Abstract
The leaf colonization strategies of two bacterial strains were investigated. The foliar pathogen Pseudomonas syringae pv. syringae strain B728a and the nonpathogen Pantoea agglomerans strain BRT98 were marked with a green fluorescent protein, and surface (epiphytic) and subsurface (endophytic) sites of bean and maize leaves in the laboratory and the field were monitored to see if populations of these strains developed. The populations were monitored using both fluorescence microscopy and counts of culturable cells recovered from nonsterilized and surface-sterilized leaves. The P. agglomerans strain exclusively colonized epiphytic sites on the two plant species. Under favorable conditions, the P. agglomerans strain formed aggregates that often extended over multiple epidermal cells. The P. syringae pv. syringae strain established epiphytic and endophytic populations on asymptomatic leaves of the two plant species in the field, with most of the P. syringae pv. syringae B728a cells remaining in epiphytic sites of the maize leaves and an increasing number occupying endophytic sites of the bean leaves in the 15-day monitoring period. The epiphytic P. syringae pv. syringae B728a populations appeared to originate primarily from multiplication in surface sites rather than from the movement of cells from subsurface to surface sites. The endophytic P. syringae pv. syringae B728a populations appeared to originate primarily from inward movement through the stomata, with higher levels of multiplication occurring in bean than in maize. A rainstorm involving a high raindrop momentum was associated with rapid growth of the P. agglomerans strain on both plant species and with rapid growth of both the epiphytic and endophytic populations of the P. syringae pv. syringae strain on bean but not with growth of the P. syringae pv. syringae strain on maize. These results demonstrate that the two bacterial strains employed distinct colonization strategies and that the epiphytic and endophytic population dynamics of the pathogenic P. syringae pv. syringae strain were dependent on the plant species, whereas those of the nonpathogenic P. agglomerans strain were not.
Diverse bacteria can colonize aerial plant leaves. Although the majority of these live as commensals, a few can alter the plant's health, such as by inducing disease symptoms or frost injury under favorable conditions. Aerial plant leaves offer a variety of habitats for microorganisms, including sites on the surface, or epiphytic sites, and sites in the internal leaf tissue, or endophytic sites. Epiphytic sites include sites on the waxy cuticle that covers the epidermal, trichome, and guard cells, and endophytic sites include the substomatal cavities, the intercellular spaces, and the mesophyll cell surfaces.
The microenvironment in surface sites probably differs dramatically from that in subsurface sites, and thus the two regions may favor colonization by distinct bacteria. For example, colonists in epiphytic sites may need to contend with large and rapid fluctuations in environmental conditions (5, 15), whereas those in endophytic sites may need to contend with plant defense responses that are induced during microbial invasion (17). Although most foliar bacterial pathogens are known to actively colonize the internal leaf tissue of susceptible plants, the extent to which individual pathogens can actively colonize epiphytic sites has rarely been examined. Foliar bacterial pathogens are often present in large populations on aerial plant surfaces, as demonstrated by their recovery in large numbers in plant washings, their sensitivity to surface sterilants, and their abundance in scanning electron micrographs (reviewed in references 5 and 15); however, these populations may have originated from multiplication in epiphytic sites or from multiplication in endophytic sites with subsequent movement to the surface. Such movement has recently been demonstrated for Pseudomonas syringae pv. tomato strain DC3000 in tomato leaves (7) and was suggested in previous studies by micrographs showing extrusion of P. syringae and Xanthomonas campestris pathovars through stomata and onto the leaf surface (reviewed in reference 3). Only one foliar pathogen, Rhodococcus fascians, has been demonstrated to multiply on leaf surfaces before entering into the plant (9); however, this pathogen is unusual in that it also induces symptoms in the absence of large endophytic populations (9).
Several diazotrophs, including strains of Herbaspirillum spp. (10, 25) and Serratia marcescens (11), have been observed in the intercellular spaces of leaves. The dynamics of the endophytic populations of these few known nonpathogenic endophytes have not been examined. Moreover, the sites that most nonphytopathogenic bacteria colonize on leaves, particularly under field conditions, are not known. Several laboratory studies have found that strains of various nonpathogenic bacterial species did not establish endophytic populations following vacuum infiltration into leaves (30, 31), indicating that they did not multiply in endophytic sites. These nonpathogenic strains also appeared to be poor at accessing endophytic sites, based on the absence of endophytic populations following inoculation onto leaf surfaces (27, 30).
A quantitative assessment of the ability of distinct bacteria to colonize surface and subsurface sites would be useful for identifying the bacterial traits that contribute to phyllosphere fitness and for understanding the evolutionary processes by which bacteria have adapted to life in or on plants. Furthermore, knowledge of the dynamics of epiphytic and endophytic populations of the bacterial brown spot pathogen P. syringae pv. syringae, which is one of the most commonly studied foliar pathogens (15), should help resolve whether this pathogen can establish and maintain endophytic populations without inducing disease symptoms, as has been shown for a few foliar pathogens (2, 8). Although P. syringae pv. syringae and other foliar pathogens are believed to induce disease symptoms only after they have invaded the leaf, epidemiological models have shown that the size of the epiphytic populations are predictive of disease (21, 26). This leads us to question whether the endophytic population sizes are also correlated with disease incidence and if the size and dynamics of the epiphytic and endophytic populations are related. Unfortunately, previous reports have not examined the dynamics of both the epiphytic and endophytic P. syringae pv. syringae populations in a given set of leaf samples.
