Abstract
Although homoacetogenic bacteria are generally considered to be obligate anaerobes, they colonize the intestinal tracts of termites and other environments that are not entirely anoxic in space or time. In this study, we investigated how homoacetogenic bacteria isolated from the hindguts of various termites respond to the presence of molecular oxygen. All strains investigated formed growth bands in oxygen gradient agar tubes under a headspace of H2-CO2. The position of the bands coincided with the oxic-anoxic interface and depended on the O2 partial pressure in the headspace; the position of the bands relative to the meniscus remained stable for more than 1 month. Experiments with dense cell suspensions, performed with Clark-type O2 and H2 electrodes, revealed a large capacity for H2-dependent oxygen reduction in Sporomusa termitida and Sporomusa sp. strain TmAO3 (149 and 826 nmol min−1 mg of protein−1, respectively). Both strains also reduced O2 with endogenous reductants, albeit at lower rates. Only in Acetonema longum did the basal rates exceed the H2-dependent rates considerably (181 versus 28 nmol min−1 mg of protein)−1). Addition of organic substrates did not stimulate O2 consumption in any of the strains. Nevertheless, reductive acetogenesis by cell suspensions of strain TmAO3 was inhibited even at the lowest O2 fluxes, and growth in nonreduced medium occurred only after the bacteria had rendered the medium anoxic. Similar results were obtained with Acetobacterium woodii, suggesting that the results are not unique to the strains isolated from termites. We concluded that because of their tolerance to temporary exposure to O2 at low partial pressures (up to 1.5 kPa in the case of strain TmAO3) and because of their large capacity for O2 reduction, homoacetogens can reestablish conditions favorable for growth by actively removing oxygen from their environment.
Homoacetogenic bacteria are generally considered to be obligately anaerobic, and pure cultures are extremely sensitive to oxygen (20, 24). Nevertheless, homoacetogens have been isolated from oxic environments, such as well-drained soils and freshly fallen leaf litter (31, 50), and the presence of reductive acetogenesis in such habitats suggests that homoacetogens may be relatively tolerant to exposure to O2 under in situ conditions (22, 33, 34). Reductive acetogenesis is also an important electron sink in the intestinal tracts of termites (3, 6, 45, 46), which have been characterized as habitats that receive large O2 fluxes per unit volume (8, 11).
Previous studies have shown that anaerobic bacteria isolated from termite guts, such as lactic acid bacteria (2, 47) and sulfate-reducing bacteria (16, 32), are not only quite tolerant to the presence of O2 but also reduce O2 at considerable rates. Also, the methanogenic archaea (Methanobrevibacter spp.) attached to the gut epithelium of the termite Reticulitermes flavipes seem to tolerate exposure to O2 (36). In this study, we investigated how growth and reductive acetogenesis of homoacetogenic bacteria isolated from the hindguts of various termites are influenced by the presence of O2.
Since this is not a trivial task, we chose a polyphasic approach to address different aspects of the problem with the most appropriate strategies. We carried out growth experiments in the presence of O2 both in liquid culture and in semisolid media, and we measured the O2 reduction rates of dense cell suspensions. In addition, we determined how reductive acetogenesis is affected by molecular oxygen when the latter is supplied at limited fluxes, and we tested for the presence of enzyme activities that catalyze the reduction of O2 or protect against toxic reduction products.
The investigations were conducted with Sporomusa termitida (7) and Acetonema longum (28), which were previously isolated from the hindguts of wood-feeding termites, and Sporomusa sp. strain TmAO3, which was recently isolated from the hindgut of the soil-feeding termite Thoracotermes macrothorax Sjöstedt (H. I. Boga, W. Ludwig, and A. Brune, submitted for publication). Acetobacterium woodii, which was isolated from anoxic sediments (1), was included for comparative purposes.
(A preliminary report of this study was presented at the Annual Meeting of the German Society for General and Applied Microbiology [VAAM] [H. Boga and A. Brune, Annu. Meet. Verein. Allgem. Angew. Mikrobiol., Biospektrum 2000, special ed., abstr. 15.P.11.33, p. 143]. At the same meeting, Karnholz et al. reported the results of a similar study, in which they investigated tolerance and metabolic responses to oxygen of different species of homoacetogenic bacteria growing on glucose or fructose [A. Karnholz, K. Küsel, and H. L. Drake, Annu. Meet. Verein. Allgem. Angew. Mikrobiol., Biospektrum 2000, special ed., abstr. 15.P.11.32, p. 142]. Their findings, including the first report on the simultaneous consumption of hydrogen and oxygen by a homoacetogenic bacterium, have recently been published [29, 35] and are complemented by the results of our study.)
MATERIALS AND METHODS
Bacterial strains and cultivation conditions.
S. termitida DSM 4440 and A. longum DSM 6540 were kindly provided by John A. Breznak, Michigan State University. Sporomusa sp. strain TmAO3 (= DSM 13326) and A. woodii DSM 1030 were obtained from our laboratory collection. All strains were cultivated in anoxic, bicarbonate-buffered mineral medium (AM-5 medium), which was based on AM-4 medium (12) but contained 4-hydroxyphenylacetic acid and 3-indolyl acetic acid (5 μM each), as well as menadione (vitamin K3; 2.5 μM) instead of naphthoquinone. The medium was routinely supplemented with 0.1% (wt/vol) yeast extract and 0.1% (wt/vol) Casamino Acids (Difco); the final pH was 7.0 to 7.2. Unless indicated otherwise, the medium was reduced with dithiothreitol (DTT) (1 mM) and contained resazurin (10 mg liter−1), which spontaneously deoxygenates to the actual redox indicator resorufin when the medium is reduced. Cultures were routinely grown on an H2-CO2 gas mixture (80:20, vol/vol) at a headspace pressure of 150 kPa in butyl rubber-stoppered serum vials or larger bottles. When an organic growth substrate (5 mM glucose or 10 mM lactate) was used, the medium was kept under an N2-CO2 atmosphere (80:20, vol/vol). Cultures were incubated at 30°C in the dark and were agitated on a rotary shaker (150 rpm).
Growth in oxygen gradients was studied by inoculating serum tubes (20 ml) containing nonreduced AM-5 medium that was supplemented with 1.5% liquid agar under an N2-CO2 atmosphere and was kept at 60°C with an exponentially growing culture of the appropriate strain (final volume, 5 ml). Immediately after inoculation, the tubes were placed in cold water, and the headspace was flushed with the H2-CO2 gas mixture. After 10 to 15 min, different volumes of pure O2 were added with a gas-tight syringe.
The same medium without agar was used to test the effects of O2 on growth in liquid culture. Cultures were agitated on a rotary shaker, and growth was monitored by measuring the optical density at 660 nm, which minimized the interference of resorufin absorption.
