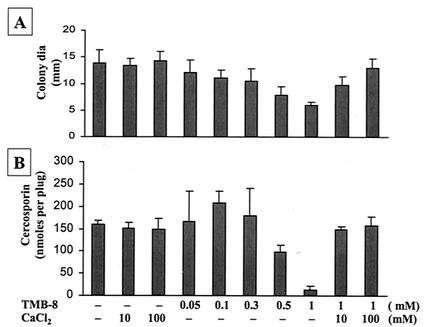
The effect of internal Ca2+ on cercosporin production was tested by using TMB-8, an antagonist of intracellular Ca2+ release. TMB-8 at low concentrations decreased fungal growth slightly but increased cercosporin production (Fig. 2). At higher concentrations (>0.5 mM), TMB-8 reduced both cercosporin production and fungal growth. The addition of Ca2+ fully reversed TMB-8 inhibition of fungal growth and cercosporin production.
FIG. 2.
Effect of TMB-8, an internal Ca2+ channel blocker, on fungal growth and cercosporin toxin production in C. nicotianae. (A) Effect on fungal growth. dia, diameter. (B) Effect on cercosporin toxin production. The data shown are means and standard errors. CaCl2 reversed TMB-8 inhibition of cercosporin biosynthesis and fungal growth.
Effects of Ca2+ ionophores on cercosporin production.
To further examine the role of Ca2+ in cercosporin production, the Ca2+ ionophores A23187 and ionomycin were used. A23187 reduced fungal growth slightly, and ionomycin had no effect, but both compounds reduced cercosporin production markedly (Table 3). The addition of EGTA partially reversed the inhibition of cercosporin biosynthesis by 50 μM A23187. EGTA, however, was unable to reverse the inhibition of cercosporin production by a high dosage of A23187 (100 μM). The addition of CaCl2 with A23187 or ionomycin did not restore cercosporin production. These data, combined with the inhibitory effect of TMB-8, indicated that the regulation of internal Ca2+ was important for cercosporin toxin production.
TABLE 3.
Effects of Ca2+ ionophores on fungal growth and cercosporin toxin production in C. nicotianaea
| Treatment | Concn | Mean colony diam (mm) ± SEM | Cercosporin (nmol per plug), mean ± SEM |
|---|---|---|---|
| None | 14.1 ± 2.5 ab | 160.0 ± 8.6 a | |
| Ethanol | 1% | 13.6 ± 1.9 ab | 152.5 ± 18.5 ab |
| DMSO | 0.1% | 14.2 ± 0.8 ab | 150.9 ± 26.4 ab |
| A23187 | 10 μM | 13.9 ± 1.4 bc | 104.2 ± 31.1 c |
| 50 μM | 11.5 ± 1.3 c | 52.2 ± 10.4 def | |
| 100 μM | 10.0 ± 1.2 d | 44.2 ± 21.9 ef | |
| 200 μM | 9.4 ± 1.0 de | 35.6 ± 25.3 ef | |
| A23187 + EGTA | 50 μM + 3 mM | 8.5 ± 0.9 f | 105.1 ± 32.9 cd |
| 100 μM + 3 mM | 8.9 ± 1.2 f | 36.9 ± 12.9 ef | |
| A23187 + CaCl2 | 100 μM + 100 mM | 10.5 ± 0.8 de | 60.9 ± 2.9 de |
| 100 μM + 200 mM | 10.0 ± 0.4 e | 22.6 ± 2.1 ef | |
| Ionomycin | 10 μM | 14.8 ± 1.2 a | 116.5 ± 6.2 bc |
| 50 μM | 14.5 ± 0.9 ab | 9.2 ± 6.2 f | |
| Ionomycin + CaCl2 | 50 μM + 10 mM | 14.5 ± 0.4 ab | 13.4 ± 6.7 f |
Fungal mycelium of C. nicotianae was grown on PDA containing various Ca2+ ionophores with or without EGTA or CaCl2, as indicated, and incubated for 7 days. Cercosporin toxin was extracted by using 5 N KOH and quantified by using a spectrophotometer. The data are the mean of at least three different experiments with five replicates of each treatment and standard error of the mean (SEM). Mean separation was done with the Waller-Duncan k ratio t test (P ≤ 0.01). Means followed by the same letter were not significantly different.
Effect of the phosphoinositide signaling system on cercosporin production.
