Abstract
The triphenyltin (TPT)-degrading bacterium Pseudomonas chlororaphis CNR15 produces extracellular yellow substances to degrade TPT. Three substances (F-I, F-IIa, and F-IIb) were purified, and their structural and catalytic properties were characterized. The primary structure of F-I was established using two-dimensional nuclear magnetic resonance techniques; the structure was identical to that of suc-pyoverdine from P. chlororaphis ATCC 9446, which is a peptide siderophore produced by fluorescent pseudomonads. Spectral and isoelectric-focusing analyses revealed that F-IIa and F-IIb were also pyoverdines, differing only in the acyl substituent attached to the chromophore part of F-I. Furthermore, we found that the fluorescent pseudomonads producing pyoverdines structurally different from F-I showed TPT degradation activity in the solid extracts of their culture supernatants. F-I and F-IIa degraded TPT to monophenyltin via diphenyltin (DPT) and degraded DPT and dibutyltin to monophenyltin and monobutyltin, respectively. The total amount of organotin metabolites produced by TPT degradation was nearly equivalent to that of the F-I added to the reaction mixture, whereas DPT degradation was not influenced by monophenyltin production. The TPT degradation activity of F-I was remarkably inhibited by the addition of metal ions chelated with pyoverdine. On the other hand, the activity of DPT was increased 13- and 8-fold by the addition of Cu2+ and Sn4+, respectively. These results suggest that metal-chelating ligands common to pyoverdines may play important roles in the Sn-C cleavage of organotin compounds in both the metal-free and metal-complexed states.
Organotin compounds, in particular tributyltin (TBT) and triphenyltin (TPT), have been extensively used as an active component in antifouling paints and agrochemicals over the last 40 years. These compounds have been introduced into aquatic systems via leaching from the antifouling paints and runoff from agricultural fields (9, 10, 18, 32), causing harmful effects on a variety of nontarget organisms, such as plankton (8, 20), gastropods (3, 16), and fish (11), even at low nanomolar aqueous concentrations. In recent years, the application of TBT and TPT in antifouling agents has been restricted in many countries, but these compounds have continued to be detected in the biota, water, and sediments because of their persistence. Thus, organotin contamination has been considered to be one of the most important ecotoxicological problems.
The slow disappearance of organotin from the environment is caused by various processes: photolysis by sunlight, chemical cleavage by strong acid or electrophilic agents, and biological degradation (12, 34). These processes involve a sequential removal of organic groups, which generally results in a reduction of toxicity. It remains unclear whether the biological degradation of organotin compounds is due to an enzymatic reaction, because no enzyme catalyzing the Sn-C cleavage reaction is known yet. The debutylation of TBT by microorganisms using polluted water, sediment samples, and pure cultures, when a sufficiently low concentration of substrate was used, has been reported extensively (7, 14, 19). Yonezawa et al. have also reported methylation and debutylation of TBT by sulfate-reducing and nitrate-reducing activities in sediment (37). On the other hand, TPT was scarcely degraded by bacteria capable of degrading TBT in estuarine water (13). Pseudomonas putida C has been found to degrade TPT under pure-culture conditions (33). Pseudomonas chlororaphis CNR15 was previously isolated from an enriched culture capable of degrading TPT (17). This strain degraded TPT to diphenyltin (DPT) concomitantly with the production of benzene; the reaction was catalyzed by a low-molecular-mass (∼1,000-Da) substance, which is expected to be one of the potent catalysts for the microbial degradation of organotins, excreted into the culture medium (17).
In the present study, we have purified and characterized three substances (F-I, F-IIa, and F-IIb) from P. chlororaphis CNR15 and have demonstrated that they are pyoverdines, a peptide siderophore produced by fluorescent pseudomonads that functions as a powerful Fe3+ chelator and an efficient Fe3+ transporter (24). Our results suggest that metal-chelating ligands common to pyoverdine are required in organotin degradation activity.
MATERIALS AND METHODS
Chemicals.
TPT chloride (98% pure), TBT chloride (95% pure), and dibutyltin dichloride (97% pure) were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). DPT dichloride (96% pure), monophenyltin trichloride (98% pure), and monobutyltin trichloride (95% pure) were from Aldrich. All other chemicals used were of analytical grade.
Bacterial strains and culture conditions.
The bacterial strains used, P. chlororaphis CNR15 (17), P. chlororaphis ATCC 9446, Pseudomonas fluorescens ATCC 13525, P. fluorescens NCIMB 10460 (also known as ATCC 17400), Pseudomonas aeruginosa ATCC 15692, P. aeruginosa NCIMB 12469 (also known as ATCC 27853), and P. aeruginosa NCIMB 5940, were grown on succinate-glycerol medium (17). The ATCC strains were obtained from the American Type Culture Collection (Manassas, Va.), and the NCIMB strains were from the National Collections of Industrial, Food and Marine Bacteria (Aberdeen, United Kingdom). All cultures were aerobically grown at 28°C under dark conditions.
Preparation of solid-phase extract of the culture supernatant.
