Abstract
The working hypotheses tested on a natural population of Microcystis sp. in Lake Wannsee (Berlin, Germany) were that (i) the varying abundance of microcystin-producing genotypes versus non-microcystin-producing genotypes is a key factor for microcystin net production and (ii) the occurrence of a gene for microcystin net production is related to colony morphology, particularly colony size. To test these hypotheses, samples were fractionated by colony size with a sieving procedure during the summer of 2000. Each colony size class was analyzed for cell numbers, the proportion of microcystin-producing genotypes, and microcystin concentrations. The smallest size class of Microcystis colonies (<50 μm) showed the lowest proportion of microcystin-producing genotypes, the highest proportion of non-microcystin-producing cells, and the lowest microcystin cell quotas (sum of microcystins RR, YR, LR, and WR). In contrast, the larger size classes of Microcystis colonies (>100 μm) showed the highest proportion of microcystin-producing genotypes, the lowest proportion of non-microcystin-producing cells, and the highest microcystin cell quotas. The microcystin net production rate was nearly one to one positively related to the population growth rate for the larger colony size classes (>100 μm); however, no relationship could be found for the smaller size classes. It was concluded that the variations found in microcystin net production between colony size classes are chiefly due to differences in genotype composition and that the microcystin net production in the lake is mainly influenced by the abundance of the larger (>100-μm) microcystin-producing colonies.
The freshwater cyanobacterium Microcystis frequently forms mass developments and surface scums in eutrophic lakes; the majority of these formations contain toxins—the hepatotoxic microcystins. The hazard posed to vertebrates, including humans, and potentially to other eucaryotic animals by these toxins necessitates assessments of their human health and environmental risk potential. Such assessments require not only rapid and reliable methods for analysis of ambient toxin concentrations but also, in particular, tools for understanding factors leading to hazardous levels of toxicity in natural populations and—ultimately—for predicting the development of toxin concentrations in water. An essential basis for these goals is a comprehensive understanding of the regulation of microcystin net production in nature.
Microcystins are members of a peptide family which have the common structure cyclo-(d-Ala-l-X-d-MeAsp-l-Z-Adda-d-Glu-Mdha), where X and Z are variable l amino acids, Addais 3-amino-9-methoxy-2,6,8-trimethyl-10-phenyl-4,6-decadi-enoic acid, d-MeAsp is 3-methyl-aspartic acid, and Mdha is N-methyldehydroalanine (3). More than 70 structural variants of microcystins are known to date. Microcystins are synthesized by thiotemplate mechanisms like those for other nonribosomal peptides (e.g., antibiotics such as gramicidin and tyrocidin) produced by bacteria and fungi (25). The large gene cluster encoding peptide synthetases and polyketide synthases for microcystin biosynthesis has a modular structure, each module activating and incorporating specific constituents of the heptapeptide (39). The mcyA, mcyB, and mcyC genes are responsible for the activation and incorporation of Mdha, d-Ala, l-X, d-MeAsp, and l-Z during microcystin biosynthesis (6, 27, 39).
With the elucidation of microcystin biosynthesis, molecular probes have been developed to detect and study the occurrence of microcystin-producing genotypes in a natural population of Microcystis sp. in Lake Wannsee (Berlin, Germany) (22). Individual colonies were selected under a microscope for identification according to the species key devised by Komárek and Anagnostidis (19) on the basis of morphological criteria and tested for the presence or absence of the mcyB gene. This approach revealed pronounced differences in the proportion of microcystin-producing genotypes between species (hereafter referred to as morphospecies): most colonies (73%) of Microcystis aeruginosa contained the mcyB gene, whereas only 16% of the colonies assigned to M. ichthyoblabe and no colonies of M. wesenbergii showed the mcyB gene. This distribution of microcystin synthesis across morphospecies has also been found by an analysis of isolates (30, 42). In addition, Kurmayer et al. (22) found a relationship to colony size: 42 to 73% of the large colonies (>500 μm) belonged to the microcystin-producing genotype, compared to only 10 to 15% of the small colonies (<500 μm). Such a difference could be explained by a difference in how breakable colonies of different morphospecies of Microcystis are (7, 32, 42). It is known that the morphospecies M. aeruginosa generally grows in firm and large colonies (32). In contrast, colonies of M. flos-aquae and M. ichthyoblabe disintegrate much more readily, e.g., by wave action, resulting in the formation of small colonies (32, 42), and therefore might be sampled as colonies smaller than those of M. aeruginosa. However, the approach followed by Kurmayer et al. (22) cannot be directly applied for a genetic analysis of the total population in the field, because it is limited to colonies large enough for individual selection and morphospecies determination and also because it is too time intensive for application to a large number of field samples.
The aims of the present study therefore were (i) to develop a quantitative approach suitable for characterizing genotype composition in entire field populations, (ii) to evaluate whether the relationship observed between colony size and occurrence of the mcyB gene for selected large colonies also holds true for the entire field population over the entire range of colony sizes, (iii) to apply this approach for assessing genotype and colony size shifts over time during the course of Microcystis population development, and (iv) to quantify the effect of the proportion of microcystin-producing genotypes on microcystin net production. These questions were addressed by fractionation of the natural Microcystis population according to colony size. Each colony size class was analyzed for cell numbers, the proportion of microcystin-producing genotypes, and microcystin concentrations. To quantify the proportion of microcystin-producing genotypes, the DNA of each size class was subjected to a series of dilutions, and each dilution step was analyzed by PCR for the presence of a control gene and the mcyB gene. It was hypothesized that if a specific size class is composed solely of microcystin-producing genotypes, then it should not be possible to dilute out the mcyB gene at a DNA concentration higher than that for the control gene. In contrast, if the proportion of microcystin-producing genotypes is low, then the mcyB gene should be diluted out of some dilution steps before the control gene.
MATERIALS AND METHODS
Sampling.
Sampling was performed in Lake Wannsee from June to September 2000. The lake is shallow (mean depth, 5.5 m; maximum depth, 8.5 m; area, 2.82 km2), polymictic, hypertrophic, and regularly dominated by Microcystis during summer. Sampling was performed weekly and included inorganic nutrients, photosynthetically active radiation, temperature, and total phytoplankton. Water samples were taken at the deepest part of the lake and integrated by collecting 2 liters every meter from the surface to the sediment. On each date, the integrated water samples were transferred from the field to the laboratory; within no more than 2 h, the water was poured gently through sieves. The sieving procedure involved the filtering of 15 to 20 liters of lake water through sieves with mesh sizes of 100 and 50 μm. The sieving process was completed within minutes. Colonies larger than 340 μm were analyzed separately. Each colony size class was resuspended in 100 ml of GF/C (Whatman, Kent, Great Britain)-filtered lake water, and aliquots of 25 ml were analyzed for Microcystis cell numbers and filtered with GF/C filters for analysis of the proportion of microcystin-producing genotypes (PCR) and for concentration of microcystins (high-pressure liquid chromatography with diode array detection [HPLC-DAD]).
Abiotic parameters.