The level of intracellular Ca2+ is usually elevated by release through phophoinositide-IP3 cycling (2). To determine whether the phophoinositide signaling system is involved in cercosporin biosynthesis in C. nicotianae, several compounds were tested. Lithium can deplete inositol, which is required for the resynthesis of IP3. U73122 blocks the activity of phospholipase C, which is involved in the hydrolysis of PIP2 to IP3. As expected, lithium and U73122 reduced cercosporin production drastically (Table 4). External Ca2+ fully reversed lithium inhibition of fungal growth but not cercosporin production. The results indicated that phophoinositide signaling leading to changes in the level of internal Ca2+ is likely involved in cercosporin biosynthesis.
TABLE 4.
Effects of phosphoinositide cycling inhibitors on fungal growth and cercosporin toxin production in C. nicotianaea
| Treatment | Concn | Mean colony diam (mm) ± SEM | Cercosporin (nmol per plug), mean ± SEM |
|---|---|---|---|
| None | 14.4 ± 2.5 bc | 154.2 ± 27.4 a | |
| DMSO | 0.1% | 14.2 ± 1.9 c | 150.9 ± 34.1 a |
| LiCl | 100 mM | 11.9 ± 1.7 d | 55.2 ± 17.4 bc |
| 150 mM | 11.5 ± 1.0 d | 14.4 ± 20.8 d | |
| 200 mM | 9.5 ± 0.6 ef | 3.2 ± 0.9 d | |
| 300 mM | 9.0 ± 0.5 fg | 2.5 ± 1.5 d | |
| LiCl + CaCl2 | 300 mM + 10 mM | 15.7 ± 0.5 b | 48.0 ± 11.7 bc |
| 300 mM + 100 mM | 17.5 ± 0.6 a | 68.2 ± 5.4 b | |
| U73122 | 10 μM | 11.3 ± 0.8 d | 159.0 ± 21.7 a |
| 30 μM | 9.7 ± 0.8 ef | 52.8 ± 13.6 bc | |
| 50 μM | 7.8 ± 0.4 g | 26.6 ± 2.5 cd |
Fungal mycelium of C. nicotianae was grown on PDA containing various phosphoinositide cycle inhibitors, as indicated, and incubated for 7 days. Toxin was extracted by using 5 N KOH and quantified by using a spectrophotometer. The data are the mean of three experiments with five replicates of each treatment and standard error of the mean (SEM). Mean separation was done with the Waller-Duncan k ratio t test (P ≤ 0.01). Means followed by the same letter were not significantly different.
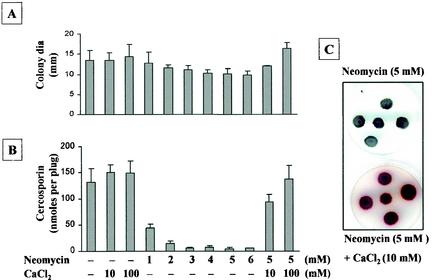
Neomycin, another potent inhibitor of phospholipase C, slightly reduced fungal growth but greatly reduced cercosporin production in a dosage-dependent manner (Fig. 3). The addition of CaCl2 nearly reversed neomycin inhibition of cercosporin production. The restoration of cercosporin production could be visualized as a distinct red pigment on PDA (Fig. 3C, bottom panel). These data provided further evidence for the involvement of phophoinositide signaling and changes in the level of internal Ca2+ in cercosporin biosynthesis.
FIG. 3.
Effect of neomycin, a potent inhibitor of phospholipase C, on fungal growth, cercosporin toxin production, and phenotypic characterization of neomycin inhibition and CaCl2 reversion of inhibition of cercosporin biosynthesis in C. nicotianae. (A) Effect on fungal growth. dia, diameter. (B) Effect on cercosporin toxin production. The data shown in panels A and B are means and standard errors. (C) Phenotypic characterization. Red indicates the presence of cercosporin toxin.
Effecta of calmodulin antagonists on cercosporin production.