To better understand the strategies that bacteria employ when colonizing leaves, we examined leaf colonization by the nonpathogen Pantoea agglomerans (formerly Erwinia herbicola) strain BRT98 and the foliar pathogen P. syringae pv. syringae strain B728a. Strains of these bacterial species are commonly found on leaf surfaces, and these strains and others have been the subject of many studies focused on phyllosphere ecology (e.g., see references 4, 6, 13, and 20). The objectives of this study were to examine the dynamics of each of these two strains on leaves, to test how plant species and environmental conditions influence their population dynamics, and to use microscopy to identify the pathway by which they colonize leaves.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
The bacterial strains P. syringae pv. syringae B728a (22) and P. agglomerans BRT98 (6) were both resistant to rifampin (RIF) and were modified by introducing plasmids that conferred kanamycin (KAN) resistance and constitutive expression of a green fluorescent protein (GFP). The resulting strains were designated P. syringae pv. syringae B728a-GFP and P. agglomerans BRT98-GFP. P. syringae pv. syringae B728a-GFP contained the plasmid pPNptGreen, which was previously constructed by inserting an nptII promoter fragment from Tn5 into the broad-host-range stable vector pPROBE-KT (24) such that an nptII-gfp fusion was formed (1). P. agglomerans BRT98-GFP contained the plasmid pVLacGreen, which was previously constructed by inserting the gfp gene into the broad-host-range stable vector pVSP61 (16, 29) such that a lacZ-gfp fusion was formed (1). P. syringae pv. syringae B728a-GFP was cultured on King's B medium (18) containing RIF and KAN (each at 50 μg ml−1) (KB RIF KAN). P. agglomerans BRT98-GFP was cultured on LB medium (23) containing RIF and KAN (each at 50 μg ml−1) (LB RIF KAN). All plates were amended with the fungicide cycloheximide (100 μg ml−1).
Plant material and plant growth conditions.
Bean (Phaseolus vulgaris L. cv. Bush Blue Lake 274) seeds were purchased from Burpee (Warminster, Pa.). Maize (Zea mays subsp. mays L. inbred B73) seeds were obtained from the North Central Plant Introduction Station (Ames, Iowa). For the laboratory experiments, seeds were planted in 11.5-cm-diameter pots containing a pasteurized, peat-vermiculite-soil (1:2:1) mixture. Plants were grown at 28°C with a 12-h photoperiod (light intensity, 350 microeinsteins m−2 s−1) in a controlled environmental chamber. Plants were fertilized with a nitrogen-phosphorous-potassium (21:5:20) mixture after the emergence of primary leaves and every other day thereafter. The field experiment was conducted from 10 to 25 September 2001 at the Horticultural Research Farm of Iowa State University near Gilbert. A permit for releasing the gfp-marked recombinant strains was obtained from the U.S. Department of Agriculture Animal and Plant Health Inspection Service. Plants were arranged in a randomized complete block design with four replications. Each plot contained approximately 50 plants in an area 0.2 by 0.9 m. The plots were separated by a 1-m unplanted zone, and the entire experimental area was surrounded by a 3-m unplanted zone. The plants were watered from below to minimize moisture on the leaves. Weather conditions were monitored using a data logger (model CR10; Campbell Scientific, Logan, Utah) and a multiplexer (model AM 416; Campbell Scientific) connected to a relative humidity (RH)-temperature sensor (Vaisala model HMP 35C; Campbell Scientific), an anemometer (model 014A; Met One Instruments, Grants Pass, Oreg.), a tipping-bucket rain gauge (Texas Electronics, Ft. Worth, Tex.), and a flat, printed-circuit leaf wetness sensor (model 237; Campbell Scientific) that had been coated with latex paint (19). The sensors were placed 30 cm above the ground. Sensor readings were taken at 1-min intervals, and 5-min averages were recorded. Rainfall accumulation in each 5-min period was recorded.
Under both laboratory and field conditions, bean plants were inoculated when the plants had fully expanded primary leaves and emerging trifoliate leaves; the primary leaves served as the sample units. Maize plants were inoculated as seedlings at approximately the time of emergence of leaf 4, with the nonleaf coleoptile designated as leaf 0; the first and second leaves served as the sample units.
Plant inoculation and sampling.
P. agglomerans BRT98-GFP inocula were prepared from cultures grown on solid LB RIF KAN for 24 h. P. syringae pv. syringae B728a-GFP inocula were prepared from cultures grown on solid KB RIF KAN for 48 h. Cells were suspended in 10 mM potassium phosphate, pH 7 (PB), washed twice in PB, and resuspended in sterile distilled water to a density of 106 cells per ml. Cell concentrations were determined turbidimetrically and were confirmed by plate counts.
Four experiments were performed under laboratory conditions, one with each strain, P. agglomerans BRT98-GFP and P. syringae pv. syringae B728a-GFP, each at a low (40 to 50%) and a high (90 to 100%) RH. In each experiment, the strain was inoculated onto maize plants in 12 pots (5 plants/pot) and bean plants in 12 pots (5 plants/pot) by applying the inoculum onto the adaxial and abaxial leaf surfaces three times using an atomizer. The atomizer was set to a gentle mist and was held approximately 25 cm from the plants. For each strain, one leaf per pot was collected immediately following inoculation (0 h), at which time the leaves were still visibly moist, and at 4 h and 1, 2, 3, and 6 days following inoculation. Six of the leaves of each plant species were used for estimating the endophytic populations of the applied strain, and six were used for estimating the epiphytic and endophytic populations of the strain, described below.
In the field experiment, each strain was applied onto four replicate plots of each plant species. Inocula were sprayed onto the adaxial and abaxial leaf surfaces using handheld spray bottles. To limit aerial dispersal, plants were inoculated under calm weather conditions and were sprayed under a moveable box that was constructed of polyethylene sheets and PVC tubes. For the plots treated with P. agglomerans BRT98-GFP and P. syringae pv. syringae B728a-GFP, leaves were collected immediately following inoculation (0 h) and at 4 h and 1, 3, 5, 7, and 15 days following inoculation. Additional plots were treated with P. agglomerans BRT98 and P. syringae pv. syringae B728a, and leaves were collected at 0 h and 15 days following inoculation. At each sampling time, 12 leaves (3 per replicate plot) were collected for estimating the endophytic populations of the applied strain, and 12 were collected for estimating the epiphytic and endophytic populations of the strain.
Evaluation of epiphytic and endophytic population dynamics.
