Abstract
Zearalenone (ZON) is a nonsteroidal estrogenic mycotoxin produced by plant-pathogenic species of Fusarium. As a consequence of infection with Fusarium culmorum and Fusarium graminearum, ZON can be found in cereals and derived food products. Since ZON is suspected to be a cause of human disease, including premature puberty syndrome, as well as hyperestrogenism in farm animals, several countries have established monitoring programs and guidelines for ZON levels in grain intended for human consumption and animal feed. We developed a low-cost method for monitoring ZON contamination in grain based on a sensitive yeast bioassay. The indicator Saccharomyces cerevisiae strain YZRM7 is unable to grow unless an engineered pyrimidine biosynthetic gene is activated by the expressed human estrogen receptor in the presence of exogenous estrogenic substances. Deletion of the genes encoding ATP-binding cassette (ABC) transporters Pdr5p and Snq2p increases net ZON uptake synergistically. Less than 1 μg of ZON per liter of medium is sufficient to allow growth of the indicator strain. To prevent interference with pyrimidines potentially present in biological samples, we also disrupted the genes FUR1 and URK1, blocking the pyrimidine salvage pathway. The bioassay strain YZRM7 allows qualitative detection and quantification of total estrogenic activity in cereal extracts without requiring further cleanup steps. Its high sensitivity makes this assay suitable for low-cost monitoring of contamination of maize and small grain cereals with estrogenic Fusarium mycotxins.
Zearalenone (ZON) (Fig. 1) is a resorcylic acid lactone (nonsteroidal) fungal metabolite with very high estrogenic activity. The mycotoxin ZON and derivatives of this compound are produced predominantly by Fusarium graminearum and Fusarium culmorum and also by several other species (7, 32). Under environmental conditions favorable for the fungus, high levels of ZON can be found in harvested maize and small grain cereals (for a review see reference 32).
FIG. 1.
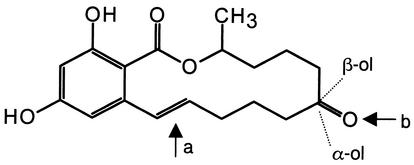
Structure of ZON [6-(10-hydroxy-6-oxo-trans-1-undencenyl)-beta-resorcylic acid lactone] and some important derivatives. Zearalanol (zeranol), which is used as an anabolic growth promoter, lacks a double bond (arrow a). In addition, the keto group (arrow b) is reduced in zeranol, as it is in α-zearalenol (see text for details).
ZON has a very high relative binding affinity for both estrogen receptor ER-α and estrogen receptor ER-β and high transactivation activity (31, 37). Studies with various animal species showed that ZON and its metabolites have potent estrogenic and anabolic activities (32). ZON has been implicated in numerous mycotoxicoses in farm animals, and swine are the most sensitive and most frequently affected species (13, 14, 18). Typical complications associated with high-level ZON ingestion are severe morphological and functional disorders of reproductive organs (39). Mild forms of hyperestrogenism have been observed in pigs exposed to ZON levels as low as 250 μg/kg in their diet (5). The ZON levels in feed implicated in poisoning can be as high as 56 mg/kg (50). Certain Fusarium strains can produce ZON in vitro in liquid cultures that yield up to 34 g/liter of medium (20).
ZON doses that are much greater than concentrations which have hormonal effects may have genotoxic and carcinogenic effects. A National Toxicology Program study found evidence of carcinogenicity in mice but not in rats (43). Likewise, species-specific formation of DNA adducts has been reported (46).
While ZON toxicoses in farm animals are widely recognized, the impact of this mycotoxin on humans is controversial (e.g., there have been proposed cases of precocious sexual development in Puerto Rico [17, 51, 53, 54]). However, high levels of ZON (up to 100 μg/liter) were reported in the sera of Hungarian children exhibiting early puberty symptoms (60, 61). Whether ZON promotes breast cancer (2, 54, 62) rather than reducing mammary tumorigenesis (21) is also controversial.
In a recent toxicological evaluation, the Scientific Committee on Food of the European Commission on Health & Consumer Protection arrived at a temporary tolerable daily intake for ZON of 0.2 μg/kg of body weight (15), while the Joint FAO/WHO Expert Committee on Food Additives recommended a provisional maximum tolerable daily intake of 0.5 μg/kg (23). No international harmonized maximum limits for ZON in grain exist yet, but several countries already have regulations, whose legal status differs (16). Austria, for instance, has had a guideline level for wheat, durum wheat, and rye intended for human consumption of 60 μg of ZON/kg (9) since 1993. A concentration of 200 μg/kg has been set as a guideline level for commodities in Brazil (maize), Uruguay (maize and barley), and France (cereals and vegetable oil) (16). Several countries have also proposed guideline levels for animal feed; the suggested guideline level for breeding pigs in Austria (33) and Germany (8) is 50 μg of ZON/kg of total feed.
In North America, contamination of grain with the Fusarium mycotoxin deoxynivalenol appears to be far more important than contamination with ZON (45, 56). In parts of Europe, delayed harvesting due to wet weather can lead to the occurrence of high ZON levels in wheat. Monitoring of data from southern Germany (42) indicated that in normal years the frequency of ZON-positive samples is less than 20%, and the observed ZON levels in most of the positive samples are well below 50 μg/kg. However, in a year in which the weather conditions favored growth of the fungus 80% of the samples were positive, and the average level of ZON detected in wheat was 178 ± 994 μg/kg; the highest levels were more than 8 mg/kg (42). Likewise, in 1998 in northern Germany 65% of the wheat samples were ZON positive (12), and the average level was 516 μg/kg (maximum level, 2.2 mg/kg).
In industrialized countries most of the Fusarium-damaged grain is likely to be consumed by farm animals rather than by humans. However, the situation may be very different in other parts in the world. In Iran, for instance, the domestic wheat consumption is now about 16 million metric tons, but only about 10 million tons is produced. The main wheat production areas are the hot and humid provinces of northern Iran (Caspian Sea littoral), where Fusarium head blight dramatically decreases yield (3). Furthermore, high levels of Fusarium toxins have been reported in Iranian wheat (63), with the ZON levels ranging from 1,266 to 5,487 μg/kg (average, 3,464 μg/kg). Notably, the provinces of northern Iran are hotspots for esophageal cancer (52), and consumption of wheat flour is among the reported risk factors for this disease (22). Regular mycotoxin monitoring thus seems to be warranted, but the high cost of monitoring grain samples is prohibitive.