Preparation of cell suspensions.
Cultures were centrifuged (10,000 × g, 30 min) in stainless steel centrifuge tubes, and cells were washed and resuspended twice in anoxic potassium phosphate buffer (0.1 M, pH 7). For measurement of O2 and H2 uptake rates and for the assays for catalase, NADH oxidase, and superoxide dismutase (SOD) activities (see below), cells were washed in N2-sparged but nonreduced buffer. For the experiments testing the effect of O2 on reductive acetogenesis (see below), the buffer was reduced with cysteine or DTT (0.1 mM), unless indicated otherwise. Cell suspensions were kept on ice and used on the days on which they were harvested.
Measurement of oxygen and hydrogen consumption.
The oxygen uptake rates of dense cell suspensions were determined by using a model 53 Clark-type oxygen meter (YSI, Yellow Springs, Ohio) with a 5-ml cuvette volume, which was operated at 25°C. The electrode was calibrated with air-saturated water (ambient pressure) and with N2-sparged, sodium dithionite-reduced water. The cuvette was first filled with H2- or N2-saturated potassium phosphate buffer (0.1 M, pH 7), to which various amounts (0.1 to 0.4 ml) of O2-saturated buffer were added. To initiate the reaction, 50 to 100 μl of a cell suspension (between 0.2 and 1 mg of protein) was added, and O2 consumption was recorded until either oxygen or the added electron donor was completely consumed.
Hydrogen uptake rates were determined with the same setup, except that the working electrode was coated with platinum black and was anodically polarized (+0.40 V) (23). The electrolyte was KCl (1 M) dissolved in HCl (1 M). The electrode was calibrated with potassium phosphate buffer (0.1 M) saturated with either N2, H2, or an N2-H2 mixture (80:20, vol/vol) at ambient pressure. The cuvette was filled with N2-H2-saturated buffer and cell suspensions (see above) before defined volumes of O2-saturated buffer (0.1 to 0.25 ml) were added to start the reaction.
Effect of oxygen on reductive acetogenesis.
The first series of experiments was carried out with dense cell suspensions (0.5 mg of protein ml−1) in reduced mineral medium (2 ml) incubated in serum vials (16 ml) under an H2-CO2 atmosphere, to which defined volumes of air were added. The vials were incubated on a rotary shaker (100 rpm). Liquid samples (0.2 ml) were taken at regular time intervals, and product formation was analyzed by high-performance liquid chromatography (HPLC).
In the second series of experiments, the cell suspensions were exposed to controlled O2 fluxes by using the diffusion-limited assay system developed by A. Tholen and A. Brune (submitted for publication). The volume of the gas reservoir was increased to 160 ml, and the bottom chamber (5 ml) had an additional sampling port for the liquid phase (Fig. 1). The diffusion properties of the membrane separating the two chambers were determined by monitoring the accumulation of O2 in the N2-flushed bottom chamber for 15 min by gas chromatography (see below). From the initial slope, the O2 permeability of the batch of membranes used in the experiments (all of which were constructed from the same Teflon sheet) was calculated to be 38 ± 3 nmol min−1 kPa−1, which means that O2 fluxes decreased by about 3% per hour due to the depletion of O2 in the reservoir.
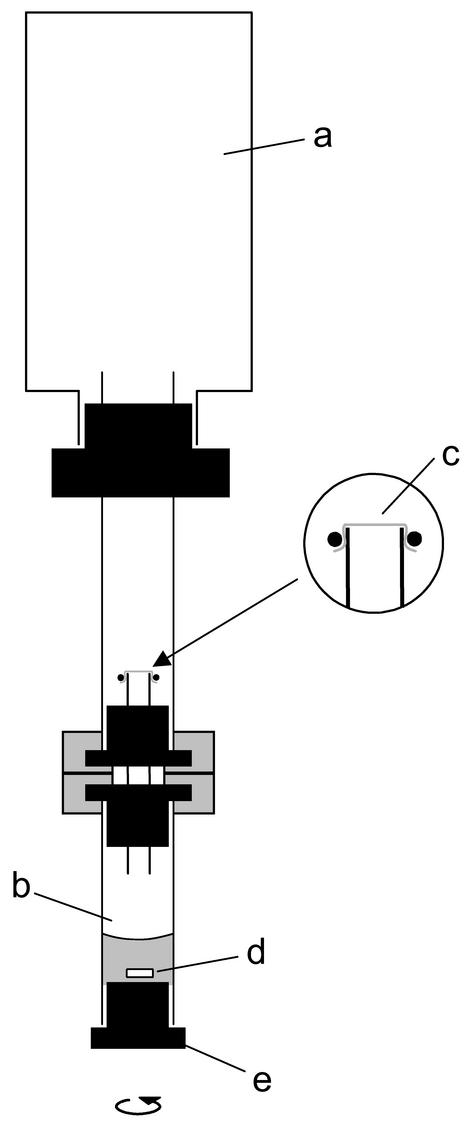
FIG. 1.
Schematic view of the diffusion-limited assay system used to expose cell suspensions of homoacetogens to controlled O2 fluxes. The reservoir (a) contained H2 and CO2 (80:20, vol/vol). The gas interchange between the reservoir and the reaction compartment (b) was controlled by a Teflon membrane (c) held in place by an O-ring. Different fluxes of O2 were achieved by adding defined amounts of O2 to the reservoir. The reaction compartment contained the cell suspension and a magnetic stir bar (d); the sampling port was fitted with a butyl rubber stopper (e).
The whole setup was preincubated for 2 days in an anaerobic glove box prior to use. Reduced potassium phosphate buffer (0.4 ml, 0.1 M), which contained DTT (0.1 mM), sodium bicarbonate (30 mM), and resazurin (10 mg liter−1) and was stored under a headspace containing N2 and CO2 (80:20, vol/vol), was added to the bottom chamber; the final pH was 7.0 to 7.2. The headspace of the gas reservoir was flushed with a mixture of H2 and CO2 (80:20, vol/vol). A freshly prepared cell suspension (0.1 ml, 1.5 to 3.0 mg of protein ml−1) was injected into the reaction compartment, and the setup was incubated at 30°C with constant stirring of the reaction compartment. Liquid samples (20 μl) were taken at regular time intervals, and acetate production was determined by gas chromatography (38). After 4 h of incubation, a defined volume of O2 was added to the upper chamber, and sampling from the bottom chamber was continued for 6 h. The remaining cell suspension (about 250 μl) was analyzed for formate and other short-chain fatty acids by HPLC.
Enzyme activity assays.
