Abstract
Anaerobic degradation of the aromatic hydrocarbon ethylbenzene was studied with sulfate as the electron acceptor. Enrichment cultures prepared with marine sediment samples from different locations showed ethylbenzene-dependent reduction of sulfate to sulfide and always contained a characteristic cell type that formed gas vesicles towards the end of growth. A pure culture of this cell type, strain EbS7, was isolated from sediment from Guaymas Basin (Gulf of California). Complete mineralization of ethylbenzene coupled to sulfate reduction was demonstrated in growth experiments with strain EbS7. Sequence analysis of the 16S rRNA gene revealed a close relationship between strain EbS7 and the previously described marine sulfate-reducing strains NaphS2 and mXyS1 (similarity values, 97.6 and 96.2%, respectively), which grow anaerobically with naphthalene and m-xylene, respectively. However, strain EbS7 did not oxidize naphthalene, m-xylene, or toluene. Other compounds utilized by strain EbS7 were phenylacetate, 3-phenylpropionate, formate, n-hexanoate, lactate, and pyruvate. 1-Phenylethanol and acetophenone, the characteristic intermediates in anaerobic ethylbenzene degradation by denitrifying bacteria, neither served as growth substrates nor were detectable as metabolites by gas chromatography-mass spectrometry in ethylbenzene-grown cultures of strain EbS7. Rather, (1-phenylethyl)succinate and 4-phenylpentanoate were detected as specific metabolites in such cultures. Formation of these intermediates can be explained by a reaction sequence involving addition of the benzyl carbon atom of ethylbenzene to fumarate, carbon skeleton rearrangement of the succinate moiety (as a thioester), and loss of one carboxyl group. Such reactions are analogous to those suggested for anaerobic n-alkane degradation and thus differ from the initial reactions in anaerobic ethylbenzene degradation by denitrifying bacteria which employ dehydrogenations.
Hydrocarbons have long been considered to be inert under anoxic conditions. During the past decade, however, enriched bacterial populations and pure cultures were shown to degrade saturated, aromatic, or unsaturated nonaromatic hydrocarbons in the absence of oxygen with nitrate, ferrous iron, or sulfate as an electron acceptor or under conditions of methanogenesis (23, 24, 37, 47, 56, 60). Anaerobic hydrocarbon degradation has been studied most extensively with toluene. Toluene belongs to the benzene-toluene-ethylbenzene-xylene group of petroleum hydrocarbons that are of particular industrial importance. On the other hand, these hydrocarbons are frequently of concern as groundwater contaminants at fuel- or solvent-contaminated sites (15, 37). The biochemistry of anaerobic toluene metabolism has been investigated mainly in denitrifying bacteria. These organisms have been shown to activate toluene by a radical-catalyzed addition to fumarate, yielding benzylsuccinate (4, 8, 17, 23, 47); the latter compound is metabolized to benzoyl coenzyme A (benzoyl-CoA) via reactions that are related to conventional β-oxidation (23, 31, 32, 47). Benzoyl-CoA undergoes ring reduction and cleavage (22). Anaerobes other than denitrifiers also apparently employ the same initial reactions for toluene degradation (3, 5, 39, 59).
Three pure cultures of anaerobes that degrade ethylbenzene have been isolated; these organisms are all denitrifying bacteria (2, 42). In contrast to toluene, ethylbenzene in these strains does not react with fumarate but is dehydrogenated, yielding (S)-1-phenylethanol; the hydroxyl group originates from water (2, 25, 26, 28, 40, 42). (S)-1-Phenylethanol is then dehydrogenated to acetophenone (2, 26, 29, 40, 42) and further metabolized, presumably via carboxylation to 3-phenyl-3-oxopropionate, thioesterification with CoA, and thiolytic cleavage into acetyl-CoA and benzoyl-CoA (2, 13, 40, 42); the latter compound is the first intermediate that this pathway has in common with the anaerobic pathway of toluene. Degradation of ethylbenzene with sulfate as the electron acceptor was demonstrated with contaminated subsurface samples and enrichment cultures (18, 35, 37). The results of analyses of metabolites were not indicative of ethylbenzene degradation via dehydrogenation reactions like those in denitrifying bacteria. Rather, identification of (1-phenylethyl)succinate (3-phenyl-1,2-butanedicarboxylate) indicated that there is an activation mechanism analogous to the mechanism for activation of toluene with fumarate as a cosubstrate (18).
The present study was undertaken to isolate sulfate-reducing bacteria that are able to utilize ethylbenzene anaerobically and to investigate the physiology of these bacteria in pure culture. Sulfate-reducing bacteria play a key role in the anaerobic mineralization of organic compounds in marine sediments due to the abundance of sulfate as an electron acceptor in seawater (concentration, 28 mM) (27, 54). Accordingly, the greatest nutritional diversity of sulfate-reducing bacteria has been observed among marine isolates (38, 55). Therefore, we used marine sediment samples as starting inocula for the present study. Use of these samples indeed led to enrichment and isolation of a metabolically novel type of sulfate-reducing bacterium that grows anaerobically with ethylbenzene as the only electron donor and source of organic carbon.
MATERIALS AND METHODS
Sources of bacteria.
Enrichment of sulfate-reducing bacteria that utilize ethylbenzene was attempted with marine sediment samples from Canale Grande in Venice (Italy), from the Bay of Arcachon (France), from the Wadden Sea in the North Sea at Horumersiel (Germany), from Eel Pond in Woods Hole, Mass., and from Guaymas Basin in the Gulf of California (Mexico) at 2,000 m depth. The Guaymas Basin sample was obtained during the R/V Atlantis II cruise guided by Holger Jannasch (who died in September 1998) in April and May 1998 by using the submersible ALVIN (53).
Media, cultivation techniques, and quantitative growth experiments.
The techniques used for preparation of media and for cultivation of sulfate-reducing bacteria under anoxic conditions have been described previously (55). Briefly, cultures were grown in defined bicarbonate-CO2-buffered (pH 7.3), strictly anoxic artificial seawater medium. The amounts of a nonchelated trace element solution (55) added (per liter of medium) were 1 ml for enrichment cultures and 2 ml for pure cultures. The culture medium and cultures were always kept in butyl rubber-sealed bottles or tubes under a headspace containing an N2-CO2 mixture (9:1, vol/vol). Enrichment cultures were grown in flat 250-ml bottles containing 200 ml of medium. For routine cultivation (including substrate tests) of the pure isolate, 20-ml tubes containing 15 ml of medium were used. Filter-sterilized (solvent-resistant cellulose filters; pore size, 0.2 μm) ethylbenzene (purity, >99%) and other hydrocarbons were diluted (0.5 to 5%, vol/vol) in sterile, anoxic 2,2,4,4,6,8,8-heptamethylnonane (HMN) as the carrier phase (4 ml per bottle; 0.5 ml per tube) to avoid toxic effects of the pure substances (41, 42). Tubes with overlaid insoluble hydrocarbon phases were incubated nearly horizontally to facilitate diffusion of the added hydrocarbons into the aqueous medium. The sealed orifices were kept below the medium surface to avoid contact of the overlying hydrocarbon phase with the stoppers (1, 41, 42). Nonhydrocarbon substrates were added from sterile aqueous stock solutions (55) in order to obtain the concentrations indicated below.
