Abstract
Class I bacteriocins (lantibiotics) and class II bacteriocins are antimicrobial peptides secreted by gram-positive bacteria. Using two lantibiotics, lacticin 481 and nisin, and the class II bacteriocin coagulin, we showed that bacteriocins can be detected without any purification from whole producer bacteria grown on plates by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF-MS). When we compared the results of MALDI-TOF-MS performed with samples of whole cells and with samples of crude supernatants of liquid cultures, the former samples led to more efficient bacteriocin detection and required less handling. Nisin and lacticin 481 were both detected from a mixture of their producer strains, but such a mixture can yield additional signals. We used this method to determine the masses of two lacticin 481 variants, which confirmed at the peptide level the effect of mutations in the corresponding structural gene.
Bacteriocins are proteinaceous antimicrobial molecules that are ribosomally synthesized and secreted by gram-positive bacteria. In the past 15 years, numerous studies have focused on bacteriocins produced by lactic acid bacteria. This interest was fuelled by the possibility of using bacteriocins as food preservatives (3). Four classes of bacteriocins have been proposed (12); whereas class III bacteriocins are large heat-labile proteins and the uncertain class IV regroups complex bacteriocins that include lipid or carbohydrate components, bacteriocins belonging to classes I and II are peptides that are also designated antimicrobial peptides (AMPs). Class I and II bacteriocins were the subjects of most of the previous studies since they are more frequently found and their stability offers greater application potential than the bacteriocins belonging to the other classes. Class I AMPs are called lantibiotics because they harbor rare amino acids, such as 2,3-didehydroalanine, 2,3-didehydrobutyrine, lanthionine, and 3-methyllanthionine (12, 16, 23). These unusual amino acids are created by enzymatic modifications of a precursor peptide; 2,3-didehydroalanine and 2,3-didehydrobutyrine are obtained by dehydration of serine and threonine, respectively, and lanthionine and 3-methyllanthionine result from the formation of a thioether bond between a dehydrated residue and a cysteine. Linear and globular lantibiotics (types A and B, respectively) are distinguished on the basis of the structure imposed by the position of the thioether bridges. Type A includes both elongated lantibiotics (subgroup AI) and AMPs with a cross-bridged C terminus and an unbridged N-terminal part (subgroup AII). Nisin (Fig. 1A), which so far is the only bacteriocin widely used in the food industry (4), and lacticin 481 (Fig. 1B) are representatives of subgroups AI and AII, respectively (16). Class II bacteriocins are nonlantibiotic heat-stable peptides (12, 17). This class has been divided into three subclasses, and subclass IIa AMPs are of particular interest because of their antilisterial activity. This subclass includes the much-studied compound pediocin PA1/AcH (17) and the closely related compound coagulin (14).
FIG. 1.
Lantibiotics nisin A and lacticin 481 and the mutations leading to the lacticin 481 variants S4T and ΔS27. (A and B) Amino acid sequences and bridging patterns of nisin A and of lacticin 481 (16) and its variants. The unusual residues are 2,3-didehydroalanine (Dha), 2,3-didehydrobutyrine (Dhb), lanthionine (alanine-S-alanine), 3-methyllanthionine (aminobutyric acid-S-alanine), and aminobutyric acid (Abu). (C) Mutations created within the lacticin 481 structural gene lctA. This gene codes for a precursor peptide, the C-terminal part of which (propeptide) yields mature lacticin 481 after creation of the unusual residues and cleavage of the N-terminal part (leader peptide) (18). Only the propeptide-encoding part of lctA is shown, and the nucleotide sequence is numbered as it is in the GenBank database (accession no. U91581). The propeptide sequence is indicated above the nucleotide sequence. Lysine +1 is the N-terminal amino acid of mature lacticin 481. The asterisks indicate stop codons. The mutated bases and amino acids are indicated by boldface type and underlining, and the changes in the DNA and peptide sequences are indicated below and above the vertical arrows, respectively.