Chemical analyses of soluble reactive phosphorus were carried out according to ISO 6878 (13). Ammonium and nitrate were analyzed in a commercial laboratory (Labor Jungfernheide, Berlin, Germany) according to DIN 38406-5 (for ammonium) (5) and ISO 10304-1 (for nitrate) (12). Depth profiles for underwater light were determined by using a spherical Licor sensor (LI-193A; LI-COR Inc., Lincoln, Nebr.). The attenuation coefficient was calculated according to the Lambert-Beers law. Data for global irradiance were measured by the Meteorological Institute (Free University, Berlin, Germany) at a station in Dahlem, Germany, 10 km from Lake Wannsee. The average light dose for a mixed water column was calculated from the global irradiance for the mixed layer as described by Riley (34) with a correction factor of 0.45 for the reflection of the water surface and the share of photosynthetically active radiation of the global irradiance as described by Behrendt and Nixdorf (1).
Determination of cell numbers and morphospecies.
Samples were fixed with Lugol's solution and enumerated with an inverted microscope by the methods of Utermöhl (40). Colonies of Microcystis were disintegrated by ultrasonication prior to counting (40 impulses per s over 4 min for a 10-ml sample). Pilot experiments with laboratory strains and microscopic counting of cells revealed no lysis of cells with sonication for up to 4 min. At least 400 specimens of Microcystis colonies were counted at a magnification of ×400, and the results from at least two transects per sedimentation chamber were averaged. To estimate algal biovolume, 20 randomly selected specimens from dominant phytoplankton species were measured on every sampling date, and the cell volume from the appropriate geometric shape was calculated. The biovolume was then calculated by multiplying the mean cell volume by the cell number (43). Individual colonies were selected from live plankton net samples (30-μm mesh size) under a microscope for identification according to the species key devised by Komárek and Anagnostidis (19) on the basis of morphological criteria and were counted on three sampling dates in July 2000. Recently, the unification of all species of Microcystis Kützing ex Lemmerman 1907 (including M. aeruginosa, M. ichthyoblabe, M. novackekii, M. viridis, and M. wesenbergii) under the Rules of the Bacteriological Code was proposed (31). Consequently, in this study, all morphospecies were considered morphological variations of individuals of one population.
DNA extraction.
Aliquots of each colony size class were filtered with GF/C filters, and extraction from frozen filters was done by using the protocol of Franche and Damerval (10). Briefly, filters were cut into pieces and subjected to osmotic shock treatment (0.5 ml of 25% [wt/vol] saccharose-50 mM Tris-HCl-100 mM EDTA [pH 8.0]) for 2 h on ice. Cell lysis was achieved by the addition of lysozyme (5 mg ml−1, 37°C, 1 h) and subsequently proteinase K (50 μg ml−1 in 2% sodium dodecyl sulfate, 60°C, 1 h). DNA extraction was achieved by the addition of phenol-chloroform-isoamyl alcohol (25:24:1 [vol/vol/vol]) twice and purification of the water phase with chloroform-isoamyl alcohol (24:1 [vol/vol]). After precipitation with 90% (vol/vol) ethanol (−20°C, 1 h) and washing with 70% (vol/vol) ethanol, the purified DNA was resuspended in 50 μl of Millipore water.
PCR analysis.
PCR was used to analyze the presence or absence of two specific gene regions: (i) the intergenic spacer region within the phycocyanin (PC) operon and (ii) the mcyB region, which encodes one step in microcystin biosynthesis (39). Both gene regions are located on the chromosome (26, 39), and the hypothesis that plasmids are involved in microcystin synthesis has not been substantiated (2). PCR amplifications were performed with a volume of 20 μl containing 2 μl of PCR buffer (Qiagen, VWR International, Vienna, Austria), 1.2 μl MgCl2 (25 mM; Qiagen), 0.6 μl of deoxynucleotide triphosphates (10 mM each; MBI Fermentas, St. Leon-Rot, Germany), 1 μl of each primer (10 pmol), 0.1 μl of Taq DNA polymerase (Qiagen), 13.6 μl of sterile Millipore water, and 0.5 μl of the sample. The PCR thermal cycling protocol included initial denaturation at 94°C for 3 min, followed by 35 cycles at 94°C for 30 s, annealing at 50°C for 30 s, and elongation at 72°C for 0.5 min. PCR products (4 μl of the reaction mixture) were visualized by ethidium bromide staining and by electrophoresis on 1.5% agarose gels in 0.5× Tris-borate-EDTA buffer.
The sequences for the PC primers were designed on the basis of the highly variable intergenic spacer region within the PC operon (26) and showed sufficient specificity for the genus Microcystis (see below). The sequences were as follows: PcMafwd, 5′-GGTCTGCGCGAAACCTATGT-3′, and PcMarev, 5′-GGTCAACACTTTAGCGGCG-3′. The primers had dissociation temperatures of 59.4 and 58.8°C, respectively, and yielded a total amplification fragment of 368 bp. The sequences for the mcyB region were as follows: McyBMafwd1, 5′-AATCAACGGTTAGTTGCTTATGT-3′, and tox4r, 5′-CACTAACCCCTATTTTGGATACC-3′. The primers had dissociation temperatures of 49.7 and 58.9°C, respectively, and yielded a total amplification fragment of 288 bp. According to tests with a number of isolates, no reactions with the corresponding gene regions of other genera of cyanobacteria (e.g., Planktothrix and Aphanizomenon) have been observed for the PC primers or for the mcyB region primers (data not shown). Both primer pairs have been tested for successful amplification of DNAs originating from various isolates from Lake Wannsee (isolates W75, W334, and W368, as reported by Rohrlack et al. [37]) as well as for type I and type II restriction fragment length polymorphisms (the two most significantly different groups of genotypes reported by Kurmayer et al. [22]).
Origin, description, and culturing of Microcystis sp. to standardize PCR analysis.
The unicellular strains HUB 5-2-4 (microcystin producing) and HUB 5-3 (non-microcystin producing) were isolated from eutrophic Lake Pehlitzsee (Brandenburg, Germany) in 1978 and kindly provided by M. Henning (Humboldt University, Berlin, Germany). Both strains were grown in batch cultures in Z medium (44) and harvested during the late logarithmic growth phase.
Specificity and sensitivity of the primers.
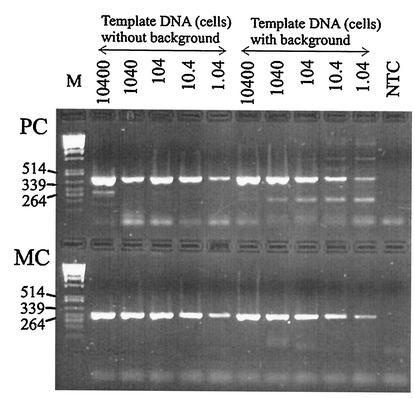
To test the specificity and sensitivity of the primers under natural conditions, 5 ml of a cell concentration of 2.08 × 107 HUB 5-2-4 cells ml−1 (determined by use of Casy 1; Schärfe Systems, Reutlingen, Germany) was filtered with a GF/C filter, and the DNA was extracted as described above. From the extract, five dilutions ranging from 1:102 to 1:106 of template DNA (equivalent to 10,400 cells to 1.04 cells) were prepared and analyzed by PCR with both primer pairs in the absence or presence of a 1:100 dilution of a natural background. To obtain the natural background, water from Lake Wannsee was filtered on 4 May 2000 through a sieve (25-μm mesh size), 300 ml was filtered with a GF/C filter, and the DNA was extracted. The counting of cyanobacteria under an inverted microscope (3.2-ml sedimentation chamber) revealed the dominance of Aphanizomenon spp., Limnothrix spp., Limnothrix redekei, and Planktothrix agardhii with biovolumes of 0.38, 1.95, 1.11, and 1.0 mm3 liter−1, respectively. No cells of Microcystis were found after careful examination of two transects of the counting chamber at a ×400 magnification. Both primer pairs showed high specificity and identical sensitivity within the range of 10,400 to 1.04 cells used as template DNA in the absence or presence of the background (Fig. 1).