To identify an optimal surface sterilization procedure, leaves that had been inoculated with P. agglomerans BRT98-GFP or P. syringae pv. syringae B728a-GFP and incubated at 95% RH and 28°C were subjected to various surface sterilization treatments, and the numbers of surviving culturable cells in leaf homogenates were compared. The treatments included submerging the leaf in a solution containing 0.02% Tween 20 and either 1% sodium hypochlorite, followed by agitation for 3 min; 2% sodium hypochlorite, followed by agitation for 3 min; or 15% hydrogen peroxide, followed by agitation for 5 min and drying in a laminar-flow hood with each side facing upward for 20 to 30 min, as described previously (30). Leaves were rinsed three times with sterile water and were homogenized using a Brinkmann's PT 10-35 homogenizer with a 20-mm-diameter generator (Brinkmann Instruments, Inc., Westbury, N.Y.). Leaf homogenates were transferred to plates of LB RIF KAN or KB RIF KAN. The effect of the sterilants on bacterial cell integrity was examined by suspending cells grown on solid media in PB to a density of 108 cells per ml, adding the sterilants, incubating the cells for 3 to 5 min, and observing the cells by fluorescence microscopy.
The number of P. agglomerans BRT98-GFP or P. syringae pv. syringae B728a-GFP cells that were located in endophytic sites in a leaf was determined by gently agitating the leaf in 0.02% Tween 20 and 2% sodium hypochlorite for 3 min; rinsing it three times with sterile water; placing it in 20 ml of washing buffer (0.1 M potassium phosphate buffer, pH 7, containing 0.1% peptone) containing 10% glycerin; storing it at −20°C for 1 to 4 weeks, as has been done previously (6); rapidly thawing it; and homogenizing it as described above. The leaf homogenate was transferred to plates of LB RIF KAN or KB RIF KAN. P. agglomerans BRT98-GFP and P. syringae pv. syringae B728a-GFP colonies were identified based on both antibiotic resistance and GFP fluorescence, which was evaluated using a handheld visible-wavelength lamp with excitation wavelengths (λex) of 420 to 500 nm and glasses that facilitated visualizing emission wavelengths (λem) greater than 520 nm. The P. agglomerans BRT98-GFP or P. syringae pv. syringae B728a-GFP cells that were recovered from epiphytic and endophytic sites were enumerated by placing a nonsterilized leaf in 20 ml of washing buffer and proceeding as for the endophytic populations.
Microscopic visualization of bacterial colonization of leaves.
At each sampling time in the laboratory studies, one leaf was collected for microscopic visualization from each of five randomly selected pots of each plant species. Four 8-mm-diameter leaf disks were removed from each leaf, with one disk being removed from each quarter of a leaf. Leaf disks were mounted on slides using 20 μl of 50% glycerin as a mounting medium. The adaxial surface of 10 leaf disks and the abaxial surface of 10 leaf disks were examined using a Nikon microscope equipped for epifluorescence. A filter set with a λex of 450 to 490 nm and a λem of ≥515 nm was used to visualize the gfp-marked bacterial strains and the plant leaf cells, and a filter set with a λex of 465 to 495 nm and a λem of 515 to 555 nm was used to visualize only the gfp-marked bacterial strains. For each leaf disk, 12 microscopic fields located along two 90° transects were observed at a magnification of ×200. The number of gfp-marked cells in each field was recorded, and the size of the epiphytic populations was calculated on a per-unit-area basis after using an optical micrometer to estimate leaf surface area in a single field of view. Images were captured using a 35-mm camera or a cooled charge-coupled device camera.
In the field study, eight bean leaves (two leaves/plot) per treatment were collected on days 3, 7, and 12 after inoculation, and eight maize leaves (two leaves/plot) per treatment were collected on days 4, 8, and 13 after inoculation. Two to three leaf disks were removed from each leaf, and the bacteria on the adaxial surface of 10 leaf disks and on the abaxial surface of 10 leaf disks were observed for each treatment at each sampling time.
Statistical methods.
Bacterial population dynamics were evaluated using the log-transformed population sizes, following confirmation that the population sizes on the leaves of each species were log-normally distributed (14). The population sizes were expressed both per gram of leaf (fresh weight) and per square centimeter of leaf (adaxial and abaxial surfaces), with the latter being estimated based on the weight of leaf models constructed from tracing leaf perimeters onto plastic transparencies. The population dynamics were similar for populations expressed on either a weight or area basis.
RESULTS
Evaluation of methods for quantifying epiphytic and endophytic populations.
All three leaf surface-sterilization treatments examined killed all of the P. agglomerans BRT98-GFP cells on bean leaves sampled 4 h after inoculation. Leaves that were sampled 3 or 5 days after inoculation with P. syringae pv. syringae B728a-GFP were predicted to have endophytic populations; on these leaves, similar numbers of cells survived the three surface sterilization treatments, indicating that the treatments were similar in invasiveness. The treatments differed in their effect on the leaves themselves: the leaves that were treated with hydrogen peroxide exhibited visible damage, whereas the leaves that were treated with sodium hypochlorite did not. The treatments also differed in their effect on the integrity of the bacterial cells. P. syringae pv. syringae B728a-GFP and P. agglomerans BRT98-GFP cells that were treated with hydrogen peroxide were not viable, based on plate counts, but all or most of the cells remained intact and fluorescent based on fluorescence microscopy. In contrast, all or most of the cells that were treated with 1 or 2% sodium hypochlorite appeared to lyse.
The surface-sterilized leaves were periodically examined using microscopy during the laboratory and field studies described below. The P. agglomerans BRT98-GFP cells were consistently visibly absent from leaves after surface sterilization. In contrast, some P. syringae pv. syringae B728a-GFP cells were visible on the leaf surfaces immediately adjacent to the stomatal openings. The proximity of these P. syringae pv. syringae B728a-GFP cells to the stomata suggested that the cells moved from internal sites to the leaf surface during the treatment and thus were not originally in epiphytic sites. Although the number of P. syringae pv. syringae B728a-GFP cells that relocated to the leaf surface during the surface-sterilization treatment appeared to be relatively small, the removal of these cells during the washings would have resulted in a slight underestimation of the endophytic population sizes in these studies.
