Abstract
The initial reactions in the cometabolic oxidation of the gasoline oxygenate, methyl tert-butyl ether (MTBE), by Mycobacterium vaccae JOB5 have been characterized. Two products, tert-butyl formate (TBF) and tert-butyl alcohol (TBA), rapidly accumulated extracellularly when propane-grown cells were incubated with MTBE. Lower rates of TBF and TBA production from MTBE were also observed with cells grown on 1- or 2-propanol, while neither product was generated from MTBE by cells grown on casein-yeast extract-dextrose broth. Kinetic studies with propane-grown cells demonstrated that TBF is the dominant (≥80%) initial product of MTBE oxidation and that TBA accumulates from further biotic and abiotic hydrolysis of TBF. Our results suggest that the biotic hydrolysis of TBF is catalyzed by a heat-stable esterase with activity toward several other tert-butyl esters. Propane-grown cells also oxidized TBA, but no further oxidation products were detected. Like the oxidation of MTBE, TBA oxidation was fully inhibited by acetylene, an inactivator of short-chain alkane monooxygenase in M. vaccae JOB5. Oxidation of both MTBE and TBA was also inhibited by propane (Ki = 3.3 to 4.4 μM). Values for Ks of 1.36 and 1.18 mM and for Vmax of 24.4 and 10.4 nmol min−1 mg of protein−1 were derived for MTBE and TBA, respectively. We conclude that the initial steps in the pathway of MTBE oxidation by M. vaccae JOB5 involve two reactions catalyzed by the same monooxygenase (MTBE and TBA oxidation) that are temporally separated by an esterase-catalyzed hydrolysis of TBF to TBA. These results that suggest the initial reactions in MTBE oxidation by M. vaccae JOB5 are the same as those that we have previously characterized in gaseous alkane-utilizing fungi.
Methyl tert-butyl ether (MTBE) is presently an important component of many gasoline formulations used in the United States and several other countries. Low concentrations of MTBE (<3% vol/vol) were first added to gasoline in the United States in the 1980s to enhance the octane rating of unleaded gasoline. More recently, MTBE has been added as one of several oxygenating compounds intended to reduce automobile emissions of carbon monoxide and smog-related air pollutants. For this purpose MTBE is added to gasoline at concentrations in excess of 10% (vol/vol). The widespread use of MTBE and its recent detection in many urban groundwater supplies (44) have prompted concerns over the human health effects of chronic exposure to this compound through contaminated water supplies (26). The U.S. Environmental Protection Agency presently classifies MTBE as a possible human carcinogen and has issued a drinking water advisory for MTBE of 20 to 40 μg/liter (parts per billion) (48).
Ether bonds (49) and branched hydrocarbon skeletons (3) are features found in many compounds that are persistent in the environment. Although initial studies suggested that MTBE was not subject to extensive biodegradation under anaerobic conditions (35, 53), it is now known to be degraded, albeit slowly, under methanogenic (51), sulfate-reducing (43), iron-reducing (13), and nitrate-reducing (5) conditions. Little is presently known about the pathway of MTBE biodegradation under any of these conditions, although it has been suggested that the anaerobic biodegradation could be initiated by a hydrolytic mechanism (39). Like many other gasoline components, the rate of MTBE degradation is higher under aerobic than anaerobic conditions. Aerobic microorganisms appear to be able to degrade MTBE using one of two ostensibly different mechanisms. Several bacterial strains have been isolated that can use MTBE as a sole source of carbon and energy for growth (14, 20, 22, 34). These organisms typically grow slowly on MTBE and often exhibit low growth yields. The pathway of MTBE oxidation in these organisms appears to involve tert-butyl alcohol (TBA) as the initial product of MTBE oxidation, although TBA often does not accumulate due to rapid biological oxidation. These organisms are thought to catalyze MTBE oxidation through the activity of inducible monooxygenases, although these enzymes have not been characterized. Present evidence from studies with Rubrivivax sp. strain PM-1 (12) and Hydrogenophaga flava ENV 735 (22) suggest that the oxidation of MTBE and TBA is catalyzed by separate enzymes, although there is some evidence that both substrates are oxidized by the same enzyme in the most recently described MTBE-metabolizing organism, Mycobacterium austroafricanum (14).
A variety of aerobic microorganisms can degrade MTBE cometabolically after growth on certain hydrocarbons. Many of the hydrocarbon cosubstrates that support MTBE cometabolism are also major gasoline components and include both normal and branched alkanes (15, 24, 30), aromatic (25, 28), and alicyclic (9) compounds. As the majority of MTBE enters the environment as part of gasoline, this range of cosubstrates raises the possibility that organisms involved in gasoline biodegradation could contribute to MTBE degradation in gasoline-impacted environments. The initial reactions in the pathway of cometabolic MTBE oxidation have been examined but incompletely characterized, in both alkane-utilizing fungi (21) and propane-oxidizing bacteria (45). Although both of these types of organisms generate TBA as an MTBE oxidation product, the fungal systems also generate tert-butyl formate (TBF). In this study we have characterized the initial reactions involved in the cometabolic oxidation of MTBE by propane-grown cells of the alkane-oxidizing bacterium, Mycobacterium vaccae JOB5. Our results show that this organism generates TBF prior to TBA and suggest that both MTBE and TBA are oxidized by the same inducible alkane monooxygenase. We conclude from our results that the initial reactions involved in MTBE oxidation by this bacterium are substantially similar to those that we have previously described for gaseous alkane-utilizing filamentous fungi (21).
MATERIALS AND METHODS
Materials.
M. vaccae JOB5 (ATCC 29678) was obtained from the American Type Culture Collection (Manassas, Va.). TBF (97% purity), tert-butyl acetate (TBAc) (>99% purity), tert-butyl propionate (TBP) (99% purity), TBA (>99% purity), 1-propanol (99.5% purity), 2-propanol (99.5% purity), MTBE (99.8% purity), and calcium carbide (∼80% purity; for acetylene generation) were obtained from Aldrich Chemical Co., Inc. (Milwaukee, Wis.). 2-Methyl 1,2-propanediol was a gift from Lyondell Chemical Co. (Houston, Tex.). Propane (instrument grade) was obtained from Matheson Gas Products, Inc. (Montgomeryville, Pa.). Other compressed gases used for gas chromatography (H2, N2, and air) were obtained from local industrial vendors.
Growth of M. vaccae JOB5.
Most of the cells of M. vaccae JOB5 used in this study were grown in batch culture at 30°C by using propane as the sole source of carbon and energy. Cells were grown in glass serum vials (160 ml) in a mineral salt medium (25 ml) (50). The vials were inoculated (initial A600 = ∼0.02) with a suspension of cells obtained from axenic cultures of M. vaccae JOB5 previously grown on casein-yeast extract-dextrose (CYD) agar plates (Difco Plate Count Agar). The inoculated vials were sealed with butyl rubber stoppers and aluminum crimp seals (Wheaton Scientific, Millville, N.J.). Propane (60 ml) was added to the sealed vials by using plastic syringes fitted with sterile filters (0.25 μm). After growth for 4 days (final A600 = ∼0.6), a sample (50 μl) of the culture was plated on CYD plates to subsequently confirm the purity of the culture. The remaining cells were harvested by centrifugation (10,000 × g, 10 min at 4°C). The supernatant was decanted, and the resulting cell pellet was resuspended in buffer (5 ml, 50 mM sodium phosphate, pH 7) and centrifuged again (10,000 × g, 10 min at 4°C). The resulting cell pellet was then resuspended in buffer (as above) at a final protein concentration of between 5 and 20 mg/ml. The harvested and washed cells were stored at 4°C and were used within 4 h. Cells grown on 1-propanol and 2-propanol were cultivated and harvested as described above except that each growth substrate was added to the culture vials to an initial concentration of 0.05% (vol/vol). Cells grown in CYD-containing liquid media were also cultivated and harvested, as described above. The media contained (per liter) casein digest (5 g), yeast extract (2.5 g), and dextrose (1 g).