Mass spectrometry (MS) methods allow accurate determination of peptide masses and have often been used to characterize class I and II bacteriocins. In most of the cases, the AMPs were ionized by an electrospray procedure, which required that they were purified before the analysis (9, 18). Matrix-assisted laser desorption ionization (MALDI) is a second ionization method that is becoming increasingly popular due to its use in proteomic studies (15). MALDI-time of flight MS (MALDI-TOF-MS) is effective for peptides and proteins with molecular masses ranging from 0.5 to 30 kDa and has been used to determine the masses of purified class I and II bacteriocins (7, 14, 26). AMP purification is, however, not absolutely required since the lantibiotics ericin S and ericin A were both detected from the same culture supernatant after only butanolic extraction (26). Furthermore, the lantibiotic subtilin and its precursors were detected from crude culture supernatants (27). In studies unrelated to bacteriocins, MALDI-TOF-MS was performed directly with whole bacterial cells in order to obtain mass spectral fingerprints that allowed taxonomic identification of bacteria (2, 10, 13). Depending on whether bacterial lysis occurs during sample preparation, the peaks appearing on the spectra result either from desorbed cell surface biomarkers or from cytoplasmic proteins (13, 22). The preparation of samples requires only very limited handling, and the greatest advantage of this technique lies in its speed. Here, we used lacticin 481, nisin, and coagulin producer strains to show that class I and II bacteriocins constitute biomarkers that are easily detected from intact bacteria by MALDI-TOF-MS.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Lactococcus lactis and Bacillus coagulans were grown in GM17 medium (Difco Laboratories, Detroit, Mich.) at 30°C and in MRS medium (Difco) at 37°C, respectively, without shaking. Solid media included 1.5% agar. L. lactis strains containing pIL252- or pIL253-based plasmids were grown in the presence of 10 μg of erythromycin ml−1. Prior to MALDI-TOF-MS analyses of bacterial colonies, L. lactis and B. coagulans were inoculated from frozen stocks into liquid media, grown to high densities, and isolated on plates. The resulting preparations were incubated until colonies that were about 1 mm in diameter were obtained (24 to 48 h for L. lactis and 72 to 120 h for B. coagulans). The culture supernatants that were used for MALDI-TOF-MS analyses and/or bacteriocin assays were recovered after centrifugation (12,000 × g for 1 min) of overnight L. lactis liquid cultures or 3-day B. coagulans liquid cultures. When required, the supernatants were diluted in the corresponding fresh medium. Escherichia coli was grown in Luria-Bertani medium (24) at 37°C with vigorous agitation in the presence of 100 μg of ampicillin ml−1 or 10 μg of tetracycline ml−1 when necessary.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference(s) or source |
|---|---|---|
| Lactococcus lactis strains | ||
| IL1403 | Plasmid-free strain, bacteriocin nonproducer | 1 |
| IL1835 | IL1403 containing pIL252 (Emr), lacticin 481 sensitive | 25 |
| LM0230 | Plasmid-free strain, bacteriocin nonproducer | 6 |
| MG1614 | Plasmid-free strain, bacteriocin nonproducer | 8 |
| CNRZ481 | Wild-type lacticin 481-producer | 18 |
| ADRIA85LO30 | Wild-type lacticin 481-producer | 20, 28 |
| SL2 | Wild-type lacticin 481-producer | 19 |
| C2102 | ADRIA85LO30 derivative, lacticin 481 producer | 5 |
| C2109 | C2102 lacking the lacticin 481 operon, lacticin 481 nonproducer | 5 |
| IL1403(pEB94) | Recombinant strain, high-level lacticin 481 producer | 21 |
| IL1403(pBS-pIL253) | IL1403 carrying the vector part of pEB94, bacteriocin nonproducer | 21 |
| IL1403(pEB170) | Recombinant strain, low-level lacticin 481 producer | 21 |
| IL1403(pEBΔS27) | Recombinant strain producing the lacticin 481 variant ΔS27 | This study |
| IL1403(pEBS4T) | Recombinant strain producing the lacticin 481 variant S4T | This study |
| NCDO1402 | Cheese starter, nisin producer | NCIMB, Aberdeen, United Kingdom |
| Bacillus coagulans strains | ||
| I4 | Wild-type coagulin producer | 11, 14 |
| CIP52.64 | Bacteriocin nonproducer | Institut Pasteur, Paris, France |
| Escherichia coli strains | ||
| JM109 | Host for cloning and plasmid propagation | 24 |
| ES1301 mutS | Mismatch repair-negative strain for site-directed mutagenesis | Promega |
| Plasmids | ||
| pALTER-1 | E. coli vector for site-directed mutagenesis, Tetr | Promega |
| pBluescript (pBS) | E. coli cloning vector, Apr | Stratagene |
| pIL252 | L. lactis cloning vector, low copy number, Emr | 25 |
| pIL253 | L. lactis cloning vector, high copy number, Emr | 25 |
| pBS-pIL253 | E. coli-L. lactis shuttle vector resulting from a fusion between the two vectors | 21 |
| pEB94 | Lacticin 481 operon carried by pBS-pIL253, high copy number in L. lactis | 21 |
| pEB170 | Lacticin 481 operon carried by a fusion between pBS and pIL252, low copy number in L. lactis | 21 |
| pTH999 | SacI-HindIII DNA fragment containing the lacticin 481 operon 5′ end (promoter region, lctA, and beginning of lctM) cloned in pALTER-1 | Hindré et al., submitted |
| pEFΔMTFEG | Lacticin 481 operon deleted of its promoter region and of lctA (lctMTFEG) in pBS | Hindré et al., submitted |
| pEBΔS27 | Lacticin 481 operon with the mutation ΔS27 in lctA, carried by pBS-pIL253 | This study |
| pEBS4T | Lacticin 481 operon with the mutation S4T in lctA, carried by pBS-pIL253 | This study |
Sample preparation and MALDI-TOF-MS analyses.