FIG. 1.
PCR amplification to test the sensitivity of both primer pairs amplifying the PC internal transcribed spacer region (PC) and a microcystin-specific region (the mcyB gene) (MC) for a microcystin-producing Microcystis sp. strain HUB 5-2-4 dilution series. In addition, the specificity of both primer pairs and the potential impact of a natural background were tested in parallel. The numbers above the lanes refer to template DNA (equivalent to 10,400 to 1.04 cells) in the presence or absence of a natural background from Lake Wannsee. For details on the preparation of the natural background, see the text. Lane M, molecular weight markers, in base pairs; lane NTC, nontemplate control.
Dilution assay.
As determined from the numbers of Microcystis cells, the extracts were diluted to a standard DNA concentration equivalent to 200 cells μl−1. This standard concentration was then diluted up to 1,000-fold through 14 dilution steps (the dilutions were at factors of 2, 6, 10, 14, 20, 25, 33, 50, 100, 200, 300, 400, 500, and 1,000, corresponding to 100, 33, 20, 14, 10, 8, 6, 4, 2, 1, 0.7, 0.5, 0.4, and 0.2 cells, respectively). Each dilution series was analyzed by PCR in the same thermal block of the thermocycler for the presence of the two gene regions (PC and mcyB) simultaneously. All dilution steps yielding a PC product but no mcyB product were considered to represent DNA extracted from non-microcystin-producing cells (genotypes). The number of PCR products (the number of bands on the gel) obtained from the dilution series for mcyB was divided by the number of bands obtained from the dilution series for PC, and a mcyB/PC ratio was calculated. After this step, the sum of non-microcystin-producing cells was calculated from all dilution steps that were negative for mcyB but positive for PC in a given sample. This is a minimum estimate, as dilution steps positive for mcyB will also contain some colonies without the mcyB gene. In relation to the standard DNA concentration (equivalent to 200 cells μl−1), the minimum number of cells without the mcyB gene is useful as an additional measure of the proportions of both genotypes. The DNA samples from the size fractionation procedure were analyzed every 2 weeks.
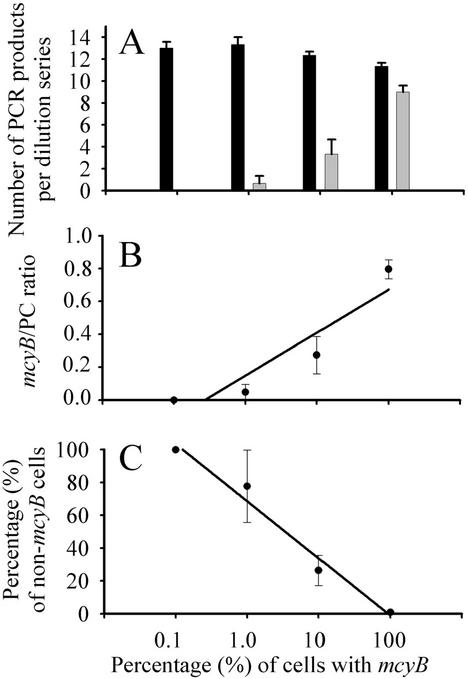
The validity of this approach was first assessed by using two Microcystis strains, HUB 5-2-4 (mcyB+) and HUB 5-3 (non-mcyB). DNA extracts equivalent to 200 cells μl−1 and originating either from strain HUB 5-2-4 (mcyB+) or from strain HUB 5-3 (mcyB) were mixed at ratios of 0.1, 1.0, 10, and 100% HUB 5-2-4 and then analyzed by the dilution assay as described above. The total number of PC products obtained by PCR was stable for the full range of the mcyB+ strain-non-mcyB strain mixture in the DNA template for three independent measurements (Fig. 2A). In contrast, the total number of mcyB products decreased accordingly with the reduction in the proportion of the mcyB+ strain in the DNA template. Consequently, the ratio of mcyB/PC products showed a significant linear positive relationship with the percentage of the mcyB+ strain in the DNA extract (Fig. 2B). In addition, there was a 1:1 relationship between the percentage of non-mcyB cells (estimated from dilution steps yielding a PC product but no mcyB product) and the percentage of the mcyB+ strain (Fig. 2C).
FIG. 2.
Test of the dilution assay. The dilution assay was tested by mixing two culture strains (with and without the mcyB gene) in different ratios but at a constant amount of DNA. The data are shown as the mean and 1 standard error (n = 3). (A) Total numbers of PC (black columns) and mcyB (microcystin; white columns) products obtained by PCR in the dilution assay with various proportions of mcyB+ cells. (B) The ratio of mcyB products to PC products obtained by PCR in the dilution assay was found to be linearly related to the proportion of DNA from mcyB+ cells (y = 0.19 + 0.11 ln x, R2 = 0.86, where y is the mcyB/PC ratio and x is the percentage of mcyB+ cells). (C) The percentage of non-mcyB cells was estimated from dilution steps yielding a PC (PCR) product but no mcyB (PCR) product and was found to be linearly related to the proportion of DNA from mcyB+ cells (y = 68.57 − 15.12 ln x, R2 = 0.98, where y is the percentage of non-mcyB cells and x is the percentage of mcyB+ cells).
Analysis of microcystins.
The extracts were analyzed for microcystins by HPLC-DAD with a LiChrosper 100, octyldecyl silane, 5-μm LiChroCART 250-4 cartridge system (Merck, Darmstadt, Germany) and a linear gradient of aqueous acetonitrile (with 0.05% [vol/vol] trifluoroacetic acid) starting with 30% (vol/vol) acetonitrile and ending after 42 min with 100% acetonitrile at a flow rate of 1 ml min−1 (8, 23). Microcystins were identified by their characteristic absorption spectra (original spectrum and first-order derivative) and retention times (8) and quantified at 240 nm by using microcystin LR (MC-LR) as an external standard (kindly provided by G. A. Codd, University of Dundee, Dundee, United Kingdom). The concentrations of other microcystin variants were determined as concentration equivalents relative to MC-LR. For quantification of cell quotas, methylated microcystins (MC-RR, MC-YR, MC-LR, and MC-WR) were summed, because these variants have been found to be the dominant ones during the analysis of single colonies (9). As shown by Kurmayer et al. (22), all methylated variants are produced by one genotype. In addition, in Lake Wannsee, the concentrations of methylated microcystin variants have been shown to correlate with the biovolume of Microcystis, whereas the concentrations of demethylated variants have been found to correlate with the biovolume of Planktothrix (8).
Statistical analyses and calculation of net production rates.