Usually, ZON levels in grains are determined by using tedious (liquid-liquid extraction) or very expensive (immunoaffinity column) cleanup procedures and subsequent separation and detection by high-performance liquid chromatography (HPLC), followed by fluorescence or UV detection (29). Our goal was to develop a sensitive and inexpensive bioassay for ZON in grain. We describe here construction of a yeast indicator strain and the first results of tests performed with this strain in a bioassay, which could become an attractive low-cost tool for monitoring ZON levels in agricultural commodities and for basic research applications.
MATERIALS AND METHODS
Plasmid construction.
Genomic DNA of S. cerevisiae strain AB1380 (10) was employed as a template for all plasmid construction steps in which PCR was used. Construction of the Δurk1::TRP1 plasmid pRM753 has been described previously (41). For construction of the Δfur1::LEU2 deletion plasmid, oligonucleotides FUR-UP (5′-AATAACAATACCTCATTCTAC-3′) and FUR-DW (5′-TACTCAAGATTTAATCTCGAC-3′) were used to amplify a 1,695-bp fragment containing the complete FUR1 gene. This fragment was digested with HindIII plus EcoRV, and the resulting 1,529-bp fragment was inserted into pUC18 digested with HindIII plus SmaI. The resulting plasmid was digested with AccI and XbaI to delete a 559-bp fragment located within the FUR1 coding region and was religated after treatment with the Klenow enzyme (fill in) in order to reconstitute the unique XbaI site. A 1,990-bp blunt-end LEU2 fragment (derived from pJJ252 [24] by SalI digestion and filling in with the Klenow enzyme) was ligated into the filled-in XbaI site to create pRM765 (Δfur1::LEU2).
Vector pCGS966 is a centromeric plasmid containing the URA3 and HIS3 genes (59).
Yeast strains.
The Saccharomyces cerevisiae strains used in this study and their relevant genotypes are shown in Table 1. The strains were derived from the pyrimidine auxotrophic strain PL3 (ura3-Δ1), in which transcription of an additional URA3 gene can be induced by substances with estrogenic activity via three estrogen-responsive elements in the promoter (trp1::3xERE-URA3) (48). Derivatives of PL3 containing the vector YEp90-HEGO (expressing the human estrogen receptor cDNA under control of the constitutive PGK1 promoter) or the empty vector YEp90 (2 μm-HIS3-PPGK1) have been described previously (35), and particular transformants were renamed for reasons of clarity (Table 1). Strains harboring Δurk1::TRP1 and Δfur1::LEU2 deletions were constructed by transforming the corresponding parental strains with the ClaI-NotI fragment of pRM753 and the HindIII-SphI fragment of pRM765, respectively. Transformations of S. cerevisiae were performed by the LiAc method (19). Correct integration of the deletion constructs was confirmed by PCR.
TABLE 1.
Genotypes of S. cerevisiae strains used in this study
| Strain | Parental strain | Plasmid (relevant change) | Genotype and relevant alterations (episomal plasmids)a |
|---|---|---|---|
| PL3 | MATα ura3-Δ1 his3-Δ200 leu2-Δ1 trp1::P3xERE-URA3b | ||
| YYM6 | PL3 | PL3 Δsnq2::hisGc | |
| YYM7 | PL3 | PL3 Δpdr5::hisGc | |
| YYM8 | YYM6 | PL3 Δsnq2::hisG Δpdr5::hisGc | |
| YZGA304 | PL3 | YEp90 | PL3 (2μm, HIS3) |
| YZGA305 | PL3 | YEp90-HEGO | PL3 (2μm, HIS3-PPGK1-hER) |
| YZGA307 | YYM6 | YEp90-HEGO | PL3 Δsnq2::hisG (2μm, HIS3-PPGK1-hER) |
| YZGA309 | YYM7 | YEp90-HEGO | PL3 Δpdr5::hisG (2μm, HIS3-PPGK1-hER) |
| YZGA310 | YYM8 | YEp90 | PL3 Δsnq2::hisG Δpdr5::hisG (2μm, HIS3) |
| YZGA311 | YYM8 | YEp90-HEGO | PL3 Δsnq2::hisG Δpdr5::hisG (2μm, HIS3-PPGK1-hER) |
| YZGA439 | YZGA311 | pRM753/ClaI+NotI | PL3 Δurk1::TRP1 Δsnq2::hisG Δpdr5::hisG (2μm, HIS3-PPGK1-hER) |
| YZRM7 | YZGA439 | pRM765/HindIII+SphI | PL3 Δfur1::LEU2 Δurk1::TRP1 Δsnq2::hisG Δpdr5::hisG (2μm, HIS3-PPGK1-hER) |
| YZGA376 | YYM8 | pCGS966 | PL3 Δsnq2::hisG Δpdr5::hisG (CEN4 HIS3 TRP1 URA3) |
Yeast media.
Yeast strains were grown in rich medium (YPD medium) or in synthetic medium (SD medium) supplemented with auxotrophic components (57) but simplified by omitting methionine, tyrosine, phenylalanine, glutamic acid, aspartic acid, valine, and serine. Uracil (catalog no. 94220; Fluka), uridine (catalog no. U-6381; Sigma), cytosine (catalog no. C-3506; Sigma), and cytidine (catalog no. C-9505; Sigma) were added as 100× aqueous stock solutions (2,000 mg/liter). Diethylstilbestrol (DES) and ZON were purchased from Sigma (catalog no. D-4628 and Z-2125).
YZRM7 (Δurk1::TRP1 Δfur1::LEU2), which is unable to utilize exogenous pyrimidines and strictly requires estrogenic compounds in the medium, was propagated on SC-HIS medium plates supplemented with DES (1 μg/liter) or ZON (10 μg/liter). For spot tests YZRM7 was pregrown in liquid ZON-containing SC-HIS medium, harvested by centrifugation, and transferred to medium without an estrogenic supplement for 6 h.
Extraction of naturally contaminated wheat and maize samples.