A culture grown for 72 h was harvested, and its supernatant was filtered (17). Aliquots (50 ml) of the cell supernatant were applied to a Sep-Pack C18 Vac 500 mg/6 cc column and then extracted with 50% (vol/vol) methanol as described previously (17). The extract was concentrated to 5 ml and stored at −20°C until it was needed. The concentrations of pyoverdines in the extract were estimated spectrophotometrically using the molar extinction coefficient (6).
Purification of F-I, F-IIa, and F-IIb.
All the purification procedures described below were carried out at 4°C using an ÄKTA purifier high-performance liquid chromatography (HPLC) system (Amersham Biosciences). The eluate was simultaneously monitored at 214, 256, and 398 nm. The solid-phase extract from P. chlororaphis CNR15 was applied to a Resource S cation-exchange column (6 ml; Amersham Biosciences) to separate F-I and F-II as described previously (17). The F-I fractions were applied to a Resource reverse-phase column (RPC) (3 ml; Amersham Biosciences) equilibrated with 10 mM potassium phosphate buffer (pH 7.2)-methanol (9:1 [vol/vol]) and eluted with 10 mM potassium phosphate buffer (pH 7.2)-methanol (1:1 [vol/vol]) (buffer B) by a linear gradient using 20 to 80% (12 ml) buffer B. The F-II fractions were also applied to a Resource RPC under the same conditions as for F-I purification, and two active peaks, F-IIa and F-IIb, were eluted with ∼25 and 60% buffer B, respectively. F-IIa was further purified with the second Resource RPC under the same conditions. F-IIb was stored at −20°C and used as a partially purified sample in isoelectric-focusing (IEF) analysis. Purity was assessed at 214, 256, and 398 nm in the single peak eluted by Resource RPC chromatography. The concentration of the purified substance was estimated from the molar extinction coefficient as described above.
Determination of organotin and inorganic tin.
The concentrations of TPT, DPT, monophenyltin, dibutyltin, and monobutyltin were determined by postcolumn HPLC, as described previously (17), with the modification of a mobile phase and a postcolumn reagent. The mobile phase used was a mixture of tetrahydrofuran-water-methanol-acetic acid (4:5:1:1 [vol/vol/vol/vol]) containing 1 mM dithiothreitol (DTT). The postcolumn reagent used consisted of 70 mM sodium succinate buffer (pH 6.5), 0.0015% (wt/vol) fisetin, and 1.5% (vol/vol) Triton X-100. The calibration graphs established from the peak areas were linear over the range of 0.3 to 10 (TPT, DPT, and monophenyltin), 0.15 to 5 (dibutyltin), and 1 to 50 (monobutyltin) μM when 20 μl of each organotin was analyzed.
Inorganic tin was eluted in a void volume by the HPLC described above and fractionated before postcolumn reaction. The sample was desiccated and then dissolved in 5 ml of 0.1 N HCl. Sn in the sample was determined by hydride generation atomic absorption spectrometry coupled with flow injection (36). The wavelength and lamp current for Sn were 286.3 nm and 15 mA, respectively.
Organotin degradation assays.
For organotin degradation assays, the reaction was performed in 20 mM MOPS [3-(N-morpholino) propanesulfonic acid] buffer (pH 7.2). Normally, the reaction mixture (400 μl) containing F-I (or F-IIa) and 200 μM organotin was incubated at 40°C for 1.5 h in a microtube. The reaction was terminated as described previously (17), and then 20 to 50 μl of the sample was injected into a postcolumn HPLC system. The activity of organotin degradation was defined as the total amount of a decomposed organotin product formed by 1 μmol of pyoverdine for an incubation time.
The effect of metal ions on the activity was investigated using a metal chloride. The reaction mixture containing 100 μM metal chloride was preincubated for 30 min, and subsequently, 200 μM TPT or DPT was added to start the reaction.
IEF analysis and chrome azurol S (CAS) overlay assay of pyoverdines.
IEF was performed with a PhastSystem (Amersham Bioscience) according to the manufacturer's recommendations. The samples and a set of pI standards (pI calibration kit 3-10; Amersham Bioscience) were deposited on PhastGel IEF 3-9. The bands in the gel were visualized under UV light at 365 nm. The gel was subsequently stained with Coomassie brilliant blue-R350 to detect the pI standards.
CAS overlay assays were performed by the method of Koedam et al. (21). In the assay, IEF was performed with PhastGel DryIEF (Amersham Bioscience) containing 3% ampholine (pH 3.5 to 10; Amersham Bioscience).
Instrumental techniques.
Fast-atom bombardment (FAB) measurement was done using a JMX SX-102A mass spectrometer (JEOL, Tokyo, Japan) in a positive-ion mode with 3-nitrobenzylalcohol as a sample matrix.
1H and 13C nuclear magnetic resonance (NMR) spectra were obtained with a Varian Unity 500 NMR spectrometer. The measurements were carried out using 8.2 mM F-I (pH 3.4) in 8% (vol/vol) D2O at 25°C. Resonance assignments of specific protons and carbon atoms were based on their chemical shifts and integrals and on data from selective homonuclear decoupling experiments (nuclear Overhauser effect spectroscopy, correlation spectroscopy, and total-correlation spectroscopy), as well as data from heteronuclear experiments (heteronuclear single-quantum coherence and heteronuclear multiple-bond correlation).