Gene (mcyB/PC) ratios, the proportions of non-microcystin-producing cells, and microcystin cell quotas were statistically compared by using Kruskal-Wallis analysis of variance on ranks. This design is essentially the same as a Mann-Whitney rank sum test, except that more than two groups are compared. When an overall significant difference (P < 0.01) was found among groups, multiple comparisons between groups were performed by using the Dunn test (P < 0.01).
To test for the reliability of the sieving procedure, the sum estimated from the colony size fractions for both cell numbers and microcystin concentrations (as a dependent variable) were regressed against the respective amount of the total sample (as an independent variable). The data were log transformed to achieve the assumptions of normal distribution, constant variance, and independence of the residuals.
In order to compare the general relationship of cell growth and division (i.e., biovolume net production) to microcystin net production between colony size classes of Microcystis, net production rates (day−1) were calculated for both parameters by using the relationship r = ln {[(C2 + 1) × 106] − ln [(C1 + 1) × 106]}/(t2 − t1), where r is the net production rate and C1 and C2 are the concentrations of cells or microcystin per volume of water at two consecutive sampling dates (t1 and t2). This method of calculation is considered useful for comparing the relationship of cell growth and division to microcystin net production between size classes. Since the export or import of cells at the sample location would be measured as net negative or positive growth, the absolute values must be interpreted with caution. The two rates were plotted against each other and tested for significance (P < 0.01) by using linear regression analysis with the microcystin net production rate as a dependent variable and the growth rate as an independent variable. The term ln [(C + 1) × 106] was used to include all zero values for microcystin concentrations as well as to achieve a normal distribution and a constant variance. A SigmaStat (version 2.03 for Windows) package was used for all statistical analyses. The linear curves were fitted by using the least-squares approximation of Sigma Plot 2000 (version 6.10).
RESULTS
Ambient abiotic factors.
During the study period, the integrated water temperature was 19.4 ± 1.4 (mean and standard deviation [SD]) (minimum, 17°C; maximum, 22°C). During the entire study period, the level of soluble reactive phosphorus never decreased below 3.6 μg liter−1 (with the limit of determination being 1 μg liter−1), and the maximum level was 764 μg liter−1. On all sampling dates, ammonium nitrogen and/or nitrate nitrogen was present in detectable amounts. However, the attenuation coefficient increased from 0.7 in winter to 3.1 in summer, and the mean daily light intensity for the total water column reached a maximum of 45 μmol m−2 s−1 in early summer (May and June) and decreased to 10 μmol m−2 s−1 afterward. It was concluded that during the study period, phytoplankton was limited by light availability (and potentially by other unknown factors) but not by phosphorus or nitrogen.
Microcystis sp. in Lake Wannsee.
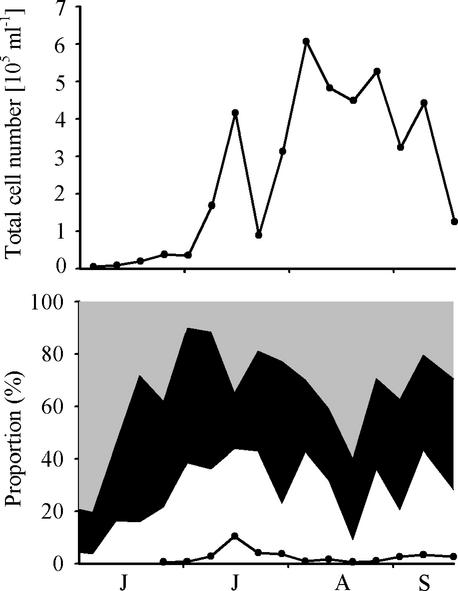
Microcystis sp. were found on each sampling date, with a minimum abundance in June (4,800 cells ml−1) and a maximum abundance in August (6.1 × 105 cells ml−1). Microcystis spp. dominated the phytoplankton biovolume during August and September. All colony size classes contributed similar proportions to the total Microcystis cell number, with the exception of the largest size class (>340 μm), which contributed only a small fraction to the total cell number (0.6 to 10%) (Fig. 3). Identification of individual colonies revealed the occurrence of five morphospecies: M. aeruginosa, M. ichthyoblabe, M. flos-aquae, M. botrys, and M. wesenbergii. Counting of 400 randomly selected colonies from live samples on three sampling dates in July 2000 showed a dominance of M. aeruginosa or M. flos-aquae (51%) and M. ichthyoblabe (41%). It was impossible to discriminate between M. aeruginosa and M. flos-aquae for the small colonies (<100 μm) during counting of the colonies under the microscope.
FIG. 3.
Population density and proportions of colony size classes. (Top) Population density of Microcystis sp. in Lake Wannsee during the summer of 2000. (Bottom) Proportion of each colony size class: <50 μm (grey area), 50 to 100 μm (black area), and >100 μm (white area). Colonies larger than >340 μm are indicated by a line; they were too sparse to be analyzed in June. Months from June to September are indicated from left to right.
Proportion of microcystin-producing genotypes.
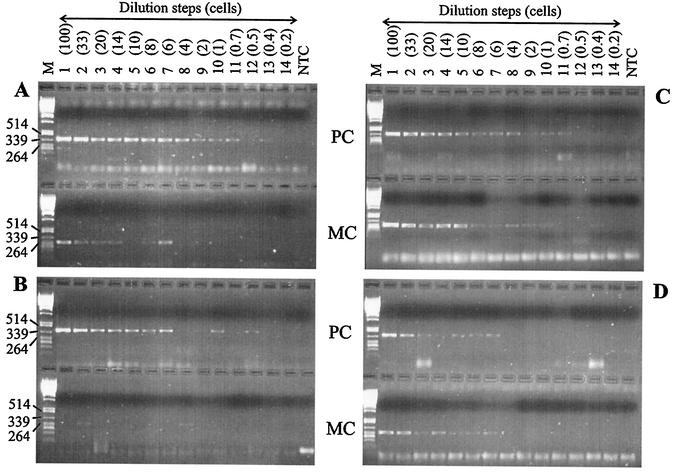
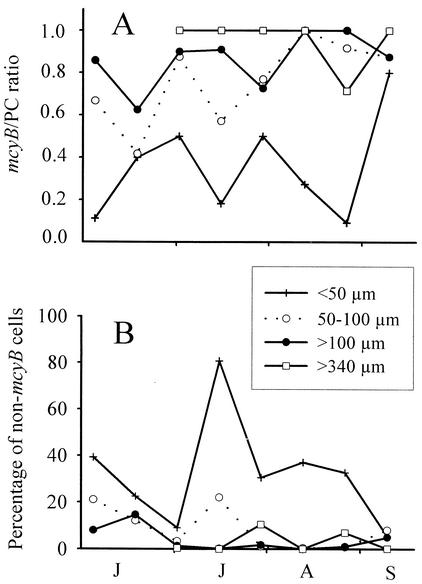
In all size classes, the mcyB gene was found during the entire year. However, the mcyB gene was quickly diluted below detection compared to the PC gene in the smallest colony size class (<50 μm) but not in the largest size class (>340 μm) (Fig. 4). Over the entire study period, the smallest size class (<50 μm) consistently revealed a mcyB/PC ratio of about 0.4 (Fig. 5A). In contrast, the two larger size classes (>100 μm and >340 μm) consistently showed mcyB/PC ratios close to 1. The difference between the smallest size class and the two larger size classes was highly significant (P < 0.001) (Table 1). In addition, the smallest size class showed a much higher proportion of non-microcystin-producing cells (Fig. 5B), which differed significantly from the proportion of non-microcystin-producing cells in the two larger size classes (P = 0.002) (Table 1). The size class of 50 to 100 μm was intermediate with regard to both parameters and did not differ significantly from either the smaller or the larger size classes.