The ZON extraction procedure used was a down-scaled version of the previously described protocol (55). Briefly, 6 g of milled wheat kernels was homogenized and extracted in 24 ml of a mixture containing 75% (vol/vol) acetonitrile and 25% water by using an Ultra Turrax T25 mixer for 3 min at 24,500 rpm in a 50-ml Greiner disposable tube. After centrifugation (5,000 × g for 5 min), the supernatant was filtered through a Whatman GF/A filter. The acetonitrile extract (200 μl) and appropriate dilutions of this extract were mixed with two 900-μl portions of SC-URA-HIS medium (in which agar was replaced by 1% agarose) in 2.2-ml Eppendorf tubes and then poured into 35-mm-diameter petri dishes. After solidification of the medium, 6-μl portions of diluted suspensions (A600 = 0.03) of the indicator yeast strain and control strains were spotted on the plates and incubated at 30°C for 4 days. The yeast growth observed on control plates containing dilutions of a ZON calibrating solution was used as a reference to estimate the ZON contents of the extracts. Initially, a ZON calibrating solution was prepared by dissolving ZON (1 mg/ml; Sigma) in ethanol, but for further analysis a certified ZON calibrating solution, prepared at the IFA Tulln Center for Analytical Chemistry, was used. The standard and the reference maize flour samples were prepared as part of a project to obtain certified reference materials for determination of the mycotoxin ZON in maize. Immunoaffinity cleanup and HPLC with fluorescence detection were used for ZON determination as described previously (55).
RESULTS
Inactivation of the genes encoding the ABC transporters Pdr5p and Snq2p improves net uptake of ZON.
It was shown previously that two plasma membrane-localized ATP-binding cassette (ABC) transporter proteins encoded by the PDR5 and SNQ2 genes mediate efflux of estradiol in yeast (35), raising the question of whether deletion of these two genes affects uptake of the structurally unrelated, estrogenic mycotoxin ZON. The test system which we used is based on the estrogen-dependent growth of strains derived from PL3 on media without a pyrimidine supplement (48). These yeast strains express the human estrogen receptor cDNA under control of the yeast PGK1 promoter (plasmid YEp90-HEGO). Binding of estrogenic substances to the receptor leads to activation of an engineered copy of the URA3 gene having three estrogen-responsive elements in the promoter (trp1::3ERE-URA3), which allows de novo pyrimidine synthesis and growth of the otherwise pyrimidine auxotrophic (ura3) strain.
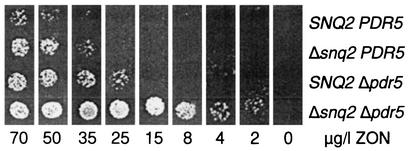
ABC transporter knockout strains derived from PL3 (Table 1), which had either a single mutation or the double mutation (Δsnq2 Δpdr5), were spotted onto plates containing different amounts of ZON. As shown in Fig. 2, the PDR5 SNQ2 wild-type strain YZGA305 required about 70 μg of ZON per liter for growth. Single disruptions of SNQ2 or PDR5 decreased the amount required about two- to threefold, whereas less than 2 μg of ZON per liter was sufficient for growth of the PL3-derived strain lacking both ABC transporter Pdr5p and ABC transporter Snq2p (YZGA311).
FIG. 2.
Yeast strains were spotted on SC-HIS-URA medium plates containing different amounts of ZON. All of the PL3 derivatives contain the estrogen-dependent URA3 gene integrated into the genome and the human estrogen receptor on the HIS3 expression plasmid, but they differ with respect to the ABC transporter genes SNQ2 and PDR5, as follows: YZGA305, wild type; YZGA307, Δsnq2; YZGA309, Δpdr5; and YZGA311, Δsnq2 Δpdr5.
Construction of a mutant unable to utilize exogenous pyrimidines.
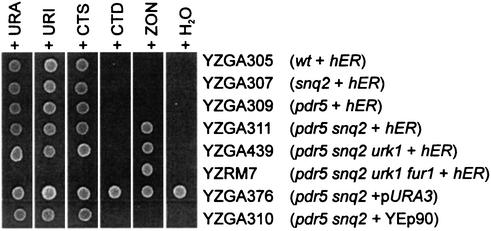
Although the Δsnq2 Δpdr5 double mutant PL3 strain allowed detection of ZON at practically relevant concentrations, the presence of pyrimidines in complex natural extracts should also allow growth of this strain. To avoid false-positive results, we inactivated the URK1 (27) and FUR1 (28) genes, which encode pyrimidine salvage pathway enzymes (Fig. 3). First, the URK1 gene was deleted by using the Δurk1::TRP1 plasmid pRM753, resulting in strain YZGA439. In the second step, the FUR1 gene was also destroyed, which yielded the final bioassay indicator strain, YZRM7. The different mutants were tested for the ability to grow on synthetic media with various pyrimidine supplements (Fig. 4). Only YZGA376 containing a URA3 gene on plasmid pCGS966 could grow without supplements. The Δsnq2 Δpdr5 double mutants, but not the single mutants, were rescued by 10 μg of ZON per liter, a process dependent on the cDNA of the expressed human estrogen receptor (hER, present on plasmid YEp90-HEGO). In accordance with the pyrimidine pathway shown in Fig. 3, the constructed strain YZRM7 (Δurk1::TRP1 Δfur1::LEU2) was unable to utilize exogenous pyrimidines but was rescued by ZON (and other estrogenic substances).
FIG. 3.
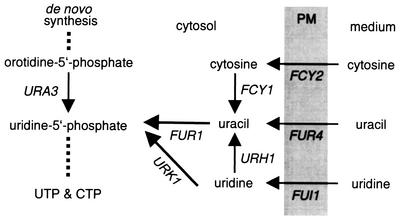
Metabolism of external pyrimidines in S. cerevisiae. The genes encoding the proteins involved in the steps are indicated. External cytosine, uracil, and uridine are taken up by purine-cytosine permease (FCY2), uracil permease (FUR4), and uridine permease (FUI1), respectively. Cytosine can be converted into uracil by cytosine deaminase (FCY1), and uridine can be converted into uracil by uridine-cytidine N-ribohydrolase (URH1). Uridine 5-phosphate, the enzymatic product of the URA3 step in pyrimidine de novo synthesis, can be formed either from uracil by uracil phosphoribosyltransferase (FUR1) or from uridine by uridine kinase (URK1). Blocking the latter two steps should therefore lead to an inability to utilize external pyrimidines. PM, plasma membrane.