RESULTS
Purification of F-I, F-IIa, and F-IIb.
At least three substances possessing TPT degradation activity, F-I, F-IIa, and F-IIb, were found during purification. F-I was separated from F-II by cation-exchange HPLC, as described previously (17), and further purified by reverse-phase HPLC. F-II was also applied to the reverse-phase HPLC column, and two active compounds eluted at ∼20% methanol (F-IIa) and ∼34% methanol (F-IIb) were obtained. The purification of F-IIa was accomplished by the second reverse-phase HPLC under the same conditions, whereas that of F-IIb was no longer included in this study, because the substance had a tendency to degrade during the purification process. The total yields of purified F-I and F-IIa were 27.3 and 9.2 mg, respectively, from 3.5 liters of culture medium.
Structural analysis of F-I.
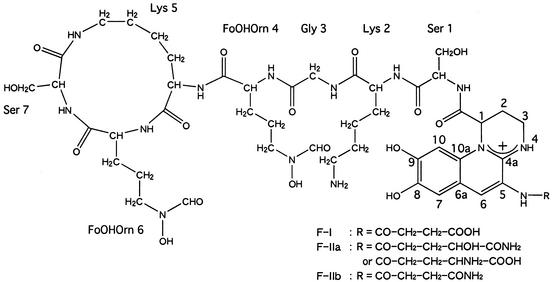
FAB-mass spectrometry (MS) of F-I gave a molecular ion (M+) at an m/z of 1,161. Total-amino-acid hydrolysis by 6 N HCl indicated that the peptide moiety of F-I consisted of Gly, Lys, and Ser (1:2.1:1.9), although the configuration of the amino acid residues was not determined in this study. Furthermore, 2 mol of Nδ-formyl-Nδ-hydroxyornithine (FoOHOrn), not quantified in the HCl hydrolysate (31), was confirmed by NMR analysis (data not shown). These data were in good agreement with those for suc-pyoverdine from P. chlororaphis ATCC 9446 (15) and P. fluorescens ATCC 13525 (22), which possess a succinate side chain bound to an amino group on C-5 of the chromophore, an 8,9-dihydroxyquinoline derivative (Fig. 1). The detailed assignment of the 1H and 13C chemical shifts of F-I was established using the results of a set of correlation spectroscopy, total-correlation spectroscopy, nuclear Overhauser effect spectroscopy, heteronuclear multiple-bond correlation, and heteronuclear single-quantum coherence experiments (data not shown). These results also did not contradict the reported data for the suc-pyoverdines of P. chlororaphis ATCC 9446, as well as the characteristic NMR data reported for some pyoverdines (1, 2, 6). We therefore concluded that F-I is identical with suc-pyoverdine (Fig. 1).
FIG. 1.
Structure of pyoverdines F-I, F-IIa, and F-IIb from P. chlororaphis CNR 15. The acyl chains (R) of F-IIa and F-IIb were deduced from FAB-MS and IEF profiles, respectively (see Discussion).
Spectral and IEF analyses.
The absorption spectra of F-I were pH sensitive and were in good agreement with that of pyoverdine from P. fluorescens, with maximal absorption at 398 (pH 7.2) and 380 (pH 5) nm, which is due to the chromophore in the pyoverdine (30, 35). In addition, pH-independent spectral changes found in the pyoverdine-iron complex were also observed in an F-I-FeCl3 mixture, with maximal absorption at 403 nm and a pronounced shoulder at 460 nm. F-IIa was found to have spectral characteristics identical to those of F-I.
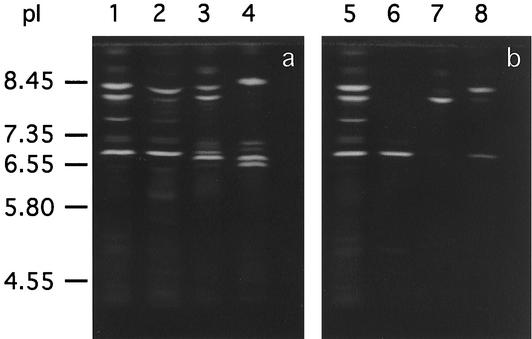
IEF analysis of pyoverdines results in the separation of the different molecular forms of pyoverdines. The profile obtained is useful not only for discriminating between strains based on the pyoverdine species but also for characterizing unknown pyoverdines produced by given fluorescent pseudomonads (24, 25, 26, 27). In IEF analysis of solid-phase extracts of culture supernatants, pyoverdines were detected on the gel as multiple fluorescent bands (Fig. 2a). The result showed that the pyoverdines of strain CNR15 provided IEF profiles identical to those of P. chlororaphis ATCC 9446 and P. fluorescens ATCC 13525, with three well-separated bands characterized by pI values of 8.3, 8.05, and 6.72, except for a band of pI 6.65 for strain ATCC 13525. Furthermore, we found that the IEF bands of F-I, F-IIa, and F-IIb were consistent with those of major pyoverdines in the solid-phase extract of strain CNR15 (Fig. 2b). Thus, F-IIa and F-IIb are also pyoverdines of strain CNR15. All IEF bands were confirmed to show siderophore activity with CAS overlay (data not shown).