Bacteria were collected by sweeping sterile plastic loops across colonies and were transferred to a target plate. Each sample was overlaid with 1 μl of a matrix solution containing 3 mg of 5-chloro-2-mercaptobenzothiazole per ml in acetonitrile-methanol-water (1:1:1). When the supernatant of a liquid culture was analyzed, the matrix solution and the supernatant were mixed (1:1), and 1 μl was spotted on the target plate and allowed to dry. MALDI-TOF-MS spectra were recorded with a MALDI-L instrument (Micromass, Manchester, United Kingdom) by using a 337-nm nitrogen laser for desorption and ionization. The mass spectrometer was operated in the linear mode at an accelerating voltage of 18 kV with an ion flight path that was 0.7 m long. The delay time was 470 ns. Matrix suppression was also used, and the mass spectra were averaged over 50 to 100 individual laser shots. The laser intensity was set just above the threshold for ion production. External calibration was performed by using the [M+H]+ signals of renin, adenocorticotropic hormone, insulin oxidized B, and bovine insulin (Sigma-Aldrich Co., St. Louis, Mo.). Calibration standard mixtures contained 10 pmol of each standard μl−1 in 0.1% trifluoroacetic acid.
Lacticin 481 bioassay.
Lacticin 481 activities in culture supernatants were assayed by the diffusion method as follows. Twenty milliliters of GM17 agar at 50°C was homogeneously mixed with 1 ml of an overnight culture of L. lactis IL1835 (lacticin 481 sensitive, erythromycin resistant) (Table 1) diluted to an optical density at 600 nm of 0.03 and poured into a plate. After agar solidification, wells were created in the medium. Twofold dilutions of the lacticin 481-containing culture supernatants were prepared in fresh GM17 medium. Portions (100 μl) of the undiluted supernatant and of each dilution were loaded into the wells, and the plate was incubated overnight at 30°C. The lacticin 481 diffusing from the wells into the agar medium prevented the growth of L. lactis IL1835 around the wells, which yielded clear zones of inhibition. The number of arbitrary units (AU) per milliliter corresponded to 10 times the dilution factor that resulted in the smallest visible inhibitory halo.
Construction of lacticin 481 operons encoding lacticin 481 variants.
The lacticin 481 structural gene lctA was subjected to site-directed mutagenesis by using the Altered Site II in vitro mutagenesis system (Promega, Madison, Wis.) as recommended by the supplier. The mutagenesis reactions were performed on the insert of pTH999 which included lctA and the promoter region of the lacticin 481 operon (Table 1). The following two mutations were independently created (Fig. 1B and C): replacement of the serine codon at the 3′ end of lctA by a stop codon, leading to loss of the C-terminal serine (mutant ΔS27), and replacement of a serine codon by a threonine codon (mutant S4T). The following oligonucleotides were used in this procedure (the nonhybridizing bases are underlined): ΔS27 (5′-GTATTTACTTGCTGCTAGTAATTTTATTGAAAAG-3′) and S4T (5′-GGTGCAAAAGGCGGCACAGGAGTTATTCATAC-3′). The mutated versions of lctA were subcloned into pEFΔMTFEG (Table 1) between the SacI and NotI restriction sites (Hindré, Haras, Le Pennec, and Dufour, submitted for publication) in order to obtain complete lacticin 481 operons (20, 21). The resulting plasmids were fused to the lactococcal vector pIL253 (25), yielding pEBΔS27 and pEBS4T, which were introduced into L. lactis IL1403 to obtain production of the lacticin 481 variants (Table 1).