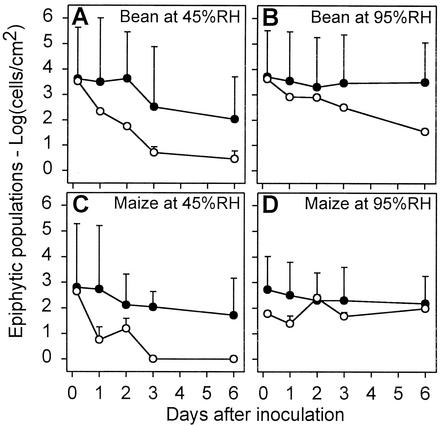
Following the introduction of the strains onto leaves during the laboratory studies described below, the number of green fluorescent cells per unit area at each sampling time was determined by counting the cells in each of 120 microscopic fields of view for each the abaxial and adaxial leaf surfaces. The population estimates of P. agglomerans BRT98-GFP based on the microscopy counts were consistently much larger than the epiphytic population estimates based on culturable cell counts (Fig. 1).
FIG. 1.
Epiphytic population sizes of P. agglomerans BRT98-GFP based on direct microscopic counts (•) and plate counts of culturable cells (○). The microscopic counts represent the mean log(cells/centimeter2 of leaf) + standard error of the mean (SE) (error bars) for 120 microscopic fields observed at a magnification of ×200 (each field was approximately 0.005 cm2). The plate counts represent the mean log(CFU/centimeter2 of leaf) + SE for six leaves and were derived from homogenates of nonsterilized leaves. The plate counts were assumed to represent the epiphytic populations because bacteria were not recovered from six additional replicate leaf samples that were surface sterilized.
Endophytic vs. epiphytic colonization by P. agglomerans BRT98-GFP.
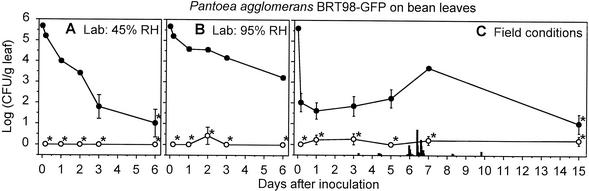
In laboratory studies, the P. agglomerans BRT98-GFP populations appeared to be composed entirely of epiphytic cells since surface sterilization consistently killed 99.9 to 100% of the P. agglomerans BRT98-GFP cells, as measured using nonsterilized leaves (Fig. 2A and B and 3A and B). These epiphytic populations exhibited much larger population decreases on leaves at 45% RH (Fig. 2A and 3A) than at 95% RH (Fig. 2B and 3B) during the 6 days following inoculation on both plant species.
FIG. 2.
Endophytic populations (○) and epiphytic and endophytic populations (•) of P. agglomerans BRT98-GFP on bean leaves under 45% RH conditions in the laboratory (A), 95% RH conditions in the laboratory (B), and field conditions (C). The endophytic populations represent the culturable cells in the homogenates of surface-sterilized leaves. The epiphytic and endophytic populations represent the culturable cells in the homogenates of nonsterilized leaves. Values shown are the mean log(CFU/gram of leaf [fresh wt]) ± SE (error bars) (n = 6 for the laboratory studies; n = 12 for the field study). Leaves for which a majority of the leaves had populations below the limit of detection are indicated with an asterisk. Vertical bars represent rainfall, with the shortest bar representing 0.1 mm of rain per h and the tallest bar representing 3.7 mm of rain per h.
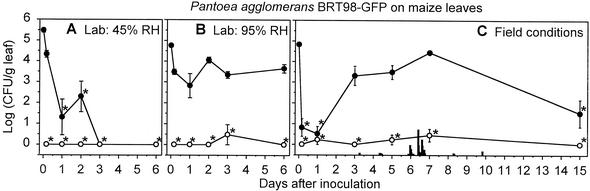
FIG. 3.
Endophytic populations (○) and epiphytic and endophytic populations (•) of P. agglomerans BRT98-GFP on maize leaves under 45% RH conditions in the laboratory (A), 95% RH conditions in the laboratory (B), and field conditions (C). The derivation of the population estimates and the definition of the symbols are described in the legend to Fig. 2.
In field studies, 96 to 100% of the P. agglomerans BRT98-GFP cells were killed by surface sterilization throughout the study (Table 1). The epiphytic P. agglomerans BRT98-GFP populations were maintained for at least 7 days on bean (Fig. 2C) and maize (Fig. 3C) plants and were detected on at least 30% of the leaves of both species at 15 days postinoculation. Although bean leaves supported larger P. agglomerans BRT98-GFP populations within the first day following inoculation, the P. agglomerans BRT98-GFP populations on maize were significantly larger than those on bean at every sampling time in the week that followed (P < 0.05 in a Student's t test). Based on the population sizes at 15 days postinoculation, P. agglomerans BRT98-GFP was identical to P. agglomerans BRT98 in colonizing exclusively epiphytic sites on bean and maize, in maintaining populations that were detectable at 15 days after inoculation on both species, and in establishing larger populations on maize than on bean (data not shown).
TABLE 1.
Colonization of epiphytic sites by P. agglomerans BRT98-GFP and P. syringae pv. syringae B728a-GFP on plants grown under field conditions
| Time p.i.b | % of population of applied strain in epiphytic sites (mean ± SEM)a
|
|||
|---|---|---|---|---|
| BRT98-GFP
|
B728a-GFP
|
|||
| Bean | Maize | Bean | Maize | |
| 4 h | 100 ± 0 | NDc | ND | 100 ± 0.0 |
| 1 day | 96.4 ± 3.6 | ND | ND | 94.4 ± 4.4 |
| 3 days | 96.4 ± 3.6 | 100 ± 0 | 72.0 ± 24.1 | 95.4 ± 2.2 |
| 5 days | 100 ± 0 | 100 ± 0 | 24.7 ± 24.7 | 99.4 ± 0.4 |
| 7 days | 100 ± 0 | 100 ± 0 | 72.9 ± 15.0 | 99.0 ± 0.5 |
| 15 days | ND | ND | 43.1 ± 25.4 | 93.4 ± 5.1 |
The percentage of cells of an applied strain that were in epiphytic sites was determined based on the difference between the endophytic population estimates, which were derived from three surface-sterilized leaves from each replicate plot, and the estimates of the epiphytic and endophytic populations, which were derived from three nonsterilized leaves from each replicate plot. Values shown are taken from four replicate plots.
p.i., postinoculation.