FIG. 4.
Results of the dilution assay of field samples. Photographs of ethidium bromide-stained gels show the amplification products of the PC internal transcribed spacer region (PC) and of a microcystin-specific region (the mcyB gene) (MC) for a Microcystis dilution series of the smallest size fraction (<50 μm) on 4 July 2000 (A) and 18 July 2000 (B) and the largest size fraction (>340 μm) on 4 July 2000 (C) and 18 July 2000 (D). The difference in the total number of PCR products for PC and mcyB can be taken as a measure of the proportion of microcystin-producing genotypes in a given amount of DNA. For example, on 4 July 2000, in the size fraction of <50 μm, the mcyB gene was detected in 6 dilution steps, whereas the PC gene was detected in 12 dilution steps (A). Lanes: 1 to 14, dilution steps (with the corresponding cell number estimate given in parentheses); NTC, nontemplate control; M, molecular weight markers, in base pairs.
FIG. 5.
Proportions of microcystin-producing genotypes in different colony size classes. (A) Ratio of the microcystin (mcyB) gene to the PC gene for each colony size class of Microcystis during the summer of 2000. The ratio of mcyB products to PC products is expected to be ∼1 if a sample consists solely of microcystin-producing genotypes and to decrease to 0.4 if the microcystin-producing genotypes constitute 10% only (see also Fig. 2). (B) Percentage of non-microcystin-producing cells found, as inferred from a PCR analysis of dilution series for each colony size class. Months from June to September are indicated from left to right.
TABLE 1.
Gene ratios, percentages of non-microcystin-producing cells, and microcystin cell quotas in Lake Wannsee during the summer of 2000 for each Microcystis size fractiona
| Colony size (μm) |
mcyB/PC ratio
|
Non-mcyB cells
|
Microcystin cell quotas
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 25%-Median-75% | Mean (95% CL) | n | 25%-Median-75% | Mean (95% CL) | n | 25%-Median-75% | Mean (95% CL) | n | |
| <50 | 0.15-0.34-0.5 a | 0.36 ± 0.2 | 8 | 15.8-31.6-38.2 a | 32.2 ± 19.4 | 8 | 0.0-0.0-3.1 a | 1.9 ± 1.5 | 16 |
| 50-100 | 0.62-0.82-0.9 ab | 0.76 ± 0.16 | 8 | 1.0-5.7-16.6 ab | 8.6 ± 7.5 | 8 | 1.2-3.7-7.9 a | 5.3 ± 2.9 | 16 |
| >100 | 0.79-0.89-0.96 b | 0.86 ± 0.11 | 8 | 0.6-1.5-6.5 b | 4.0 ± 4.2 | 8 | 16.5-18.5-22 b | 23.7 ± 7.9 | 16 |
| >340 | 1.0-1.0-1.0 b | 0.95 ± 0.12 | 6 | 0.0-0.3-7.0 b | 3.0 ± 4.8 | 6 | 31.7-59.4-91.7 b | 62.5 ± 20.4 | 16 |
| Total | ND | ND | ND | ND | ND | ND | 3.1-4.5-8.6 a | 5.7 ± 2 | 16 |
The differences were tested by using Kruskal-Wallis one-way analysis of variance on ranks followed by the Dunn multiple-comparison procedure. Letters a and b indicate subsets whose highest and lowest medians were not significantly different (P > 0.01). P values (overall significance) for mcyB/PC ratios, non-mcyB cells, and microcystin cell quotas were <0.001, 0.002, and <0.001, respectively. ND, not determined; 25% and 75% are percentiles.
Microcystin cell quotas.
Microcystin concentrations and cell densities were determined directly, i.e., in nonfractionated samples, and as a sum of all colony size fractions. The results of both approaches correlated closely for both cell densities [R2 = 0.95, ln y = 0.934 × ln x + 0.537, P < 0.001, n = 16] and microcystin concentrations [R2 = 0.92, ln y = 0.683 × ln x + 2.284, P < 0.001, n = 13], where y is the sum of the colony size fractions and x is the total amount determined directly. These results indicate that the sieving procedure closely matched the actual situation in the water samples.
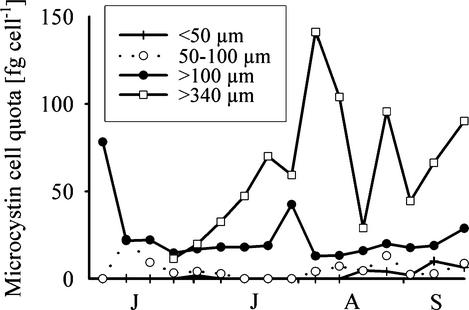
Corresponding to the lower proportion of the mcyB gene and the higher proportion of non-microcystin-producing cells found in the two smaller colony size classes (<100 μm) (Fig. 5), the microcystin cell quotas for these fractions were rather low (Fig. 6). The difference in cell quotas between the smaller size classes and the two larger size classes (>100 μm and >340 μm) was highly significant (P < 0.001) (Table 1). The two larger size classes also differed significantly from the total water sample (Table 1). On 9 of 16 sampling dates, no microcystins were detected in the smallest size class (<50 μm) by HPLC-DAD, whereas on all sampling dates for the larger size classes (>100 μm), at least one microcystin variant was detected. Averaged over the entire study period, the differences in microcystin cell quotas between the smallest size class (<50 μm) and the two larger size classes (>100 μm and >340 μm) varied by factors of 13- and 33-fold, respectively. Colonies of >100 μm accounted for 83% ± 34% (95% confidence limits) of the total microcystin concentration.
FIG. 6.
Microcystin cell quotas (sum of MC-RR, MC-YR, MC-LR, and MC-WR) for each size class of Microcystis in Lake Wannsee. Months from June to September are indicated from left to right.
Relationship of cell growth to microcystin net production.
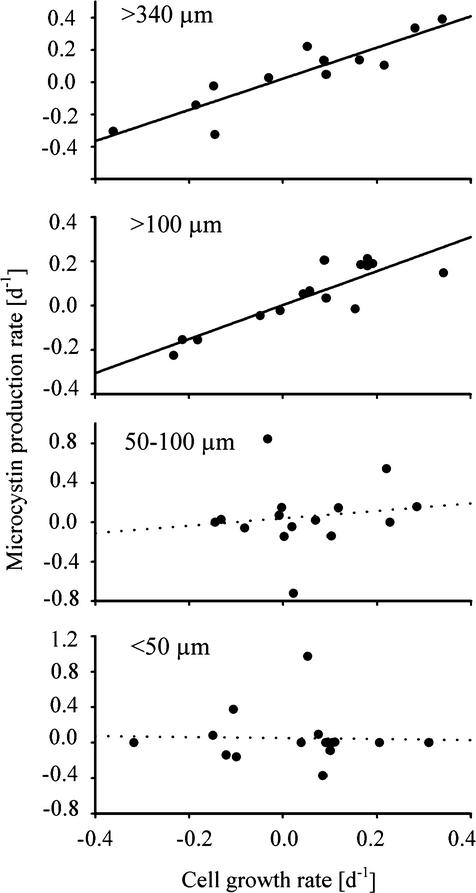
The linear regression analysis revealed a significant positive relationship between the growth rate for a colony size fraction and its microcystin net production rate per day for the two larger colony size classes (>340 μm and >100 μm) and also for the total water sample (Fig. 7). Both significant regression lines had a slope close to 1 and an origin near 0 (Table 2). The coefficient of determination (R2) was highest in the two larger size classes, indicating that 82% (>340 μm) and 77% (>100 μm) of the variability in the microcystin net production rate could be explained by the growth rate of the cells. The relationship was much weaker for colonies of <100 μm, and the slope of the regression line for the smallest size class was 0; for both of the small colony size classes, the relationship between cell growth and microcystin net production was not significant (Table 2).