The time course of sulfate reduction to sulfide with ethylbenzene as the electron donor was measured in the flat bottles described above. Aliquots for determination of the optical density at 660 nm and sulfide content were withdrawn from the closed bottles via the stoppers by using sterile syringes. Consumption of sulfate and ethylbenzene and formation of sulfide, cell protein, and excreted metabolites by strain EbS7 were quantified in separate growth experiments by using butyl rubber-sealed 100-ml bottles containing 70 ml of medium, 2 ml of HMN, and the amounts of ethylbenzene indicated below (see Table 2).
TABLE 2.
Quantification of substrates consumed and products formed during growth of strain EbS7 with ethylbenzenea
| Expt | Amt of ethylbenzene added (μmol) | Amt of ethylbenzene that disappeared (μmol)b | Amt of sulfate that disappeared (μmol) | Amt of 4-phenyl- pentanoate formed (μmol) | Amt of cell dry mass formed (mg)c | Amt of electrons available for sulfate reduction (μmol)d | Amt of electrons consumed by sulfate reduction (μmol)e | Electron balancef |
|---|---|---|---|---|---|---|---|---|
| Cells with limiting amount of ethylbenzene | 44 | 44 | 223 | 1.5 | 1.4 | 1,628 | 1,520 | 1.07/1 |
| Cells with excess ethylbenzene | 244 | 168 | 683 | 27.4 | 3.8 | 5,042 | 5,200 | 0.97/1 |
| Cells without ethylbenzene (control) | 0 | 0 | 33 | 1.1 | <0.2 | |||
| Sterile medium without cells | 242.6 | 0 | 0 | 0 |
Incubation experiments (separate from the growth experiment whose results are shown in Fig. 4) were carried out in anoxic flat bottles with a culture volume of 70 ml. The medium was overlaid with 2 ml of HMN as the carrier phase for ethylbenzene.
Difference between the amount of ethylbenzene added and the amount of ethylbenzene recovered at the end of incubation in the carrier phase and the aqueous phase.
The amount of cell dry mass was determined by measuring the amount of protein, assuming that the protein accounted for 50% (wt/wt) of the dry mass. The amount of cell mass present at the beginning of the experiment was subtracted.
The electrons available for sulfate reduction were the electrons derived from consumed ethylbenzene, corrected by subtraction of the electrons present in the 4-phenylpentanoate excreted and the cell mass formed. Stoichiometrically, 1 mol of ethylbenzene corresponds to 42 mol of electrons, whereas 1 mol of 4-phenylpentanoate corresponds to 54 mol of electrons. One milligram (dry weight) of cells (approximate net formula, C4H7O3) contains 165 μmol of electrons. Before calculation of the electrons available for sulfate reduction, the values for 4-phenylpentanoate and cell dry mass were corrected by subtraction of the corresponding values obtained for the control without ethylbenzene.
Stoichiometrically, 8 mol of electrons is required for reduction of 1 mol of SO42− to H2S. The correct amount of sulfate reduced was obtained by subtraction of the amount consumed in the control without ethylbenzene.
Ratio of the amount of electrons from dissimilated ethylbenzene to the amount of electrons consumed by sulfate reduction.
For characterization of metabolites, strain EbS7 was cultivated in butyl rubber-sealed flat 500-ml glass bottles containing 400 ml of artificial seawater medium. The cultures contained 4% (vol/vol) ethylbenzene in HMN (10 ml per bottle). In control experiments, strain EbS7 was incubated with 3 mM 3-phenylpropionate or 4 mM n-hexanoate instead of ethylbenzene, or ethylbenzene was incubated with sterile medium.
Isolation, purity control, and maintenance.
Strain EbS7 was isolated by serial dilution by using anoxic medium mixed with molten agar as the gelling agent (55). The dilution of the artificial seawater medium with agar that had been prepared with pure water was compensated for by addition of a concentrated salt mixture to each tube (55). The solidified agar medium (9 ml) in each tube was overlaid with 0.5 ml of HMN containing 2% (vol/vol) ethylbenzene. All agar tubes were kept anoxic. In subsequent agar dilution series, ethylbenzene was replaced by 3-phenylpropionate (3 mM) as a soluble substrate that was used without a carrier phase.
The purity of the isolated strain was routinely checked by phase-contrast microscopy of cultures grown with ethylbenzene and other utilizable substrates. In addition, inoculated media containing glucose (5 mM), fructose (5 mM), or AC medium (3 g/liter; Difco Laboratories, Detroit, Mich.) were incubated and checked by microscopy. For maintenance, strain EbS7 was grown on ethylbenzene or 3-phenylpropionate and stored at 4°C; transfers were made every 3 months.
Sequence analyses of the 16S rRNA gene.
DNA was released from cells by repeated rapid freezing and thawing (45). The previously described primers 8F (5′-AGAGTTTGATCMTGG-3′) and 1492R (5′-TACCTTGTTACGACTT-3′) were used for PCR amplification of the 16S rRNA gene (10). The purified PCR product was sequenced by GAG BioScience (Bremen, Germany) with an automated sequencer (ABI 377; Perkin-Elmer, Langen, Germany) by using dye-labeled dideoxynucleotides (14).
The retrieved sequence was added to the 16S rRNA gene database of the Technical University of Munich (release December 1998) by using the program package ARB (50). The ARB_ALIGN tool was used for automatic sequence alignment. The resulting alignments were checked by eye and corrected manually by considering the secondary structure of the rRNA molecule. The ARB database was supplemented by importing all 16S rRNA gene sequences that are available for the δ subclass of Proteobacteria. Tree topologies were evaluated by performing maximum-parsimony, neighbor-joining, and maximum-likelihood analyses. Only sequences that were at least 90% complete were used for tree construction. Trees were constructed either without filters or with a 50% filter. This filter takes into account only positions at which at least 50% of the sequences in a selected cluster have the same residue.
Chemical and other analyses.
A simple test with CuSO4 (5 mM in 50 mM HCl) that yielded CuS (16) was routinely used for detection of formed sulfide in enrichment cultures compared to controls without an organic substrate. Sulfide contents in quantitative growth experiments were determined colorimetrically by using the methylene blue formation reaction in a microassay (1). Sulfate contents were determined gravimetrically as BaSO4 precipitated in a microassay (1).
Ethylbenzene contents in the carrier phase and in the aqueous phase were measured by gas chromatography and by high-performance liquid chromatography (HPLC), respectively, as described previously for xylenes (21).
Possible production of short-chain fatty acids was examined directly in filtered (pore size, 0.2 μm) culture supernatant by using an HPLC system (Sykam, Gilching/Munich, Germany) equipped with an SS-100 H+ column (7.8 by 300 mm; Sierra Separations, Sparks, Nev.). Samples were eluted with 5 mM H2SO4 in water at a flow rate of 0.6 ml min−1 and a column temperature of 60°C. Fatty acids were detected by UV absorption at 210 nm (Sykam).