Crude cell extracts were prepared by repeatedly passing cell suspensions through a French pressure cell at 138 MPa, followed by centrifugation to remove cell debris. Anoxic conditions were maintained throughout the procedure; samples were kept on ice until they were used. All enzyme assays were performed at 25°C in 1-cm cuvettes with 1 ml of reaction mixture containing crude cell extract (5 to 150 μg of protein ml−1). A linear relationship between enzyme activity and protein concentration was routinely verified. Heat-inactivated cell extracts were used as controls.
Catalase activity was assayed photometrically by monitoring the decomposition of H2O2 (2). SOD activity was assayed by using both the xanthine-xanthine oxidase assay (2) and the nitroblue tetrazolium-salt reduction assay (26). Commercial SOD from bovine erythrocytes (Sigma) was used as a positive control.
NAD(P)H oxidase activity was assayed spectrophotometrically as described previously (44), except that the assay mixture contained only 0.1 mM β-NAD(P)H. Cell extracts were also preincubated with NADH (0.2 mM) at room temperature for 1 to 4 h and were tested for the production of hydrogen peroxide by using the methods of Stanton and Jensen (44) and Green and Hill (26).
Carbon monoxide dehydrogenase and hydrogenase activities were assayed by monitoring the increase in absorbance at 578 nm caused by the reduction of benzyl viologen (BV) (19). N2-gassed cuvettes contained the assay buffer, which was saturated with CO or H2, and BV (2 mM) was added. Before the cell suspension was added, the assay mixture was slightly reduced with sodium dithionite (40 μM). Hexadecyltrimethylammonium bromide (0.02%) was added to permeabilize the cells and to start the reaction. Values obtained with N2-saturated buffer were subtracted. One unit of activity was defined as reduction of 2 μmol of BV per min.
Other analytical techniques.
O2 in gas samples was assayed by gas chromatography by using helium (18 ml min−1) as the carrier gas, a 5-Å molecular sieve column (2 m by 2 mm; Porapak N; 60/80 mesh; Serva, Heidelberg, Germany), and a thermal conductivity detector. The column, detector, injector, and filament temperatures were 60, 180, 150, and 270°C, respectively. Oxygen gradients in agar tubes were measured with Clark-type O2 microsensors (39) constructed in our laboratory and calibrated as described previously (9).
Short-chain fatty acids and other fermentation products were analyzed by HPLC (47). The protein concentration was determined according to the manufacturer's instructions by using a Bradford protein assay kit (Bio-Rad) for cell extracts and a bicinchoninic acid protein assay kit (enhanced protocol; Pierce) for cell suspensions, with bovine serum albumin as the standard.
All results were routinely verified by repeating the experiment at least once with a different cell suspension. Unless mentioned otherwise, all values given below are averages ± standard deviations based on at least triplicate assays.
RESULTS
Effect of oxygen on growth in agar tubes.
In agar tubes containing nonreduced medium under an H2-CO2 headspace, all strains formed discrete growth bands directly below the meniscus. The medium was always reduced within 5 to 10 min of inoculation, as indicated by the color change of the redox indicator resorufin, which was pink when it was oxidized and colorless when it was reduced (E0′ = −51 mV [5]), regardless of whether the inoculum contained a reducing agent. When O2 was added to the headspace, a sharp redox boundary developed (Fig. 2A). The position of this boundary coincided with the position of the oxic-anoxic interface, as verified with O2 microsensors (data not shown), and it remained stable for more than 1 month. In controls inoculated with reduced sterile medium, O2 penetrated to the bottom of the tube within 2 to 3 days.
FIG. 2.
Influence of the O2 partial pressure in the gas headspace on the position of the oxic-anoxic interface in agar tubes incubated under an H2-CO2 gas headspace. (A) Series of tubes inoculated with Sporomusa sp. strain TmAO3. The dark color of the agar (actually pink) is caused by oxidized resorufin. Bacterial growth bands (not visible) were located immediately below the redox transition zone or, in the oxygen-free tube, below the meniscus. The control tube (tube C) was inoculated with reduced sterile medium. (B) Results obtained for all strains tested (○, Sporomusa sp. strain TmAO3; ▪, A. longum; □, A. woodii; •, S. termitida). In each case a 20% inoculum was used. The error bars indicate standard deviations from the mean for two independent series.
In all cases, the bacteria grew as a narrow band immediately below the oxidized zone. The absolute positions of the growth bands in tubes incubated under H2 depended on the O2 partial pressure in the headspace (Fig. 2B). All species except A. longum showed an almost linear relationship between O2 partial pressure and the distance of the band from the meniscus, which indicates that the O2 consumption rates of the growth bands were rather constant. At identical O2 partial pressures in the gas headspace, strain TmAO3 always grew significantly closer to the agar surface than the other strains, while the growth bands of S. termitida were always located deepest in the agar.
Although the distance of a band from the meniscus should be directly proportional the O2 flux into the band (37), this was not a reliable indicator for the specific rates of oxygen consumption of all of the strains tested (see below). Control experiments showed that band position was not totally independent of cell density in the inoculum (2.5 to 20%) and seemed to be influenced by differences in the growth rate and mobility of the cells within the agar (data not shown).
Growth bands also formed in the absence of the redox indicator and when nonreduced precultures were used (thus excluding all traces of reducing agent from the medium). When H2 in the gas headspace was replaced with N2, no stable redox boundary was established, although the time required for O2 to penetrate the whole tube (5 to 10 days) was longer than that in the uninoculated controls. Only in the case of A. longum, which exhibited high endogenous rates of O2 reduction (see below), did the redox boundary remain stable for about 1 week in the absence of H2.
Effect of oxygen on growth in liquid medium.
When cultures of strain TmAO3 growing under an H2-CO2 headspace (agitated at 150 rpm) were transferred into fresh, nonreduced medium (20% inoculum), resorufin was reduced within 10 min of inoculation, and exponential growth commenced without a noticeable lag phase. However, when O2 was included in the headspace, the cultures exhibited a distinct lag phase, which increased with the initial O2 partial pressure (0 to 4 h), and growth resumed only after resorufin was reduced (data not shown). The same phenomenon was observed with cultures of A. woodii (agitated at 100 rpm), except that the lag phases were much longer (0 to 50 h) and resorufin turned colorless long before growth commenced. With both strains, the observed phenomenon was independent of the presence of reducing agents in the preculture.
The highest headspace O2 partial pressures tolerated were 1.5 kPa for strain TmAO3 and 0.8 kPa for A. woodii; at pressures above these values, the medium remained oxidized throughout the incubation period (4 days). No growth occurred in the absence of H2, but both strains were still able to reduce the medium at O2 partial pressures up to 0.5 kPa. Resorufin always remained oxidized in uninoculated controls under H2.
Oxygen reduction by dense cell suspensions.