RESULTS AND DISCUSSION
Detection of lacticin 481 from bacterial colonies.
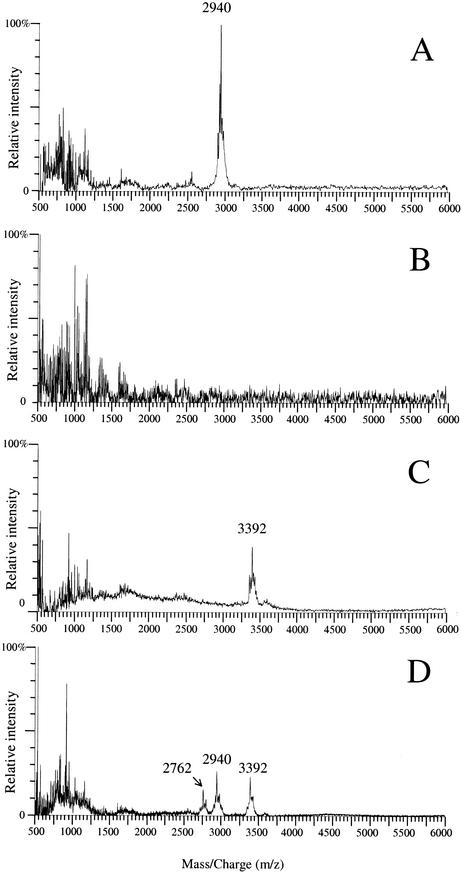
Lacticin 481 is a 2,901-Da lantibiotic (Fig. 1B) produced by some L. lactis strains (Table 1) (18, 19, 20, 28). When whole bacteria grown on a plate were analyzed by MALDI-TOF-MS, a peak cluster covering the expected m/z value (m/z is the mass/charge ratio, where z is usually 1) was observed in the spectra from three different wild-type strains that produce lacticin 481, strains ADRIA85LO30 (Fig. 2A), CNRZ481, and SL2 (data not shown) (Table 2). In contrast, no peak appeared in the m/z range from 2,000 to 6,000 in spectra recorded for three L. lactis strains that are not bacteriocin producers, strains IL1403 (Fig. 2B), LM0230, and MG1614 (data not shown) (Table 2). The peak cluster specific for lacticin 481 producers was composed of three main peaks at m/z 2,902, 2,924, and 2,940, and the m/z 2,940 peak was the most intense (Fig. 3). These values were repeatedly obtained with a standard deviation of 1 and are consistent with the masses of the molecular ion [M+H]+ and the adduct ions [M+Na]+ and [M+K]+ of lacticin 481. To verify that these peaks were indeed due to lacticin 481, we compared the following two pairs of strains: L. lactis C2102 and C2109 (Table 1), both of which were obtained from the wild-type lacticin 481 producer L. lactis ADRIA85LO30 (20, 28) and which differ by the presence (C2102) or the absence (C2109) of the lacticin 481 operon (5); and L. lactis IL-1403(pEB94) and IL1403(pBS-pIL253), which carry the lacticin 481 operon on a recombinant plasmid and only its vector portion, respectively (21) (Table 1). The expected peak cluster appeared in the spectra for the two lacticin 481-producing strains but not in the spectra for their nonproducing counterparts (data not shown) (Table 2). This unambiguously demonstrated that the lacticin 481 peaks are the most intense peaks in the m/z range from 2,000 to 6,000 for colonies of producing strains. After its secretion, lacticin 481 is probably not strongly bound to the cell surface, and it should thus be more efficiently desorbed by MALDI than cellular components, explaining why lacticin 481 constitutes one of the strongest MALDI-TOF-MS biomarkers of the producing strains.
FIG. 2.