ND, not determined.
Endophytic versus epiphytic colonization by P. syringae pv. syringae B728a-GFP.
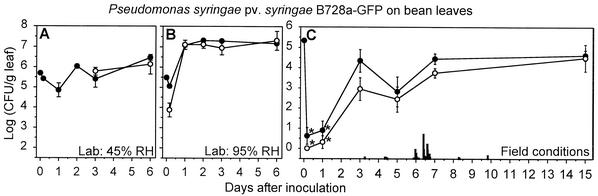
P. syringae pv. syringae B728a-GFP established populations that survived surface sterilization on bean leaves under all of the conditions tested (Fig. 4). Microscopic examination of surface-sterilized leaves indicated that the populations that survived the sterilization were in the substomatal chambers and surrounding intercellular spaces. This endophytic colonization occurred within 3 days on bean leaves (Fig. 4), with the 95% RH conditions in the laboratory supporting endophytic colonization within only 1 day. In addition to maintaining endophytic populations, P. syringae pv. syringae B728a-GFP maintained epiphytic populations on bean throughout the 15-day field study (Table 1).
FIG. 4.
Endophytic populations (○) and epiphytic and endophytic populations (•) of P. syringae pv. syringae B728a-GFP on bean leaves under 45% RH conditions in the laboratory (A), 95% RH conditions in the laboratory (B), and field conditions (C). The derivation of the population estimates and the definition of the symbols are described in the legend to Fig. 2.
P. syringae pv. syringae B728a-GFP exhibited poor survival on maize leaves in the two laboratory studies but established endophytic and epiphytic populations on maize in the field (Fig. 5). Endophytic P. syringae pv. syringae B728a-GFP populations were detectable in maize leaves within 1 day following inoculation; however, they consistently composed less than 6% of the total P. syringae pv. syringae B728a-GFP populations associated with the leaves (Table 1). The P. syringae pv. syringae B728a-GFP populations on maize leaves were more constant in size than those on bean leaves, with 30-fold fewer cells being recovered from maize than from bean 15 days after inoculation. Based on the population sizes at 15 days postinoculation, P. syringae pv. syringae B728a-GFP was identical to P. syringae pv. syringae B728a in establishing epiphytic and endophytic populations on bean and maize, and in establishing much larger endophytic populations in bean than in maize (data not shown).
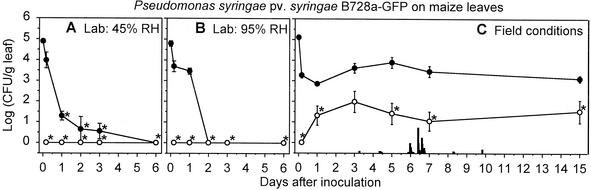
FIG. 5.
Endophytic populations (○) and epiphytic and endophytic populations (•) of P. syringae pv. syringae B728a-GFP on maize leaves under 45% RH conditions in the laboratory (A), 95% RH conditions in the laboratory (B), and field conditions (C). The derivation of the population estimates and the definition of the symbols are described in the legend to Fig. 2.
Influence of rain on endophytic versus epiphytic colonization by P. agglomerans BRT98-GFP and P. syringae pv. syringae B728a-GFP.
Rain events in which the rainfall rate was less than 1 mm per 5 min occurred on days 3, 4, 5, 6, 8, and 9, and these events were not clearly associated with specific population dynamics of either strain. A rain event in which 3.4 mm of rain accumulated in only 5 min occurred during a period in which the epiphytic P. agglomerans BRT98-GFP populations increased on bean and maize (Fig. 2C and 3C) and the epiphytic and endophytic P. syringae pv. syringae B728a-GFP populations increased on bean (Fig. 4C). The epiphytic and endo phytic P. syringae pv. syringae B728a-GFP populations on maize did not increase during this period (Fig. 5C).
The other weather parameters that were monitored indicated that the leaves were exposed to a wide range of physical conditions during the study. These conditions included warm (maximum temperature, 25 to 30°C), dry (minimum RH, 30 to 40%) conditions on days 0, 1, and 2, with free water being mostly absent in the nights. During the remainder of the experiment, the weather conditions followed three primary trends: cool on days 3 to 8 (daytime maximum temperature, 13 to 20°C, daytime minimum RH, 55 to 75%), slightly warmer on days 9 to 12 (daytime maximum temperature, 21 to 25°C; daytime minimum RH, 60 to 75%), and much cooler on days 13 to 15 (daytime maximum temperature, 13 to 14°C; daytime minimum RH, 40 to 75%).
Leaf colonization patterns based on microscopy.
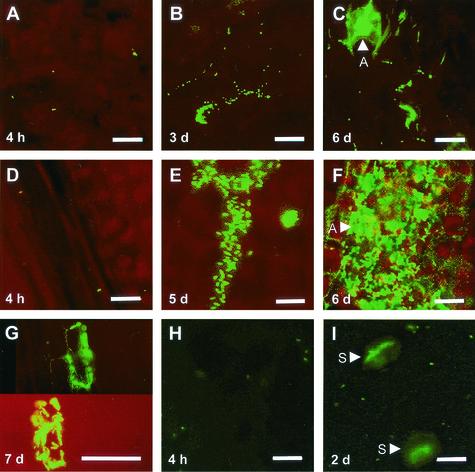
Both bacterial strains were present as individual cells on the surface of both plant species immediately after inoculation and at 4 h postinoculation in all of the laboratory studies (Fig. 6A, D, and H). Groups of distinct P. agglomerans BRT98-GFP cells were visible within 3 days on both plant species under conditions of both 45 and 95% RH in the laboratory (Fig. 6B), as well as at 7 and 12 days postinoculation under field conditions. These groups of P. agglomerans BRT98-GFP cells were typically located in the epidermal cell junctions and sometimes surrounded individual maize epidermal cells (Fig. 6G). Under the 95% RH conditions, P. agglomerans BRT98-GFP eventually formed cellular aggregates on both maize and bean (Fig. 6C and F), although they were particularly abundant on maize. These aggregates often extended over multiple epidermal cells. Throughout the studies, P. agglomerans BRT98-GFP cells were not observed in the stomata.