FIG. 2.
IEF profiles of pyoverdines (a) and purified F-I, F-IIa, and F-IIb (b). The sample containing pyoverdines was prepared by solid-phase extraction of the culture supernatant. The bands were visualized under UV light at 365 nm. Lanes 1 and 5, P. chlororaphis CNR 15; lane 2, P. chlororaphis ATCC 9446; lane 3, P. fluorescens ATCC 13525; lane 4, P. aeruginosa ATCC 15692; lane 6, F-I; lane 7, F-IIa; lane 8, F-IIb.
TPT degradation by various types of pyoverdines.
A total of close to 40 different peptide structures for pyoverdine have been established so far (24). F-I can be structurally classified as a member of a group of pyoverdines characterized by a C-terminal cyclic part consisting of three or four amino acids. The culture supernatant of P. aeruginosa ATCC 15692 containing a pyoverdine that belongs to this group also showed TPT degradation activity as described in a previous study (17). To investigate whether this reaction is catalyzed by only a particular form of pyoverdine, the TPT degradation activity was examined in the solid-phase extracts containing two types of pyoverdines which are structurally different from F-I: one belongs to a group possessing cyclo-Nδ-hydroxyornithine (cOHOrn) as a C-terminal amino acid (P. fluorescens NCIMB 10460 and P. aeruginosa NCIMB 12469), and the other belongs to a group composed of a linear structure containing no FoOHOrn (P. aeruginosa NCIMB 5940) (Table 1). The presence of pyoverdines in solid-phase extracts was confirmed by the IEF profile with UV detection and spectral analysis. In all the extracts, TPT was significantly degraded, probably due to the pyoverdine contained in the sample (Table 1). The result suggests that TPT degradation by pyoverdine occurs without requiring a particular peptide structure.
TABLE 1.
TPT degradation by solid-phase extract from fluorescent pseudomonads
| Fluorescent pseudomonad | TPT degradation activitya (μmol of product/μmol of pyoverdines) | Peptide sequence of pyoverdineb | Reference |
|---|---|---|---|
| P. chlororaphis CNR 15 | 0.37 | Chr-S-K-G-FoOHOrn-c(K-FoOHOrn-S)c | This study |
| P. chlororaphis ATCC 9446 | 0.23 | Chr-S-K-G-FoOHOrn-c(K-FoOHOrn-S) | 15 |
| P. fluorescens ATCC 13525 | 0.55 | Chr-S-K-G-FoOHOrn-c(K-FoOHOrn-S) | 22 |
| P. aeruginosa ATCC 15692 | 0.20 | Chr-S-R-S-FoOHOrn-c(K-FoOHOrn-T-T) | 6 |
| P. aeruginosa NCIMB 5940 | 0.28 | Chr-S-Dab-FoOHOrn-Q-O-FoOHOrn-G | 26 |
| P. aeruginosa NCIMB 12469 | 0.43 | Chr-S-FoOHOrn-Orn-G-aT-S-cOHOrn | 26 |
| P. fluorescens NCIMB 10460 | 0.10 | Chr-A-K-G-G-OHAsp-O/Dab-S-A-cOHOrn | 5 |
The reaction mixture (400 μl) containing the solid-phase extract and 200 μM TPT was incubated for 12 h, and the total amount of DPT and monophenyltin produced was determined as the product. The concentration of pyoverdines in the solid-phase extract was estimated spectrophotometrically as described in Materials and Methods.
Italicized amino acid residues correspond to a d-configuration. Chr, common chromophore part of pyoverdine; aT, allothreonine; Q/Dab, a condensation product of 2,4-diaminobutyric acid and glutamine; c(amino acids), cyclic structure for the amino acids in parentheses.
The configurations of the amino acid residues were not determined in this study.
Catalytic properties of F-I and F-IIa.
To investigate the degradation activity of pyoverdine for phenyl and butyltin compounds, we developed a modified postcolumn HPLC analysis to determine organotin metabolites, especially mono-organotin compounds. Effective chromatographic separation of mono- and diorganotins was achieved by the addition of thiol compounds to the mobile phase, such as 1 mM DTT, 1 mM dithioerythritol, and 10 mM 2-mercaptoethanol. The mobile phase containing 1 mM DTT was used in this study, and the postcolumn HPLC conditions were optimized as described in Materials and Methods. The retention times of TPT, DPT, monophenyltin, dibutyltin, and monobutyltin were 11.4, 7.6, 4.6, 8.0, and 4.7 min, respectively.