FIG. 4.
Logarithmically growing cultures of strains (relevant genotypes are indicated in parentheses) were spotted onto SC-HIS-URA medium plates supplemented with (final concentrations) 20 mg of uracil (URA) per liter, 20 mg of uridine (URI) per liter, 20 mg of cytosine (CTS) per liter, 20 mg of cytidine (CTD) per liter, or 10 μg of ZON per liter. Strain YZRM7 is unable to utilize external pyrimidines.
Characterization of indicator strain YZRM7 and tests with maize and wheat.
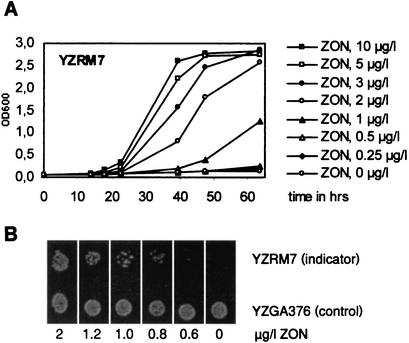
To test whether ZON-dependent growth of bioassay strain YZRM7 can be monitored by simple turbidimetric measurement, this organism was grown in SC-HIS-URA liquid medium containing 0 to 10 μg of ZON per liter. Growth curves were determined by measuring A600, as shown in Fig. 5. The growth of the indicator strain was fairly slow, but it was strictly ZON dependent. The minimum amount of ZON sufficient to give detectable growth after 48 h was less than 1 μg/liter, and it was more than 0.5 μg/liter in liquid culture. Similarly, growth was observed on SC-HIS medium plates containing ZON concentrations higher than 0.8 μg/liter (Fig. 5). The change from growth to no growth within a narrow concentration range thus allowed determination of ZON concentrations by simple titration and comparison with a ZON calibrating solution.
FIG. 5.
ZON-dependent growth of strain YZRM7 at 30°C. (A) Turbidimetric measurement of growth in liquid SC-HIS-URA medium containing different amounts of ZON. OD600, optical density at 600 nm. (B) Growth observed on SC-HIS-URA medium plates after 4 days of incubation. The positive control strain YZGA376 contains a URA3 gene (plasmid pCGS966).
Since ZON is usually extracted from grain samples by using acetonitrile as the main solvent component, we determined the acetonitrile concentration necessary to inhibit growth of strain YZRM7. On solid media, growth was completely unaffected by final acetonitrile concentrations as high as 15%. In contrast, acetonitrile severely inhibited growth in liquid cultures, and the 50% inhibitory concentration was about 2.5% (data not shown). Therefore, we focused on development of the bioassay in which the agar plate format was used.
The bioassay was first tested with a reference maize flour (30) with a ZON concentration of 60 μg/kg. Assuming that the ZON present in the maize flour was diluted fourfold during the extraction procedure and that the extract was diluted 10-fold in the yeast growth medium, the final ZON concentration should have been 1.5 μg/liter, which is about twice the concentration required to induce YZRM growth. We added 300, 200, 100, and 50 μl of unpurified extract to 2 ml of agar and spotted indicator strain YZRM7 and several controls on the resulting plates. Growth of YZRM7 was clearly observed on the plates containing 200 and 300 μl of maize flour extract, there was very slight growth on the plate containing 100 μl, and there was no growth on the plate containing 50 μl or the solvent controls (data not shown). We concluded that our indicator strain can detect the presence of ZON in crude extracts of maize without a loss of sensitivity due to matrix effects.
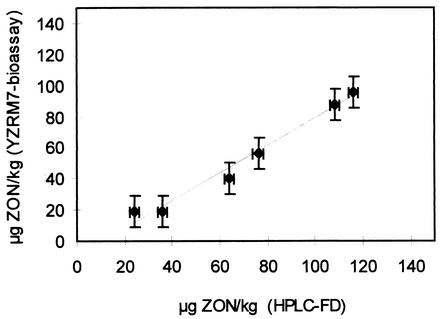
To test whether the bioassay can also detect ZON in wheat flour, we analyzed six grain samples derived from different wheat cultivars that were artificially inoculated at anthesis with the same F. graminearum strain. Extracts were prepared from the ground grains, and a series of twofold dilutions of the extracts was initially tested to determine the ZON concentration range. The highest dilution which still clearly gave a positive result in this test was defined as 100%, and further dilutions in 10% steps were then analyzed with the bioassay. For comparison, the extracts were also analyzed by conventional reversed-phase HPLC with fluorescence detection, as shown in Fig. 6. The calculated correlation coefficient for this experiment was 0.988, and the constant error as estimated by using the y intercept was −9.7 μg/kg. We subsequently analyzed more wheat and maize samples and confirmed that the bioassay is a very useful tool for detecting maize and wheat that is naturally contaminated with ZON and for evaluating the contents of grain samples. For instance, we analyzed kernels from wheat field trials performed in Iran, in which the concentration in only one of seven samples fell below the detection limit of 30 μg of ZON equivalents per kg, as shown in Table 2.
FIG. 6.
Comparison of the ZON contents of wheat samples determined by the bioassay and by the HPLC method with fluorescence detection (HPLC-FD). The proportional error, determined by the slope, is about 11% and was primarily caused by overestimating the ZON concentration in the sample with the lowest content.
TABLE 2.
ZON contents of Iranian wheat samples
| Location | Wheat cultivar | Growth responsesa
|
ZON content (μg/kg of grain)b | |||
|---|---|---|---|---|---|---|
| Undiluted | 1/2 Dilution | 1/4 Dilution | 1/8 Dilution | |||
| Karaj | Falat Ic | − | − | − | − | 25 |
| Karaj | Falat II | ++++ | ++++ | ++++ | − | 197 |
| Parsabad Moghan | Sisonz | ++++ | ++++ | ++++ | (+) | 219 |
| Parsabad Moghan | Bacanoura | ++++ | ++++ | +++ | +++ | 347 |
| Parsabad Moghan | Kaspard | ++++ | ++++ | +++ | − | 190 |
| Parsabad Moghan | Siren I | ++++ | ++++ | ++ | − | 136 |
| Parsabad Moghan | Siren II | +++ | ++ | (−) | − | 95 |
Qualitative growth responses of the indicator strain were determined on plates containing 200-μl portions of undiluted acetonitrile extract (undiluted) or twofold dilutions of the extract. (+), Slight growth above the titration point; (−), slight growth below the titration point. A positive result for the 1/4 dilution indicates the possible presence of estrogenic activity equivalent to twice the Austrian ZON guideline level (60 μg/kg).