All strains tested showed high rates of O2 consumption in the presence of H2 (Table 1). The oxygen consumption rates at H2 saturation were independent of the O2 partial pressure within the range tested (0 to 5 kPa) and were linearly dependent on the density of the cell suspension. The oxygen reduction rates of strain TmAO3 were about fourfold higher than those of the other species. The cell suspensions of all strains reduced O2 in the absence of H2, apparently by using endogenous reductants. The rates were much lower than those in the presence of H2. Only in A. longum were the basal rates almost as high as the uncorrected rates of O2 reduction observed in the presence of H2. We incidentally found that the O2 consumption of strain TmAO3 was considerably lower (360 ± 2.6 nmol of O2 min−1 mg of protein−1) when the cells were pregrown on medium reduced with cysteine instead of DTT; the basal rates were not affected.
TABLE 1.
Oxygen uptake by washed cell suspensions of homoacetogenic bacteria in the presence and in the absence of hydrogen
| Headspace gas | O2 uptake rates (nmol min−1 mg of protein−1)a
|
|||
|---|---|---|---|---|
| Strain TmAO3 | S. termitida | A. longum | A. woodii | |
| H2b | 843 ± 95 (n = 9) | 181 ± 52 (n = 5) | 209 ± 21 (n = 6) | 138 ± 14 (n = 6) |
| N2 | 17 ± 14 (n = 5) | 32 ± 5 (n = 3) | 181 ± 33 (n = 5) | 14 ± 9 (n = 3) |
All cultures were grown in reduced medium on H2-CO2. The values are means ± standard deviations for several replicates with at least two different cell suspensions.
The basal rates of O2 consumption under an N2 headspace were not subtracted.
Addition of organic substrates, such as glucose (5 mM), lactate (10 mM), or ethylene glycol (10 mM), did not stimulate O2 reduction above the basal rates with any strain, even when the cultures had been grown on the respective substrate. In contrast, cells pregrown on organic substrates were always induced for H2-dependent oxygen reduction. Heat-inactivated cell suspensions or filter-sterilized culture supernatant did not catalyze this reaction. Oxygen reduction by strain TmAO3, S. termitida, or A. longum was completely inhibited by addition of 5 mM KCN, regardless of whether the cyanide was added to the assay mixture before or after the cells were added. Only in the case of A. woodii was the rate of O2 reduction not affected by KCN.
Since the assays were carried out in CO2-free buffer, there was no H2 consumption due to reductive acetogenesis, and none of the strains consumed H2 with endogenous oxidants. However, upon addition of O2, cell suspensions of A. longum and S. termitida consumed H2 at rates of 196 ± 18 and 250 ± 32 nmol min−1 mg of protein−1, respectively. Strain TmAO3 oxidized H2 at a much higher rate (1,570 ± 139 nmol of H2 min−1 mg of protein−1). The decreases in H2 concentration after the addition of defined volumes of O2-saturated buffer allowed determination of the stoichiometric ratio of oxygen and hydrogen consumption. The H2-to-O2 ratios obtained for strain TmAO3 and S. termitida were 2.1 ± 0.1 and 1.9 ± 0.1, respectively, which are close to the expected ratio for O2 reduction to water. The value for A. longum was consistently lower (1.1 ± 0.1), underlining the importance of an endogenous reductant(s) in O2 reduction by this strain.
Influence of oxygen on reductive acetogenesis.
Sporomusa sp. strain TmAO3 was chosen for further study because it had the highest O2 reduction rates of all the strains tested and also exhibited the shortest lag phase when it was inoculated into oxic media, which indicated that it had a high oxygen tolerance. In the first series of experiments, dense cell suspensions incubated under an H2-CO2 gas headspace formed acetate at a rate of 201 ± 3 nmol min−1 mg of protein−1. Except for the lowest O2 partial pressure tested (0.2 kPa), the rate of acetate formation decreased progressively with incubation time at increasing O2 partial pressures in the headspaces of the vials, and formate accumulated at increasing rates (140 ± 11 nmol min−1 mg of protein−1 at 1.6 kPa). However, when the reducing agent was omitted, acetate formation was strongly inhibited even at an O2 partial pressure of 0.2 kPa, and formate accumulated in all cases (data not shown).
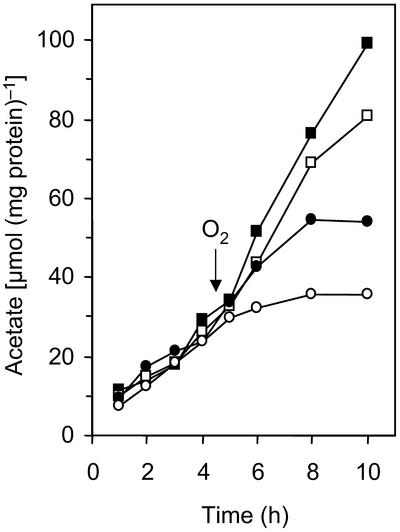
To determine whether the observed effects were due only to a detrimental accumulation of O2 in the cell suspensions, we performed a second series of experiments using a diffusion-limited assay system, which restricted O2 flux to the cells by means of a Teflon membrane (Fig. 1). Since the initial rates of acetate formation, even in oxygen-free controls, were not reproducible unless the cell suspensions were prepared in a reduced buffer, it was necessary to include low levels of a reducing agent (0.1 mM DTT) in the assay mixture. The acetate formation rates in oxygen-free controls (239 ± 56 nmol min−1 mg of protein−1) were in the same range as those in the conventional assays, indicating that the flux of H2 across the membrane was not limiting, and they decreased with increasing O2 fluxes (Fig. 3). Acetate production came to a complete standstill within 4 h after the onset of O2 fluxes of 100 nmol min−1 mg of protein−1 or higher. However, only in this case did formate accumulate in the assay mixture.
FIG. 3.
Influence of controlled O2 fluxes on reductive acetogenesis in cell suspensions of Sporomusa sp. strain TmAO3, determined by using the diffusion-limited assay system shown in Fig. 1. The oxygen fluxes were 0 (▪), 52 (□), 103 (•), and 367 (○) nmol min−1 mg of protein−1. The arrow indicates when O2 was added to the reservoir. The results were reproduced in at least five sets of experiments, but for reasons of clarity only a representative data set is shown.