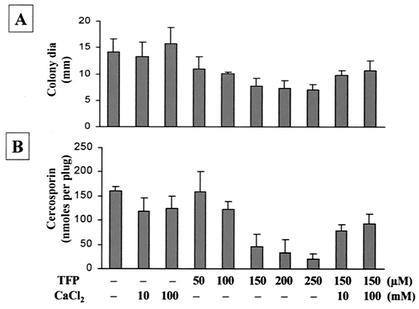
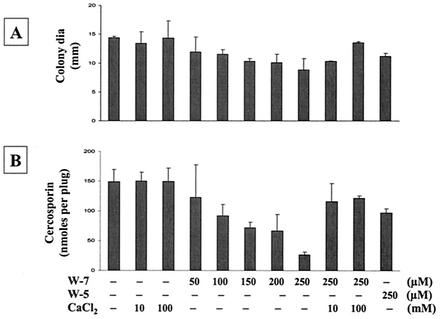
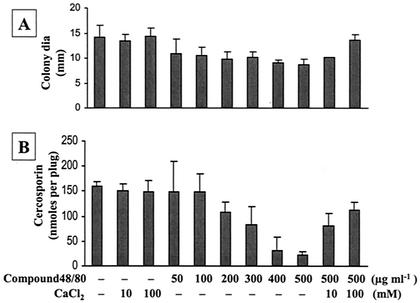
Ca2+ signaling in cells is mainly mediated by CaM, a potent Ca2+ binding protein. To determine whether CaM is involved in cercosporin production, several CaM inhibitors, i.e., TFP (Fig. 4), W-5 and W-7 (Fig. 5), compound 48/80 (Fig. 6), and chlorpromazine (Table 5), were tested. All CaM inhibitors except for W-5 (at 250 μM) inhibited cercosporin production in a dosage-dependent manner. W-5 at 250 μM did not alter fungal growth and only slightly reduced cercosporin production (Fig. 5). The addition of Ca2+ only partially restored fungal growth and cercosporin production inhibited by TFP (Fig. 4). The addition of CaCl2 reversed W-7 inhibition of fungal growth and cercosporin production (Fig. 5). The inhibition of fungal growth and cercosporin production caused by compound 48/80 was also completely reversed by the addition of CaCl2 (Fig. 6). Thus, Ca2+ signaling via the CaM transduction pathway may play a significant role in cercosporin biosynthesis.
FIG. 4.
Effect of TFP, a CaM antagonist, on fungal growth and cercosporin toxin production in C. nicotianae. (A) Effect on fungal growth. dia, diameter. (B) Effect on cercosporin toxin production. The data shown are means and standard errors. CaCl2 only partially relieved TFP inhibition of fungal growth and toxin production.
FIG. 5.
Effects of W-5 and W-7, potent CaM antagonists, on fungal growth and cercosporin toxin production in C. nicotianae. (A) Effect on fungal growth. dia, diameter. (B) Effect on cercosporin toxin production. The data shown are means and standard errors. CaCl2 reversed W-7 inhibition of cercosporin biosynthesis and fungal growth.
FIG. 6.
Effect of compound 48/80, a potent CaM antagonist, on fungal growth and cercosporin toxin production in C. nicotianae. (A) Effect on fungal growth. dia, diameter. (B) Effect on cercosporin toxin production. The data shown are means and standard deviations. CaCl2 reversed compound 48/80 inhibition of cercosporin biosynthesis.
TABLE 5.
Effect of chlorpromazine on fungal growth and cercosporin toxin production in C. nicotianaea
| Treatment | Concn (μM) | Mean colony diam (mm) ± SEM | Cercosporin (nmol per plug), mean ± SEM |
|---|---|---|---|
| None | 12.6 ± 0.4 a | 143.9 ± 20.7 a | |
| Chlorpromazine | 20 | 12.7 ± 1.7 a | 134.1 ± 16.5 a |
| 60 | 11.5 ± 1.7 b | 127.2 ± 19.3 a | |
| 100 | 10.8 ± 1.4 b | 86.6 ± 6.8 b |
Fungal mycelium of C. nicotianae was grown on PDA containing chlorpromazine, as indicated, and incubated for 7 days. Toxin was extracted by using 5 N KOH. The data are the mean of three different experiments with five replicates of each treatment and standard error of the mean (SEM). Mean separation was done with the Waller-Duncan k ratio t test (P ≤ 0.01). Means followed by the same letter were not significantly different.
Estimation of Ca2+ within hyphae by using Fluo-3 fluorescence.