Degradation experiments.
All experiments investigating the degradation of MTBE and MTBE oxidation products were conducted in glass serum vials (10 ml). The reaction vials contained sodium phosphate buffer (50 mM, pH 7; 0.9 to 1.0 ml) and were sealed with butyl rubber stoppers and aluminum crimp seals. Substrates and inhibitors were added directly to the sealed vials either as gases or from aqueous stock solutions. The vials were placed in a shaking water bath (30°C, 150 rpm) for 5 min to allow equilibration of the reactants between the gas and liquid phases. The reactions were initiated by the addition of aliquots of the harvested cell suspension (100 μl [∼0.5 to 2.0 mg of total protein]) to give a final aqueous reaction volume of 1 ml. The reaction vials were then returned to the shaking water bath and were sampled at the times indicated in each experiment. Whenever possible, the results of the individual experiments described in this study were derived by using cells harvested from the same batch culture.
Analytical methods.
The concentrations of all reactants and products were determined by gas chromatography (GC). In all cases aqueous samples (2 μl) of reaction media or calibration standards were directly injected into a gas chromatograph (Shimadzu model GC-8A) (Kyoto, Japan) fitted with a flame ionization detector. A stainless steel column (0.3 by 183 cm) filled with Porapak Q (60/80 mesh) (Waters Associates, Framingham, Mass.) was used at a temperature of 160°C for the quantification of MTBE, TBF, and TBA. The injection and detector temperatures were 200 and 220°C, respectively, and nitrogen was used as carrier gas at a flow rate of 15 ml/min. The gas chromatograph was interfaced to a Hewlett-Packard HP3395 (Palo Alto, Calif.) integrator for data collection. To account for possible absorption effects, the concentrations of all reactants and products were determined from calibration plots (6 to 10 point) developed by using incubation vials (see above) containing cells (1 mg total protein) that had been heat treated (1 h at 95°C).
Cell protein concentrations were determined with the biuret protein assay (16) after solubilization of cell material for 30 min at 65°C in 3 N NaOH and sedimentation of insoluble material by centrifugation (14,000 × g, 5 min). Bovine serum albumin was used as a standard. The concentration of MTBE in saturated aqueous solution at room temperature (23°C) was taken as 0.544 M (21). The dimensionless Henry's constant for MTBE at 30°C was taken as 0.0255 (32), and the aqueous solubility of propane at 1 atm and 30°C was taken as 1.41 mM (31). Kinetic constants (Ks and Vmax) were determined by nonlinear regression with the hyperbolic, one-site-binding equation [Y = Vmax · X/(Ks + X)] in GraphPad Prism, version 3.0a for Macintosh (GraphPad Software, San Diego, Calif.).
RESULTS
Products of MTBE oxidation.
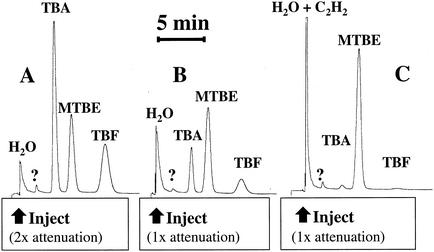
When propane-grown cells of M. vaccae JOB5 were incubated with MTBE (1 μmol) for 1 h, a GC analysis revealed that two oxidation products had accumulated in the reaction medium (Fig. 1A). These products were identified by coelution with authentic standards as TBF and TBA (Fig. 1B). The specific rate of MTBE oxidation (determined from total MTBE consumption) was estimated at ∼13 nmol min−1 mg of protein−1. Only trace quantities of TBA and TBF were generated when cells were incubated with MTBE in the presence of acetylene (10% [vol/vol], gas phase), a potent inactivator of alkane monooxygenase activity in M. vaccae JOB5 (18, 19) (Fig. 1C). Low concentrations of both TBF and TBA were also observed when cells grown on either 1-propanol or 2-propanol were incubated with MTBE (1 μmol) and when the accumulation of these products was also inhibited by acetylene (not shown). The specific rates of MTBE oxidation under these conditions were estimated at 0.5 and 2.5 nmol min−1 mg of protein−1 for 1-propanol and 2-propanol-grown cells, respectively. Neither TBA nor TBF was detected in incubations conducted with MTBE and cells grown on CYD media (not shown). The detection limits for TBF and TBA in our GC analysis were ∼20 and ∼3 nmol ml−1, respectively.
FIG. 1.
GC analysis of reaction products of MTBE oxidation by propane-grown cells of M. vaccae JOB5. The chromatogram in panel A shows the chromatographic separation of TBA, MTBE, and TBF and the relative response of the flame ionization detector for aqueous samples (2 μl) taken from reaction vials incubated at 30°C. The vials (10 ml) contained buffer (1 ml) and 1 μmol of each compound. Panel B shows the accumulation of TBF and TBA when propane-grown cells (0.83 mg of total protein) were incubated with MTBE (1 μmol) for 1 h at 30°C, as described in Materials and Methods. Panel C shows the effect of acetylene (10% [vol/vol], gas phase) on the oxidation of MTBE and the production of TBA and TBF under the conditions described for panel B.
Kinetics of TBF and TBA production.
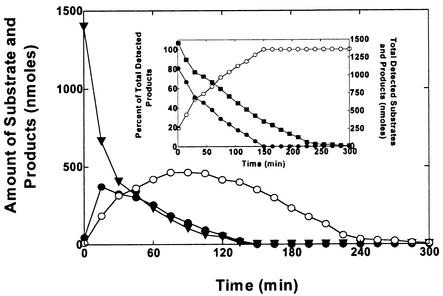
The time course of MTBE oxidation and TBF and TBA accumulation was examined using propane-grown cells incubated with MTBE (1.4 μmol). In this incubation, MTBE was rapidly oxidized without a lag phase, indicating that the enzyme(s) responsible for MTBE oxidation was present in propane-grown cells. In this experiment TBF rapidly accumulated during the early part of the reaction time course (<30 min) and then slowly disappeared over the following 2 h (Fig. 2). Once the MTBE had been completely consumed (∼130 min), TBF was no longer detected in the reaction vial. Compared to TBF, TBA accumulated more slowly during the early portion of the reaction time course and only reached a maximum concentration after ∼100 min, at a point when ∼90% of the MTBE had been consumed. Over the remainder of the incubation, the concentration of TBA slowly decreased and was no longer detected after ∼300 min. No other oxidation products were observed in this incubation under the GC conditions used in this study. A further analysis of the percentage of detected products accounted for by TBF and TBA revealed that TBF accounted for ∼81% of the products observed at the first time point (1 min) and steadily declined to zero as the MTBE was consumed during the reaction time course (Fig. 2, inset).