Although reductive acetogenesis by strain TmAO3 was affected by the lowest O2 fluxes, the suspensions remained reduced throughout the entire incubation period (6 h) as long as H2 was present in the gas headspace, which is a safe indicator that O2 did not accumulate in the reaction compartment. Even when the cells were incubated under an N2-CO2 atmosphere, the assay buffer remained reduced for more than 1 h. In contrast, all cell-free controls were oxidized within minutes after the addition of O2 to the gas reservoir, which underlines the fact that the reducing agent alone was not responsible for O2 consumption in the reaction compartment. Using the O2 partial pressure in the reservoir, the O2 permeability of the Teflon membrane, and the time that elapsed after the onset of the oxygen flux, we estimated that cell suspensions incubated under an N2-CO2 atmosphere apparently consumed about 15 times more O2 than the DTT-reduced buffer consumed before their endogenous reductants were exhausted. When the experiment was terminated after 6 h, cells incubated under an H2-CO2 atmosphere had consumed about 5 times more O2 than cells incubated under an N2-CO2 atmosphere and 75 times more than the control (Table 2).
TABLE 2.
O2 consumption by cell suspensions of Sporomusa sp. strain TmAO3 under H2-CO2 and N2-CO2 headspaces and O2 consumption by cell-free controls, as determined by using the diffusion-limited assay systema
| Assay mixture | Headspace | O2 flux (nmol min−1)b | Time period (min)c | Amt of O2 consumed (μmol)d |
|---|---|---|---|---|
| Buffer only | N2-CO2 | 170 ± 13 | 6.5 ± 0.5 | 1.1 ± 0.1 |
| Buffer + cellse | N2-CO2 | 224 ± 18 | 73.0 ± 2.5 | 16.4 ± 1.4 |
| Buffer + cellse | H2-CO2 | 224 ± 18 | >360f | >80.6 |
See Fig. 1.
Calculated by determining the product of the O2 partial pressure in the reservoir and the O2 permeability of the Teflon membrane.
Time period between the onset of the O2 flux and the color change of the redox indicator. The values are means ± mean deviations for two independent experiments.
Calculated by determining the product of the O2 flux and time before the color change occurred.
The assay mixtures with cells contained 0.6 mg of protein.
Buffer remained reduced during the whole experiment.
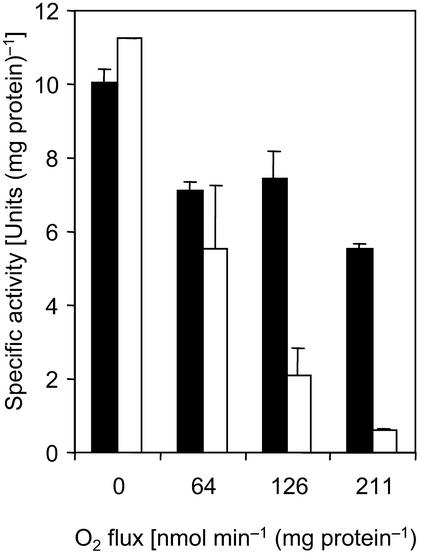
Cell suspensions of strain TmAO3 that had been exposed to defined O2 fluxes for 4.5 h were tested for hydrogenase and CO dehydrogenase activities (Fig. 4). In all cases, hydrogenase activity was significantly reduced compared to the activity in cell suspensions that were not exposed to O2. However, the activity did not decrease below 50% of that in the oxygen-free controls even at the highest O2 flux tested (211 nmol min−1 mg of protein−1), whereas almost 95% of the original activity of CO dehydrogenase was lost.
FIG. 4.
Activities of CO dehydrogenase (open bars) and hydrogenase (solid bars) in cell suspensions of Sporomusa sp. strain TmAO3 exposed to controlled O2 fluxes for 4.5 h in experiments identical to the experiment whose results are shown in Fig. 3.
Enzyme activities.
Lactate-grown cell suspensions of strain TmAO3 and S. termitida exhibited low NADH oxidase activities (<0.01 μmol min−1 mg of protein−1) but high catalase activities (9.7 and 78 μmol min−1 mg of protein−1, respectively). Hydrogen peroxide did not accumulate during O2 reduction, and SOD activity was not detected in either strain.
DISCUSSION
Our study shows that the homoacetogenic bacteria S. termitida, A. longum, and Sporomusa sp. strain TmAO3, all of which were isolated from termite hindguts, and also A. woodii, which was isolated from sediment, can reduce O2 at high rates by using H2 or endogenous reductants. Because of this evidence, together with the evidence for oxygen consumption recently presented by Drake and coworkers for A. woodii, Clostridium magnum, Moorella thermoacetica, Sporomusa silvacetica, and Thermoanaerobacter kivui during growth on glucose or fructose (29) and for Clostridium glycolicum strain RD-1 during growth on H2-CO2 (35), it has now been unequivocally established that homoacetogenic bacteria, which generally are considered to be strictly anaerobic and sensitive to molecular oxygen, are in fact quite tolerant to temporary exposure to oxygen and can reestablish conditions favorable for growth by actively removing O2 from their environments. Nevertheless, the results obtained in this study with strain TmAO3 underline the fact that reductive acetogenesis from H2-CO2 is extremely sensitive to the presence of O2 and is inhibited by even the smallest O2 fluxes.
Growth in semisolid medium.
The results obtained with oxygen gradient agar tubes indicate that in a structured environment, even pure cultures of homoacetogens can cope with considerable O2 fluxes over an extended time period, provided that H2 is present as a reductant. Also, in the study of Karnholz et al. (29), all of the homoacetogenic bacteria tested gave rise to steep oxygen gradients in agar tubes, and growth was apparent only in the portions of the tubes in which O2 was not detectable. Since the bacteria used organic substrates supplied via the medium, the oxic-anoxic interface migrated deeper into the agar during incubation (albeit more slowly than in the uninoculated controls), whereas in our study the bacterial band remained at a stable position for several weeks at any given O2 partial pressure as long as H2 was present in the headspace, a consequence of the quasiconstant source concentration of H2 at the meniscus.
Oxygen reduction by cell suspensions.
Most of the species of homoacetogens included in this study had a large capacity for H2-dependent oxygen reduction; the only exception was A. longum, which already exhibited high basal rates, apparently caused by an endogenous reductant(s). The specific rates (Table 1) are considerably higher than the glucose-dependent rates of O2 reduction by lactic acid bacteria isolated from termite guts (2). In the case of strain TmAO3, they are surpassed only by the O2 reduction rates reported for several Desulfovibrio spp. that use H2 as an electron donor (30, 32).
The capacity for H2-dependent oxygen reduction seems to be constitutive in all of the strains tested since it was also present in cells pregrown on glucose or lactate. Addition of organic substrates did not increase the O2 uptake rate above the background rates, which are apparently caused by other, endogenous electron donors. This observation is in agreement with the results of Karnholz et al. (29), who found that O2 consumption by several other homoacetogenic bacteria during growth on glucose did not alter the 1:3 glucose-to-acetate stoichiometry typical of homoacetogenesis. Therefore, it can be speculated that O2 consumption by homoacetogenic bacteria is due to endogenous electron donors that are not directly derived from the catabolic pathway. Also, Desulfovibrio spp. reduce O2 with endogenous reductants, and it has been shown that polyglucose acts as an electron donor during O2 reduction by Desulfovibrio salexigens (49).