ZON content as determined by HPLC with fluorescence detection.
Most wheat samples were obtained in 2001; the only exception is the Falat I sample, which was obtained in 2000.
DISCUSSION
We developed a sensitive and low-cost yeast bioassay for ZON and other estrogenic compounds, which is based on the estrogen receptor-dependent growth of an engineered yeast strain described previously (35, 48). The yeast growth response in this bioassay is very robust and reproducible, and the bioassay does not require preparation of cell extracts, chromogenic substrates, or expensive instrumentation, in contrast to conventional yeast two-plasmid systems, which rely on measurement of the expression of a reporter gene (for a review see reference 11), and especially in contrast to assays based on mammalian cells (36). Two findings were important for the development of the bioassay. The first was the observation that disruption of the PDR5 and SNQ2 genes, encoding two highly expressed ABC transporter proteins, improves intracellular retention of ZON. The second important observation was that inactivation of the yeast pyrimidine salvage pathway genes URK1 and FUR1 successfully prevents false-positive growth on pyrimidine substrates present in biological samples.
Disruption of PDR5 and SNQ2 leads to greatly improved uptake not only of 17-β estradiol (35) and ZON but also of the ZON derivatives α-zearalenol, β-zearalenol, α-zearalanol, and β-zearalanol and several other estrogenic compounds (data not shown). By inactivating the two ABC transporters, the limit of detection could be lowered to the concentration range that is relevant with respect to existing or proposed national health and safety regulations.
A potential problem with Δsnq2 Δpdr5 mutants is that they are more sensitive to (accompanying) toxic substances that are also substrates of the broad-specificity transporters. We have previously shown that PDR5 is responsible for the high trichothecene resistance of yeast (1), and more than 120 mg of the main Fusarium mycotoxin deoxynivalenol per liter (120 ppm) is tolerated by Δpdr5 mutants. In view of the dilution steps involved in the assay procedure (40-fold), unrealistic amounts of deoxynivalenol would have to be present in a grain sample to produce an extract that inhibits the growth of the indicator strain. False-negative results are easily detected, since growth of the control strain YZGA376 is also inhibited.
As shown in Fig. 4, false-positive growth of the indicator strain is prevented by inactivating genes of the pyrimidine salvage pathway (URK1 and FUR1). Since cytidine, which can be converted to uridine by cytidine deaminase (CDD1), does not support growth of the original PL3 strain (41), this pathway is not shown in Fig. 3. The inability of the Δurk1 Δfur1 double mutant to grow on uracil, uridine, and cytosine shows that no other yeast gene products (e.g., YNL129w protein) have overlapping activity in the pyrimidine pathway.
The indicator strain YZRM7 can be propagated only on media containing estrogenic substances. However, if this strain is pregrown in the presence of high levels of potent estrogenic compounds (e.g., 1 μg of DES per liter), several cell divisions can occur before growth arrest occurs. To avoid a high background level on the bioassay plates, it is advisable to inoculate the plates with highly diluted indicator yeast suspensions and to pregrow the strain for some time on a medium lacking an estrogenic supplement. Mutants of the indicator yeast strain able to grow without any estrogenic substance in the medium are observed after extended incubation periods. This phenotype could arise from mutations leading to expression of constitutively activating, truncated forms of the estrogen receptor (47).
The bioassay allows detection of estrogenic activity but not determination of the identity or concentration of a particular compound. Fusarium isolates produce not only ZON but also other derivatives (49). For instance, α-zearalenol, which has 36-fold-higher estrogenic activity than ZON in mammalian cells (58), was found in wheat grain together with ZON (42), albeit at much lower concentrations. Our bioassay allows determination of only the total estrogenic activity in a sample, and the method is also limited to matrices without other interfering phytoestrogens. Soy flour samples, for instance, gave positive results. We also found that a honey-frosted corn flake sample was positive in the bioassay, but no ZON was detected in this sample by HPLC (40). One could speculate that the estrogenic activity originated from phytoestrogens present in clover honey. The presence of phytoestrogens in hops (38) also prevents use of the bioassay for beer.
Conventional ZON analysis procedures require sample cleanup before detection and quantification of ZON are possible (29). In our bioassay, the acetonitrile extracts can be used directly without further purification. If the ZON present in the sample is diluted 40-fold, the limit of detection for ZON in grain is about 30 μg/kg, which is well below the Austrian guideline level approved for wheat and rye for human consumption (60 μg/kg). With wheat it is possible to increase the volume of acetonitrile extract added to the plates, and the detection limit can be lowered accordingly. The high fat content of maize extracts limits this approach.
The limit of detection of the bioassay compares favorably with the limits of detection of previously described antibody-based detection systems and commercially available kits (4, 6, 29, 34). A major disadvantage is the long time required for growth of the yeast indicator strain. In Austria, the bioassay would be a useful screening tool for identifying rare samples in which the concentration may exceed the ZON guideline level for grain intended for human consumption, which can then be confirmed by conventional analytical procedures. The few Iranian grain samples that we have analyzed so far confirm the presence of ZON, although the levels observed (Table 2) are much lower than the levels reported for the 1996 samples (63). The bioassay should be useful for obtaining a clearer picture of the situation in Iran.
The assay is very cost-effective, making it attractive for screening cheap grain commodities intended for feed use. In Europe, at least in theory, resorcylic acid lactones are illegal feed ingredients according to EC directive 96/22, appendix 1 (44). The import of meat from animals treated with zearalanol (zeranol) is banned, while feeding farm animals with naturally Fusarium-infected grain can also lead to the presence of zearalanol residues in pigs and cattle (26, 64). In view of increased public awareness of the problem of xenohormones or endocrine disrupters (25) and the need to monitor agricultural products for estrogenic Fusarium mycotoxins, we think that the bioassay which we developed may provide a valuable and economic screening tool. It is difficult to understand why the presence of estrogenic Fusarium mycotoxins in staple food should receive less attention than the presence of zeranol residues in hormone-treated beef or other environmental contaminants, which are orders of magnitude less potent than ZON.