FIG. 2.
Time course of MTBE oxidation by propane-grown cells of M. vaccae JOB5. Propane-grown cells (1 mg of total protein) were incubated with MTBE (1.4 μmol) in glass serum vials, as described in Materials and Methods. The figure shows the time course for consumption of MTBE (▾) and production of TBF (•) and TBA (○), as determined by GC. The inset panel shows the total amount of reactants and products detected (▪) and the percentage of total detected products present as TBF (•) and TBA (○) at each time point.
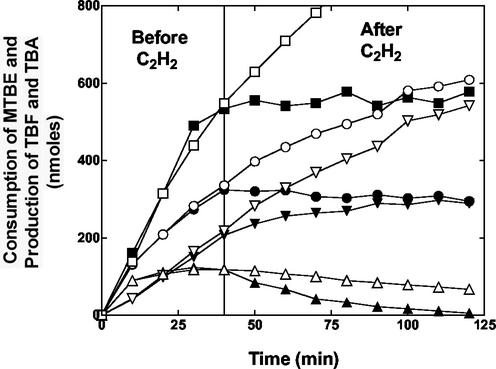
The kinetics of TBF and TBA accumulation in relation to MTBE consumption were further investigated using two initially identical reactions. In one reaction the degradation of MTBE (1.4 μmol) by propane-grown cells was allowed to proceed unhindered over the entire reaction time course (2 h). In the second reaction acetylene was added after 40 min. In the first reaction the cells consumed approximately 60% of the total added MTBE over the 2-h incubation period (Fig. 3). The time course of TBF accumulation again involved an initial rapid phase over the first 15 min, followed by a slow and limited decrease in the quantity of detected TBF over the remainder of the reaction time course. The rate of production of TBA production was again initially lower than the rate of TBF production over the initial 15 min of the reaction. After this, the rate of TBA accumulation reached a maximum and remained nearly constant over the next 60 min. Finally, the rate of TBA accumulation slowly declined in the later stages of the reaction. In the second reaction, the time course of MTBE consumption and product accumulation over the first 40 min of the reaction were indistinguishable from those in the first reaction. When acetylene was added, several effects were observed. First, there was an immediate and complete inhibition of further MTBE consumption. Second, there was an immediate inhibition of further increases in the total concentration of products detected ([TBA] + [TBF]). However, the individual concentrations of these two products continued to change over the remainder of the reaction time course. The rate of disappearance of TBF was higher in the acetylene-treated reaction than in the uninhibited reaction, and the time course of TBA production followed kinetics similar to those of the disappearance of TBF.
FIG. 3.
Effects of acetylene of MTBE oxidation by propane-grown cells of M. vaccae JOB5. Propane-grown cells (1.8 mg of total protein) were incubated with MTBE (1.4 μmol) in glass serum vials, as described in Materials and Methods. The figure shows the amount of MTBE consumed (▪, □) and the amount of TBF (▴, ▵), TBA (▾, ▿), and total detected products (TBA plus TBF) (•, ○) detected by GC plotted against time. The open symbols are for a control reaction that was allowed to proceed without intervention. The closed symbols are for an identical reaction to which acetylene (10% [vol/vol], gas phase) was added after 40 min (solid line).
Degradation of TBF.
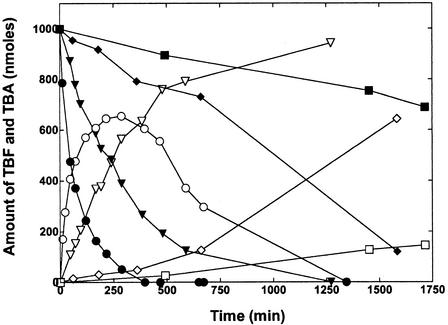
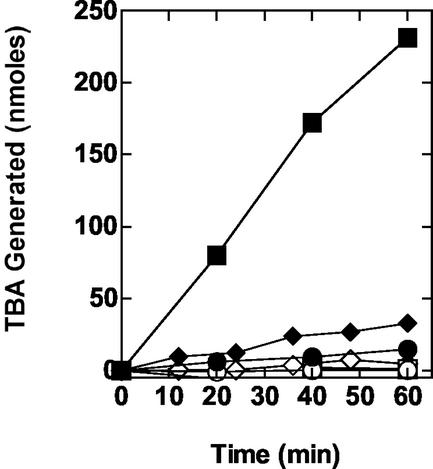
The results of the experiments described above suggested to us that the oxidation of MTBE by propane-grown cells gives rise to TBF and TBA as sequential products. To further define the relationship between TBF degradation and TBA accumulation, we examined the time course of TBF degradation in the absence of MTBE. Propane-grown cells rapidly converted TBF to TBA as the sole detected product (Fig. 4). The initial rate (0 to 50 min) of TBF degradation was estimated at 4.9 nmol min−1 mg of protein−1. In this reaction, TBA accumulation in the reaction medium was substoichiometric with respect to TBF disappearance due to TBA consumption during the reaction. Propane-grown cells also rapidly transformed TBF to TBA in the presence of acetylene. The initial rate on TBF consumption (0 to 50 min) was estimated at 1.2 nmol min−1 mg of protein−1. While the presence of acetylene clearly slowed the degradation of TBF, the amount of TBA that accumulated over the reaction time course was close to equivalent (≥95%) to the original amount of TBF added to the reaction. This suggested that the enzyme responsible for TBA consumption was fully inhibited by the presence of acetylene. A low rate of TBF consumption (∼0.2 nmol min−1) and TBA accumulation was observed when TBF was incubated in the absence of cells. Surprisingly, a more-than-twofold-higher rate of TBF degradation (∼0.5 nmol min−1 mg of protein−1) and a correspondingly higher rate of TBA accumulation were observed when heat-treated cells (95°C for 30 min) were incubated with TBF in the absence of acetylene.
FIG. 4.
Time course of biological and chemical hydrolysis of TBF. The reactions were conducted in glass serum vials, and TBA and TBF were detected by GC, as described in Materials and Methods. The figure shows the amount of TBF consumed (closed symbols) and TBA produced (open symbols) in reactions with propane-grown cells (2.1 mg of total protein) incubated with TBF (1 μmol) in the absence (•, ○) or presence (▾, ▿) of acetylene (10% [vol/vol], gas phase). The figure also shows the time course for the amount of TBF consumed (closed symbols) and TBA produced (open symbols) for a reaction containing heat-treated (30 min at 95°C) propane-grown cells (♦, ⋄) or for a reaction containing no cells (▪, □).