Biochemical basis of oxygen reduction.
The mechanism of O2 reduction in homoacetogens is not clear. Differences in the sensitivity to cyanide among the strains tested in this study indicate that there are at least two different biochemical pathways. Oxygen reduction by A. woodii, which reportedly lacks cytochromes (41), was not affected by 5 mM KCN. In lactic acid bacteria, cyanide-insensitive O2 reduction is catalyzed mainly by NAD(P)H oxidases and pyruvate oxidases (15), and in many Desulfovibrio species, the activities of NADH oxidases surpass the O2 reduction by cell suspensions (49). The oxygen-reducing electron transport system in Desulfovibrio gigas consists of a combination of NADH-rubredoxin oxidoreductase (13) and rubredoxin oxidase (14, 25), which is also insensitive to cyanide. The genomes of methanogenic archaea and other bacteria (51), including the homoacetogenic organism M. thermoacetica (17), contain homologs of the genes involved in this pathway, which may indicate that there are similar mechanisms for oxygen removal.
In contrast, O2 reduction by all termite gut isolates was completely inhibited in the presence of cyanide. Although other homoacetogenic bacteria possess significant levels of NADH oxidase activity (29, 35), the activities in S. termitida and in strain TmAO3 were much too low to account for the high rates of H2-dependent oxygen reduction (this study). Since S. termitida, A. longum, and strain TmAO3, in contrast to A. woodii, contain membrane-bound type b cytochromes (7, 28; Boga et al., submitted), it is possible that membrane-associated processes are involved in the electron transport to O2.
Inhibition of growth and reductive acetogenesis.
Although both Sporomusa sp. strain TmAO3 and A. woodii were able to initiate growth when they were transferred into oxic medium, growth did not start before the medium was reduced. Strain TmAO3, which exhibited the highest capacity for O2 reduction in dense cell suspensions, started to grow immediately after resorufin turned colorless, and the slight differences in the length of the lag phase merely reflect the time needed by resting cells to remove all of the O2 present in the tubes. The significantly longer lag phases observed with A. woodii are in agreement with the considerably lower capacity of this organism for O2 reduction but may also in part reflect a higher sensitivity to oxidative stress, which may explain why the redox indicator turned colorless long before growth commenced.
Also, Karnholz et al. (29) found that the lag phases of several homoacetogenic bacteria cultivated in the presence of O2 increased with the O2 concentration, but they reported that growth was initiated while O2 was still present in the headspace of the tubes. This does not necessarily contradict the results of our study since the cultures of Karnholz et al. were shaken vigorously only before optical densities were measured (i.e., once every 2 to 3 h); the limitation of mass transfer between headspace and medium may have allowed the cells to establish anoxic conditions at least in a portion of the culture volume. In our experiments, we observed that in cultures incubated in the presence of O2 in the headspace, the resorufin indicated that there were reducing conditions in the bulk of the liquid phase within minutes after shaking was stopped.
In the case of the recently isolated C. glycolicum strain RD-1 (35), however, O2 reduction concurrent with growth in agitated cultures has been unequivocally established. The study of Küsel et al. (35), which was the first study to document the simultaneous consumption of hydrogen and oxygen by a homoacetogenic bacterium, also showed that H2-dependent acetogenesis by C. glycolicum strain RD-1 was more sensitive to O2 than fermentation was and demonstrated that when cells growing on glucose were exposed to oxygen, they switched from partially homoacetogenic metabolism to purely fermentative pathways.
Also, our results obtained with dense cell suspensions of Sporomusa sp. strain TmAO3 document that homoacetogenic metabolism is severely compromised by the presence of O2 in the medium. Even under flux-limited conditions, which prevented accumulation of O2 in the buffer and allowed the cells to maintain a negative oxidation-reduction potential throughout the incubation, acetate production was inhibited by even the smallest O2 fluxes. The concomitant inhibition of CO dehydrogenase activity in these suspensions indicates that inhibition of homoacetogenesis can be (at least in part) attributed to the extreme O2 sensitivity of CO dehydrogenase (24).
The activity of hydrogenase and apparently also the activity of formate dehydrogenase seem to be less affected by low O2 fluxes than is the activity of CO dehydrogenase. This explains the H2-dependent accumulation of formate in cell suspensions of strain TmAO3 exposed to high O2 fluxes. Formate accumulation has also been observed in cultures of A. woodii when CO dehydrogenase activities are reduced due to the depletion of nickel (18).
Protection from toxic metabolites.
The fact that homoacetogens can tolerate the transient presence of O2 implies that they must possess protective mechanisms to eliminate toxic products of O2 reduction, such as hydrogen peroxide and superoxide anion radicals, which would otherwise cause serious damage to proteins, lipids, and DNA (48). It is therefore not astonishing that catalase and SOD are found in many obligately anaerobic bacteria (40), including oxygen-tolerant Desulfovibrio species (16, 21) and, in the case of catalase, also in some methanogens (36, 42, 43).
The situation in homoacetogens is somewhat unclear. The presence of catalase activities in S. termitida (7), A. longum (28), and strain TmAO3 (this study) indicates that homoacetogens have mechanisms for elimination of hydrogen peroxide, but SOD activity was found neither in S. termitida nor in strain TmAO3 (this study). Neither catalase nor SOD has been detected in S. silvacetica, M. thermoacetica, and C. magnum (29), whereas SOD, but not catalase, is present in C. glycolicum strain RD-1 (35).
It may be noteworthy that biochemical characterization of the apparent SOD activity in the hyperthermophilic anaerobe Pyrococcus furiosus has revealed the presence of a superoxide reductase (SOR), which seems to use reduced rubredoxin, provided by an NAD(P)H-dependent oxidoreductase, as the physiological electron donor (27). Since the genome of M. thermoacetica (synonym, Clostridium thermoaceticum) contains homologs of rub (rubredoxin), rbo (rubredoxin oxidoreductase), and other genes whose products have been implicated in oxidative stress protection in other anaerobic bacteria and archaea (17), it is possible that homoacetogens use SOR to remove superoxide anion radicals. Unlike SOD, SOR does not produce O2, which would be advantageous for oxygen-sensitive microorganisms.
Ecological implications.
Although termite guts experience a significant O2 influx (8), reductive acetogenesis has been detected in the guts of termites belonging to all major feeding guilds (3, 4). In R. flavipes, O2 diffusion into the hindgut does not decrease the in situ rate of reductive acetogenesis, whereas the fate of lactate is strongly affected (46). It has been pointed out that the significance of the capacity of a gut bacterium to reduce O2 depends on its position relative to the oxygen gradient (11). Therefore, it is important to establish the exact spatial distribution of such bacteria within their habitats in order to understand the ecological implications of this phenomenon.