FIG. 7.
Relationships between the net population growth rate (per day) and the net microcystin production rate (per day) for the four colony size fractions of Microcystis in Lake Wannsee. The data include the months of June to September 2000. The curves were fitted by a linear regression calculation with least-squares approximation. Only the solid regression lines were found to be significant. Table 2 shows details of the regression analysis.
TABLE 2.
Regression parameters for the relationship between population growth rate and microcystin production rate for each Microcystis colony size classa
| Colony size (μm) | A ± SEb | B ± SEb | R2 | P | n |
|---|---|---|---|---|---|
| >340 | 0.97 ± 0.15 | 0.02 ± 0.03 | 0.82 | <0.001 | 12 |
| >100 | 0.77 ± 0.12 | 0.002 ± 0.02 | 0.77 | <0.001 | 15 |
| 50-100 | 0.38 ± 0.73 | 0.04 ± 0.095 | 0.02 | 0.61 | 15 |
| <50 | −0.06 ± 0.53 | 0.05 ± 0.08 | <0.001 | 0.92 | 15 |
| Total | 1.13 ± 0.36 | 0.04 ± 0.04 | 0.43 | 0.008 | 15 |
The data were fitted as the linear relationship y = Ax + B, where x is the daily change in cell number and y is the daily change in microcystin concentration. Both rates were calculated between successive sampling dates as described in the text.
Data are reported as mean and SE.
DISCUSSION
Effects of the abundance of microcystin-producing genotypes on microcystin net production.
Recent limnological field studies addressed the significance of direct environmental influences on microcystin concentrations (14, 20, 28). Chorus et al. (4) interpreted their data as proposing that the abundance of microcystin producers is the overriding mechanism governing the average cellular microcystin content of a given field population, although genotypes were not differentiated in their study. In the study presented here, we made an effort to quantify the proportions of genotypes with and without the mcyB gene and thus the effects of genetic diversity on microcystin net production in a field population. This approach had two major results. (i) The sample fractions chiefly containing large colonies contained a substantially larger amount of mcyB genes than those chiefly containing small colonies. (ii) The increase or decrease in microcystin concentrations was related nearly 1:1 to the growth rate of the cells originating from the larger colonies (>100 μm). In contrast, the microcystin concentrations in the smaller colony size fractions (<100 μm) were not related to the corresponding change in cell numbers. This result means that the wax and wane of microcystin concentrations can be ascribed to the population development of the larger colonies; in addition, it confirms the working hypothesis that the larger colony size classes (>100 μm) consisted mainly of microcystin-producing genotypes, whereas in the smaller colony size classes (<100 μm), microcystin-producing genotypes were less abundant and cooccurred with a larger number of non-microcystin-producing genotypes.
Direct effects of environmental factors on microcystin net production.
The linear regressions between the net rate of growth of Microcystis cells and the net rate of microcystin production implied that microcystin net production is constantly coupled to the increase or decrease in cell numbers throughout the growth cycle. For all significant regression lines in this study, the slopes were close to 1 and the intercepts were close to 0. These results support the conclusion of Orr and Jones (29), confirmed by Long et al. (24) (Fig. 4), that the influence of nutrients and other growth-limiting factors (light, temperature, and so forth) on the net production of microcystin is chiefly indirect, i.e., through their effects on growth and cell division. Nonetheless, the contrary hypothesis—i.e., that the variations in cell quotas between colony size classes in this study could be caused by environmental effects alone—is relevant because larger colonies are known to perform more pronounced vertical migration via buoyancy regulation than smaller colonies and are able to use light more efficiently (11, 41) than smaller colonies. Although the advantage of buoyancy diminishes with the degree of mixing in the water column, this effect of larger colony size is considered significant even in shallow eutrophic lakes (11, 41).
In Lake Wannsee, light is likely to be an important environmental factor having an impact on phytoplankton growth and physiology. Kaebernick et al. (17) showed direct effects of high to low irradiance on the transcription of the mcyB gene, encoding one step in microcystin biosynthesis; however, in their experiments even cells grown under low light continued in the transcription of the mcyB gene. The range of variation in the amount of transcripts in relation to the light dose did not exceed three- to fourfold, corresponding to the general range of variation that has been found for individual strains in the laboratory under different environmental conditions (38). In contrast to the light-induced changes observed in the laboratory (17), the range of variation observed between size classes in this study reached up to 30-fold. Assuming the influence of light to be the major factor, even small colonies experiencing low-light conditions would be expected to show at least some relationship between microcystin net production and cell growth. It was further proposed that the limitation of Microcystis sp. by more than one factor (light and iron, trace elements, and so forth) might induce more than 10-fold variations in microcystin cell quotas. This possibility cannot be fully excluded, but as the lake is a nonstratified hypertrophic system heavily loaded with sewage treatment plant effluents, a role of limiting factors other than light is unlikely.
Size fractions of Microcystis sp. and interactions with grazers.
It is possible that transitions between colony size classes occurred, e.g., by fragmentation of larger colonies during the growth cycle (33). Consequently, each size class still contained more than one Microcystis genotype, as shown by the PCR results. However, the difference in the proportions of microcystin-producing versus non-microcystin-producing genotypes between size classes was found to be highly significant and also appeared to be rather stable over the season (Fig. 5). The increase in cell numbers from the beginning of the season to the seasonal maximum was pronounced (128-fold), and small numbers of large M. aeruginosa colonies also were found in the water column during the winter between 1999 and 2000 (22). In conclusion, it was proposed that the largest and smallest size categories proliferated more or less separately from each other and that the seasonal maximum of the smallest size class (<50 μm) during the growth season occurs through the growth of the smallest colonies rather than through fragmentation of the larger colonies.