Metabolites that could give hints concerning the initial reactions in ethylbenzene degradation were searched for as described previously (44), with slight modifications. Heat-inactivated cultures (400 ml) of strain EbS7 were extracted three times at pH 1.5 (acidified with 5 M HCl) by using dichloromethane (80 ml) instead of diethyl ether. Organic extracts were concentrated by evaporation to 4 ml and were methylated by using an etheral solution of diazomethane (44) that was freshly prepared from Diazald (Sigma-Aldrich, Deisenhofen, Germany). Methylated extracts were analyzed by gas chromatography-mass spectrometry as described previously (44). The identity of the major metabolite with an authentic standard of 4-phenylpentanoic acid (Sigma-Aldrich, Milwaukee, Wis.) was verified by coinjection of the methyl esters into two different fused silica capillary columns (with the hydrophobic coating purely aliphatic or partly aromatic) as described previously (44). The structure of the standard was confirmed by 1H and 13C nuclear magnetic resonance spectroscopy (44).
The 4-phenylpentanoate in culture supernatants for calculation of the electron balance (see Table 2) was quantified by HPLC as described previously for other polar aromatic compounds (43). Identification was based on the retention time and the absorption spectrum compared to those of the standard.
Whole-cell protein contents were determined by using the Coomassie brilliant blue dye binding method (9). To solubilize proteins prior to the assay, cells from 1 ml of culture medium were centrifuged (11,000 × g, 40 min) and mixed with 0.5 ml of a solution containing 0.25 M NaOH and 5 mM EDTA. The cell suspension was incubated for 10 min at 95°C. Bovine serum albumin was used as the standard.
The G+C content of isolated DNA (12) was determined by HPLC (34) at the German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany.
Nucleotide sequence accession number.
The nearly complete 16S rRNA gene sequence of strain EbS7 has been deposited in the EMBL database under accession number AJ 430774.
RESULTS
Enrichment and isolation.
Enrichment of ethylbenzene-degrading sulfate-reducing bacteria was attempted with anoxic marine sediment samples from five different locations in Western Europe and North America. Distinct production of sulfide (≥4 mM) in the presence of ethylbenzene compared to sulfide production in controls (around 3 mM) was observed after 2 to 3 months (samples from Venice and Arcachon Bay) and after 6 to 7 months (other samples). After transfer of aliquots (inoculum size, 10% [vol/vol]) to fresh media, ethylbenzene-dependent sulfide production became more rapid (10 mM within 6 to 8 weeks), whereas controls without ethylbenzene no longer produced sulfide. In all cases, oval cells with gas vesicles were revealed by microscopy. Growth of cells became most rapid in subcultures originating from Guaymas Basin sediment, which yielded a sediment-free enrichment culture with homogeneous cell turbidity after four transfers. Only this enrichment culture was used for further experiments.
Agar dilutions of the enrichment culture from Guaymas Basin sediment yielded small yellowish to brownish colonies after 3 months. Agar tubes with low dilution factors (≥1/100) contained distinct bands of tiny bacterial colonies (diameter, <0.1 mm) that were 1 to 2 mm from the overlying hydrocarbon phase but did not contain colonies deeper in the agar. In contrast, agar tubes with higher dilution factors (≤1/1,000) contained fewer and bigger colonies (diameter, 0.1 to 0.5 mm) throughout the agar. Obviously, the high cell density and resulting high numbers of colonies at low dilutions resulted in complete scavenging of ethylbenzene in the upper parts of the agar columns. Eight colonies were transferred to liquid medium (15 ml) with ethylbenzene; they all exhibited growth within 2 months and consistently yielded the cell type observed in the enrichment cultures. To avoid the use of a carrier phase for further purification in agar, preliminary growth tests were carried out with benzoate, phenylacetate, and 3-phenylpropionate (3 mM each) as polar aromatic compounds that could be directly added to the medium. The results showed that 3-phenylpropionate allowed more rapid growth than ethylbenzene and phenylacetate, whereas benzoate was not used. 3-Phenylpropionate was therefore used to prepare subsequent agar dilution series. All colonies isolated from these dilution series were able to grow with the original substrate, ethylbenzene, in liquid medium. One strain, designated EbS7, was used for further characterization.
Morphological and other characteristics.
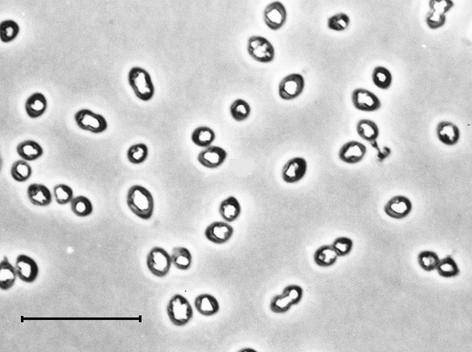
Cells of strain EbS7 were rod shaped and nonmotile and formed gas vesicles towards the end of growth (Fig. 1). The cells were 1.5 by 2 to 2.3 μm. Growth of strain EbS7 was observed at temperatures ranging from 12 to 35°C (lower temperatures were not tested), and the optimum temperature was 31 to 32°C; the pH range for growth was 6.1 to 7.8, and the optimum pH was around 7.5. Growth was also observed if the original concentrations of NaCl and MgCl2 in the artificial seawater medium (26.4 and 5.7 g per liter, respectively [55]) were decreased to 10 and 1.7 g per liter, respectively, but growth was not observed in a freshwater medium containing only 1 g of NaCl and 0.4 g of MgCl2 per liter. The most rapid growth was observed with 2, 3, and 5% (vol/vol) ethylbenzene in the carrier phase (HMN). When the concentration of ethylbenzene in the carrier phase was 2%, the concentration in the aqueous phase was around 25 μM.
FIG. 1.
Phase-contrast photomicrograph of the newly isolated marine sulfate-reducing strain EbS7 grown with ethylbenzene. Cells contain gas vesicles that are formed towards the end of growth. Bar, 10 μm.
Relationships based on 16S rRNA gene sequences.
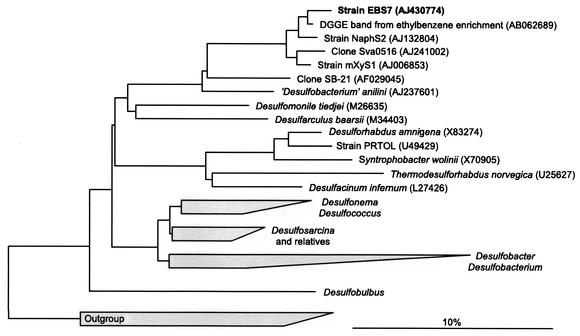
Analyses of the 16S rRNA gene sequence revealed that strain EbS7 is affiliated with the δ subclass of the class Proteobacteria (Fig. 2). The closest relatives are sulfate-reducing strains NaphS2 and mXyS1 (97.6 and 96.2% similarity, respectively), which were isolated with naphthalene (19) and m-xylene (21), respectively. Furthermore, the database revealed identity with 550 bp of a 16S rRNA gene sequence retrieved from an enrichment culture containing ethylbenzene and sediment from Tokyo Bay (35). There was even similarity (95.8%) with a cloned 16S rRNA gene retrieved from arctic sediment (45).