To further characterize the degradation of TBF, cells grown on either propane, 1-propanol, 2-propanol, or dextrose were incubated with TBF (1 μmol) and the rate of TBA generation over 1 h was determined by GC. Acetylene was also added to these incubations to prevent TBA consumption by these cells. The specific rates of TBF degradation by these cell types were determined to be 0.6, 0.6, 0.3, and 0.6 nmol min−1 mg of protein−1, respectively, while the abiotic degradation rate was estimated at 0.1 nmol min−1. To investigate whether the enzyme(s) responsible for the biological degradation of TBF was specific for TBF, the rates of TBA accumulation were determined for acetylene-treated, propane-grown cells incubated with other tert-butyl esters (Fig. 5). Low rates of TBA accumulation were observed for cells incubated with either TBF (0.7 nmol min−1 mg of protein−1) or tert-butyl acetate (TBAc) (0.3 nmol min−1 mg of protein−1), while a higher rate of TBA accumulation was observed with TBP (4.8 nmol min−1 mg of protein−1). The rates of abiotic degradation of TBF, TBAc, and TBP to TBA were all lower than the rates of biotic degradation (in the presence of acetylene) and were estimated at 0.1, 0.01 and 0.02 nmol min−1, respectively. We also examined whether TBF, TBAc, or TBP could serve as a growth substrate for M. vaccae JOB5 when supplied as the sole source of carbon and energy. No growth was observed over 7 days for cells incubated with TBF or TBAc (0.05% [vol/vol]), while strong growth was observed with the same concentration of TBP. We also used the relatively high rate of biological TBP degradation (Fig. 5) to further investigate the apparent heat stability of the TBF-degrading activity of propane-grown cells. By following TBA accumulation from TBP degradation in short-term assays (1 h), we observed that acetylene-treated, propane-grown cells retained ∼25% of their TBP-degrading activity after prior heating at 95°C for 40 min.
FIG. 5.
Degradation of tert-butyl esters by propane-grown cells of M. vaccae JOB5. The figure shows the time course of TBA production from three tert-butyl esters by propane-grown cells of M. vaccae JOB5. Cells (0.4 mg of total protein) were incubated with TBF, TBAc, and TBP (1 μmol) in glass serum vials in the presence of acetylene (10% [vol/vol], gas phase). The production of TBA was determined by GC, as described in Materials and Methods. The figure shows the amount of TBA accumulated plotted against time for reactions conducted with cells (closed symbols) and without cells (open symbols) in the presence of TBF (♦, ⋄), TBAc (•, ○), and TBP (▪, □).
Kinetics of MTBE and TBA oxidation.
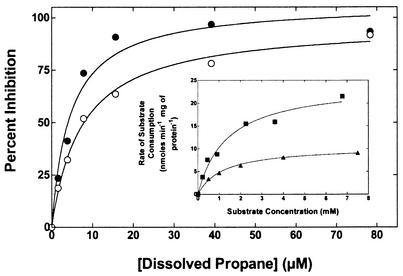
The initial rates of MTBE and TBA oxidation (0 to 30 min) by propane-grown cells of M. vaccae JOB5 were determined, and the results were computer fitted by nonlinear regression analysis to determine Ks and Vmax values. The analyses provided good fits in both cases (MTBE: r2 = 0.976; TBA: r2 = 0.997), and values for Ks of 1.36 mM (standard deviation [SD] = 0.32) and 1.18 mM (SD = 0.10) and for Vmax of 24.4 (SD = 2.00) and 10.4 (SD = 0.28) nmol min−1 mg of protein−1 were determined for MTBE and TBA, respectively (Fig. 6, inset). Three separate determinations of the Ks for MTBE by this approach yielded an average value of 1.48 mM (SD = 0.11). Additional time course experiments with cells incubated with TBA (1 mM) demonstrated that 1-propanol- and 2-propanol-grown cells also consumed TBA (0.4 and 1.21 nmol min−1 mg of protein−1, respectively), while no TBA oxidation was observed with dextrose-grown cells. The consumption of TBA was also fully inhibited by acetylene in propane-, 1-propanol-, and 2-propanol-grown cells. In all instances when TBA oxidation was observed, no further oxidation products derived from TBA were detected by the GC analysis used in this study. Additional kinetic experiments were also conducted to investigate whether MTBE and TBA oxidation could be accounted for by the activity of a single enzyme. We examined whether the rate of oxidation of MTBE (450 μM aqueous- phase concentration) was influenced by a TBA concentration up to 2 mM (aqueous- phase concentration). The presence of 1 and 2 mM TBA decreased the initial rate (0 to 30 min) of MTBE oxidation by 9 and 45%, respectively. No inhibitory effect of TBA on the amount of MTBE oxidized was observable after incubation for 3 h. In contrast to the limited effects of TBA, propane was a potent inhibitor of both MTBE and TBA oxidation by propane-grown cells. The level of inhibition of either MTBE or TBA oxidation at each concentration of propane was plotted versus propane concentration and was computer fitted to a hyperbola by nonlinear regression (Fig. 6). In both cases good fits were obtained (r2 > 0.97) and apparent Ki (Kiapp) values for propane of 4.6 (SD = 1.0 μM) and 7.4 μM (SD = 0.75 μM) were derived for the experiments conducted with MTBE and TBA, respectively.
FIG. 6.
Kinetics of MTBE and TBA oxidation by propane-grown cells of M. vaccae JOB5. The figure shows the effect of various concentrations of propane on the rate of MTBE and TBA oxidation. Propane-grown cells (∼1 mg of total protein) were incubated for 30 min with a fixed initial concentration of either MTBE (0.9 mM aqueous concentration) or TBA (1 mM aqueous concentration) in the presence of the indicated aqueous concentrations of propane. The aqueous concentrations of propane were estimated from the partial pressure of propane added to the gas phase of the reaction vials by assuming that the small volumes of gas added (≤5% of gas phase) had a negligible effect on the total gas pressure within the reaction vial. The plot shows the percent inhibition of MTBE (○) and TBA (•) consumption at each propane concentration, compared to the amount of each substrate consumed in the absence of propane. The figure shows the resulting curves generated when the values for percent inhibition were fitted by computer, as described in Materials and Methods. The inset shows the rates of MTBE and TBA oxidation by propane-grown cells of M. vaccae JOB5 plotted versus initial substrate concentration. For each datum point, cells (0.8 mg total protein) were incubated with the indicated concentration of MTBE or TBA and the initial rate (0 to 30 min) of substrate consumption was determined by GC, as described in Materials and Methods. The figure shows the resulting curves generated when the calculated rates of substrate consumption for MTBE (▪) and TBA (▴) were fitted by computer, as described in Materials and Methods.
DISCUSSION
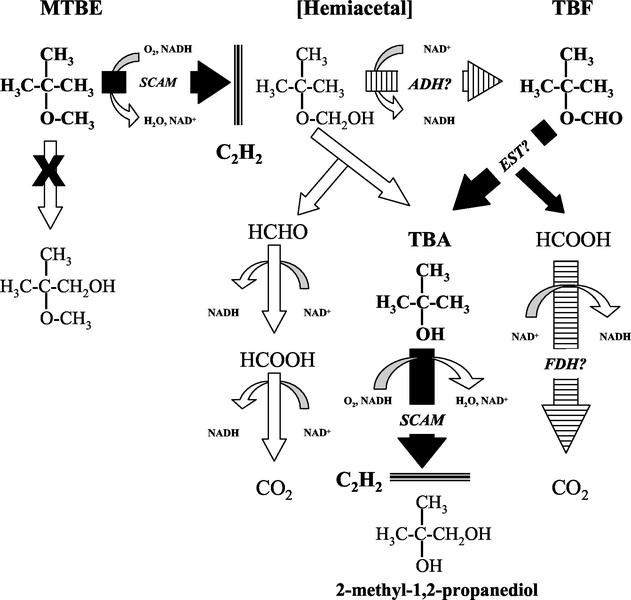
The results of this study suggest that there are at least three key reactions involved in the initial steps of MTBE oxidation propane-grown cells of M. vaccae JOB5. These steps are, in sequence, an initial monooxygenase-catalyzed oxidation of MTBE, a hydrolytic reaction that converts TBF to TBA, and a second monooxygenase-catalyzed reaction responsible for the further oxidation of TBA (Fig. 7). The evidence for each of these, and other potential reactions, is discussed below.