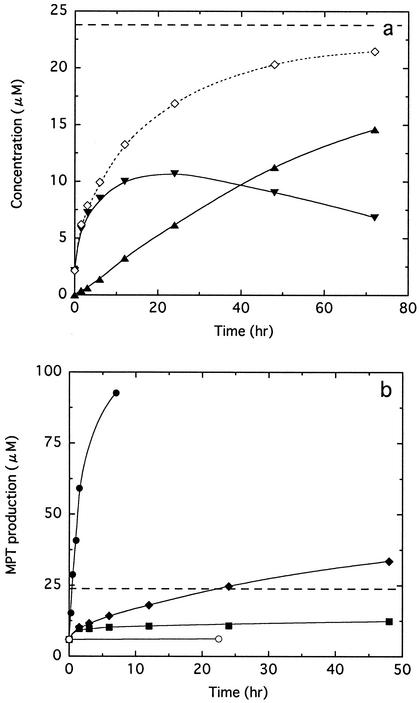
The substrate specificities for the organotin degradation activities of pyoverdines F-I and F-IIa are summarized in Table 2. We found that DPT and dibutyltin were degraded to the corresponding mono-organotin compounds, monophenyltin and monobutyltin, respectively. In addition, the production of butane that occurs in dibutyltin degradation was confirmed by gas chromatography (data not shown). Inorganic tin was not detected in all degradation reactions. A long-term incubation of the TPT degradation reaction mixture resulted in further degradation of the DPT produced and simultaneous accumulation of monophenyltin (Fig. 3a). The total amount of the products (DPT and monophenyltin) obtained after a 72-h incubation was nearly consistent with that of the F-I added to the reaction mixture. In contrast, when the reaction was performed using DPT as a substrate, the production of monophenyltin exceeded the initial concentration of F-I added (Fig. 3b).
TABLE 2.
Substrate specificities of pyoverdines F-I and F-IIa
| Substrate | Organotin degradation activity (μmol of product/μmol of pyoverdine)a
|
|
|---|---|---|
| F-I | F-IIa | |
| TPT | 0.16 | 0.18 |
| DPT | 0.17 | 0.22 |
| TBT | NDb | ND |
| Dibutyltin | 0.014 | 0.033 |
The reaction mixture (400 μl) containing 23.8 μM F-I or 28.8 μM F-IIa and 200 μM substrate was incubated for 1.5 h. DPT, monophenyltin, and monobutyltin produced by TPT, DPT, and dibutyltin degradation, respectively, were determined as the product.
ND, not detected
FIG. 3.
Time course of F-I activity for TPT (a) and DPT (b). These reactions were performed at 40°C in a mixture containing 23.8 μM F-I and 200 μM TPT or DPT. The concentration of F-I used is drawn as an additional dashed line. (a) Concentrations of DPT (▾) and monophenyltin (▴) produced were determined using postcolumn HPLC. The total amounts (⋄) of the products during the reaction were calculated from the amounts of DPT and monophenyltin produced. (b) Concentrations of monophenyltin produced in reaction mixtures containing 100 μM CuCl2 (•), FeCl3 (▪), and no metal ion (♦) were determined. The control (○) contained CuCl2 and DPT in the reaction mixture, except for F-I.
Effects of metal ions on TPT and DPT degradation.
Pyoverdine is known to chelate various metal ions, such as Cu2+ and Al3+, as well as Fe3+. To test the involvement of the metal-chelating site of pyoverdine in organotin degradation, the effects of metal ions on tri- and diorganotin degradation activities were investigated. When TPT degradation by F-I was carried out in a reaction mixture supplemented with 100 μM metal ion, the activity was markedly inhibited by all metal ions used except for Mg2+ and Ca2+ (Table 3). On the other hand, these metal ions had no effect on DPT degradation activity during a short-term incubation (1.5 h). Furthermore, addition of CuCl2 and SnCl4 caused 13- and 8-fold increases in DPT degradation activity, respectively (Table 3 and Fig. 3b). The concentration of these metals required for maximal activation was greater than or equal to that of F-I in the reaction mixture. In a long-term incubation of the reaction mixture containing FeCl2, FeCl3, and AlCl3, however, the final amount of monophenyltin produced by DPT degradation was approximately half of the F-I concentration added to these reaction mixtures (Table 3 and Fig. 3b).
TABLE 3.
Effects of various metal ions on F-I activity
| Metal ion | Degdradation activitya (μmol of product/μmol of F-I)
|
|
|---|---|---|
| TPTb | DPTc | |
| None | 0.17 | 0.79 |
| 0.17b | ||
| Sn4+ | NDd | 1.26b |
| Al3+ | ND | 0.32 |
| Fe3+ | ND | 0.31 |
| Fe2+ | ND | 0.33 |
| Cu2+ | ND | 2.24b |
| Co2+ | 0.014 | 0.73 |
| Mn2+ | 0.0084 | 0.72 |
| Zn2+ | 0.0063 | 0.87 |
| Ca2+ | 0.17 | 0.77 |
| Mg2+ | 0.16 | 0.80 |
The reaction mixture (400 μl) containing 23.8 μM F-I and 200 μM substrate was incubated with 100 μM metal chloride for 1.5 or 24 h. DPT and monophenyltin were determined as the product in TPT and DPT degradation, respectively.
The reaction mixture was incubated for 1.5 h.
The reaction mixture was incubated for 24 h.
ND, not detected.