In addition to grain testing, the bioassay is a valuable research tool, and we have used it to screen for ZON-degrading microorganisms and insertion mutants of F. graminearum which have lost the ability to produce ZON.
Acknowledgments
This work was supported by grants from the Austrian Federal Ministry of Agriculture and Forestry (BMLF Zl. 24.002/51-IIA/97) and from the Austrian Federal Ministry for Education, Science and Culture (bm:bwk GZ 309.007/3-VIII/B/8b/2000). The work on reference materials was supported by the European Community (grant EU SMT4-CT98-2228). We are grateful for a small grant from the City of Vienna (Hochschuljubiläumsstiftung H-00199/97), which allowed G.A. to start the work on ZON. K.K. was supported by grant FWF P-1266 from the Austrian Science Foundation. N.S. is a recipient of fellowships from the Iranian Presidential Technology Cooperation Office and Ministry of Science, Research and Technology.
We thank A. Alizadeh (Cereal Research Department, Seed and Plant Improvement Institute, Karaj, Iran) for providing Iranian wheat samples and G. Wiesenberger and V. Cameron-Mills for critically reading the manuscript.
REFERENCES
- 1.Adam, G., R. Mitterbauer, A. Raditschnig, B. Poppenberger, T. Karl, S. Goritschnig, H. Weindorfer, and J. Glössl. 2001. Molecular mechanisms of deoxynivalenol resistance in the yeast Saccharomyces cerevisiae. Mycotoxin Res. 17:19-23. [DOI] [PubMed] [Google Scholar]
- 2.Ahamed, S., J. S. Foster, A. Bukovsky, and J. Wimalasena. 2001. Signal transduction through the Ras/Erk pathway is essential for the mycoestrogen zearalenone-induced cell-cycle progression in MCF-7 cells. Mol. Carcinog. 30:88-98. [DOI] [PubMed] [Google Scholar]
- 3.Alizadeh, A., and A. Saidi (ed.). 1999. Fusarium head blight of wheat and the possibility of its control in Iran. Progress Report of the National Fusarium Project. Seed and Plant Improvement Institute, Karaj, Iran. (In Farsi.)
- 4.Barna-Vetro, I., A. Gyongyosi, and L. Solti. 1994. Monoclonal antibody-based enzyme-linked immunosorbent assay of Fusarium T-2 and zearalenone toxins in cereals. Appl. Environ. Microbiol. 60:729-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer, J., K. Heinritzi, M. Gareis, and B. Gedek. 1987. Changes in the genital tract of female swine after feeding with practice-relevant amounts of zearalenone. Tieraerztl. Prax. 15:33-36. [PubMed] [Google Scholar]
- 6.Bennett, G. A., T. C. Nelson, and B. M. Miller. 1994. Enzyme-linked immunosorbent assay for detection of zearalenone in corn, wheat, and pig feed: collaborative study. J. AOAC Int. 77:1500-1509. [PubMed] [Google Scholar]
- 7.Botallico, A. 1998. Fusarium diseases of cereals: species complex and related mycotoxin profiles in Europe. J. Plant Pathol. 80:85-103. [Google Scholar]
- 8.Bundesministerium für Ernährung, Landwirtschaft und Forsten. 30 June 2000, posting date. Orientierungswerte für die Beurteilung der Gehalte an Deoxynivalenol und Zearalenon in Futtermitteln im Rahmen des §3 des Futtermittelgesetzes im Futter von Schwein, Rind und Huhn. BML Rdschr. 2000-324-3830/323. [Online.] http://www.verbraucherministerium.de/landwirtschaft/futtermittel/orientierungswerte.htm.
- 9.Bundesministerium für Gesundheit, Sport und Konsumentenschutz. 1993. Richtwerte für Mykotoxine in Getreide für den menschlichen Genuß (GZ 32.110/1-III/B/1b/93). Mitt. Oesterr. Sanitaetsverwalt. (Vienna) 94:360. [Google Scholar]
- 10.Burke, D. T., F. G. Carle, and M. V. Olsen. 1987. Cloning of large segments of exogenous DNA into yeast by means of artificial chromosome vectors. Science 236:806-812. [DOI] [PubMed] [Google Scholar]
- 11.Diel, P., K. Smolnikar, and H. Michna. 1999. In vitro test systems for the evaluation of the estrogenic activity of natural products. Planta Med. 65:197-203. [DOI] [PubMed] [Google Scholar]
- 12.Ellner, F. M. 1999. 1998. Ein Jahr für Fusariumtoxine, p. 1-4. In H. Rosner and P. Kielstein (ed.), Proceedings of the 21st Mycotoxin Workshop. German Federal Institute of Health Protection of Consumers and Veterinary Medicine, Berlin, Germany.
- 13.Etienne, M., and J.-Y. Dourmad,. 1994. Effects of zearalenone or glucosinolates in the diet in reproduction in sows: a review. Livest. Prod. Sci. 40:99-113. [Google Scholar]
- 14.Etienne, M., and M. Jemmali. 1982. Effects of zearalenone (F-2) on estrous activity and reproduction in gilts. J. Anim. Sci. 55:1-10. [DOI] [PubMed] [Google Scholar]
- 15.European Commission Scientific Committee on Food. 22 July 2000, posting date. Opinion on Fusarium toxins. Part 2: Zearaleonone (ZEA). [Online.] Health and Consumer Directorate-General, European Commission, Brussels, Belgium. http://europa.eu.int/comm/food/fs/sc/scf/out65._en.pdf.