FIG. 6.
Fluorescence microscopy images of P. agglomerans BRT98-GFP on bean leaves (A to C), P. agglomerans BRT98-GFP on maize leaves (D to G), and P. syringae pv. syringae B728a-GFP on bean leaves (H and I). The leaves were sampled from plants incubated at 95% RH in the laboratory for 4 h to 7 days following inoculation. Images S were captured using a long-pass emission filter (wavelength ≥ 515 nm) (A to G) or a band-pass filter (wavelengths, 515 to 555 nm) (H and I). The leaf cells appear red or black, and the GFP-labeled bacterial cells appear green. The bars represent 20 μm. Abbreviations: A, aggregate; S, stomatal opening.
P. syringae pv. syringae B728a-GFP colonization of bean leaves under both 95% and 45% RH conditions was characterized by the appearance of cells in the stomatal openings, and thus presumably in the substomatal chambers (Fig. 6I). P. syringae pv. syringae B728a-GFP cells appeared in the stomata as early as 24 h postinoculation under the 95% RH conditions. Their appearance in the stomata under the 45% RH conditions was less evident than at 95% RH based on the lower number of colonized stomata and the lower fluorescence intensity of these stomatal openings at 24 and 48 h postinoculation under 45% RH. After 3 days under the 95% RH conditions, the P. syringae pv. syringae B728a-GFP cells had migrated into the intercellular spaces. After 6 days under the 95% RH conditions, bacterial streaming was observed in the intercellular spaces, particularly in areas where the leaf exhibited water soaking. Bacterial brown spot lesions appeared after incubating the plants longer than 6 days in 95% RH. The number of P. syringae pv. syringae B728a-GFP cells that were visible on the leaf surface decreased dramatically over time in the laboratory studies. For example, the average number of P. syringae pv. syringae B728a-GFP cells that were present at 4 h, 2 days, and 6 days was 4,300, 170, and 100 cells per cm2, respectively, under 95% RH conditions and was only 3,600, 28, and 10 cells per cm2, respectively, under 45% RH conditions. In the field study, the P. syringae pv. syringae B728a-GFP cells were visible on the bean leaf surfaces at 3 days but were not visible at 7 or 12 days. At 7 and 12 days postinoculation, however, they were clearly visible in the intercellular spaces. Bacterial brown spot lesions were not observed in the 15-day field study.
The P. syringae pv. syringae B728a-GFP cells were present in very low numbers on the maize leaves in the laboratory studies. For example, the average number of bacteria present at 4 h, 2 days, and 6 days was 100, 13, and 2.5 cells per cm2, respectively, under 95% RH conditions and only 330, 12, and 0 cells per cm2, respectively, under 45% RH conditions. P. syringae pv. syringae B728a-GFP cells were present in similarly low numbers on the leaves in the field study.
DISCUSSION
Differences among bacterial strains in leaf colonization strategies have not been well documented. In this study we demonstrated that the pathogenic strain P. syringae pv. syringae B728a-GFP and the nonpathogenic strain P. agglomerans BRT98-GFP differ in how they colonize leaves. Previously, we hypothesized that endophytic colonization is primarily a property of pathogens (5). This hypothesis was supported by the results of Wilson et al. (30), who found that in laboratory studies endophytic populations in bean leaves increased for several pathogens but remained very low or undetectable for several nonpathogens. The present study extended these results by demonstrating that (i) the nonpathogenic strain P. agglomerans BRT98-GFP colonized exclusively epiphytic sites on two plant species under field and laboratory conditions and (ii) the pathogenic strain P. syringae pv. syringae B728a-GFP established epiphytic and endophytic populations on asymptomatic leaves of the two species in the field, with the majority of the cells in epiphytic sites on maize leaves for at least 15 days but a majority in endophytic sites on bean leaves by 15 days.
Techniques for distinguishing cells in endophytic versus epiphytic sites can involve some ambiguity in the exact location of the cells. One ambiguity with surface sterilization treatments, including chemical sterilants and UV radiation exposure (e.g., see references 8, 28, and 30), is whether they kill all of the cells on the leaf surface. In this study, the elimination of all P. agglomerans BRT98-GFP cells by surface sterilization, even at times when visible aggregates were present, provided evidence for complete surface killing. A second ambiguity is whether surface sterilization kills cells that are not on the surface, such as by penetrating the substomatal cavities or by promoting release from these cavities prior to killing the bacteria. In this study, the P. syringae pv. syringae B728a-GFP cells were primarily in the substomatal cavities of bean leaves at 95% RH. If the surface sterilization treatment had killed large numbers of subsurface cells, then the number of cells recovered from the nonsterilized leaves should have differed from the number recovered from sterilized leaves. The finding that these numbers did not differ indicates that the surface sterilization treatment did not kill detectable numbers of subsurface cells, although it is possible that it killed some.
The microscopy studies showed that P. agglomerans BRT98-GFP colonization involved the appearance of increasing numbers of cells on the surface of leaves of both plant species and, when the conditions were favorable, i.e., under the 95% RH conditions, the formation of aggregates. These aggregates formed at a time when the P. agglomerans BRT98-GFP counts remained steady or decreased, indicating that this aggregation resulted either from the amassing of surviving cells or, more likely, from the multiplication of some cells simultaneous with the death of many others. The exclusively epiphytic populations of P. agglomerans BRT98-GFP were strongly influenced by the environmental conditions, as indicated by their distinct dynamics under conditions of 95 versus 45% RH.