FIG. 7.
Proposed pathway for the initial reactions involved in MTBE oxidation by propane-grown cells of M. vaccae JOB5. The figure shows the proposed pathway for the initial reactions involved in MTBE oxidation by propane-grown cells of M. vaccae JOB5. The structures of hypothesized transient intermediates and other undetected products are in normal type, whereas structures of detected products are in boldface. The reactions with filled arrows were characterized during this study and represent the proposed dominant pathway of MTBE oxidation. The putative enzymes responsible for each reaction are in italics (SCAM = short-chain alkane monooxygenase; EST = esterase). Reactions with hash-marked arrows are predicted but uncharacterized reactions. The putative enzymes responsible for each of these reactions are also in italics (ADH = alcohol dehydrogenase; FDH = formate dehydrogenase). Open arrows represent either abiotic reactions or possible alternative reactions. The reaction arrow marked with an X indicates reactions for which no evidence was obtained. The three thick horizontal lines on the bottom beside C2H2 indicate reactions that are sensitive to complete inhibition by acetylene (10% [vol/vol], gas phase).
Oxidation of MTBE.
Several observations suggest that the enzyme responsible for the first reaction in the proposed sequence, the oxidation of MTBE, is the alkane monooxygenase that initiates propane oxidation by propane-grown cells of M. vaccae JOB5. This conclusion is in agreement with several previous studies of bacterial MTBE cometabolism (15, 24, 30, 45) but is based on a more comprehensive set of observations than those presented in most previous studies. These observations include (i) the differences in MTBE-oxidizing activity in propane-grown cells and cells grown on other substrates, (ii) the immediate MTBE-oxidizing activity observed with propane-grown cells, (iii) the inhibitory effect of acetylene on MTBE consumption and product generation (Fig. 1 and 3), and (iv) the inhibitory effect of propane on MTBE-oxidizing activity (Fig. 6). In a cometabolic process the fortuitous transformation of a non-growth-supporting substrate is catalyzed by an enzyme or cofactor that is expressed by the cell for an alternative, and often growth-related, purpose. It therefore follows that transformation of a fortuitous substrate should commence immediately at the time that the cells are challenged with that substrate and that the rate of oxidation should be proportional to the level of the key enzyme or cofactor within the cell at that time. If, as we suggest, MTBE oxidation is catalyzed by alkane monooxygenase, the highest rates of MTBE oxidation should be observed in cells with high levels of alkane monooxygenase activity and the activity should be absent in cells grown under conditions where alkane-oxidizing activity is repressed. This is the behavior that we observed in this study for propane- and CYD-grown cells, respectively (Fig. 1 and 2). The low levels of MTBE oxidation in cells grown on 1- and 2-propanol also agree with previous studies with this organism showing that alcohol-grown cells exhibit low levels of alkane-oxidizing activity (40). Various propane-oxidizing bacteria, including M. vaccae JOB5, have been shown to slowly degrade MTBE after growth on a variety of propane oxidation intermediates, including alcohols (45). However, the extended time course of these experiments (24 h) raises the possibility of inducing effects of MTBE (or its metabolites) on alkane-oxidizing activity. Our observation that low levels of MTBE-oxidizing activity were observed in short-term assays (1 h) by using cells previously grown on either 1-propanol or 2-propanol is less ambiguous with respect to these potential inducing effects. Our results are also fully compatible with the expected proportionality between MTBE oxidation rates and levels of alkane monooxygenase activity.
Our other evidence supporting a role for alkane monooxygenase in MTBE oxidation is more biochemical. The potent mechanism-based inactivating effects of acetylene on many bacterial monooxygenases are well recognized (19, 23, 52). In the case of M. vaccae JOB5, acetylene is known to fully inhibit chloroform oxidation by n-butane-grown cells (18) and exposure of n-butane-grown cells to 14C2H2 results in the covalent modification of two polypeptides (30 and 55 kDa) thought to be components of alkane monooxygenase (19). Although acetylene appears to partially inhibit other processes in M. vaccae JOB5 (Fig. 4), the complete inhibition of MTBE oxidation caused by acetylene (Fig. 3) provides strong additional evidence for a role for alkane monooxygenase in MTBE degradation. This is further bolstered by our observation that propane inhibits MTBE oxidation by propane-grown cells (Fig. 6).
Production of TBF.
Although previous studies have shown that TBF is a dominant oxidation product in the O3- and H2O2-catalyzed degradation of MTBE (1, 33), this compound has not been frequently identified as a product of biological MTBE oxidation. Our kinetic studies (Fig. 2 and 3) indicate that TBF production accounts for a large proportion (≥80%) of MTBE consumption. This suggests that the initial oxidation of MTBE by M. vaccae JOB5 is directed overwhelmingly toward the methoxy group rather than toward the methyl groups in the tert-butyl structure (Fig. 7). However, while TBF is clearly an important intermediate, it is unlikely that TBF is the immediate product of MTBE oxidation by alkane monooxygenase in M. vaccae JOB5. The expected product of the monooxygenation of the methoxy group of MTBE is a hemiacetal that is expected to undergo rapid dismutation to TBA and formaldehyde (49) (Fig. 7). However, oxidation of this hemiacetal would be expected to yield TBF as a stable product. The rapid oxidation of hydroxylated products clearly occurs during the oxidation of alkanes, as alcohols rarely accumulate when alkane-grown cells are exposed to alkanes. A precedent for hemiacetal oxidation reactions exists in methanol-grown yeasts (42). Methyl formate synthase generates methyl formate (CH3OCHO) from the NAD+-dependent oxidation of the hemiacetal species (CH3OCH2OH) generated at equilibrium in aqueous-methanol-formaldehyde mixtures (29, 36). Methyl formate synthase has been purified and shown to be a Zn-containing, NAD+-reducing alcohol dehydrogenase that oxidizes primary alcohols from ethanol to n-octanol (37). Notably, both organisms that we have shown to generate TBF during MTBE oxidation (M. vaccae JOB5 [this study] and a Graphium sp. [21]) were grown on alkanes and have high levels of alcohol dehydrogenase activity due to the important role of this enzyme in the alkane oxidation pathway. The other organism that is known to generate TBF during MTBE oxidation, M. austroafricanum, is a methylotroph. This organism may therefore have enzyme activities similar to those of the methylotrophic yeasts in which methyl formate synthase activity was first characterized (14). It is important that the production of TBF does not eliminate the concurrent operation of more than one MTBE oxidation pathway in M. vaccae JOB5. Our current experiments lack the definition to eliminate the possibility that a fraction of the putative hemiacetal product of MTBE oxidation is not “captured” as TBF but undergoes rapid dismutation to yield TBA and formaldehyde.
Hydrolysis of TBF.