- 16.Food and Agriculture Organization. 1997. Worldwide regulations for mycotoxins 1995. A compendium. Food Nutr. Pap. 64:1-43. [PubMed] [Google Scholar]
- 17.Freni-Titulaer, L. W., J. F. Cordero, L. Haddock, G. Lebron, R. Martinez, and J. L. Mills. 1986. Premature thelarche in Puerto Rico. A search for environmental factors. Am. J. Dis. Child. 140:1263-1267. [DOI] [PubMed] [Google Scholar]
- 18.Gaumy, J. L., J. D. Bailly, G. Benard, and P. Guerre. 2001. Zearalenone: origin and effect on farm animals. Rev. Med. Vet. 152:123-136. [Google Scholar]
- 19.Gietz, R. D., R. H. Schiestl, A. R. Willems, and R. A. Woods. 1995. Studies on the transformation of intact yeast cells by the LiAc/ss-DNA/PEG procedure. Yeast 11:355-360. [DOI] [PubMed] [Google Scholar]
- 20.Hidy, P. H., R. S. Baldwin, R. L. Greasham, C. L. Keith, and J. R. McMullen. 1977. Zearalenone and some derivatives: production and biological activities. Adv. Appl. Microbiol. 22:59-82. [DOI] [PubMed] [Google Scholar]
- 21.Hilakivi-Clarke, L., I. Onojafe, M. Raygada, E. Cho, T. Skaar, I. Russo, and R. Clarke. 1999. Prepubertal exposure to zearalenone or genistein reduces mammary tumorigenesis. Br. J. Cancer 80:1682-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hormozdiari, H., N. E. Day, B. Aramesh, and E. Mahboubi. 1975. Dietary factors and esophageal cancer in the Caspian littoral of Iran. Cancer Res. 35:3493-3498. [PubMed] [Google Scholar]
- 23.Joint FAO/WHO Expert Committee on Food Additives. January 2000, posting date. Joint FAO/WHO Expert Committee on Food Additives: position paper on zearalenone. Publication CX/FAC00/19. [Online.] Codex Alimentarius Commission, Rome, Italy. http://www.who.int/fsf/Chemicalcontaminants/ZearalenonePP00_19.pdf.
- 24.Jones, J. S., and L. Prakash. 1990. Yeast Saccharomyces cerevisiae selectable markers in pUC18 polylinkers. Yeast 6:363-366. [DOI] [PubMed] [Google Scholar]
- 25.Juberg, D. R. 2000. An evaluation of endocrine modulators: implications for human health. Ecotoxicol. Environ. Saf. 45:93-105. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy, D. G., J. D. G. McEvoy, S. A. Hewitt, A. Cannavan, W. J. Blanchflower, and C. T. Elliott. 1998. Zeranol is formed from Fusarium ssp. toxins in cattle in vivo. Food Addit. Contam. 15:393-400. [DOI] [PubMed] [Google Scholar]
- 27.Kern, L. 1990. The URK1 gene of Saccharomyces cerevisiae encoding uridine kinase. Nucleic Acids Res. 18:5279.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kern, L., J. de Montigny, R. Jund, and F. Lacroute. 1990. The FUR1 gene of Saccharomyces cerevisiae: cloning, structure and expression of wild-type and mutant alleles. Gene 88:149-157. [DOI] [PubMed] [Google Scholar]
- 29.Krska, R., and R. Josephs. 2001. The state-of-the-art in the analysis of estrogenic mycotoxins in cereals. Fresenius J. Anal. Chem. 369:469-476. [DOI] [PubMed] [Google Scholar]
- 30.Krska, R., R. Josephs, H. Pettersson, and S. McDonald. 2001. ZONMAIZE: EU-SMT project for the production of certified reference materials (CRM) for the determination of zearalenone in maize. Mycotoxin Res. 17:92-96. [DOI] [PubMed] [Google Scholar]
- 31.Kuiper, G. G. J. M., J. G. Lemmen, B. Carlsson, J. C. Corton, S. H. Safe, P. T. van der Saag, B. van der Burg, and J.-Å. Gustafsson. 1998. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 139:4252-4263. [DOI] [PubMed] [Google Scholar]
- 32.Kuiper-Goodman, T., P. M. Scott, and H. Watanabe. 1987. Risk assessment of the mycotoxin zearalenone. Regul. Toxicol. Pharmacol. 7:253-306. [DOI] [PubMed] [Google Scholar]
- 33.Lew, H. 15 March 2000, revision date. Empfohlenes Richtwerteschema für Desoxynivalenol (Vomitoxin) und Zearalenon im Futter. Foerderungsdienst 47:157. [Online.] http://www.lebensministerium.at/download/dlpublik/FD0599.PDF.
- 34.Liu, M. T., B. P. Ram, L. P. Hart, and J. J. Pestka. 1985. Indirect enzyme-linked immunosorbent assay for the mycotoxin zearalenone. Appl. Environ. Microbiol. 50:332-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahe, Y., Y. Lemoine, and K. Kuchler. 1996. The ATP-binding cassette transporters Pdr5 and Snq2 of Saccharomyces cerevisiae can mediate transport of steroids in vivo. J. Biol. Chem. 271:25167-25172. [DOI] [PubMed] [Google Scholar]
- 36.Mayer, U., A. Butsch, and S. Schneider. 1992. Validation of two in vitro test systems for estrogenic activities with zearalenone, phytoestrogens and cereal extracts. Toxicology 74:135-149. [DOI] [PubMed] [Google Scholar]
- 37.Miksicek, R. J. 1994. Interaction of naturally occurring nonsteroidal estrogens with expressed recombinant human estrogen receptor. J. Steroid Biochem. Mol. Biol. 49:153-160. [DOI] [PubMed] [Google Scholar]
- 38.Milligan, S., J. Kalita, V. Pocock, A. Heyerick, L. De Cooman, H. Rong, and D. De Keukeleire. 2002. Estrogenic activity of the hop-phytoestrogen 8-prenylnaringenin. Reproduction 123:235-242. [PubMed] [Google Scholar]
- 39.Mirocha, C. J., and C. M. Christensen. 1974. Estrogenic mycotoxins synthesized by Fusarium, p. 129-148. In I. F. H. Purchase (ed.), Mycotoxins. Elsevier, Amsterdam, The Netherlands.
- 40.Mitterbauer, R. 2000. Molecular mechanisms of trichothecene resistance and development of a yeast-based bioassay for zearalenone. Ph.D. thesis. University of Agricultural Sciences, Vienna, Austria.