FIG. 2.
Phylogenetic 16S rRNA-based tree reflecting the relationships of the newly isolated sulfate-reducing strain EbS7 to selected members of the δ subclass of the class Proteobacteria. Only nearly full-length sequences (>1,300 bases) were used for the calculations. The tree was corrected by considering the different results obtained with various tree reconstruction algorithms. Members of the α, β, and γ subclasses of the Proteobacteria were used as an outgroup. The scale bar indicates an estimated sequence divergence of 10%. Clones Sva0516 and SB-21 were retrieved from arctic sediment (45) and a sulfate-reducing enrichment culture with benzene (37), respectively. Strain PRTOL is a toluene-degrading sulfate-reducing bacterium (6); strain Tol2, another toluene-degrading sulfate-reducing bacterium, belongs to the Desulfobacter-Desulfobacterium cluster (41). Desulfarculus baarsii is the revised name of Desulfovibrio baarsii (55). The genus name of the former Desulfobacterium anilini is no longer valid; the line of descent of this species should be renamed. DGGE, denaturing gradient gel electrophoresis.
Substrate utilization.
The results of growth tests of strain EbS7 with various aromatic and aliphatic compounds are shown in Table 1 (several properties are compared to those of the related strains NaphS2 and mXyS1). Of the hydrocarbons tested, strain EbS7 utilized only ethylbenzene for growth. The most rapid growth and highest cell densities and concentrations of sulfide produced were observed when 3-phenylpropionate was the organic substrate. With 4 mM 3-phenylpropionate, a sulfide concentration of 18 mM was reached in 4 to 6 weeks, whereas with ethylbenzene the sulfide concentrations were never higher than 9 mM in 10 to 12 weeks (inoculum size, 10% [vol/vol]).
TABLE 1.
Characteristics of the newly isolated sulfate-reducing strain EbS7 and comparison of selected properties of this strain to those of the phylogenetically closely related strains NaphS2 and mXyS1
| Strain | Cell size (μm) | G + C content of DNA (mol %) | Utilization of electron donors and carbon sourcesd
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toluene (1 and 2%) | Ethylbenzene (≤10%) | m-Xylene (1 and 2%) | m-Ethyltoluene (1 and 2%) | Naphthalene (1 and 2%) | Benzoate | Phenylacetate (1 and 4 mM) | 3-Phenylpropionate (1 and 4 mM) | Formate(10 and 20 mM)e | Acetate (5 and 10 mM) | n-Hexanoate (1 and 4 mM) | Lactate (1 and 5 mM) | Pyruvate (1 and 5 mM) | |||
| EbS7 | 1.5 by 2-2.3 | 51.8 | − | + | − | − | − | − | + | + | + | − | + | + | + |
| NaphS2a | 1.3 by 1.0-1.9 | 53.2c | − | − | − | − | + | + | +c | +c | − | + | ND | − | + |
| mXyS1b | 0.6-1 by 1-2 | 49.0 | + | − | + | + | − | + | ND | ND | + | + | ND | − | + |
Data from reference 19.
Data from reference 22.
Data from A. Galushko (personal communication).
The test concentrations indicated are the concentrations used only in the present study with strain EbS7. The concentrations expressed as percentages are the concentrations in the HMN carrier phase. The following other compounds were tested with strain EbS7 but were not utilized: benzene, o-ethyltoluene, p-ethyltoluene, n-propylbenzene, and ethylcyclohexane (each at concentrations of 1 and 2% in the carrier phase); styrene (0.5 and 2%); 1-phenylethanol (0.5 and 2 mM); acetophenone (0.5 mM); 2-phenylpropionate (1 and 2 mM); 4-phenylbutyrate (1 and 4 mM); 4-phenylpentanoate (0.5, 1, and 2 mM); mandelate (0.5 and 2 mM); phenol (0.5 and 2 mM); propionate (5 and 10 mM); butyrate (3 and 6 mM); hexadecanoate (0.5 and 1 mM); succinate (1 and 5 mM); fumarate (1 and 5 mM); malate (1 and 5 mM); ethanol (1 and 5 mM); 1-propanol (1 and 5 mM); 2-propanol (1 and 5 mM); glucose (5 mM) and fructose (5 mM); and H2 (mixed with 20% CO2 in the headspace; medium with 1 mM acetate as the carbon source). +, growth observed; −, no growth observed; ND, not determined.
Without an additional organic carbon source in the medium.
Inhibition of ethylbenzene utilization by 1-phenylethanol.
1-Phenylethanol, which was not utilized as a growth substrate, was a specific inhibitor of growth of strain EbS7 on ethylbenzene. No growth occurred if 0.5 mM 1-phenylethanol was added to cultures containing 2% ethylbenzene in the carrier phase. In contrast, the growth of strain EbS7 on pyruvate (5 mM) was not affected by 1-phenylethanol (other substrates were not tested). Acetophenone (0.5 mM) did not inhibit growth with ethylbenzene or pyruvate.
Identification of metabolites.
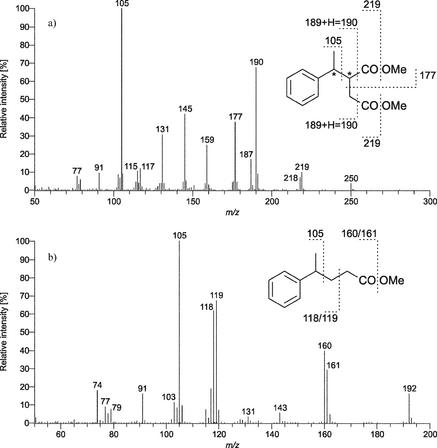
Gas chromatography-mass spectrometry analysis of methylated extracts from ethylbenzene-grown cultures of strain EbS7 revealed three metabolites. These metabolites could not be detected in cultures grown on n-hexanoate or 3-phenylpropionate or in sterile control medium incubated with ethylbenzene. Two of the metabolites detected eluted close to each other and yielded essentially the same mass spectrum (Fig. 3a). Because of its characteristic fragment ions at m/z 250, 219, 190, 187, 177, 159, 145, 131, and 105, the mass spectrum resembled that of dimethyl esters of (methylbenzyl)succinic acids that have been identified as anaerobic activation products of xylenes (4, 57). The peak at m/z 145, which is also derived from the dimethyl ester of benzylsuccinic acid formed during toluene degradation (4, 5), indicates that a succinic acid moiety (as a dimethyl ester) is present. The base peak at m/z 105 is consistent with the presence of an ethylbenzene-derived moiety (isomeric with a xylene-derived moiety). Consistent with the detection of (1-phenylethyl)succinate (analyzed as the trimethylsilyl derivative) in contaminated aquifer sediment incubated with ethylbenzene and sulfate (18), the metabolite observed in this study is expected to be the same compound (for interpretation of the two peaks see below). The other metabolite, which had a shorter retention time, yielded a different fragmentation pattern but again produced a base peak at m/z 105 indicative of a conserved ethylbenzene-derived moiety. The fragment ions were in agreement with the structure of 4-phenylpentanoic acid methyl ester (Fig. 3b). This interpretation was confirmed by using an authentic standard of this compound that yielded the same fragmentation pattern and also coeluted with the metabolite during gas chromatography on two different columns (data not shown).