The second reaction that we have characterized (Fig. 7) is the conversion of TBF to TBA. As TBA is generated from TBF, as well as TBAc and TBP (Fig. 5), we conclude that the biological degradation of TBF by M. vaccae JOB5 is a hydrolytic reaction. Several dehydrogenases hydrolyze esters, and perhaps the most relevant of these reactions to the present study is the hydrolysis of the tert-butyl ester p-nitropivalate (trimethylacetate) by aldehyde dehydrogenase (27). Alkane-grown bacteria typically have high levels of aldehyde dehydrogenase activity due to the important role of aldehyde production in the alkane oxidation pathway. However, as our results suggest that the specific rate of TBF hydrolysis is largely unaffected by growth substrate, we conclude that it is unlikely that aldehyde dehydrogenase is the enzyme responsible for TBF hydrolysis in M. vaccae JOB5. A more reasonable candidate enzyme for tert-butyl ester hydrolysis is a nonspecific, cell-associated esterase. Esterases are common in mycobacteria and have been recognized in these organisms for over a century (7). Staining for esterase activity after starch gel electrophoresis has been used to differentiate pathogenic strains (6), and esterases are recognized as mycobacteriocins (41). Esterase activity also underlies the widely used Tween 80 hydrolysis test (11) in which M. vaccae JOB5 tests strongly positive (C. A. Smith and M. R. Hyman, unpublished results). Several studies have also demonstrated a high thermal stability for some mycobacterial esterases (2, 17, 46, 47), another feature suggested by our results (Fig. 4). Although tert-butyl ester-hydrolyzing esterases are uncommon (54, 55), these enzymes are expected to hydrolyze TBF, TBAc, and TBP to TBA and the corresponding acid. We have not quantified formate production from TBF hydrolysis, but we have confirmed that M. vaccae JOB5 does not grow on formate, MTBE, TBA, TBF, or formaldehyde as sole sources of carbon and energy. However, we and others (40) have established that M. vaccae JOB5 grows readily on acetate, propionate, and many other organic acids. This suggests that our observation that M. vaccae JOB5 grows on TBP but not on TBAc may reflect the substantially higher rate of biological TBP hydrolysis that we observed for this compound (Fig. 5). The low rate of TBF hydrolysis by M. vaccae JOB5 clearly has important implications for the overall kinetics of MTBE oxidation, as the combined biotic and abiotic rate of hydrolysis dictates the rate at which TBA will accumulate and become available for further oxidation. The issue of TBF hydrolysis also potentially impacts studies of MTBE oxidation by other organisms. For example, the use of acid-preserved samples or reactors with high biomass levels may mask the production of TBF during MTBE oxidation by accelerating either the chemical or biological hydrolysis of this compound, respectively.
Further oxidation of TBA.
The third reaction that we have characterized is the further oxidation of TBA. Our results suggest that TBA is also oxidized by the same alkane monooxygenase responsible for both MTBE and alkane propane oxidation. Several previous studies have also recognized this possibility (15, 30, 45) but have generally provided data to substantiate this that are more limited than the data for the role of this enzyme in the initial oxidation of MTBE. Our evidence for the role of this enzyme in this reaction is substantially similar to that discussed earlier for MTBE oxidation. This evidence includes the proportionality between alkane monooxygenase levels and the rate of TBA oxidation and the immediacy of TBA oxidation in propane-grown cells, as well as the inhibitory effects of both propane and acetylene. The most meaningful method for comparing MTBE and TBA as substrates for alkane monooxygenase is given by the ratio of Vmax/Ks. Using the values for Ks and Vmax derived from our kinetic studies (Fig. 6), MTBE (Vmax/Ks = 1.79 × 10−5) can be seen to be a better overall substrate than TBA (Vmax/Ks = 8.8 × 10−6). This difference may help rationalize why MTBE appears to be preferentially oxidized over TBA in several of our experiments, even though the Ks values for these two compounds are similar. A value for Vmax/Ks of 2.8 × 10−4 for MTBE degradation can be derived from a recent report describing the cometabolic, monooxygenase-catalyzed oxidation of MTBE by a butane-grown Arthrobacter sp. (30). This suggests that considerable variability exists in the specificity of alkane monooxygenases toward MTBE. It is notable that this Arthrobacter sp. was not reported to generate TBF during MTBE oxidation, and it may be that TBF production has unforeseen effects on the kinetics of MTBE oxidation.
Although differences in Vmax/Ks values for MTBE and TBA can rationalize substrate utilization patterns by whole cells, they do not establish that the same enzyme is responsible for oxidizing both compounds. However, our kinetic analysis of the inhibitory effects of propane on the oxidation of MTBE and TBA (Fig. 6) strongly suggests that this is the case. To determine the true Ki for propane, the Kiapp values that we derived (Fig. 6) have to be modified to account for the concentration of MTBE and TBA in the reactions and their respective Ks values, according to the following equation:
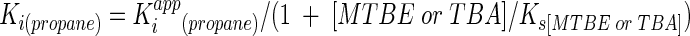 |
Based on initial dissolved concentrations of 0.9 mM (MTBE) and 1 mM (TBA) and Ks values of 1.36 mM (MTBE) and 1.18 mM (TBA), the Ki for propane was estimated to be 3.3 and 4.4 μM for the experiments conducted with MTBE and TBA, respectively (Fig. 6). The close similarity in these derived Ki values suggests that propane competitively displaces both MTBE and TBA from the same site on the same enzyme. It should also be noted that the Ki for a competitive inhibitor is equivalent to the Ks if the inhibitor is actually a substrate for the target enzyme (10). This suggests that the estimated Ks for propane is approximately 250- to 400-fold lower than for either MTBE or TBA.
Significance of proposed pathway.
During this study we have not observed any additional products of MTBE oxidation other than TBF and TBA. Studies of MTBE oxidation in mammalian systems have identified 2-methyl 1,2-propanediol and 2-hydroxyisobutyric acid (HIBA) as products of TBA oxidation (4, 38), as well as formaldehyde (8). A previous study of MTBE cometabolism by propane-oxidizing bacteria (45) and a recent study of bacterial MTBE metabolism (14) have also suggested a similar reaction sequence. The GC analysis used in this study can resolve 2-methyl 1,2-propanediol and HIBA from MTBE, TBA, and TBF but has a high minimum detection level for HIBA. It is likely that our failure to observe other products derived from TBA oxidation reflect the rapid further oxidation of 2-methyl 1,2-propanediol to HIBA by alcohol and aldehyde dehydrogenase activities present in alkane-grown cells. As a final point it is notable that the reactions that we have described in the present study are very similar to those that we have previously described for the cometabolic degradation of MTBE by the filamentous fungus Graphium (21). In particular, both organisms generate TBF as a major product of MTBE oxidation, and as indicated earlier, this may be due to the presence of distinct alcohol dehydrogenases capable of oxidizing hemiacetals. Further studies will be required to investigate this hypothesis. Additional studies will also be required to determine whether this fungus, like M. vaccae JOB5, also has the ability to oxidize TBA and whether both organisms generate 2-methyl 1,2-propanediol as products of this reaction.
Acknowledgments
This work was supported by funding to M.R.H. from ChevronTexaco and the U.S. Environmental Protection Agency-sponsored Western Region Hazardous Substances Research Center.
The opinions expressed are ours and not necessarily those of the funding agencies.