MALDI-TOF spectra of the lacticin 481-producing strain L. lactis ADRIA85LO30 (A), of L. lactis IL1403, which does not produce any bacteriocin (B), of the nisin-producing strain L. lactis NCDO1402 (C), and of a mixture of the lacticin 481- and nisin-producing strains L. lactis ADRIA85LO30 and NCDO1402 (D).
TABLE 2.
Detection of lacticin 481 by MALDI-TOF-MS
| L. lactis strain | Lacticin 481 production | Lacticin 481 detection from:
|
Lacticin 481 activity in SN (AU · ml−1)b | |
|---|---|---|---|---|
| Colonies | SNa | |||
| IL1403 | No | − | NTc | |
| LM0230 | No | − | NT | |
| MG1614 | No | − | NT | |
| CNRZ481 | Yes | + | + | 160 |
| ADRIA85LO30 | Yes | + | + | 160 |
| SL2 | Yes | + | − | 40 |
| C2102 | Yes | + | NT | |
| C2109 | No | − | NT | |
| IL1403(pEB94) | Yes | + | + | 1,280 |
| IL1403(pBS-pIL253) | No | − | NT | |
| IL1403(pEB170) | Yes | + | − | 20 |
SN, crude supernatants of overnight liquid cultures. The same supernatants were used for lacticin 481 detection by MALDI-TOF-MS and for antimicrobial activity assays.
The values are representative of the values obtained in several experiments.
NT, not tested.
FIG. 3.
Detail of the peak cluster shown in Fig. 2A. The peaks at m/z 2,902, 2,924, and 2,940 correspond to the ions [M+H]+, [M+Na]+, and [M+K]+ of lacticin 481, respectively.
Lacticin 481 is more efficiently detected in solid cultures than in liquid cultures.
As shown in Table 2, lacticin 481 was detected by MALDI-TOF-MS in untreated supernatants of overnight liquid cultures of two wild-type strains, L. lactis CNRZ481 and ADRIA85LO30, and of the recombinant strain IL1403(pEB94). The supernatants of L. lactis CNRZ481 and ADRIA85LO30 cultures had lacticin 481 activities of 160 AU · ml−1, whereas IL1403(pEB94) exhibited an eightfold-higher activity (Table 2), since it carries the lacticin 481 operon on a high-copy-number plasmid (21) (Table 1). After the CNRZ481 and ADRIA85LO30 supernatants were diluted 1/2 and the IL1403(pEB94) supernatant was diluted 1/16, lacticin 481 was not detected by MALDI-TOF-MS or detection was very difficult, showing that the lacticin 481 activity had to be more than 80 AU · ml−1 to yield obvious peaks in mass spectra. Consistently, we were unable to detect lacticin 481 from the undiluted culture supernatants of the weaker producing strains, L. lactis SL2 (wild type) and L. lactis IL1403(pEB170), which carries the lacticin 481 operon on a low-copy-number plasmid (21) (Tables 1 and 2). In contrast, we were able to clearly detect lacticin 481 from colonies of these two strains (Table 2). MALDI-TOF-MS detection of lacticin 481 from bacterial colonies thus not only requires less sample preparation but is more efficient than MALDI-TOF-MS detection of lacticin 481 from liquid culture supernatants. A simple explanation for this observation is that AMP diffusion is limited on solid media. AMPs thus likely accumulate at the cell surface in a colony, whereas they are diluted in the whole medium of liquid cultures.
Nisin detection.
We examined if nisin A (molecular mass, 3,353 Da [23]), a subgroup AI lantibiotic (Fig. 1A), could also be detected from whole cells by MALDI-TOF-MS. Like lacticin 481, nisin is produced by L. lactis strains. Compared to other L. lactis strains (Fig. 2A and B), the nisin-producing strain L. lactis NCDO1402 (Table 1) produced a specific peak cluster, with the main peak corresponding to the nisin A [M+K]+ ion (m/z 3,392) (Fig. 2C). We were not able to detect the nisin peaks from crude supernatant of an overnight liquid culture of L. lactis NCDO1402. This confirms that bacteriocin detection from bacterial colonies is more efficient than bacteriocin detection from culture supernatants.
Detection of lacticin 481 and nisin from a mixture of strains.