FIG. 3.
Mass spectra of the metabolites that are specifically produced by cells of strain EbS7 during growth on ethylbenzene. (a) Metabolite tentatively identified as (1-phenylethyl)succinic acid dimethyl ester. (b) Metabolite identified as 4-phenylpentanoic acid methyl ester. An authentic standard yielded the same fragmentation pattern.
Quantitative growth experiments.
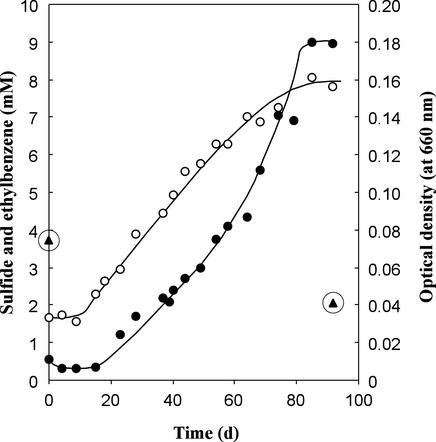
The growth and the time course of sulfide production of strain EbS7 with ethylbenzene are shown in Fig. 4. There was no pronounced exponential growth phase; rather, the increases in cell density and sulfide content appeared to be nearly linear during the whole growth phase. A semilogarithmic plot (data not shown) of points from the early growth phase revealed much scattering, so that only rough estimation of the doubling time (two experiments) was possible; the estimates of doubling time were between 7 and 10 days.
FIG. 4.
Increases in cell density (•) and sulfide concentration (○) during anaerobic growth of strain EbS7 with ethylbenzene. Ethylbenzene (▴) was quantified only at the beginning and end of growth. Ethylbenzene was dissolved in a carrier phase of HMN, but for convenience the concentration indicated was calculated by attributing it to the aqueous phase. The growth experiment was carried out in a flat 250-ml bottle containing 200 ml of medium and 0.13 ml of ethylbenzene dissolved in 6 ml of HMN as a carrier phase. The experiment was carried out separately from the growth experiments whose results are shown in Table 2.
The consumption of organic substrates and sulfate and the formation of cell protein and free metabolites during growth of strain EbS7 were quantified in separate experiments with small and large amounts of ethylbenzene. The results are shown in Table 2. The small amount of ethylbenzene was completely consumed. 4-Phenylpentanoate was detectable in the supernatants of the cultures, and the concentration of this metabolite (see above) was especially high in the culture with the large amount of ethylbenzene. The amount of ethylbenzene-derived electrons available for sulfate reduction (viz., the dissimilated portion) was calculated by subtracting the amount of electrons present in excreted 4-phenylpentanoate and the biomass formed (estimated from the amount of cell protein formed). This amount of electrons was close to the amount needed for the observed reduction of sulfate to sulfide; viz., the electron balance was nearly complete. Formation of acetate, propionate, and benzoate as end products was not detectable in the supernatants of ethylbenzene-grown cultures.
DISCUSSION
Strain EbS7 isolated from Guaymas Basin sediment is the first pure-culture strain of a sulfate-reducing bacterium that grows with the aromatic hydrocarbon ethylbenzene as the electron donor and carbon source. Morphologically very similar gas vesicle-containing cells were enriched from marine sediment samples from other locations, indicating that this type of sulfate-reducing bacterium is widespread in marine sediments. This conclusion is supported by the recent enrichment of an ethylbenzene-degrading sulfate-reducing culture from Tokyo Bay sediment (35); some of the cells of this culture also contained gas vesicles (which were smaller than those in strain EbS7). The sequence of a 16S rRNA gene fragment retrieved from this enrichment culture was identical to a sequence in the 16S rRNA gene of strain EbS7. In oil-contaminated sediment from the coastal area of Kuwait, the corresponding rRNA sequence accounted for 4.3% of the extractable rRNA (30). Guaymas Basin sediment is naturally rich in aliphatic and aromatic hydrocarbons which are formed by geothermal transformation of sedimented detritus (46). However, the actual abundance (number of cells per volume of sediment) of the sulfate reducer which we isolated remains unknown due to the use of batch cultures for the enrichment studies.
Strain EbS7 and two other closely related sulfate-reducing bacteria, strains NaphS2 and mXyS1 (Fig. 2), which utilize naphthalene and m-xylene, respectively, may be considered members of the same genus. From a taxonomic point of view, the high levels of similarity of the 16S rRNA genes, particularly those of strains EbS7 and NaphS2 (97.6%), would even justify considering these isolates members of the same species (20, 48). With respect to hydrocarbon utilization, however, the three strains exhibited striking differences (Table 1). For definite classification, additional characteristics, especially DNA-DNA hybridization data, have to be taken into consideration.
A striking morphological property of strain EbS7, as well as the cells observed in the other enrichment cultures with ethylbenzene, is the formation of gas vesicles. Gas vesicles have been observed in a few other sulfate-reducing bacteria (55). Usually, gas vesicles are assumed to cause and regulate cell buoyancy (52). However, in the case of sulfate-reducing bacteria that thrive in anoxic sediments, such a role appears to be questionable. In particular, at the high hydrostatic pressure (20 MPa) that prevails in Guaymas Basin sediment, the capacity for gas vesicle formation does not seem to offer any advantage because the formation of the gas vesicles is physically impeded (52). We speculate that this capacity is a genetically relatively stable, conserved trait.
Like an enriched ethylbenzene-degrading sulfate-reducing population in aquifer sediment (18), marine strain EbS7 apparently activates ethylbenzene by addition to fumarate and not via dehydrogenation, as in denitrifiers (25, 26, 28). This hypothesis is also in agreement with the results of substrate tests. In contrast to denitrifying strains that have the capacity to degrade ethylbenzene (2, 42), sulfate-reducing strain EbS7 does not grow on 1-phenylethanol and acetophenone (Table 1). If it is assumed that these compounds diffuse into the cell due to their hydrophobic character, the results suggest that strain EbS7 cannot metabolize 1-phenylethanol and acetophenone. Like n-alkanes, ethylbenzene is activated not at the methyl group but at a secondary carbon atom. In particular, the secondary carbon atom of ethylbenzene (benzyl carbon atom) is relatively reactive, due to its juxtaposition with the π-electron system. The C—H bond dissociation energy at this position is 358 kJ per mol, whereas the dissociation energy at the methyl group would be close to 410 kJ per mol (the value for the methyl group of n-alkanes). For comparison, the C—H bond dissociation energies at the methyl group of toluene and at the secondary carbon of an n-alkane are 368 and 398 kJ per mol, respectively (33). The presence of the tentatively identified compound (1-phenylethyl)succinate in two peaks during gas chromatography suggests that these peaks are diastereomers. One explanation for their formation is that the secondary carbon of ethylbenzene reacts with relaxed stereospecificity, as discussed previously in more detail for anaerobic activation of n-hexane (44).