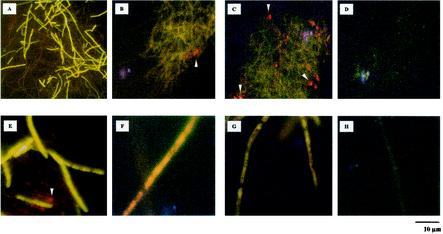
Since all Ca2+/CaM antagonists were developed originally in mammalian cells, their specificities in C. nicotianae were examined by using Fluo-3 fluorescent dye as a Ca2+ indicator to estimate the cytoplasmic Ca2+ content (7). Fluo-3 is a nonfluorescent dye but produces green fluorescence at wavelengths of 506 to 520 nm after Ca2+ binding. The intensities of fluorescence represent the relative amounts of free Ca2+ in the cytoplasm. Fluorescence microscopy indicated that C. nicotianae hyphae were successfully loaded with Fluo-3 dye (Fig. 7). No autofluorescence was observed in fungal hyphae without Fluo-3. However, strong green fluorescence was observed in the hyphal cytoplasm after treatment with Fluo-3 when C. nicotianae was grown on PDA alone (Fig. 7A and E). The green fluorescence decreased greatly when the fungus was grown in a medium with neomycin (Fig. 7B and F), compound 48/80 (Fig. 7C and G), nifedipine (Fig. 7D and H), or nicardipine, LiCl, or amiloride (data not shown). Since cercosporin shows absorbance at a wavelength of 480 nm, the accumulation of cercosporin as red crystals was also observed. The results strongly indicated that Ca2+/CaM antagonists may have similar specificities in C. nicotianae.
FIG. 7.
Fluorescence studies. Images obtained simultaneously with a fluorescence microscope at magnifications of ×200 (A to D) and ×1,000 (E to H) indicated the fluorescence of the Ca2+ probe Fluo-3 in C. nicotianae hyphae. Mycelial fragments were transferred from PDA medium alone (A and E) or medium containing 3 mM neomycin (B and F), 400 μg of compound 48/80 ml−1 (C and G), or 2 mM nifedipine (D and H) to acidic PDB medium containing 150 μM Fluo-3 fluorescent dye. The intensities of green fluorescence represent the relative amounts of free Ca2+ in the cytoplasm. The red crystals (indicated by arrowheads) are cercosporin. Bar, 10 μm.
DISCUSSION
Signal transduction through Ca2+/CaM, linked to IP3 metabolism, has been well documented to be involved in various cellular responses to external stimuli in animal cells (2-4). Studies with fungi have indicated that a similar framework may also occur in fungi (14, 19, 35, 45). The regulation of cercosporin toxin by environmental cues has been well studied; however, signal regulation at the cellular level has never been addressed. Cercosporin toxin is easily produced on thin PDA medium by the phytopathogenic fungus C. nicotianae (17), providing an opportunity to examine the role of Ca2+/CaM in cercosporin biosynthesis. The striking red color of cercosporin facilitates primary screening for the effects of inhibitors on its biosynthesis. The simple KOH extraction method for cercosporin toxin (17, 18) has also facilitated the measurement and qualification of cercosporin in large factorial experiments. The results derived from this study indicated that Ca2+/CaM signaling plays a key role in cercosporin biosynthesis in C. nicotianae.
The production of cercosporin toxin was usually associated with fungal growth, and Ca2+ appeared to be required for both fungal growth and toxin production. However, the reduction of cercosporin production by Ca2+/CaM inhibitors was not due mainly to the reduction of fungal growth, since growth was not altered or was even promoted by many of the inhibitors. C. nicotianae required large amounts of Ca2+ for vegetative growth. In contrast, the role of Ca2+ in the regulation of cercosporin biosynthesis was more complex. A dynamic equilibrium of cellular Ca2+ was critical for cercosporin biosynthesis. The addition of Ca2+ to the medium increased fungal growth but slightly decreased cercosporin production. Two inorganic Ca2+ blockers (gadolinium and lanthanum) and a voltage-dependent calcium blocker (nifedipine) slightly stimulated cercosporin production at low concentrations. Ca2+ channel blockers at low concentrations promoted cercosporin production, probably by prohibiting Ca2+ influx into the cytosol and thus reducing the cytosolic Ca2+ level. Blocking of Ca2+ influx by use of higher doses of Ca2+ channel blockers, however, resulted in severe inhibition of growth as well as cercosporin production. These data indicated that the requirements of Ca2+ for fungal growth and toxin biosynthesis are apparently not identical.