- 41.Mitterbauer, R., T. Karl, and G. Adam. 2002. Saccharomyces cerevisiae URH1 (encoding uridine-cytidine N-ribohydrolase): functional complementation by a nucleoside hydrolase from a protozoan parasite and by a mammalian uridine phosphorylase. Appl. Environ. Microbiol. 68:1336-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Müller, H. M., J. Reimann, U. Schumacher, and K. Schwadorf. 1997. Fusarium toxins in wheat harvested during six years in an area of southwest Germany. Nat. Toxins 5:24-30. [DOI] [PubMed] [Google Scholar]
- 43.National Toxicology Program. 16 December 2002, revision date. National Toxicology Program carcinogenesis bioassay of zearalenone in F344/N rats and F6C3F1 mice. National Toxicology Program Technical Report Series, vol. 235. [Online.] Department of Health and Human Services, Research Triangle Park, N.C. http://ntp-server.niehs.nih.gov/htdocs/Results_status/ResstatZ/10770.-C.html.
- 44.Official Journal of the European Communities 1996. Council directive 96/22/EC of 29 April 1996 concerning the prohibition on the use in stockfarming of certain substances having a hormonal or thyrostatic action and of β-agonists. Off. J. Eur. Communities L125:10-32.
- 45.Park, D. L., and T. C. Troxell. 2002. U.S. perspective on mycotoxin regulatory issues. Adv. Exp. Med. Biol. 504:277-285. [DOI] [PubMed] [Google Scholar]
- 46.Pfohl-Leszkowicz, A., L. Chekir-Ghedira, and H. Bacha. 1995. Genotoxicity of zearalenone, an estrogenic mycotoxin: DNA adduct formation in female mouse tissues. Carcinogenesis 16:2315-2320. [DOI] [PubMed] [Google Scholar]
- 47.Pierrat, B., D. M. Heery, P. Chambon, and R. Losson. 1994. A highly conserved region in the hormone binding domain of the human estrogen receptor functions as an efficient transactivation domain in yeast. Gene 143:193-200. [DOI] [PubMed] [Google Scholar]
- 48.Pierrat, B., D. M. Heery, Y. Lemoine, and R. Losson. 1992. Functional analysis of the human estrogen receptor using a phenotypic transactivation assay in yeast. Gene 119:237-245. [DOI] [PubMed] [Google Scholar]
- 49.Richardson, K. E., W. M. Hagler, and C. J. Mirocha. 1985. Production of zearalenone, α- and β-zearalenol, and α- and β-zearalanol by Fusarium spp. in rice culture. J. Agric. Food. Chem. 33:862-866. [Google Scholar]
- 50.Rocco, J. A. 1988. Hyperestrogenism in swine due to natural poisoning with zearalenone. Rev. Argent. Microbiol. 20:119-123. [PubMed] [Google Scholar]
- 51.Saenz de Rodriguez, C. A., A. M. Bongiovanni, and L. Conde de Borrego. 1985. An epidemic of precocious development in Puerto Rican children. J. Pediatr. 107:393-396. [DOI] [PubMed] [Google Scholar]
- 52.Saidi, F., A. Sepehr, S. Fahimi, M. J. Farahvash, P. Salehian, A. Esmailzadeh, M. Keshoofy, N. Pirmoazen, M. Yazdanbod, and M. K. Roshan. 2000. Oesophageal cancer among the Turkomans of northeast Iran. Br. J. Cancer 83:1249-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoental, R. 1983. Precocious sexual development in Puerto Rico and oestrogenic mycotoxins (zearalenone). Lancet i:573. [DOI] [PubMed]
- 54.Schoental, R. 1985. Trichothecenes, zearalenone and other carcinogenic metabolites of Fusarium and related microfungi. Adv. Cancer Res. 45:217-290. [DOI] [PubMed] [Google Scholar]
- 55.Schuhmacher, R., R. Krska, M. Grasserbauer, W. Edinger, and H. Lew. 1998. Immuno-affinity columns versus conventional clean-up: a method-comparison study for the determination of zearalenone in corn. Fresenius J. Anal. Chem. 360:241-245. [Google Scholar]
- 56.Scott, P. M. 1997. Multi-year monitoring of Canadian grains and grain-based foods for trichothecenes and zearalenone. Food Addit. Contam. 14:333-339. [DOI] [PubMed] [Google Scholar]
- 57.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 58.Shier, W. T., A. C. Shier, W. Xie, and C. J. Microcha. 2001. Structure-activity relationships for human estrogenic activity in zearalenone mycotoxins. Toxicon 39:1435-1438. [DOI] [PubMed] [Google Scholar]
- 59.Smith, D. R., A. P. Smyth, and D. T. Moir. 1990. Amplification of large artificial chromosomes. Proc. Natl. Acad. Sci. USA 87:8242-8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szuets, P., A. Mesterhazy, T. Bartok, and G. Y. Falkay. 1998. Premature thelarche/mastopathy and Fusarium toxin contamination of foods: key to a mysterious disease. Pediatr. Res. 43:86. [Google Scholar]
- 61.Szuets, P., A. Mesterhazy, G. Y. Falkay, and T. Bartok. 1997. Early thelarche symptoms in children and their relations to zearalenone contamination in foodstuffs. Cereal Res. Commun. 25:429-436. [Google Scholar]
- 62.Tomaszewski, J., R. Miturski, A. Semczuk, J. Kotarski, and J. Jakowicki. 1998. Tissue zearalenone concentration in normal, hyperplastic and neoplastic human endometrium. Ginekol. Pol. 69:363-366. [PubMed] [Google Scholar]
- 63.Yazdanpanah, H., M. J. Khoshnood-Mansourkhani, A. Shafaati, H. Rahimian, H. R. Rasekh, K. Gilani, and M. Moradkhani. 1997. Evaluation of natural occurrence of Fusarium toxins in wheat fields of northern Iran. Cereal. Res. Commun. 25:337-341. [Google Scholar]
- 64.Zöllner, P., J. Jodlbauer, M. Kleinova, H. Kahlbacher, T. Kuhn, W. Hochsteiner, and W. Lindner. 2002. Concentration levels of zearalenone and its metabolites in urine, muscle tissue, and liver samples of pigs fed with mycotoxin contaminated oats. J. Agric. Food Chem. 50:2494-2501. [DOI] [PubMed] [Google Scholar]