The finding that microcystins are mainly produced by larger colonies has important implications for interactions between Microcystis sp. and grazers. Microcystin has been shown to be a strong factor in shaping zooplankton communities (21); however, the role of microcystin as a feeding deterrent is much less important (36). A number of other factors have been shown to overrule the poisoning effect of microcystin, such as the composition of the mucilage (35) or the size of the colonies. It is known from earlier studies that the size limit for large daphnids (the zooplankton organisms with the highest grazing rates) to feed efficiently on Microcystis sp. is <100 μm (15). Consequently, in Lake Wannsee, the colonies with the lowest microcystin content would be the ones grazed upon by zooplankton. In contrast, larger colonies with the highest microcystin cell quotas would not have a negative impact on Daphnia, as they would not be grazed upon. However, this suggestion does not necessarily mean that Microcystis is not harmful to Daphnia in Lake Wannsee. For example, Jungmann and Benndorf (16) demonstrated a plankton sample from Lake Wannsee to be highly toxic to Daphnia, although microcystins were not detected. In addition, an array of protease inhibitors has been detected in Microcystis sp., e.g., aeruginosin, cyanopeptolin, and microginin (9). A toxic effect of those compounds in non-microcystin-producing cyanobacteria on Daphnia has been suggested by Kaebernick et al. (18) and might contribute to grazing resistance similar to or even more pronounced than that of microcystin.
Applied aspects.
The finding that shifts of microcystin genotype proportions between and within size classes of Microcystis sp. are stable over the growing season might have useful implications for the surveillance of water bodies. This means that for one water body, microcystin concentrations could be inferred from regular cell counts of the total population but only occasional examination of the populations for mean microcystin content. For example, cell counts could be determined on a weekly basis by the authority responsible for surveillance, whereas microcystin analyses could be carried out at substantially longer time intervals, e.g., once at the beginning of the “cyanobacterial season” (or bathing season, depending on the setting and water body use) and once during the cyanobacterial population maximum.
The colony size distributions of microcystin-producing genotypes found in Lake Wannsee in the present study and by Kurmayer et al. (22) were confirmed by the results of a study with Microcystis samples from central European countries (The Netherlands, Germany, Czech Republic, and Austria) (L. Via-Odorika et al., unpublished data); that study showed that 70% of all M. aeruginosa colonies contained microcystins, whereas only <20% of the colonies of the fragile morphospecies assigned to M. ichthyoblabe were found to produce microcystins. Consequently, the observation that larger colonies of Microcystis are the chief microcystin producers in Lake Wannsee may be more generally valid.
Acknowledgments
We thank Karina Laskus and Sara Monka for assistance in phytoplankton counting. The help of Jutta Fastner, Inge Flieger, Ingrid Klinkmueller, and Claudia Preuβ in HPLC-DAD analysis of microcystins is gratefully acknowledged. Bettina Reinhold did the phosphorus analysis. Many thanks are due to Elke Pawlitzky for help with filtration in the laboratory and Liselotte Eisl for help with DNA extraction and PCR analysis. We are grateful to the crew of the Wasserschiffahrtsamt (Harald Becker) for providing the boat and captain for sampling. M. Hahn provided valuable comments on an earlier version of this article. Two anonymous reviewers contributed to the improvement of this article.
This work was financed by the EU TMR (Training and Mobility of Researchers) network TOPIC (TOxin Production In Cyanobacteria), coordinated by Luc Mur (CT 38-0246).
REFERENCES
- 1.Behrendt, H., and B. Nixdorf. 1993. The carbon balance of phytoplankton production and loss processes based on in situ measurement in a shallow lake. Int. Rev. Hydrobiol. 78:439-458.
- 2.Bolch, C. J. S., S. I. Blackburn, G. J. Jones, P. T. Orr, and P. M. Grewe. 1997. Plasmid content and distribution in the toxic cyanobacterial genus Microcystis Kützing ex Lemmermann (Cyanobacteria: Chroococcales). Phycologia 36:6-11. [Google Scholar]
- 3.Carmichael, W. W., V. Beasly, D. L. Bunner, J. N. Eloff, I. Falconer, P. Gorham, K.-I. Harada, T. Krishnamurty, Y. Min-Juan, R. E. Moore, K. Rinehart, M. Runnegar, O. M. Skulberg, and M. Watanabe. 1988. Naming cyclic heptapeptide toxins of cyanobacteria (blue-green algae). Toxicon 26:971-973. [DOI] [PubMed] [Google Scholar]
- 4.Chorus, I., V. Niesel, J. Fastner, C. Wiedner, B. Nixdorf, and K.-E. Lindenschmidt. 2001. Environmental factors and microcystin levels in water bodies, p. 159-177. In I. Chorus (ed.), Cyanotoxins: occurrences, causes, consequences. Springer-Verlag KG, Berlin, Germany.
- 5.Deutsches Institut für Normung. 1983. Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung; Kationen (Gruppe E); Bestimmung des Ammoniumstickstoffs (E5). DIN 38406-5. Beuth Verlag, Berlin, Germany.
- 6.Dittmann, E., B. A. Neilan, M. Erhard, H. von Döhren, and T. Börner. 1997. Insertional mutagenesis of a peptide synthetase gene that is responsible for hepatotoxin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Mol. Microbiol. 26:779-787. [DOI] [PubMed] [Google Scholar]
- 7.Doers, M. P., and D. L. Parker. 1988. Properties of Microcystis aeruginosa and M. flos-aquae (Cyanophyta) in culture: taxonomic implications. J. Phycol. 24:502-508. [Google Scholar]
- 8.Fastner, J., M. Erhard, W. W. Carmichael, F. Sun, K. L. Rinehart, H. Rönicke, and I. Chorus. 1999. Characterization and diversity of microcystins in natural blooms and strains of the genera Microcystis and Planktothrix from German freshwaters. Arch. Hydrobiol. 145:147-163. [Google Scholar]
- 9.Fastner, J., M. Erhard, and H. von Döhren. 2001. Determination of oligopeptide diversity within a natural population of Microcystis spp. (Cyanobacteria) by typing single colonies by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 67:5069-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franche, C., and T. Damerval. 1988. Test on nif probes and DNA hybridizations. Methods Enzymol. 167:803-808. [Google Scholar]
- 11.Ibelings, M. W., L. R. Mur, and A. E. Walsby. 1991. Diurnal changes in buoyancy and vertical distribution in populations of Microcystis in two shallow lakes. J. Plankton Res. 13:419-437. [Google Scholar]
- 12.International Organisation for Standardization. 1992. Water quality. Determination of dissolved fluoride, chloride, nitrite, orthophosphate, bromide, nitrate and sulfate ions, using liquid chromatography of ions. Part 1. Method for water with low contamination. ISO 10304-1. International Organisation for Standardization, Geneva, Switzerland.
- 13.International Organisation for Standardization. 1998. Water quality. Spectrometric determination of phosphorus using ammonium molybdate. ISO 6878. International Organisation for Standardization, Geneva, Switzerland.
- 14.Jacoby, J. M., D. C. Collier, E. B. Welch, F. J. Hardy, and M. Crayton. 2000. Environmental factors associated with a toxic bloom of Microcystis aeruginosa. Can. J. Fish. Aquat. Sci. 57:231-240. [Google Scholar]
- 15.Jarvis, A. C., R. C. Hart, and S. Combrink. 1987. Zooplankton feeding on size fractionated Microcystis colonies and Chlorella in a hypertrophic lake (Hartbeesport Dam, South Africa): implications to resource utilization and zooplankton succession. J. Plankton Res. 9:1231-1249. [Google Scholar]
- 16.Jungmann, D., and J. Benndorf. 1994. Toxicity to Daphnia of a compound extracted from laboratory and natural Microcystis spp., and the role of microcystins. Freshw. Biol. 32:13-20. [Google Scholar]
- 17.Kaebernick, M., B. A. Neilan, T. Börner, and E. Dittmann. 2000. Light and the transcriptional response of the microcystin biosynthesis gene cluster. Appl. Environ. Microbiol. 66:3387-3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaebernick, M., T. Rohrlack, K. Christoffersen, and B. A. Neilan. 2001. A spontaneous mutant of microcystin biosynthesis: genetic characterization and effect on Daphnia. Environ. Microbiol. 3:669-679. [DOI] [PubMed] [Google Scholar]
- 19.Komárek, J., and K. Anagnostidis. 1999. Cyanoprokaryota, 1. Teil Chroococcales, p. 225-236. Gustav Fischer Verlag, Jena, Germany.