REFERENCES
- 1.Acero, J. L., S. B. Haderlein, T. C. Schmidt, M. J. Suter, and U. Gunten. 2001. MTBE oxidation by conventional ozonation and the combination ozone/hydrogen peroxide: efficiency of the process and bromate formation. Environ. Sci. Technol. 35:4252-4259. [DOI] [PubMed] [Google Scholar]
- 2.Akoa, T., T. Kusaka, and K. Kobashi. 1981. Two esterases from Mycobacterium smegmatis for the hydrolysis of long chain Acyl-CoAs and Tween. J. Biochem. 90:1661-1669. [DOI] [PubMed] [Google Scholar]
- 3.Alexander, M. 1973. Nonbiodegradable and other recalcitrant molecules. Biotechnol. Bioeng. 15:611-647. [Google Scholar]
- 4.Bernauer, U., A. Amberg, D. Scheutzow, and W. Decant. 1998. Biotransformation of 12C-and 2-13C-labeled methyl tert-butyl ether, ethyl tert-butyl ether and tert-butyl alcohol in rats: identification of metabolites in urine by 13C nuclear magnetic resonance and gas chromatography/mass spectrometry. Chem. Res. Toxicol. 11:651-658. [DOI] [PubMed] [Google Scholar]
- 5.Bradley, P. M., F. H. Chapelle, and J. E. Landmeyer. 2001. Methyl t-butyl ether mineralization in surface-water sediment microcosms under denitrifying conditions. Appl. Environ. Microbiol. 67:1975-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cann, D. C., and M. E. Wilcox. 1965. Analysis of multimolecular enzymes as an aid to the identification of certain rapidly growing mycobacteria, using starch gel electrophoresis. J. Appl. Bacteriol. 28:165-173. [Google Scholar]
- 7.Carrière, G. 1901. Sur l'existence d'un ferment soluble dans les cultures de bacilles de Koch. C. R. Soc. Biol. 53:320-322. [Google Scholar]
- 8.Cederbaum, A. I., and G. Cohen. 1980. Oxidative demethylation of t-butyl alcohol by rat liver microsomes. Biochem. Biophys. Res. Commun. 97:730-736. [DOI] [PubMed] [Google Scholar]
- 9.Corcho, D., R. J. Watkinson, and D. N. Lerner. 2000. Cometabolic degradation of MTBE by a cyclohexane-oxidizing bacteria, p. 183-189. In G. B. Wickramanayake, A. R. Gavaskar, B. C. Alleman, and V. S. Magar (ed.), Bioremediation and phytoremediation of chlorinated and recalcitrant compounds. Battelle Press, Columbus, Ohio.
- 10.Cornish-Bowden, A. 1979. Fundamentals of enzyme kinetics. Butterworths, London, United Kingdom.
- 11.Cox, F. R., C. E. Slack, M. E. Cox, E. L. Pruden, and J. R. Martin. 1978. Rapid Tween 80 hydrolysis test for mycobacteria. J. Clin. Microbiol. 7:104-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeb, R. A., S. Nishino, J. Spain, H.-Y. Hu, K. Scow, and L. Alvarez-Cohen. 2000. MTBE and benzene biodegradation by a bacterial isolate via two independent monooxygenase-initiated pathways, p. 280-282. In D. L. Drogos and A. L. Diaz (ed.), Exploring the environmental issues of mobile, recalcitrant compounds in gasoline. American Chemical Society, San Francisco, Calif.
- 13.Finneran, K. T., and D. R. Lovley. 2001. Anaerobic degradation of methyl-tert-butyl ether (MTBE) and tert-butyl ether (TBA). Environ. Sci. Technol. 35:1785-1790. [DOI] [PubMed] [Google Scholar]
- 14.François, A., H. Mathis, D. Godeefroy, P. Pivateau, F. Fayolle, and F. Monot. 2002. Biodegradation of methyl tert-butyl ether and other fuel oxygenates by a new strain, Mycobacterium austroafricanum IFP 2102. Appl. Environ. Microbiol. 68:2754-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garnier, P., R. Auria, C. Auger, and S. Revah. 1999. Cometabolic biodegradation of methyl t-butyl ether by Pseudomonas aeruginosa grown on pentane. Appl. Microbiol. Biotechnol. 51:498-503. [DOI] [PubMed] [Google Scholar]
- 16.Gornall, A. G., C. J. Bardawill, and M. M. David. 1949. Determination of serum proteins by means of the Biuret reaction. J. Biol. Chem. 177:751-766. [PubMed] [Google Scholar]
- 17.Grange, J. M. 1977. A fluorigenic substrate for the rapid differentiation of Mycobacterium fortuitum from Mycobacterium chelonei on the basis of heat stable esterase activity. Tubercle 58:147-150. [DOI] [PubMed] [Google Scholar]
- 18.Hamamura, N., C. Page, T. Long, L. Semprini, and D. J. Arp. 1997. Chloroform cometabolism by butane-grown CF8, Pseudomonas butanovora, and Mycobacterium vaccae JOB 5 and methane-grown Methylosinus trichosporium OB3b. Appl. Environ. Microbiol. 63:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamamura, N., R. T. Storfa, L. Semprini, and D. J. Arp. 1999. Diversity in butane monooxygenases among butane-grown bacteria. Appl. Environ. Microbiol. 65:4586-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanson, J. R., C. E. Ackerman, and K. M. Scow. 1999. Biodegradation of methyl tert-butyl ether by a bacterial pure culture. Appl. Environ. Microbiol. 65:79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardison, L. K., S. S. Curry, L. M. Ciuffetti, and M. R. Hyman. 1997. Metabolism of diethyl ether and cometabolism of methyl tert-butyl ether by a filamentous fungus, a Graphium sp. Appl. Environ. Microbiol. 63:3059-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatzinger, P. B., K. McClay, S. Vainberg, M. Tugusheva, C. W. Condee, and R. J. Steffan. 2001. Biodegradation of methyl tert-butyl ether by a pure bacterial culture. Appl. Environ. Microbiol. 67:5601-5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyman, M. R., and D. J. Arp. 1988. Acetylene inhibition of metalloenzymes. Anal. Biochem. 173:207-220. [DOI] [PubMed] [Google Scholar]
- 24.Hyman, M. R., C. Taylor, and K. O'Reilly. 2000. Cometabolic degradation of MTBE by iso-alkane-utilizing bacteria from gasoline-impacted soils, p. 149-155. In G. B. Wickramanayake, A. R. Gavaskar, B. C. Alleman, and V. S. Magar (ed.), Bioremediation and phytoremediation of chlorinated and recalcitrant compounds. Battelle Press, Columbus, Ohio.
- 25.Hyman, M., C. Smith, and K. O'Reilly. 2001. Cometabolism of MTBE by an aromatic hydrocarbon-oxidizing bacterium, p. 145-152. In V. S. Magar, J. T. Gibbs, K. T. O'Reilly, M. R. Hyman, and A. Leeson (ed.), Bioremediation of MTBE, alcohols, and ethers. Battelle Press, Columbus, Ohio.
- 26.Johnson, R., J. Pankow, D. Bender, C. Price, and J. Zigorski. 2000. MTBE: to what extent will past releases contaminate community water supply wells? Environ. Sci. Technol. 34:210A-217A. [DOI] [PubMed]
- 27.Kitson, T. M. 1989. Kinetics of p-nitrophenyl pivalate hydrolysis catalyzed by cytoplasmic aldehyde dehydrogenase. Biochem. J. 257:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koenigsberg, S., C. Sandefur, W. Mahaffey, M. Deshusses, and N. Fortin. 1999. Peroxygen mediated bioremediation of MTBE, p. 13-18. In B. C. Alleman and A. Leeson (ed.), Proceedings of the Fifth International In Situ and On-Site Bioremediation Symposium, vol. 3. Battelle Press, Columbus, Ohio.