DISCUSSION
In this study, we revealed that purified F-I, F-IIa, and F-IIb correspond to major pyoverdines from P. chlororaphis CNR15. For a given fluorescent-pseudomonad strain, it is well known that several forms of pyoverdine (isoforms), differing only in the acyl substituent bound to the chromophore, are produced (6, 22). P. fluorescens ATCC 13525, possessing a pyoverdine IEF profile similar to that of strain CNR15, produces five isoforms of pyoverdines, as well as suc-pyoverdine identical to F-I (22). Thus, the structures of F-IIa and F-IIb are also suggested to be isoforms in which the succinic acid chain of F-I has been altered. Furthermore, FAB-MS of F-IIa gave a molecular ion (M+) at an m/z of 1,190, suggesting that F-IIa corresponds to pyoverdine 1d from P. fluorescens ATCC 13525 (22) or a glutamate isoform (24) (Fig. 1). Because F-I and F-IIa showed similar substrate specificities for organotin degradation activities, the acyl chain difference appears to have no effect on degradation (Table 2). F-IIb (pI 8.3) was an unstable compound, and its artifact during the purification procedure showed the same pI value (6.72) as F-I by IEF analysis (Fig. 2b). This result suggests that F-IIb is a succinamide isoform (Fig. 1), which is known to produce the succinic acid form by hydrolysis of the amide group during culture (6).
The Sn-C cleavage of TPT by F-I may be interpreted as a kind of metal complexation reaction which is derived from the chelating capability of pyoverdine for various metal ions. The absorption spectrum of the F-I-TPT complex showed a little quenching of the 400-nm peak, whereas that of the F-I-DPT complex had a maximum at 406 nm and shoulder at 265 nm (data not shown). This result suggests that at least the catechol-like group in the chromophore interacts with organotin. In addition, the inhibition of TPT degradation activity by metal ions also supports the idea that the metal-chelating site of F-I may play an important role in the activity (Table 3). F-I seems to degrade TPT to monophenyltin without releasing an intermediate, DPT (Fig. 3a). The DPT and monophenyltin produced were also water soluble and showed no adsorption on the tube wall. These results suggest that F-I forms a stable complex with organotin metabolites, although it remains unknown whether F-I is irreversibly inactivated during TPT complexation. Reactivation and decomplexation studies of the inactivated F-I (or F-I complex) are in progress. On the other hand, benzene was detected as another product in TPT degradation (17), suggesting that the Sn-C bond is directly broken with some residues of F-I. Pyoverdines possess the common hydroxamate groups involved in ferric complexation, such as FoOHOrn, cOHOrn, and β-threo-hydroxyaspartic acid, as well as the catechol-like group of the chromophore. These ligands may participate in the Sn-C cleavage of TPT by a ligand displacement reaction (Table 1).
DPT degradation activity should be considered a catalytic reaction followed by metal complexation (Fig. 3b). In addition, metal ions that inhibited TPT degradation activity, Zn2+, Co2+, and Mn2+, had no apparent effect on DPT degradation (Table 3). It is not clear whether the F-I-monophenyltin or F-I-metal complex formed is easily replaced with DPT or coexists with DPT as a binuclear complex during the reaction. Interestingly, the Cu electron paramagnetic resonance spectrum of Cu-F-I-DPT was different from the spectrum of Cu-F-I (unpublished data). This result has suggested that Cu2+ chelated by F-I possesses an alternative coordination sphere by the addition of DPT, although the coordinate structure and the interaction of Cu2+ with DPT are unknown. Thus, pyoverdine is involved in DPT degradation in both the metal-free and metal-complexed states. It has been reported that the Sn-phenyl cleavages of TPT and DPT occur with the coordination of chelating agents, such as acetylacetone and 8-hydroxy quinoline, at 100 to 200°C (23, 28). In a comparison of these reactions with F-I activity, pyoverdine is expected to have a catalytic advantage over a general chelating agent as a kind of metal-complexation catalyst detected at room temperature under neutral conditions. The new function of pyoverdine that was found may be available for a model of an artificial enzyme to degrade organometallic compounds.
The species producing the pyoverdines belong to Pseudomonas RNA homology group I and include the species P. aeruginosa, P. fluorescens, P. chlororaphis, P. putida, Pseudomonas tolaasii, and Pseudomonas syringae (29). Furthermore, recent results based on polyphasic taxonomy have added several newly described species, among them Pseudomonas jessenii, Pseudomonas mandelii, Pseudomonas monteilii, Pseudomonas rhodesiae, and Pseudomonas veronii, to the list of pyoverdine-producing species (24). These fluorescent pseudomonads are widely distributed in the environment and are predicted to be potential organotin-degrading bacteria, because TPT degradation seems to be independent of the peptide structure of pyoverdine (Table 1). It should be noted that pyoverdines are generally produced in response to iron starvation. This suggests that organotin degradation by pyoverdine may be considered a kind of cometabolism, although it remains unknown whether the degradation system directly contributes to cell growth.
Azotobactin from Azotobacter vinelandii, a peptide siderophore similar to pyoverdine but with a different type of chromophore (4), may also possess the same catalytic function as pyoverdine. Pyoverdines from strain CNR15 did not show degradation activity for TBT (Table 2), whereas we have confirmed both TBT and TPT degradation activities in a culture supernatant of newly isolated bacteria in which no pyoverdine was detected by IEF analysis (unpublished data). Therefore, the degradation reaction of organotin compounds is likely to occur by chelation of certain types of siderophores. In the debutylation of TBT and dibutyltin by several strains of microorganisms, the monobutyltin formed has been observed in solution rather than in the biomass (7). These reactions also appear to proceed by a mechanism similar to that in our results.