Homoacetogenic bacteria also occur in many other habitats that are not permanently anoxic (22, 29, 35). The same is true for sulfate-reducing bacteria belonging to the genus Desulfovibrio (16, 49), which even seem to be able to exhibit energy metabolism involving O2 (16). However, there is no conclusive evidence that strictly anaerobic microorganisms are able to grow in the presence of O2. Nevertheless, the oxygen status of many habitats is subject to strong temporal fluctuations (10). The large capacity of homoacetogens to scavenge O2 entering their environment, together with the apparent tolerance of these organisms to toxic O2 reduction products, not only should enable them to survive temporary exposure to O2 but also should allow them to actively reestablish conditions favorable for growth. It seems to be time to develop a more differentiated concept of anaerobiosis among microorganisms.
Acknowledgments
This study was supported by a grant from Deutsche Forschungsgemeinschaft (DFG). H. Boga was supported by a scholarship from the Deutscher Akademischer Austauschdienst (DAAD).
REFERENCES
- 1.Balch, W. E., S. Schoberth, R. S. Tanner, and R. S. Wolfe. 1977. Acetobacterium woodii, a new genus of hydrogen-oxidizing, carbon dioxide-reducing, anaerobic bacteria. Int. J. Syst. Bacteriol. 27:355-361. [Google Scholar]
- 2.Bauer, S., A. Tholen, J. Overmann, and A. Brune. 2000. Characterization of abundance and diversity of lactic acid bacteria in the hindgut of wood- and soil-feeding termites by molecular and culture-dependent techniques. Arch. Microbiol. 173:126-137. [DOI] [PubMed] [Google Scholar]
- 3.Brauman, A., M. D. Kane, M. Labat, and J. A. Breznak. 1992. Genesis of acetate and methane by gut bacteria of nutritionally diverse termites. Science 257:1384-1387. [DOI] [PubMed] [Google Scholar]
- 4.Breznak, J. A. 1994. Acetogenesis from carbon dioxide in termite guts, p. 303-330. In H. L. Drake (ed.), Acetogenesis. Chapman and Hall, New York, N.Y.
- 5.Breznak, J. A., and R. Costilow. 1994. Physicochemical factors in growth, p. 134-137. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 6.Breznak, J. A., and J. M. Switzer. 1986. Acetate synthesis from H2 plus CO2 by termite gut microbes. Appl. Environ. Microbiol. 52:623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breznak, J. A., J. M. Switzer, and H. J. Seitz. 1988. Sporomusa termitida sp. nov., an H2/CO2-utilizing acetogen isolated from termites. Arch. Microbiol. 150:282-288. [Google Scholar]
- 8.Brune, A. 1998. Termite guts: the world's smallest bioreactors. Trends Biotechnol. 12:16-21. [Google Scholar]
- 9.Brune, A., D. Emerson, and J. A. Breznak. 1995. The termite gut microflora as an oxygen sink: microelectrode determination of oxygen and pH gradients in guts of lower and higher termites. Appl. Environ. Microbiol. 61:2681-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brune, A., P. Frenzel, and H. Cypionka. 2000. Life at the oxic-anoxic interface: microbial activities and adaptations. FEMS Microbiol. Rev. 24:691-710. [DOI] [PubMed] [Google Scholar]
- 11.Brune, A., and M. Friedrich. 2000. Microecology of the termite gut: structure and function on a microscale. Curr. Opin. Microbiol. 3:263-269. [DOI] [PubMed] [Google Scholar]
- 12.Brune, A., E. Miambi, and J. A. Breznak. 1995. Roles of oxygen and the intestinal microflora in the metabolism of lignin-derived phenylpropanoids and other monoaromatic compounds by termites. Appl. Environ. Microbiol. 61:2688-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, L., M.-Y. Liu, J. LeGall, P. Fareleira, H. Santos, and A. V. Xavier. 1993. Purification and characterization of an NADH-rubredoxin oxidoreductase involved in the utilization of oxygen by Desulfovibrio gigas. Eur. J. Biochem. 216:443-448. [DOI] [PubMed] [Google Scholar]
- 14.Chen, L., M.-Y. Liu, J. LeGall, P. Fareleira, H. Santos, and A. V. Xavier. 1993. Rubredoxin oxidase, a new flavo-hemo-protein, is the site of oxygen reduction to water by the “strict anaerobe” Desulfovibrio gigas. Biochem. Biophys. Res. Commun. 193:100-105. [DOI] [PubMed] [Google Scholar]
- 15.Condon, S. 1987. Responses of lactic acid bacteria to oxygen. FEMS Microbiol. Rev. 46:269-280. [Google Scholar]
- 16.Cypionka, H. 2000. Oxygen respiration by Desulfovibrio species. Annu. Rev. Microbiol. 54:827-848. [DOI] [PubMed] [Google Scholar]
- 17.Das, A., E. D. Coulter, D. M. Kurtz, Jr., and L. G. Ljungdahl. 2001. Five-gene cluster in Clostridium thermoaceticum consisting of two divergent operons encoding rubredoxin oxidoreductase-rubredoxin and ruberythrin-type A flavoprotein-high-molecular-weight rubredoxin. J. Bacteriol. 183:1560-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diekert, G., and M. Ritter. 1982. Nickel requirement of Acetobacterium woodii. J. Bacteriol. 152:1043-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diekert, G., and R. K. Thauer. 1978. Carbon monoxide oxidation by Clostridium thermoaceticum and Clostridium formicoaceticum. J. Bacteriol. 136:597-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diekert, G., and G. Wohlfarth. 1994. Metabolism of homoacetogens. Antonie Leeuwenhoek 66:209-221. [DOI] [PubMed] [Google Scholar]
- 21.Dos Santos, W. G., I. Pacheco, M. Y. Liu, M. Teixeira, A. V. Xavier, and J. LeGall. 2000. Purification and characterization of an iron superoxide dismutase and a catalase from the sulfate-reducing bacterium Desulfovibrio gigas. J. Bacteriol. 182:796-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drake, H. L., S. L. Daniel, K. Küsel, C. Matthies, C. Kuhner, and S. Braus-Stromeyer. 1997. Acetogenic bacteria: what are the in situ consequences of their diverse metabolic versatilities? Biofactors 61:13-24. [DOI] [PubMed] [Google Scholar]
- 23.Ebert, A., and A. Brune. 1997. Hydrogen concentration profiles at the oxic-anoxic interface: a microsensor study of the hindgut of the wood-feeding lower termite Reticulitermes flavipes (Kollar) Appl. Environ. Microbiol. 63:4039-4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferry, J. G. 1995. CO dehydrogenase. Annu. Rev. Microbiol. 49:305-333. [DOI] [PubMed] [Google Scholar]