Amiloride, a T-type-specific Ca2+ channel blocker as well as a Ca2+-activated Na+ channel blocker (21), also had markedly inhibitory effects on cercosporin production. Ca2+ only partially reversed amiloride inhibition of cercosporin production, implying that Na+ might also be involved in cercosporin production. In contrast to external Ca2+, the disturbance of intracellular Ca2+ had a profound effect on cercosporin production, as indicated by inhibition by an internal Ca2+ channel blocker, TMB-8. Although TMB-8 also inhibits protein kinase C, involved in other signaling pathways (36), TMB-8 inhibition of cercosporin biosynthesis and fungal growth was relieved completely by CaCl2, suggesting that the primary effect of TMB-8 is to directly alter the Ca2+ concentration in the cytosol.
In many cells, cytosolic Ca2+ can be regulated by Ca2+ influx channels in the cell membrane and/or the release of Ca2+ from intracellular stores. A23187 and ionomycin can release Ca2+ from intracellular stores by acting as Ca2+ carriers (32). In this study, factors that disturbed Ca2+ homeostasis, especially the endogenous Ca2+ level, had marked effects on cercosporin production. The growth of C. nicotianae on a medium containing A23187 or ionomycin resulted in a severe reduction in cercosporin biosynthesis. A combination of A23187 with excess Ca2+ might cause a severe disturbance of Ca2+ regulation, thereby reducing cercosporin production. A depletion of external Ca2+ by EGTA or a disturbance of internal Ca2+ by A23187 or ionomycin altered cercosporin production and was not reversed by Ca2+, indicating that cercosporin biosynthesis requires a sustained Ca2+ concentration in the cytosol.
The important role of intracellular Ca2+ in cercosporin biosynthesis was further explored by using several inhibitors of the phosphoinositide signaling system. IP3 generated by phospholipase C can serve as an intracellular Ca2+ channel activator (39). Therefore, inhibition of phospholipase C activity will impair the IP3-dependent signal transduction pathway due to the depletion of cellular inositol (1) and decreased cytosolic Ca2+. In this study, three phospholipase C inhibitors, lithium, U73122, and neomycin, exerted strong inhibition on cercosporin production. U73122 inhibits the coupling of G protein to phospholipase C, thus blocking the hydrolysis of PIP2 to IP3 (38). Excess CaCl2 only partially reversed lithium inhibition of cercosporin production, likely due to the multiple activities of lithium. Lithium has an inhibitory effect on diverse enzymes in cells. Lithium can suppress inositol-1-phosphatase activity (15), glycogen synthase kinase-3β, and other enzymes not related directly to signal transduction, such as fructose-1,6-bisphosphatase, phosphoglucomutase, and 3′(2′),5′-bisphosphate nucleotidase (20, 24-26).
Neomycin is an aminoglycoside antibiotic and also has multiple inhibitory effects on Ca2+ signaling. It can serve as a nonspecific phospholipase C inhibitor (13, 31) and can block voltage-sensitive Ca2+ channels without affecting the Na+-Ca2+ antiporter (6). Neomycin affects Ca2+ release and inositol phospholipid turnover by inhibiting phosphoinositide kinase (37, 44) and also inhibits the activity of phosphatidylcholine-phospholipase D (23). Neomycin markedly reduced the cytoplasmic Ca2+ content and inhibited cercosporin production. CaCl2 reversed neomycin inhibition of cercosporin, suggesting that the main action of neomycin is in blocking Ca2+ signaling and cycling in C. nicotianae. These results suggested that the biosynthesis of cercosporin toxin is regulated by IP3-related Ca2+ homeostasis.
CaM is a highly conserved Ca2+ binding protein and has been found to be involved in many Ca2+-dependent signal pathways in various cells (12). A close relationship between Ca2+ and CaM in cercosporin biosynthesis was demonstrated by the inhibitory effect of CaM antagonists, such as W-7, W-5, TFP, compound 48/80, and chlorpromazine. All of the CaM inhibitors, except for chlorpromazine and W-5, exhibited strong inhibition of cercosporin production. Compound W-5 has a structure similar to that of compound W-7 but has a much lower affinity for CaM. W-7, but not W-5, at 250 μM exhibited strong inhibition of cercosporin production, indicating that CaM is involved in cercosporin biosynthesis. The inhibitory effects of W-7 and compound 48/80 on cercosporin production were reversed by the addition of CaCl2. Unlike the situation in mammalian systems, the addition of CaCl2 could partially reverse TFP inhibition of fungal growth and cercosporin production, likely due to the lower specificity of TFP. It is noteworthy that TFP also inhibits Mg2+-dependent ATPase (43) and could also play a role. Alternatively, excess CaCl2 competes with TFP for binding to CaM, therefore relieving TFP inhibition of cercosporin production.