Acknowledgments
We thank the MS laboratory of the Faculty of Agriculture, Okayama University, for performing the FAB-MS analysis.
REFERENCES
- 1.Amann, C., K. Taraz, H. Budzikiewicz, and J. M. Meyer. 2000. The siderophores of Pseudomonas fluorescens 18.1 and the importance of cyclopeptidic substructures for the recognition at the cell surface. Z. Naturforsch. 55C:671-680. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson, R. A., A. L. M. Salah El Din, B. Kieffer, J. F. Lefèvre, and M. A. Abdallah. 1998. Bacterial iron transport: 1H NMR determination of the three-dimensional structure of the gallium complex of pyoverdin G4R, the peptidic siderophore of Pseudomonas putida G4R. Biochemistry 37:15965-15973. [DOI] [PubMed] [Google Scholar]
- 3.Bryan, G. W., P. E. Gibbs, and G. R. Burt. 1988. Comparison of the effectiveness of tri-n-butyltin chloride and five other organotin compounds in promoting the development of imposex in the dog-whelk Nucella lapillus. J. Mar. Biolog. Assoc. U. K. 68:733-744. [Google Scholar]
- 4.Demange, P., A. Bateman, A. Dell, and M. A. Abdallah. 1988. Structure of azotobactin D, a siderophore of Azotobacter vinelandii strain D (CCM 289). Biochemistry 27:2745-2752. [Google Scholar]
- 5.Demange, P., A. Bateman, J. K. Macleod, A. Dell, and M. A. Abdallah. 1990. Bacterial siderophores: unusual 3,4,5,6-tetrahydropyrimidine-based amino acids in pyoverdins from Pseudomonas fluorescens. Tetrahedron Lett. 31:7611-7614. [Google Scholar]
- 6.Demange, P., S. Wendenbaum, C. Linget, C. Mertz, M. T. Cung, A. Dell, and M. A. Abdallah. 1990. Bacterial siderophores: structure and NMR assignment of pyoverdins Pa, siderophores of Pseudomonas aeruginosa ATCC 15692. Biol. Metals 3:155-170. [Google Scholar]
- 7.Errécalde, O., M. Astruc, G. Maury, and R. Pinel. 1995. Biotransformation of butyltin compounds using pure strains of microorganisms. Appl. Organomet. Chem. 9:23-28. [Google Scholar]
- 8.Fargasova, A., and J. Kizlink. 1996. Effect of organotin compounds on the growth of the freshwater alga Scenedesmus quadricauda. Ecotoxicol. Environ. Saf. 34:156-159. [DOI] [PubMed] [Google Scholar]
- 9.Fent, K. 1996. Organotin compounds in municipal wastewater and sewage sludge: contamination, fate in treatment process and ecotoxicological consequences. Sci. Total Environ. 185:151-159. [Google Scholar]
- 10.Fent, K., and J. Hunn. 1991. Phenyltins in water, sediment, and biota of freshwater marinas. Environ. Sci. Technol. 25:956-963. [Google Scholar]
- 11.Fent, K., and W. Meier. 1994. Effects of triphenyltin on fish early life stages. Arch. Environ. Contam. Toxicol. 27:224-231. [DOI] [PubMed] [Google Scholar]
- 12.Gadd, G. M. 2000. Microbial interactions with tributyltin compounds: detoxification, accumulation, and environmental fate. Sci. Total Environ. 258:119-127. [DOI] [PubMed] [Google Scholar]
- 13.Harino, H., M. Fukushima, Y. Kurokawa, and S. Kawai. 1997. Susceptibility of bacterial populations to organotin compounds and microbial degradation of organotin compounds in environmental water. Environ. Pollut. 98:157-162. [Google Scholar]
- 14.Harino, H., M. Fukushima, Y. Kurokawa, and S. Kawai. 1997. Degradation of the tributyltin compounds by the microorganisms in water and sediment collected from the harbour area of Osaka City, Japan. Environ. Pollut. 98:163-167. [Google Scholar]
- 15.Hohlneicher, U., R. Hartmann, K. Taraz, and H. Budzikiewicz. 1995. Pyoverdin, ferribactin, azotobactin: a new triad of siderophores from Pseudomonas chlororaphis ATCC 9446 and its relation to Pseudomonas fluorescens ATCC 13525. Z. Naturforsch. 50C:337-344.