- 20.Kotak, B. G., A. K. Y. Lam, E. E. Prepas, and S. E. Hrudey. 2000. Role of chemical and physical variables in regulating microcystin-LR concentration in phytoplankton of eutrophic lakes. Can. J. Fish. Aquat. Sci. 57:1584-1593. [Google Scholar]
- 21.Kurmayer, R., and F. Jüttner. 1999. Strategies for the co-existence of zooplankton with the toxic cyanobacterium Planktothrix rubescens in Lake Zürich. J. Plankton Res. 21:659-683. [Google Scholar]
- 22.Kurmayer, R., E. Dittmann, J. Fastner, and I. Chorus. 2002. Diversity of microcystin genes within a population of the toxic cyanobacterium Microcystis spp. in Lake Wannsee (Germany). Microb. Ecol. 43:107-118. [DOI] [PubMed] [Google Scholar]
- 23.Lawton, L. A., C. Edwards, and G. A. Codd. 1994. Extraction and high-performance liquid chromatographic method for the determination of microcystins in raw and treated waters. Analyst 119:1525-1530. [DOI] [PubMed] [Google Scholar]
- 24.Long, B. M., G. J. Jones, and P. T. Orr. 2001. Cellular microcystin content in N-limited Microcystis aeruginosa can be predicted from growth rate. Appl. Environ. Microbiol. 67:278-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marahiel, M. A., T. Stachelhaus, and H. D. Mootz. 1997. Modular peptide synthetases involved in non-ribosomal peptide synthesis. Chem. Rev. 97:2651-2673. [DOI] [PubMed] [Google Scholar]
- 26.Neilan, B. A., D. Jacobs, and A. E. Goodman. 1995. Genetic diversity and phylogeny of toxic cyanobacteria determined by DNA polymorphisms within the phycocyanin locus. Appl. Environ. Microbiol. 61:3875-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishizawa, T., M. Asayama, K. Fujii, K. Harada, and M. Shirai. 1999. Genetic analysis of the peptide synthetase genes for a cyclic heptapeptide microcystin in Microcystis spp. J. Biochem. 126:520-529. [DOI] [PubMed] [Google Scholar]
- 28.Oh, H.-M., S. J. Lee, J.-H. Kim, H.-S. Kim, and B.-D. Yoon. 2001. Seasonal variation and indirect monitoring of microcystin concentrations in Daechung Reservoir, Korea. Appl. Environ. Microbiol. 67:1484-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orr, P. T., and G. J. Jones. 1998. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol. Oceanogr. 43:1604-1614. [Google Scholar]
- 30.Otsuka, S., S. Suda, R. Li, M. Watanabe, H. Oyaizu, S. Matsumoto, and M. M. Watanabe. 1999. Phylogenetic relationships between toxic and non-toxic strains of the genus Microcystis based on 16S to 23S internal transcribed spacer sequence. FEMS Microbiol. Lett. 172:15-21. [DOI] [PubMed] [Google Scholar]
- 31.Otsuka, S., S. Suda, S. Shibata, H. Oyaizu, S. Matsumoto, and M. M. Watanabe. 2001. A proposal for the unification of five species of the cyanobacterial genus Microcystis Kützing ex Lemmermann 1907 under the rules of the bacteriological code. Int. J. Syst. E vol. Microbiol. 51:873-879. [DOI] [PubMed] [Google Scholar]
- 32.Parker, D. L. 1982. Improved procedures for the cloning and purification of Microcystis cultures (Cyanophyta). J. Phycol. 18:471-477. [Google Scholar]
- 33.Reynolds, C. S., G. H. M. Jaworski, H. A. Cmiech, and G. F. Leedale. 1981. On the annual cycle of the blue-green alga Microcystis aeruginosa Kütz. Emend. Elenkin. Philos. Trans. R. Soc. Lond. B 293:390-477. [Google Scholar]
- 34.Riley, G. A. 1957. Phytoplankton in the north central Sargasso Sea 1950-1952. Limnol. Oceanogr. 2:252-272. [Google Scholar]
- 35.Rohrlack, T., M. Henning, and J.-G. Kohl. 1999. Mechanisms of the inhibitory effect of the cyanobacterium Microcystis aeruginosa on Daphnia galeata's ingestion rate. J. Plankton Res. 21:1489-1500. [Google Scholar]
- 36.Rohrlack, T., E. Dittmann, M. Henning, T. Börner, and J.-G. Kohl. 1999. Role of microcystins in poisoning and food ingestion inhibition of Daphnia galeata caused by the cyanobacterium Microcystis aeruginosa. Appl. Environ. Microbiol. 65:737-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rohrlack, T., M. Henning, and J. G. Kohl. 2001. Isolation and characterization of colony-forming Microcystis aeruginosa strains, p. 152-158. In I. Chorus (ed.), Cyanotoxins: occurrences, causes, consequences. Springer-Verlag KG, Berlin, Germany.
- 38.Sivonen, K., and G. Jones. 1999. Cyanobacterial toxins, p. 41-111. In I. Chorus and J. Bartram (ed.), Toxic cyanobacteria in water. E. & F. N. Spon, London, England.
- 39.Tillett, D., E. Dittmann, M. Erhard, H. von Döhren, T. Börner, and B. A. Neilan. 2000. Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: an integrated peptide-polyketide synthetase system. Chem. Biol. 7:753-764. [DOI] [PubMed] [Google Scholar]
- 40.Utermöhl, H. 1958. Zur Vervollkommnung der quantitativen Phytoplanktonmethodik. Mitt. Int. Ver. Theor. Angew. Limnol. 2:1-38. [Google Scholar]
- 41.Wallace, B. B., M. C. Bailey, and D. P. Hamilton. 2000. Simulation of vertical position of buoyancy regulating Microcystis aeruginosa in a shallow eutrophic lake. Aquat. Sci. 62:320-333. [Google Scholar]
- 42.Watanabe, M. 1996. Isolation, cultivation and classification of bloom-forming Microcystis in Japan, p. 13-34. In M. F. Watanabe, K. I. Harada, W. W. Carmichael, and H. Fujiki (ed.), Toxic Microcystis. CRC Press, Inc., Boca Raton, Fla.
- 43.Wetzel, R. G., and G. E. Likens. 2000. Limnological analyses, 3rd ed., p. 147-175. Springer-Verlag, New York, N.Y.
- 44.Zehnder, A., and P. R. Gorham. 1960. Factors influencing the growth of Microcystis aeruginosa Kütz. Emend. Elenkin. Can. J. Microbiol. 6:645-660. [DOI] [PubMed] [Google Scholar]