Despite the fact that many pharmacological inhibitors of Ca2+ and CaM lack target specificity, the use of a wide array of inhibitors provides an initial indication of the involvement of the Ca2+/CaM signaling system in cercosporin production. To more precisely determine their roles in cercosporin biosynthesis, molecular approaches, such as the cloning of genes encoding CaM, CaM kinase, or phospholiase C in the signaling pathway, followed by functional disruption or antisense suppression, may be necessary. Nevertheless, different types of Ca2+/CaM antagonists exhibited similar physiological inhibition of cercosporin production, demonstrating that there is a common mode of action in effects on Ca2+/CaM signaling. Fluorescence microscopy with Fluo-3 dye as a Ca2+ indicator also indicated that the addition of Ca2+/CaM antagonists tended to decrease the free Ca2+ content in the fungal cytoplasm, further confirming their specificity in fungal cells. Moreover, the inhibitory effects of Ca2+/CaM inhibitors on cercosporin production were reversed by the addition of Ca2+, indicating that Ca2+/CaM is likely involved in cercosporin toxin biosynthesis in the phytopathogenic fungus C. nicotianae. The production of cercosporin toxin is affected by many environmental cues that may completely or partially activate the Ca2+/CaM signaling pathway. Thus, the involvement of Ca2+/CaM in early signal transduction may trigger the expression of a set of genes involved in cercosporin regulation and biosynthesis.
Acknowledgments
I am indebted to J. K. Burns, M. E. Parish, L. W. Timmer, and two anonymous reviewers for comments. I also thank T. Shilts for technical assistance.
This research was supported by the Florida Agricultural Experiment Station.
Footnotes
Approved for publication as journal series no. R-08884 of the Florida Agricultural Experiment Station.
REFERENCES
- 1.Berridge, M. J., C. P. Downes, and M. R. Hanley. 1989. Neural and developmental actions of lithium: a unifying hypothesis. Cell 59:411-419. [DOI] [PubMed] [Google Scholar]
- 2.Berridge, M. J. 1993. Inositol triphosphate and calcium signaling. Nature 361:315-325. [DOI] [PubMed] [Google Scholar]
- 3.Berridge, M. J., P. Lipp, and M. D. Bootman. 2000. The versatility and university of calcium signaling. Nat. Rev. Mol. Cell. Biol. 1:11-21. [DOI] [PubMed] [Google Scholar]
- 4.Bootman, M. D., T. J. Collins, C. M. Peppiatt, L. S. Prothero, L. MacKenzie, P. De Smet, M. Travers, S. C. Tovey, J. T. Seo, M. J. Berridge, F. Ciccolini, and P. Lipp. 2001. Calcium signaling—an overview. Semin. Cell Dev. Biol. 12:3-10. [DOI] [PubMed] [Google Scholar]
- 5.Callahan, T. M., M. S. Rose, M. J. Meade, M. Ehrenshaft, and R. G. Upchurch. 1999. CFP, the putative cercosporin transporter of Cercospora kikuchii, is required for wild type cercosporin production, resistance, and virulence on soybean. Mol. Plant-Microbe Interact. 12:901-910. [DOI] [PubMed] [Google Scholar]
- 6.Canzoniero, L. M., M. Taglialatela, G. Di Renzo, and L. Annunziato. 1993. Gadolinium and neomycin block voltage-sensitive Ca2+ channels without interfering with the Na+-Ca2+antiporter in brain nerve endings. Eur. J. Pharmacol. 245:97-103. [DOI] [PubMed] [Google Scholar]
- 7.Chandra, S., G. M. Leinhos, G. Morrison, and H. C. Hoch. 1999. Imaging of total calcium in urediospore germlings of Uromyces by ion microscopy. Fungal Genet. Biol. 27:77-87. [DOI] [PubMed] [Google Scholar]
- 8.Daub, M. E. 1982. Peroxidation of tobacco membrane lipids by the photosensitizing toxin, cercosporin. Plant Physiol. 69:1361-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daub, M. E., and R. P. Hangarter. 1983. Production of singlet oxygen and superoxide by the fungal toxin, cercosporin. Plant Physiol. 73:855-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daub, M. E., and M. Ehrenshaft. 2000. The photoactivated Cercospora toxin cercosporin: contributions to plant disease and fundamental biology. Annu. Rev. Phytopathol. 38:437-466. [DOI] [PubMed] [Google Scholar]