P. syringae pv. syringae B728a-GFP established and maintained endophytic populations on leaves of both plant species under field conditions. These endophytic populations developed within 3 days following inoculation and were present for at least another 12 days. The proportion of the P. syringae pv. syringae B728a-GFP population that was endophytic in the first 3 days on beans in the field was similar to that observed in previous studies on bean leaves in the laboratory (4, 30). The absence of visible symptoms on the bean leaves under field conditions indicates that, similar to a few other foliar pathogens (2, 8), P. syringae pv. syringae B728a-GFP can establish and maintain endophytic populations without inducing disease symptoms. The expansion of the zones of fluorescence beneath the stomatal openings to the surrounding intercellular spaces of bean leaves provides evidence that the stomata were a primary point of entry for P. syringae pv. syringae B728a-GFP into these leaves, as is commonly believed. Furthermore, the development of endophytic populations under conditions of 95% RH earlier than that under conditions of 45% RH suggests that a high RH promoted entry into the leaf and/or multiplication in the endophytic sites. The smaller size of the endophytic populations in maize leaves than in bean leaves in the field was consistent with the common laboratory observation that pathogenic bacteria establish smaller populations following infiltration into nonhost plants than into susceptible host plants. We assume that maize is not a host plant for P. syringae pv. syringae B728a based on the lack of induction of holcus spot (D. C. Gross, personal communication) or any other known disease.
Although P. syringae pv. syringae B728a-GFP established populations that were primarily endophytic on bean leaves in the laboratory, it established populations that contained significant numbers of epiphytic cells on bean and maize leaves under field conditions. During periods in which these epiphytic populations increased, the increases were too large to have resulted solely from the movement of endophytic cells to the surface. For example, between 5 and 7 days postinoculation, the endophytic populations in bean increased from an average of 274 cells per g to 5,572 cells per g, whereas the epiphytic populations on bean appeared to increase from 381 cells per g to 22,289 cells per g. These observations provide evidence for P. syringae pv. syringae B728a-GFP multiplication in epiphytic sites on bean and maize leaves.
The microscopy-based population estimates of P. agglomerans BRT98-GFP cells on leaves were larger than the culture-based estimates of cells recovered from leaves. The presence of cells that retained their cellular integrity and fluorescence on leaves but were not culturable could explain this difference. In a recent study in which we enumerated the P. agglomerans BRT98-GFP cells in suspensions recovered from leaves using both fluorescence microscopy and culturing, we found that only 30 to 40% of the visible cells in the suspension were cultured (data not shown). The presence of up to 70% nonculturable cells of the applied strain, however, does not fully explain the difference in the population estimates derived from in planta microscopy versus from culturing cells recovered from leaves. An additional explanation is based on the highly heterogeneous distribution of P. agglomerans BRT98-GFP cells on the leaves. Specifically, the sampling plan of counting cells in 12 fields of view along each of two 90° transects across the leaf section likely permitted an inadvertent bias toward selecting fields of view that contained P. agglomerans BRT98-GFP cells. This possibility is supported by the observation that the extent to which the P. agglomerans BRT98-GFP populations were overestimated increased over time under conditions that favored aggregation (Fig. 1 and data not shown).
Hirano et al. (12) demonstrated that high-momentum rains are associated with large increases in the P. syringae pv. syringae populations associated with bean leaves. In this study, both the epiphytic and endophytic P. syringae pv. syringae B728a-GFP populations increased following exposure of field-grown bean leaves to a high-momentum rain. Epiphytic populations of P. agglomerans BRT98-GFP on bean leaves similarly increased following this rain event. These observations suggest that the primary mechanism underlying the rain-induced population increases of these P. agglomerans and P. syringae pv. syringae strains was not enhanced ingression. Alternative mechanisms include the release of nutrients (12), the removal of inhibitors from the leaf surface (12), and the dissemination and subsequent growth of bacteria due to the disruption of bacterial aggregates. Interestingly, neither the epiphytic nor endophytic P. syringae pv. syringae B728a-GFP populations increased on maize leaves during the rain event, and the P. agglomerans BRT98-GFP population increases on maize were smaller than those on bean. These observations suggest that maize leaves are poorer at promoting rain-induced bacterial growth enhancement of these strains than bean leaves.
In conclusion, the dynamics of the surface and subsurface populations of these bacterial strains on two plant species illustrate the elements of two major leaf colonization strategies. The P. agglomerans strain established populations only on the plant surface, with the dynamics of these populations being fairly sensitive to environmental conditions but independent of the plant species. In contrast, the P. syringae pv. syringae strain established populations on the plant surface and in subsurface sites, with the subsequent growth of the subsurface populations being highly dependent on the nature of the host plant.
Acknowledgments
This work was supported by grant 99-35303-8301 from the USDA CSREES National Research Initiative Competitive Grants Program and by the Hatch Act and State of Iowa funds as Iowa Agriculture and Home Economics Experiment Station, Ames, project 3588.
We thank M. L. Gleason and J. C. Batzer for the generous use of their weather monitoring instruments and for sharing their expertise with these instruments. We also thank C. A. Axtell, J. R. Seibel, and L. N. Schulz for assistance with sampling and L. J. Halverson and C. A. Axtell for helpful comments on the manuscript.