- 16.Horiguchi, T., H. Shiraishi, M. Shimizu, and M. Morita. 1997. Effects of triphenyltin chloride and five other organotin compounds on the development of imposex in the rock shell Thais clavigera. Environ. Pollut. 95:85-91. [DOI] [PubMed] [Google Scholar]
- 17.Inoue, H., O. Takimura, H. Fuse, K. Murakami, K. Kamimura, and Y. Yamaoka. 2000. Degradation of triphenyltin by a fluorescent pseudomonad. Appl. Environ. Microbiol. 66:3492-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kannan, K., and R. F. Lee. 1996. Triphenyltin and its degradation products in foliage and soils from sprayed pecan orchards and in fish from adjacent ponds. Environ. Toxicol. Chem. 15:1492-1499. [Google Scholar]
- 19.Kawai, S., Y. Kurokawa, H. Harino, and M. Fukushima. 1998. Degradation of tributyltin by a bacterial strain isolated from polluted river water. Environ. Pollut. 102:259-263. [Google Scholar]
- 20.Kline, E. R., A. W. Jarvinen, and M. L. Knuth. 1989. Acute toxicity of triphenyltin hydroxide to three cladoceran species. Environ. Pollut. 56:11-17. [DOI] [PubMed] [Google Scholar]
- 21.Koedam, N., E. Wittouck, A. Gaballa, A. Gillis, M. Hofte, and P. Cornelis. 1994. Detection and differentiation of microbial siderophores by isoelectric focusing and chrome azurol S overlay. Biometals 7:287-291. [DOI] [PubMed] [Google Scholar]
- 22.Linget, C., P. Azadi, J. K. MacLeod, A. Dell, and M. A. Abdallah. 1992. Bacterial siderophores: the structures of the pyoverdins of Pseudomonas fluorescens ATCC 13525. Tetrahedron Lett. 33:1737-1740. [Google Scholar]
- 23.Martin, D. F., and R. D. Walton. 1966. Kinetics of phenyl-tin cleavage by a chelating agent. J. Organomet. Chem. 5:57-62. [Google Scholar]
- 24.Meyer, J. M. 2000. Pyoverdines: pigments, siderophores and potential taxonomic markers of fluorescent Pseudomonas species. Arch. Microbiol. 174:135-142. [DOI] [PubMed] [Google Scholar]
- 25.Meyer, J. M., A. Stintzi, V. Coulanges, S. Shivaji, J. A. Voss, K. Taraz, and H. Budzikiewicz. 1998. Siderotyping of fluorescent pseudomonads: characterization of pyoverdines of Pseudomonas fluorescens and Pseudomonas putida strains from Antarctica. Microbiology 144:3119-3126. [DOI] [PubMed] [Google Scholar]
- 26.Meyer, J. M., A. Stintzi, D. De Vos, P. Cornelis, R. Tappe, K. Taraz, and H. Budzikiewicz. 1997. Use of siderophores to type pseudomonads: the three Pseudomonas aeruginosa pyoverdine systems. Microbiology 143:35-43. [DOI] [PubMed] [Google Scholar]
- 27.Munsch, P., V. A. Geoffroy, T. Alatossava, and J. M. Meyer. 2000. Application of siderotyping for characterization of Pseudomonas tolaasii and “Pseudomonas reactans” isolates associated with brown blotch disease of cultivated mushrooms. Appl. Environ. Microbiol. 66:4834-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson, W. H., and D. F. Martin. 1965. Phenyl-tin cleavage by chelating agents. J. Organomet. Chem. 4:67-73. [Google Scholar]
- 29.Palleromi, N. J. 1984. Gram-negative aerobic rods and cocci, p. 140-219. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. The Williams & Wilkins Co., Baltimore, Md.
- 30.Philson, S. B., and M. Llinás. 1982. Siderochromes from Pseudomonas fluorescens. I. Isolation and characterization. J. Biol. Chem. 257:8081-8085. [PubMed] [Google Scholar]
- 31.Salah El Din, A. L. M., P. Kyslík, D. Stephan, and M. A. Abdallah. 1997. Bacterial iron transport: structure elucidation by FAB-MS and by 2D NMR (1H, 13C, 15N) of pyoverdin G4R, a peptidic siderophore produced by a nitrogen-fixing strain of Pseudomonas putida. Tetrahedron 53:12539-12552. [Google Scholar]
- 32.Stäb, J. A., W. P. Cofino, B. van Hattum, and U. A. T. Brinkman. 1994. Assessment of transport routes of triphenyltin used in potato culture in the Netherlands. Anal. Chim. Acta 286:335-341. [Google Scholar]
- 33.Visoottiviseth, P., K. Kruawan, A. Bhumiratana, and P. Wilairat. 1995. Isolation of bacterial culture capable of degrading triphenyltin pesticides. Appl. Organomet. Chem. 9:1-9. [Google Scholar]
- 34.White, J. S., J. M. Tobin, and J. J. Cooney. 1999. Organotin compounds and their interactions with microorganisms. Can. J. Microbiol. 45:541-554. [PubMed] [Google Scholar]
- 35.Xiao, R., and W. S. Kisaalita. 1998. Fluorescent pseudomonad pyoverdines bind and oxidize ferrous ion. Appl. Environ. Microbiol. 64:1472-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto, M., M. Yasuda, and Y. Yamamoto. 1985. Hydride-generation atomic absorption spectrometry coupled with flow injection analysis. Anal. Chem. 57:1382-1385. [Google Scholar]
- 37.Yonezawa, Y., M. Fukui, T. Yoshida, A. Ochi, T. Tanaka, Y. Noguti, T. Kowata, Y. Sato, S. Masunaga, and Y. Urushigawa. 1994. Degradation of tri-n-butyltin in Ise Bay sediment. Chemosphere 29:1349-1356. [DOI] [PubMed] [Google Scholar]