- 29.Kusano, M., Y. Sakai, N. Kato, H. Yoshimoto, H. Sone, and Y. Tamai. 1998. Hemiacetal dehydrogenase activity of alcohol dehydrogenase in Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 62:1956-1961. [DOI] [PubMed] [Google Scholar]
- 30.Liu, C. Y., G. E. Speitel, Jr., and G. Georgiou. 2001. Kinetics of methyl t-butyl ether cometabolism at low concentrations by pure cultures of butane-degrading bacteria. Appl. Environ. Microbiol. 67:2197-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackay, D., and W. Y. Shiu. 1981. A critical review of Henry's law constants for chemicals of environmental interest. J. Phys. Chem. Ref. Data 10:1175-1199. [Google Scholar]
- 32.Miller, M. E., and J. D. Stuart. 2000. Measurement of aqueous Henry's law constants for oxygenates and aromatics found in gasolines by static headspace method. Anal. Chem. 72:622-625. [DOI] [PubMed] [Google Scholar]
- 33.Mitani, M. M., A. A. Keller, C. A. Bunton, R. G. Rinker, and O. C. Sandall. 2002. Kinetics and products of reactions of MTBE with ozone and ozone/hydrogen peroxide in water. J. Hazard. Mater. 89:197-212. [DOI] [PubMed] [Google Scholar]
- 34.Mo, K., C. O. Lora, A. E. Wanken, M. Javarnmardian, X. Yang, and C. F. Kulpa. 1997. Biodegradation of methyl-t-butyl ether by pure bacterial cultures. Appl. Microbiol. Biotechnol. 47:69-72. [DOI] [PubMed] [Google Scholar]
- 35.Mormile, M. R., S. Liu, and J. M. Suflita. 1994. Anaerobic biodegradation of gasoline oxygenates: extrapolation of information to multiple sites and redox conditions. Environ. Sci. Technol. 28:1727-1732. [DOI] [PubMed] [Google Scholar]
- 36.Murdanoto, A. P., Y. Sakai, L. Sembiring, Y. Tani, and N. Kato. 1997. Ester synthesis by NAD+-dependent dehydrogenation of hemiacetal: production of methyl formate by cells of methylotrophic yeasts. Biosci. Biotechnol. Biochem. 61:1391-1393. [DOI] [PubMed] [Google Scholar]
- 37.Murdanoto, A. P., Y. Sakai, T. Konishi, F. Yasuda, Y. Tania, and N. Kato. 1997. Purification and properties of methyl formate synthase, a mitochondrial alcohol dehydrogenase, participating in formaldehyde oxidation in methylotrophic yeasts. Appl. Environ. Microbiol. 63:1715-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nihlén, A., S. C. J. Sumner, A. Löf, and G. Johanson. 1999. 13C2-labelled methyl tert-butyl ether: toxicokinetics and characterization of urinary metabolites in humans. Chem. Res. Toxicol. 12:822.:830. [DOI] [PubMed]
- 39.O'Reilly, K. T., M. E. Moir, C. D. Taylor, C. A. Smith, and M. R. Hyman. 2001. Hydrolysis of tert-butyl methyl ether (MTBE) in dilute aqueous acid. Environ. Sci. Technol. 19:3954-3961. [DOI] [PubMed] [Google Scholar]
- 40.Perry, J. J. 1968. Substrate specificity in hydrocarbon utilizing microorganisms. Antonie Leeuwenhoek 34:27-36. [DOI] [PubMed] [Google Scholar]
- 41.Saito, H., H. Tomioka, T. Watanabe, and T. Yoneyama. 1983. Mycobacteriocins produced by rapidly growing mycobacteria are Tween-hydrolyzing esterases. J. Bacteriol. 153:1294-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakai, Y., A. P. Murdanoto, L. Sembiring, Y. Tani, and N. Kato. 1995. A novel formaldehyde oxidation pathway in methylotrophic yeasts: methylformate as a possible intermediate. FEMS Microbiol. Lett. 127:229-234. [DOI] [PubMed] [Google Scholar]
- 43.Somsamak, P., R. M. Cowan, and M. M. Haggblom. 2001. Anaerobic biotransformation of fuel oxygenates under sulfate-reducing conditions. FEMS Microbiol. Ecol. 37:259-264. [Google Scholar]
- 44.Squillace, P. J., J. S. Zogorski, W. G. Wilbur, and C. V. Price. 1996. Primary assessment of the occurrence and possible sources of MTBE in groundwater in the United States, 1993-1994. Environ. Sci. Technol. 30:1721-1730. [Google Scholar]
- 45.Steffan, R. J., K. McClay, S. Vainberg, C. W. Condee, and D. Zhang. 1997. Biodegradation of the gasoline oxygenates methyl tert-butyl ether, ethyl tert-butyl ether, and tert-amyl methyl ether by propane-oxidizing bacteria. Appl. Environ. Microbiol. 63:4216-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeya, K., M. Shimamoto, and Y. Mizuguchi. 1978. Physiochemical and biological properties of mycobacteriocin M12 produced by Mycobacterium smegmatis ATCC 25855. J. Gen. Microbiol. 109:215-223. [DOI] [PubMed] [Google Scholar]
- 47.Tomioka, H. 1983. Purification and characterization of the Tween-hydrolyzing esterase of Mycobacterium smegmatis. J. Bacteriol. 155:1249-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U.S. Environmental Protection Agency. 1997. Drinking water advisory: consumer acceptability advice and health effects analysis on methyl tertiary- butyl ether (MtBE). EPA-822-F-97-009. Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
- 49.White, G. F., N. J. Russell, and E. C. Tidswell. 1996. Bacterial scission of ether bonds. Microbiol. Rev. 60:216-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiegant, W. W., and J. A. M. deBont. 1980. A new route for ethylene glycol metabolism in Mycobacterium E44. J. Gen. Microbiol. 120:325-331. [Google Scholar]
- 51.Wilson, J. T., J. S. Cho, B. H. Wilson, and J. A. Vardy. 2000. Natural attenuation of MTBE in the subsurface under methanogenic conditions. EPA/600/R-00/006. U.S. Environmental Protection Agency, Washington, D.C.
- 52.Yeager, C. M., P. J. Bottomley, D. J. Arp, and M. R. Hyman. 1999. Inactivation of toluene 2-monooxygenase in Burkholderia cepacia G4 by alkynes. Appl. Environ. Microbiol. 65:632-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeh, C. K., and J. T. Novak. 1994. Anaerobic biodegradation of gasoline oxygenates in soils. Wat. Environ. Res. 66:744-752. [Google Scholar]
- 54.Yeo, S.-H., T. Nihira, and Y. Yamada. 1998. Screening and identification of a novel lipase from Burkholderia sp. YY62 which hydrolyzes t-butyl esters. J. Gen. Appl. Microbiol. 44:147-152. [DOI] [PubMed] [Google Scholar]
- 55.Yeo, S.-H., T. Nihira, and Y. Yamada. 1998. Purification and characterization of a tert-butyl ester-hydrolyzing lipase from Burkholderia sp. YY62. Biosci. Biotechnol. Biochem. 62:2312-2317. [DOI] [PubMed] [Google Scholar]