- 11.Donaldson, S. P., and J. W. Deacon. 1992. Role of calcium in adhesion and germination of zoospore cysts of Pythium: a model to explain infection of host plants. J. Gen. Microbiol. 138:2051-2059. [Google Scholar]
- 12.Hoeflich, K. P., and M. Ikura. 2002. Calmodulin in action. Diversity in target recognition and activation mechanisms. Cell 108:739-742. [DOI] [PubMed] [Google Scholar]
- 13.Hu, G. F. 1998. Neomycin inhibits angiogenin-induced angiogenesis. Proc. Natl. Acad. Sci. USA 95:9791-9795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyde, G. J., and I. B. Heath. 1997. Ca2+ gradients in hyphae and branches of Saprolegnia ferax. Fungal Genet. Biol. 21:238-251. [DOI] [PubMed] [Google Scholar]
- 15.Inhorn, R. C., and P. W. Majerus. 1988. Properties of inositol polyphosphate 1-phosphatase. J. Biol. Chem. 263:14559-14565. [PubMed] [Google Scholar]
- 16.Irving, H. R., and B. R. Grant. 1984. The effect of calcium on zoospore differentiation in Phytophthora cinnamomi. J. Gen. Microbiol. 130:1569-1576. [Google Scholar]
- 17.Jenns, A. E., M. E. Daub, and R. G. Upchurch. 1989. Regulation of cercosporin accumulation in culture by medium and temperature manipulation. Phytopathology 79:213-219. [Google Scholar]
- 18.Jenns, A. E., D. L. Scott, E. F. Bowden, and M. E. Daub. 1995. Isolation of mutants of the fungus Cercospora nicotianae altered in their response to singlet-oxygen-generating photosensitizers. Photochem. Photobiol. 61:488-493. [Google Scholar]
- 19.Kim, Y., D. Li, and P. E. Kolattukudy. 1998. Induction of Ca2+-calmodulin signaling by hard-surface contact primes Colletotrichum gloeosporioides conidia to germinate and form appressoria. J. Bacteriol. 180:5144-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein, P. S., and D. A. Melton. 1996. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 93:8455-8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleyman, T. R., and E. J. Cragoe, Jr. 1988. Amiloride and its analogs as tools in the study of ion transport. J. Membr. Biol. 105:1-21. [DOI] [PubMed] [Google Scholar]
- 22.Lee, S., and Y. Lee. 1998. Calcium/calmodulin-dependent signaling for appressorium formation in the plant pathogenic fungus Magnaporthe grisea. Mol. Cell 8:698-704. [PubMed] [Google Scholar]
- 23.Liscovitch, M., V. Chalifa, M. Danin, and Y. Eli. 1991. Inhibition of neural phospholipase D activity by aminoglycoside antibiotics. Biochem. J. 279:319-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcus, F., and M. M. Hosey. 1980. Purification and properties of liver fructose 1,6-bisphosphatase from C57BL/KsJ normal and diabetic mice. J. Biol. Chem. 255:2481-2486. [PubMed] [Google Scholar]
- 25.Masuda, C. A., M. A. Xavier, K. A. Mattos, A. Galina, and M. Montero-Lomelí. 2001. Phosphoglucomutase is an in vivo lithium target in yeast. J. Biol. Chem. 276:37794-37801. [DOI] [PubMed] [Google Scholar]
- 26.Murguía, J. R., J. M. Bellés, and R. Serrano. 1995. A salt-sensitive 3′(2′),5′-bisphosphate nucleotidase involved in sulfate activation. Science 267:232-234. [DOI] [PubMed] [Google Scholar]
- 27.Muthukumar, G., and K. W. Nickerson. 1984. Ca(II)-calmodulin regulation of fungal dimorphism in Ceratocystis ulmi. J. Bacteriol. 159:390-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muthukumar, G., E. C. Jensen, A. W. Nickerson, M. K. Eckles, and K. W. Nickerson. 1991. Photomorphogenesis in Penicillium isariaeforme: exogenous calcium substitutes for light. Photochem. Photobiol. 53:287-291. [Google Scholar]