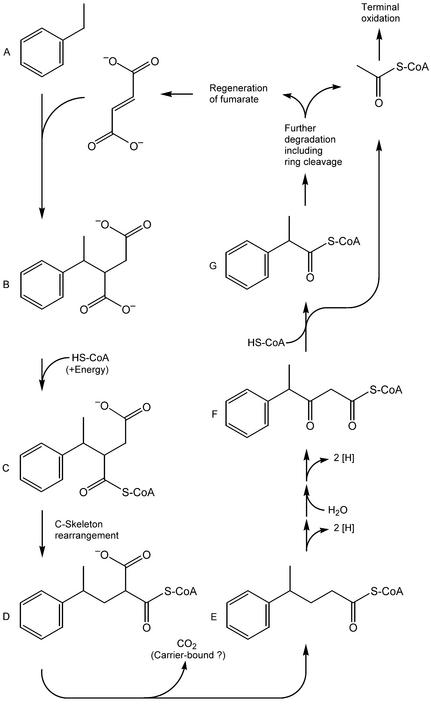
The detection of 4-phenylpentanoic acid in ethylbenzene-grown cultures of strain EbS7 indicates that the subsequent reactions for the degradation of (1-phenylethyl)succinate are analogous to those suggested for (1-methylpentyl)succinate during the anaerobic metabolism of n-hexane (58). The addition of fumarate at a subterminal position leads to a tertiary carbon atom that excludes an oxidation sequence, as in anaerobic toluene degradation (23, 31, 32, 47). For the degradation of (1-methylpentyl)succinate formed from n-hexane, a C skeleton rearrangement (possibly coenzyme B12 dependent) of the thioester followed by α-decarboxylation was suggested (58). An analogous reaction of (1-phenylethyl)succinate would lead to 4-phenylpentanoyl-CoA (Fig. 5). During sample preparation for analysis, this intermediate would give rise to the methyl ester that has been identified.
FIG. 5.
Proposed pathway for the degradation of ethylbenzene in the sulfate-reducing strain EbS7. A, ethylbenzene; B, (1-phenylethyl)-succinate; C, (1-phenylethyl)succinyl-CoA; D, (2-phenylpropyl)malonyl-CoA; E, 4-phenylpentanoyl-CoA; F, 3-oxo-4-phenyl-pentanoyl-CoA; G, 2-phenylpropionyl-CoA.
At present, there is no experimental evidence concerning further degradation of 4-phenylpentanoyl-CoA. A conventional β-oxidation step and thiolytic cleavage would lead to 2-phenylpropionyl-CoA (Fig. 3). The methyl branch in this compound prevents a second round of β-oxidation, leading to a situation like that in isobutyryl-CoA degradation. A coenzyme B12-dependent interconversion between isobutyryl-CoA and n-butyryl-CoA has been shown to occur in anaerobic catabolic reactions (36, 49, 51), as well as in biosynthetic reactions (11). By an analogous mechanism, 2-phenylpropionyl-CoA could be converted to 3-phenylpropionyl-CoA, which could undergo conventional β-oxidation to benzoyl-CoA. The finding that strain EbS7 did not utilize 4-phenylpentanoate, 2-phenylpropionate (in contrast to 3-phenylpropionate), or benzoate does not contradict the hypothesized pathway but could have been due to a lack of specifically activating enzymes. On the other hand, 2-phenylpropionate may also be further metabolized via 2-phenylmalonic semialdehyde to phenylacetate, analogous to reactions in the metabolism of isobutyrate in the sulfate-reducing organism Desulfococcus multivorans (49).
The inhibition of growth on ethylbenzene by 1-phenylethanol not only confirms the assumed diffusion of this alcohol into the cell (see above) but also supports the hypothesis that the initial reaction with ethylbenzene in strain EbS7 is analogous to the initial reaction with toluene in various anaerobes. The metabolism of toluene is specifically inhibited by benzyl alcohol (7, 8, 41, 43). Likewise, 1-phenylethanol, a structural analogon of benzyl alcohol, may interfere with the activation of ethylbenzene, a structural analogue of toluene.
The use of two completely different initial reaction sequences for the anaerobic degradation of ethylbenzene in denitrifiers and sulfate reducers can be explained from a bioenergetic point of view. For the oxidation of ethylbenzene to 1-phenylethanol that occurs in denitrifiers, the reported midpoint potentials (at pH 7) are −0.012 V (26) and 0.03 V (28). For effective conversion of ethylbenzene to 1-phenylethanol, the electron acceptor is expected to have a more positive redox potential. In extracts of the denitrifiers, benzoquinone and ferricenium, which have midpoint potentials of 0.293 V (26) and 0.38 V (28), respectively, could serve as artificial electron acceptors; the natural electron acceptor may have a similar redox potential, resulting in an irreversible net reaction. Electron acceptors with such positive redox potentials may not be compatible with the metabolism of a sulfate-reducing bacterium, in which reactions on average occur at redox potentials more negative than 0 V (38). In contrast, an alternative mechanism of activation of ethylbenzene involving hydrocarbon addition to fumarate by a radical reaction would also be feasible or even be favored at low redox potentials. The reactive residue in the hydrocarbon-activating radical enzyme is generated via the reduction of S-adenosylmethionine, yielding methionine and an adenosyl radical (23, 47, 56).
The electron balance between the amount of ethylbenzene consumed corrected for organic products (excreted 4-phenylpentanoate and cell mass) and the amount of sulfate reduced is close to 1.0 (Table 2). This can only be explained by concluding that there is complete oxidation in the energy metabolism according to the following equation: C6H5C2H5 + 5.25 SO42− + 3 H2O → 8 HCO3− + 5.25 HS− + 2.75 H+. This conclusion is further supported by the lack of acetate, propionate, or benzoate formation upon growth with ethylbenzene; these compounds have been observed in the case of incomplete oxidation of aliphatic and phenyl-substituted compounds by sulfate-reducing bacteria (37). The inability of strain EbS7 to grow with free acetate does not contradict the assumed capacity for complete substrate oxidation. Several sulfate-reducing bacteria which oxidize organic compounds other than acetate readily and completely to CO2 are unable to grow on free acetate (38), possibly due to the lack of a mechanism for acetate activation.
Acknowledgments
We are indebted to the crew of R/V Atlantis (Woods Hole, Mass.) and to Karsten Zengler (Bremen, Germany) for providing sediment samples from Guaymas Basin. We thank Manabu Fukui (Tokyo, Japan) for stimulating discussions, Alexander Galushko for providing unpublished data, and Carsten Bolm (Aachen, Germany) for providing nuclear magnetic resonance spectra for the 4-phenylpentanoate standard.
This work was supported by EU grant EVK3-1999-00043 (MATBIOPOL) and by the Max-Planck-Gesellschaft.