- 25.Gomes, C. M., G. Silva, S. Oliveira, J. LeGall, M. Y. Liu, A. V. Xavier, C. Rodrigues-Pousada, and M. Teixeira. 1997. Studies on the redox centers of the terminal oxidase from Desulfovibrio gigas and evidence for its interaction with rubredoxin. J. Biol. Chem. 272:22502-22508. [DOI] [PubMed] [Google Scholar]
- 26.Green, M. J., and H. A. O. Hill. 1984. Chemistry of dioxygen. Methods Enzymol. 105:3-105. [DOI] [PubMed] [Google Scholar]
- 27.Jenney, F. E., Jr., M. F. J. M. Verhagen, X. Cui, and M. W. W. Adams. 1999. Anaerobic microbes: oxygen detoxification without superoxide dismutase. Science 286:306-309. [DOI] [PubMed] [Google Scholar]
- 28.Kane, M. D., and J. A. Breznak. 1991. Acetonema longum gen. nov., sp. nov., an H2/CO2 acetogenic bacterium from the termite Pterotermes occidentis. Arch. Microbiol. 156:291-298. [DOI] [PubMed] [Google Scholar]
- 29.Karnholz, A., K. Küsel, A. Gößner, A. Schramm, and H. L. Drake. 2002. Tolerance and metabolic response of acetogenic bacteria toward oxygen. Appl. Environ. Microbiol. 68:1005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krekeler, D., and H. Cypionka. 1995. The preferred electron acceptor of Desulfovibrio desulfuricans CSN. FEMS Microbiol. Ecol. 17:271-278. [Google Scholar]
- 31.Kuhner, C., C. Frank, A. Grieshammer, M. Schmittroth, G. Acker, A. Gößner, and H. L. Drake. 1997. Sporomusa silvacetica sp. nov., an acetogenic bacterium isolated from aggregated soil. Int. J. Syst. Bacteriol. 47:352-358. [DOI] [PubMed] [Google Scholar]
- 32.Kuhnigk, T., J. Branke, D. Krekeler, H. Cypionka, and H. König. 1996. A feasible role of sulfate-reducing bacteria in the termite gut. Syst. Appl. Microbiol. 19:139-149. [Google Scholar]
- 33.Küsel, K., and H. L. Drake. 1995. Effects of environmental parameters on the formation and turnover of acetate by forest soils. Appl. Environ. Microbiol. 61:3667-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Küsel, K., and H. L. Drake. 1996. Anaerobic capacities of leaf litter. Appl. Environ. Microbiol. 62:4216-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Küsel, K., A. Karnholz, T. Trinkwalter, R. Devereux, G. Acker, and H. L. Drake. 2001. Physiological ecology of Clostridium glycolicum RD-1, an aerotolerant acetogen isolated from sea grass roots. Appl. Environ. Microbiol. 67:4734-4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leadbetter, J. R., and J. A. Breznak. 1996. Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipes. Appl. Environ. Microbiol. 62:3620-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marschall, C., P. Frenzel, and H. Cypionka. 1993. Influence of oxygen on sulfate reduction and growth of sulfate-reducing bacteria. Arch. Microbiol. 159:168-173. [Google Scholar]
- 38.Platen, H., and B. Schink. 1987. Methanogenic degradation of acetone by enriched culture. Arch. Microbiol. 149:136-141. [DOI] [PubMed] [Google Scholar]
- 39.Revsbech, N. P. 1989. Diffusion characteristics of microbial communities determined by use of oxygen microsensors. J. Microbiol. Methods 9:111-122. [Google Scholar]
- 40.Rolfe, R. D., D. J. Hentges, B. J. Campbell, and J. T. Barrett. 1978. Factors related to the oxygen tolerance of anaerobic bacteria. Appl. Environ. Microbiol. 36:306-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schink, B., and M. Bomar. 1992. The genera Acetobacterium, Acetogenium, Acetoanaerobium and Acetitomaculum, p. 1925-1936. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer-Verlag, New York, N.Y.
- 42.Shima, S., A. Netrusov, M. Sordel, M. Wicke, G. C. Hartmann, and R. K. Thauer. 1999. Purification, characterization, and primary structure of a monofunctional catalase from Methanosarcina barkeri. Arch. Microbiol. 171:317-323. [DOI] [PubMed] [Google Scholar]
- 43.Shima, S., M. Sordel-Klippert, A. Brioukhanov, A. Netrusov, D. Linder, and R. K. Thauer. 2001. Characterization of a heme-dependent catalase from Methanobrevibacter arboriphilus. Appl. Environ. Microbiol. 67:3041-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanton, T. B., and N. S. Jensen. 1993. Purification and characterization of NADH oxidase from Serpulina (Treponema) hyodysenteriae. J. Bacteriol. 175:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tholen, A., and A. Brune. 1999. Localization and in situ activities of homoacetogenic bacteria in the highly compartmentalized hindgut of soil-feeding higher termites (Cubitermes spp.). Appl. Environ. Microbiol. 65:4497-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tholen, A., and A. Brune. 2000. Impact of oxygen on metabolic fluxes and in situ rates of reductive acetogenesis in the hindgut of the wood-feeding termite Reticulitermes flavipes. Environ. Microbiol. 2:436-449. [DOI] [PubMed] [Google Scholar]
- 47.Tholen, A., B. Schink, and A. Brune. 1997. The gut microflora of Reticulitermes flavipes, its relation to oxygen, and evidence for oxygen-dependent acetogenesis by the most abundant Enterococcus sp. FEMS Microbiol. Ecol. 24:137-149. [Google Scholar]
- 48.Valentine, J. S., D. L. Wertz, T. J. Lyons, L.-L. Liou, J. J. Goto, and E. B. Gralla. 1998. The dark side of dioxygen biochemistry. Curr. Opin. Chem. Biol. 2:253-262. [DOI] [PubMed] [Google Scholar]
- 49.van Niel, E. W. J., and J. C. Gottschal. 1998. Oxygen consumption by Desulfovibrio strains with and without polyglucose. Appl. Environ. Microbiol. 64:1034-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner, C., A. Grießhammer, and H. L. Drake. 1996. Acetogenic capacities and the anaerobic turnover of carbon in Kansas prairie soil. Appl. Environ. Microbiol. 62:494-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wasserfallen, A., S. Ragettli, Y. Jouanneau, and T. Leisinger. 1998. A family of flavoproteins in the domain archaea and bacteria. Eur. J. Biochem. 254:325-332. [DOI] [PubMed] [Google Scholar]