- 29.Okubo, A., S. Yamazak, and K. Fuwa. 1975. Biosynthesis of cercosporin. Agric. Biol. Chem. 39:1173-1175. [Google Scholar]
- 30.Osherov, N., and G. S. May. 2001. The molecular mechanisms of conidial germination. FEMS Microbiol. Lett. 199:153-160. [DOI] [PubMed] [Google Scholar]
- 31.Phillippe, M. 1994. Neomycin inhibition of hormone-stimulated smooth muscle contractions in myometrial tissue. Biochem. Biophys. Res. Commun. 205:245-250. [DOI] [PubMed] [Google Scholar]
- 32.Pressman, B. C. 1976. Biological applications of ionophores. Annu. Rev. Biochem. 45:501-530. [DOI] [PubMed] [Google Scholar]
- 33.Rao, J. P., and C. Subramanyam. 1999. Requirement of Ca2+ for aflatoxin production: inhibitory effect of Ca2+ channel blockers on aflatoxin production by Aspergillus parasiticus NRRL 2999. Lett. Appl. Microbiol. 28:85-90. [DOI] [PubMed] [Google Scholar]
- 34.Robson, G. D., M. G. Wiebe, and A. P. J. Trinci. 1991. Involvement of Ca2+ in the regulation of hyphal extension and branching in Fusarium graminearum A3/5. Exp. Mycol. 15:263-272. [Google Scholar]
- 35.Shaw, B. D., and H. C. Hoch. 2000. Ca2+ regulation of Phyllosticta ampelicida pycnidiospore germination and appressorium formation. Fungal Genet. Biol. 31:43-53. [DOI] [PubMed] [Google Scholar]
- 36.Simpson, A. W. M., T. J. Hallam, and T. J. Rink. 1984. TMB-8 inhibits secretion evoked by phenol ester at basal cytoplasmic free calcium in Quin-2-loaded platelets much more effectively than it inhibits thrombin-induced mobilization. FEBS Lett. 176:139-143. [DOI] [PubMed] [Google Scholar]
- 37.Smith, M. J., M. Globus, and S. Vethamany-Globus. 1995. Nerve extracts and substance P activate the phosphatidylinositol signaling pathway and mitogenesis in newt forelimb regenerates. Dev. Biol. 167:239-251. [DOI] [PubMed] [Google Scholar]
- 38.Tatrai, A., S. K. Lee, and P. H. Stern. 1994. U-73122, a phospholipase C antagonist, inhibits effects of endothelin-1 and parathyroid hormone on signal transduction in UMR-106 osteoblastic cells. Biochim. Biophys. Acta 1224:575-582. [DOI] [PubMed] [Google Scholar]
- 39.Taylor, C. W., and P. Thorn. 2001. Calcium signaling: IP3 rises again…and again. Curr. Biol. 11:R352-R355. [DOI] [PubMed]
- 40.Upchurch, R. G., D. C. Walker, J. A. Rollins, M. Ehrenshaft, and M. E. Daub. 1991. Mutants of Cercospora kikuchii altered in cercosporin synthesis and pathogenicity. Appl. Environ. Microbiol. 57:2940-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Upchurch, R. G., M. S. Rose, and M. Eweida. 2001. Over-expression of the cercosporin facilitator protein, CFP, in Cercospora kikuchii up-regulates production and secretion of cercosporin. FEMS Microbol. Lett. 204:89-93. [DOI] [PubMed] [Google Scholar]
- 42.Upchurch, R. G., M. S. Rose, M. Eweida, and T. M. Callahan. 2002. Transgenic assessment of CFP-mediated cercosporin export and resistance in a cercosporin-sensitive fungus. Curr. Genet. 41:25-30. [DOI] [PubMed] [Google Scholar]
- 43.Veigl, M. L., T. C. Vanaman, and W. D. Sedwick. 1984. Calcium and calmodulin in cell growth and transformation. Biochim. Biophys. Acta 738:21-48. [DOI] [PubMed] [Google Scholar]
- 44.Wang, J. P., D. H. Needleman, A. B. Seryshev, B. Aghdasi, K. J. Slavik, S. Q. Liu, S. E. Pedersen, and S. L. Hamilton. 1996. Interaction between ryanodine and neomycin binding sites on Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 271:8387-8393. [DOI] [PubMed] [Google Scholar]
- 45.Warwar, V., and M. B. Dickman. 1996. Effects of calcium and calmodulin on spore germination and appressorium development in Colletotrichum trifolii. Appl. Environ. Microbiol. 62:74-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamazaki, S., and T. Ogawa. 1972. The chemistry and stereochemistry of cercosporin. Agric. Biol. Chem. 36:1707-1718. [Google Scholar]