REFERENCES
- 1.Aeckersberg, F., F. Bak, and F. Widdel. 1991. Anaerobic oxidation of saturated hydrocarbons to CO2 by a new type of sulfate reducing bacterium. Arch. Microbiol. 156:5-14. [Google Scholar]
- 2.Ball, H. A., H. A. Johnson, M. Reinhard, and A. M. Spormann. 1996. Initial reactions in anaerobic ethylbenzene oxidation by a denitrifying bacterium, strain EB1. J. Bacteriol. 178:5755-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beller, H. R., and E. A. Edwards. 2000. Anaerobic toluene activation by benzylsuccinate synthase in a highly enriched methanogenic culture. Appl. Environ. Microbiol. 66:5503-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beller, H. R., and A. M. Spormann. 1997. Anaerobic activation of toluene and o-xylene by addition to fumarate in denitrifying strain T. J. Bacteriol. 179:670-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beller, H. R., and A. M. Spormann. 1997. Benzylsuccinate formation as a means of anaerobic toluene activation by sulfate-reducing strain PRTOL1. Appl. Environ. Microbiol. 63:3729-3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beller, H. R., A. M. Spormann, P. K. Sharma, J. R. Cole, and M. Reinhard. 1996. Isolation and characterization of a novel toluene-degrading, sulfate-reducing bacterium. Appl. Environ. Microbiol. 62:1188-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biegert, T., and G. Fuchs. 1995. Anaerobic oxidation of toluene (analogues) to benzoate (analogues) by whole cells and by cell extracts of a denitrifying Thauera sp. Arch. Microbiol. 163:407-417. [DOI] [PubMed] [Google Scholar]
- 8.Biegert, T., G. Fuchs, and J. Heider. 1996. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur. J. Biochem. 238:661-668. [DOI] [PubMed] [Google Scholar]
- 9.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 10.Buchholz-Cleven, B. E. E., B. Rattunde, and K. L. Straub. 1997. Screening for genetic diversity of isolates of anaerobic Fe(II)-oxidizing bacteria using DGGE and whole-cell hybridization. Syst. Appl. Microbiol. 20:301-309. [Google Scholar]
- 11.Burkhardt, K., N. Philippon, and J. A. Robinson. 1998. Isobutyryl-CoA mutase from streptomycetes, p. 265-271. In B. Kräutler, D. Arigoni, and B. T. Golding (ed.), Vitamin B12 and B12-proteins. Wiley-VCH, Weinheim, Germany.
- 12.Cashion, P., M. A. Holder-Franklin, J. McCully, and M. Franklin. 1977. A rapid method for the base ratio determination of bacterial DNA. Anal. Biochem. 81:461-466. [DOI] [PubMed] [Google Scholar]
- 13.Champion, K. M., K. Zengler, and R. Rabus. 1999. Anaerobic degradation of ethylbenzene and toluene in denitrifying strain EbN1 proceeds via independent substrate induced pathways. J. Mol. Microbiol. Biotechnol. 1:157-164. [PubMed] [Google Scholar]
- 14.Chen, E. Y., and P. H. Seeburg. 1985. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA 4:165-170. [DOI] [PubMed] [Google Scholar]
- 15.Coates, J. D., and R. T. Anderson. 2000. Emerging techniques for anaerobic bioremediation of contaminated environments. Trends Biotechnol. 18:408-412. [DOI] [PubMed] [Google Scholar]
- 16.Cord-Ruwisch, R. 1985. A quick method for the determination of dissolved and precipitated sulfides in cultures of sulfate-reducing bacteria. J. Microbiol. Methods 4:33-36. [Google Scholar]
- 17.Coschigano, P. W., T. S. Wehrmann, and L. Y. Young. 1998. Identification and analysis of genes involved in anaerobic toluene metabolism by strain T1: putative role of a glycine free radical. Appl. Environ. Microbiol. 64:1650-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elshahed, M. S., L. M. Gieg, M. J. McInerney, and J. M. Suflita. 2001. Signature metabolites attesting to the in situ attenuation of alkylbenzenes in anaerobic environments. Environ. Sci. Technol. 35:682-689. [DOI] [PubMed] [Google Scholar]
- 19.Galushko, A., D. Minz, B. Schink, and F. Widdel. 1999. Anaerobic degradation of naphthalene by a pure culture of a novel type of marine sulphate-reducing bacterium. Environ. Microbiol. 1:415-420. [DOI] [PubMed] [Google Scholar]
- 20.Goodfellow, M., G. P. Manfio, and J. Chun. 1997. Towards a practical species concept for cultivable bacteria, p. 25-59. In M. F. Claridge, H. A. Dawah, and M. R. Wilson (ed.), The units of biodiversity. Chapman & Hall, London, United Kingdom.
- 21.Harms, G., K. Zengler, R. Rabus, F. Aeckersberg, D. Minz, R. Rossello-Mora, and F. Widdel. 1999. Anaerobic oxidation of o-xylene, m-xylene, and homologous alkylbenzes by new types of sulfate-reducing bacteria. Appl. Environ. Microbiol. 65:999-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harwood, C. S., G. Burchardt, H. Herrmann, and G. Fuchs. 1999. Anaerobic metabolism of aromatic compounds via the benzoyl-CoA pathway. FEMS Microbiol. Rev. 22:439-458. [Google Scholar]
- 23.Heider, J., A. M. Spormann, H. R. Beller, and F. Widdel. 1999. Anaerobic bacterial metabolism of hydrocarbons. FEMS Microbiol. Rev. 22:459-473. [Google Scholar]
- 24.Hylemon, P. B., and J. Harder. 1999. Biotransformation of monoterpenes, bile acids, and other isoprenoids in anaerobic ecosystems. FEMS Microbiol. Rev. 22:475-488. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, H. A., D. A. Pelletier, and A. M. Spormann. 2001. Isolation and characterization of anaerobic ethylbenzene dehydrogenase, a novel Mo-Fe-S enyzme. J. Bacteriol. 183:4536-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, H. A., and A. M. Spormann. 1999. In vitro studies on the initial reactions of anaerobic ethylbenzene mineralization. J. Bacteriol. 181:5662-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jørgensen, B. B. 1982. Mineralization of organic matter in the sea bed—the role of sulphate reduction. Nature 296:643-645. [Google Scholar]
- 28.Kniemeyer, O., and J. Heider. 2001. Ethylbenzene dehydrogenase, a novel hydrocarbon-oxidizing molybdenum/iron-sulfur/heme enzyme. J. Biol. Chem. 276:21381-21386. [DOI] [PubMed] [Google Scholar]
- 29.Kniemeyer, O., and J. Heider. 2001. (S)-1-Phenylethanol dehydrogenase of Azoarcus sp. strain EbN1, an enzyme of anaerobic ethylbenzene catabolism. Arch. Microbiol. 176:129-135. [DOI] [PubMed] [Google Scholar]
- 30.Koizumi, Y., J. K. Kelly, T. Nakagawa, H. Urakawa, S. El-Fantroussi, S. Al-Muzaini, M. Fukui, Y. Urushigawa, and D. Stahl. 2002. Parallel characterization of anaerobic toluene- and ethylbenzene-degrading microbial consortia by PCR-denaturing gradient gel electrophoresis, RNA-DNA membrane hybridization, and DNA microarray technology. Appl. Environ. Microbiol. 68:3215-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leuthner, B., and J. Heider. 2000. Anaerobic toluene catabolism of Thauera aromatica: the bbs operon codes for enzymes of β oxidation of the intermediate benzylsuccinate. J. Bacteriol. 182:272-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leutwein, C., and J. Heider. 2001. Succinyl-CoA:(R)-benzylsuccinate CoA-transferase: an enzyme of the anaerobic toluene catabolic pathway in denitrifying bacteria. J. Bacteriol. 183:4288-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMillen, D. F., and D. M. Golden. 1982. Hydrocarbon bond dissociation energies. Annu. Rev. Phys. Chem. 33:493-532. [Google Scholar]
- 34.Mesbah, M., U. Premachandran, and W. B. Whitman. 1989. Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int. J. Syst. Bacteriol. 39:159-167. [Google Scholar]
- 35.Nakagawa, T., S. Sato, Y. Yamamoto, M. Fukui. 2001. Successive changes in community structure of an ethylbenzene-degrading sulfate-reducing consortium. Water Res. 36:2813-2823. [DOI] [PubMed] [Google Scholar]
- 36.Oude Elferink, S. J. W. H., P. N. L. Lens, C. Dijkema, and A. J. M. Stams. 1996. Isomerization of butyrate to isobutyrate by Desulforhabdus amnigenus. FEMS Microbiol. Lett. 142:237-241. [Google Scholar]
- 37.Phelps, C. D., and L. Y. Young. 2001. Biodegradation of BTEX under anaerobic conditions: a review. Adv. Agron. 70:329-357. [Google Scholar]
- 38.Rabus, R., T. Hansen, and F. Widdel. 2000. Dissimilatory sulfate- and sulfur-reducing prokaryotes. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes—an evolving electronic resource for the microbiological community. [Online.] Springer, New York, N.Y. http://www.prokaryotes.com.