We examined if lacticin 481 and nisin could both be detected when colonies of the producing strains were mixed before the bacteria were transferred to the target plate and the matrix solution was added. Figure 2D shows that the two peak clusters specific for lacticin 481 and nisin A were produced by a mixture of L. lactis ADRIA85LO30 and NCDO1402 cells. An additional peak cluster with the main peak at m/z 2,762 was observed in spectra obtained for the strain mixture (Fig. 2D) but not in spectra obtained for the two strains examined separately (Fig. 2A and C). This novel peak cluster did not appear when the control strain L. lactis IL1403 was mixed with either L. lactis ADRIA85LO30 or L. lactis NCDO1402 (data not shown), but it was observed after the nisin-producing strain L. lactis NCDO1402 was mixed with L. lactis C2109, which was derived from L. lactis ADRIA85LO30 but is unable to produce lacticin 481 (Table 1) (data not shown). The peak cluster at m/z 2,762 is therefore strain dependent but lacticin 481 independent. It is thus possible to detect several bacteriocins in a mixture of producer strains, but one should be cautious when interpreting the resulting spectra as this procedure can generate additional signals.
Detection of coagulin, a class II bacteriocin.
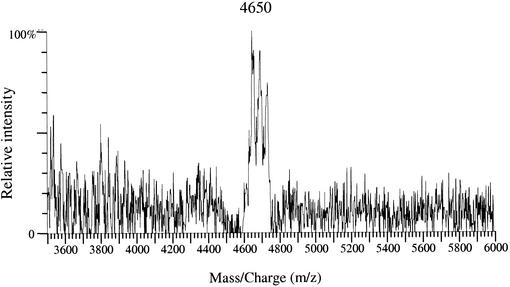
Coagulin (molecular mass, 4,612 Da [14]) was chosen as a representative of the subclass IIa bacteriocins to examine if the method could be applied to nonlantibiotic bacteriocins. When analyzing the coagulin-producing strain B. coagulans I4 (11) by MALDI-TOF-MS, we observed that the MRS medium was responsible for numerous intense peaks between m/z 800 and 2,500. In order to observe the coagulin peaks, we narrowed the m/z window to the m/z 3,500 to 6,000 range. This allowed detection of a peak cluster in which the major peak was at m/z 4,650 (Fig. 4) and corresponded to the coagulin [M+K]+ ion. This peak cluster did not appear in spectra of B. coagulans strain CIP52.64 (Table 1), which does not produce coagulin (data not shown). As in the nisin analysis, we did not detect the coagulin peaks from crude liquid culture supernatants.
FIG. 4.
MALDI-TOF spectrum of the coagulin-producing strain B. coagulans I4. The peak at m/z 4,650 corresponds to the ion [M+K]+ of coagulin.
Application of the method to determine the masses of lacticin 481 mutant molecules.
The three AMPs that we tested were thus easily detected by MALDI-TOF-MS directly from producing bacteria. This suggests that in general this method can be used for lantibiotics and class II bacteriocins, as long as their peaks are not masked by signals due to other biomarkers of the strains or to medium components. The main advantage of the procedure lies in its speed: once the bacteria have been grown on a plate, only few minutes is required to prepare the sample and complete the MALDI-TOF-MS analysis. In addition, up to 96 samples can be prepared simultaneously and spotted on a single target plate, and the subsequent analyses can be performed automatically, which allows screening of numerous bacteria in a single day. Two types of information are provided: whether a given bacteriocin is produced can be determined, and the AMP molecular mass can be determined. Many applications can be anticipated; the method could be used to screen colonies for the loss or gain of production of a particular AMP, or it could be used as a first step in the characterization of unknown bacteriocins. We used this method to determine the masses of two lacticin 481 variants obtained by site-directed mutagenesis of the structural gene lctA (18, 20), as described in Materials and Methods. One mutation consisted of replacement of the serine codon at the 3′ end of lctA by a stop codon, leading to loss of the C-terminal serine (ΔS27), whereas the other mutation consisted of replacement of a serine codon by a threonine codon (S4T) (Fig. 1B and C). Both constructions induced the production of active AMPs, as tested with L. lactis IL1835 (data not shown). The ΔS27 and S4T lacticin 481 variants were detected by MALDI-TOF-MS from bacterial colonies, and the m/z values of their [M+K]+ adduct ions were 2,854 and 2,954, respectively (Fig. 5). The differences between these values and the value for the lacticin 481 [M+K]+ ion (m/z 2,940) correspond to the predicted differences for deletion of the C-terminal serine (−87 Da) and replacement of a serine by a threonine (+14 Da). This confirmed the mutations at the peptide level and showed that four residues were dehydrated in the two lacticin 481 variant precursors (the same number as in wild-type lacticin 481) and that cleavage of the N-terminal part of the precursors occurred at the normal site (18). We intend to characterize a larger set of lacticin 481 mutant molecules. In the case of the loss of antimicrobial activity after mutagenesis, MALDI-TOF-MS should allow us to examine if the mutation prevents bacteriocin production or if an inactive AMP is produced.