REFERENCES
- 1.Axtell, C. A., and G. A. Beattie. 2002. Construction and characterization of a proU-gfp transcriptional fusion that measures water availability in a microbial habitat. Appl. Environ. Microbiol. 68:4604-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashan, Y., M. Azaizeh, S. Diab, H. Yunis, and Y. Okon. 1985. Crop loss of pepper plants artificially infected with Xanthomonas campestris pv. vesicatoria in relation to symptom expression. Crop Prot. 4:77-84. [Google Scholar]
- 3.Beattie, G. A., and S. E. Lindow. 1999. Bacterial colonization of leaves: a spectrum of strategies. Phytopathology 89:353-359. [DOI] [PubMed] [Google Scholar]
- 4.Beattie, G. A., and S. E. Lindow. 1994. Comparison of the behavior of epiphytic fitness mutants of Pseudomonas syringae under controlled and field conditions. Appl. Environ. Microbiol. 60:3799-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beattie, G. A., and S. E. Lindow. 1995. The secret life of foliar bacterial pathogens on leaves. Annu. Rev. Phytopathol. 33:145-172. [DOI] [PubMed] [Google Scholar]
- 6.Beattie, G. A., and L. M. Marcell. 2002. Comparative dynamics of adherent and non-adherent bacterial populations on maize leaves. Phytopathology 92:1015-1023. [DOI] [PubMed] [Google Scholar]
- 7.Boureau, T., J. Rouuttu, E. Roine, S. Taira, and M. Romantschuk. 2002. Localization of hrpA-induced Pseudomonas syringae pv. tomato DC3000 in infected tomato leaves. Mol. Plant Pathol. 3:451-460. [DOI] [PubMed] [Google Scholar]
- 8.Cafati, C. R., and A. W. Saettler. 1980. Effect of host on multiplication and distribution of bean common blight bacteria. Phytopathology 70:675-679. [Google Scholar]
- 9.Cornelis, K., T. Ritsema, J. Nijsse, M. Holsters, K. Goethals, and M. Jaziri. 2001. The plant pathogen Rhodococcus fascians colonizes the exterior and interior of the aerial parts of plants. Mol. Plant-Microbe Interact. 14:599-608. [DOI] [PubMed] [Google Scholar]
- 10.Elbertagy, A., K. Nishioka, T. Sato, H. Suzuki, B. Ye, T. Hamada, T. Isawa, H. Mitsui, and K. Minamisawa. 2001. Endophytic colonization and in planta nitrogen fixation by a Herbaspirillum sp. isolated from wild rice species. Appl. Environ. Microbiol. 67:5285-5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gyaneshwar, P., E. K. James, N. Mathan, P. M. Reddy, B. Reinhold-Hurek, and J. K. Ladha. 2001. Endophytic colonization by a diazotrophic strain of Serratia marcescens. J. Bacteriol. 183:2634-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano, S. S., L. S. Baker, and C. D. Upper. 1996. Raindrop momentum triggers growth of leaf-associated populations of Pseudomonas syringae on field-grown snap bean plants. Appl. Environ. Microbiol. 62:2560-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano, S. S., A. O. Charkowski, A. Collmer, D. K. Willis, and C. D. Upper. 1999. Role of the Hrp type III protein secretion system in growth of Pseudomonas syringae pv. syringae B728a on host plants in the field. Proc. Natl. Acad. Sci. USA 96:9851-9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirano, S. S., E. V. Nordheim, D. C. Arny, and C. D. Upper. 1982. Lognormal distribution of epiphytic bacterial populations on leaf surfaces. Appl. Environ. Microbiol. 44:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirano, S. S., and C. D. Upper. 2000. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae—a pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 64:624-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoh, Y., J. M. Watson, D. Haas, and T. Leisinger. 1984. Genetic and molecular characterization of the Pseudomonas plasmid pVS1. Plasmid 11:206-220. [DOI] [PubMed] [Google Scholar]
- 17.Jakobek, J. L., J. A. Smith, and P. B. Lindgren. 1993. Suppression of bean defense responses by Pseudomonas syringae. Plant Cell 5:57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King, E. O., M. K. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 44:301-307. [PubMed] [Google Scholar]
- 19.Lau, Y. F., M. L. Gleason, N. Zriba, and P. N. Hinz. 2000. Effects of coating, deployment angle, and compass orientation on performance of electronic wetness sensors during dew periods. Plant Dis. 84:192-197. [DOI] [PubMed] [Google Scholar]
- 20.Leveau, J. H. J., and S. E. Lindow. 2001. Appetite of an epiphyte: quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proc. Natl. Acad. Sci. USA 98:3446-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindemann, J., D. C. Arny, and C. D. Upper. 1984. Epiphytic populations of Pseudomonas syringae pv. syringae on snap bean and nonhost plants and the incidence of bacterial brown spot disease in relation to cropping patterns. Phytopathology 74:1329-1333. [Google Scholar]
- 22.Loper, J. E., and S. E. Lindow. 1987. Lack of evidence for in situ fluorescent pigment production by Pseudomonas syringae pv. syringae on bean leaf surfaces. Phytopathology 77:1449-1454. [Google Scholar]
- 23.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 24.Miller, W. G., J. H. Leveau, and S. E. Lindow. 2000. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol. Plant-Microbe Interact. 13:1243-1250. [DOI] [PubMed] [Google Scholar]
- 25.Olivares, F. L., V. L. K. Baldani, V. M. Reis, J. I. Baldani, and J. Döbereiner. 1996. Occurrence of the endophytic diazotrophs Herbaspirillum spp. in roots, stems, and leaves, predominantly of Gramineae. Biol. Fertil. Soils 21:197-200. [Google Scholar]
- 26.Rouse, D. I., E. V. Nordheim, S. S. Hirano, and C. D. Upper. 1985. A model relating the probability of foliar disease incidence to the population frequencies of bacterial plant pathogens. Phytopathology 75:505-509. [Google Scholar]
- 27.Sharon, E., Y. Okon, Y. Bashan, and Y. Henis. 1982. Detached leaf enrichment: a method for detecting small numbers of Pseudomonas syringae pv. tomato and Xanthomonas campestris pv. vesicatoria in seed and symptomless leaves of tomato and pepper. J. Appl. Bacteriol. 53:371-377. [Google Scholar]
- 28.Stadt, S. J., and A. W. Saettler. 1981. Effect of host genotype on multiplication of Pseudomonas phaseolicola. Phytopathology 71:1307-1310. [Google Scholar]
- 29.van der Bij, A. J., L. A. de Weger, W. T. Tucker, and B. J. J. Lugtenberg. 1996. Plasmid stability in Pseudomonas fluorescens in the rhizosphere. Appl. Environ. Microbiol. 62:1076-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson, M., S. S. Hirano, and S. E. Lindow. 1999. Location and survival of leaf-associated bacteria in relation to pathogenicity and potential for growth within the leaf. Appl. Environ. Microbiol. 65:1435-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young, J. M. 1974. Development of bacterial populations in vivo in relation to plant pathogenicity. N. Z. J. Agric. Res. 17:105-113. [Google Scholar]