- 39.Rabus, R., and J. Heider. 1998. Initial reactions of anaerobic metabolism of alkylbenzenes in denitrifying and sulfate-reducing bacteria. Arch. Microbiol. 170:377-384. [Google Scholar]
- 40.Rabus, R., M. Kube, A. Beck, F. Widdel, and R. Reinhardt. 2002. Genes involved in the anaerobic degradation of ethylbenzene in a denitrifying bacterium, strain EbN1. Arch. Microbiol. 178:506-516. [DOI] [PubMed] [Google Scholar]
- 41.Rabus, R., R. Nordhaus, W. Ludwig, and F. Widdel. 1993. Complete oxidation of toluene under strictly anoxic conditions by a new sulfate-reducing bacterium. Appl. Environ. Microbiol. 59:1444-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabus, R., and F. Widdel. 1995. Anaerobic degradation of ethylbenzene and other aromatic hydrocarbons by new denitrifying bacteria. Arch. Microbiol. 163:96-103. [DOI] [PubMed] [Google Scholar]
- 43.Rabus, R., and F. Widdel. 1995. Conversion studies with substrate analogues of toluene in a sulfate-reducing bacterium, strain Tol2. Arch. Microbiol. 164:448-451. [DOI] [PubMed] [Google Scholar]
- 44.Rabus, R., H. Wilkes, A. Behrends, A. Armstroff, T. Fischer, A. J. Pierik, and F. Widdel. 2001. Anaerobic initial reaction of n-alkanes in a denitrifying bacterium: evidence for (1-methylpentyl)succinate as initial product and for involvement of an organic radical in n-hexane metabolism. J. Bacteriol. 183:1707-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravenschlag, K., K. Sahm, J. Pernthaler, and R. Amann. 1999. High bacterial diversity in permanently cold marine sediments. Appl. Environ. Microbiol. 65:3982-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simoneit, B. R. T., O. E. Kawka, and M. Brault. 1988. Origin of gases and condensates in the Guaymas basin hydrothermal system (Gulf of California). Chem. Geol. 71:169-182. [Google Scholar]
- 47.Spormann, A. M., and F. Widdel. 2000. Metabolism of alkylbenzenes, alkanes, and other hydrocarbons in anaerobic bacteria. Biodegradation 11:85-105. [DOI] [PubMed] [Google Scholar]
- 48.Stackebrandt, E., and B. M. Goebel. 1994. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846-849. [Google Scholar]
- 49.Stieb, M., and B. Schink. 1989. Anaerobic degradation of isobutyrate by methanogenic enrichment cultures and by a Desulfococcus multivorans strain. Arch. Microbiol. 151:126-132. [Google Scholar]
- 50.Strunk, O., O. Gross, B. Reichel, M. May, S. Hermann, N. Stuckmann, B. Nonhoff, M. Lenke, T. Ginhart, A. Vilbig, T. Ludwig, A. Bode, K.-H. Schleifer, and W. Ludwig. 2002. ARB: a software environment for sequence data. [Online.] Department of Microbiology, Technische Universität München, Munich, Germany. http://www.arb-home.de.
- 51.Tholozan, J.-L., E. Samain, and J.-P. Grivet. 1988. Isomerization between n-butyrate and isobutyrate in enrichment cultures. FEMS Microbiol. Ecol. 53:187-191. [Google Scholar]
- 52.Walsby, A. E. 1994. Gas vesicles. Microbiol. Rev. 58:94-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber, A., and B. B. Jørgensen. 2001. Bacterial sulfate reduction in hydrothermal sediments of the Guaymas Basin of California, Mexico. Deep-Sea Res. Part I 49:827-841. [Google Scholar]
- 54.Widdel, F. 1988. Microbiology and ecology of sulfate- and sulfur reducing bacteria, p. 469-585. In A. J. B. Zehnder (ed.), Biology of anaerobic microorganisms. John Wiley & Sons, New York, N.Y.
- 55.Widdel, F., and F. Bak. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p. 3352-3378. In A. Balows, H. G. Trüper, W. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer, Berlin, Germany.
- 56.Widdel, F., and R. Rabus. 2001. Anaerobic biodegradation of saturated and aromatic hydrocarbons. Curr. Opin. Biotechnol. 12:259-276. [DOI] [PubMed] [Google Scholar]
- 57.Wilkes, H., C. Boreham, G. Harms, K. Zengler, and R. Rabus. 2000. Anaerobic degradation and carbon isotopic fractionation of alkylbenzenes in crude oil by sulphate-reducing bacteria. Org. Geochem. 31:101-115. [Google Scholar]
- 58.Wilkes, H., R. Rabus, T. Fischer, A. Armstroff, A. Behrends, and F. Widdel. 2002. Anaerobic degradation of n-hexane in a denitrifying bacterium: further degradation of the initial intermediate (1-methylpentyl)succinate via C-skeleton rearrangement. Arch. Microbiol. 177:235-243. [DOI] [PubMed] [Google Scholar]
- 59.Zengler, K., J. Heider, R. Rossello-Mora, and Widdel, F. 1999. Phototrophic utilization of toluene under anoxic conditions by a new strain of Blastochloris sulfovirides. Arch. Microbiol. 172:204-212. [DOI] [PubMed] [Google Scholar]
- 60.Zwolinski, M. D., R. F. Harris, and W. J. Hickey. 2000. Microbial consortia involved in the anaerobic degradation of hydrocarbons. Biodegradation 11:141-158. [DOI] [PubMed] [Google Scholar]