FIG. 5.
MALDI-TOF spectra of L. lactis IL1403(pEBΔS27) (A) and of L. lactis IL1403(pEBS4T) (B), which produce lacticin 481 mutant molecules which lack the C-terminal serine (ΔS27) or in which the serine at position 4 is replaced by a threonine (S4T).
Acknowledgments
We are grateful to D. Thuault (ADRIA, Quimper, France), C. Le Marrec (Université de Bordeaux I, Talence, France), B. Mollet (NESTEC Ltd., Lausanne, Switzerland), and J.-C. Piard (INRA, Jouy-en-Josas, France) for providing strains.
T.H. is the recipient of a doctoral fellowship from the Ministère de l'Education Nationale, de la Recherche et de la Technologie, France. This work was supported by the Région Bretagne, the Centre de Génie Industriel (Guidel, France), and European FEDER funds.
REFERENCES
- 1.Chopin, A., M. C. Chopin, A. Moillo-Batt, and P. Langella. 1984. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid 11:260-263. [DOI] [PubMed] [Google Scholar]
- 2.Claydon, M. A., S. N. Davey, V. Edwards-Jones, and D. B. Gordon. 1996. The rapid identification of intact microorganisms using mass spectrometry. Nat. Biotechnol. 14:1584-1586. [DOI] [PubMed] [Google Scholar]
- 3.Cleveland, J., T. J. Montville, I. F. Nes, and M. L. Chikindas. 2001. Bacteriocins: safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 71:1-20. [DOI] [PubMed] [Google Scholar]
- 4.Delves-Broughton, J., P. Blackburn, R. J. Evans, and J. Hugenholtz. 1996. Applications of the bacteriocin, nisin. Antonie Leeuwenhoek 69:193-202. [DOI] [PubMed] [Google Scholar]
- 5.Dufour, A., D. Thuault, A. Boulliou, C. M. Bourgeois, and J.-P. Le Pennec. 1991. Plasmid-encoded determinants for bacteriocin production and immunity in a Lactococcus lactis strain and purification of the inhibitory peptide. J. Gen. Microbiol. 137:2423-2429. [DOI] [PubMed] [Google Scholar]
- 6.Efstathiou, J. D., and L. L. McKay. 1977. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J. Bacteriol. 130:257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferchichi, M., J. Frère, K. Mabrouk, and M. Manai. 2001. Lactococcin MMFII, a novel class IIa bacteriocin produced by Lactococcus lactis MMFII, isolated from a Tunisian dairy product. FEMS Microbiol. Lett. 205:49-55. [DOI] [PubMed] [Google Scholar]
- 8.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guyonnet, D., C. Fremaux, Y. Cenatiempo, and J. M. Berjeaud. 2000. Method for rapid purification of class IIa bacteriocins and comparison of their activities. Appl. Environ. Microbiol. 66:1744-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holland, R. D., J. G. Wilkes, F. Rafii, J. B. Sutherland, C. C. Persons, K. J. Voorhees, and J. O. Lay, Jr. 1996. Rapid identification of intact whole bacteria based on spectral patterns using matrix-assisted laser desorption/ionization with time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 10:1227-1232. [DOI] [PubMed] [Google Scholar]
- 11.Hyronimus, B., C. Le Marrec, and M. C. Urdaci. 1998. Coagulin, a bacteriocin-like inhibitory substance produced by Bacillus coagulans I4. J. Appl. Microbiol. 85:42-50. [DOI] [PubMed] [Google Scholar]
- 12.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-86. [DOI] [PubMed] [Google Scholar]
- 13.Lay, J. O., Jr. 2001. MALDI-TOF mass spectrometry of bacteria. Mass Spectrom. Rev. 20:172-194. [DOI] [PubMed] [Google Scholar]
- 14.Le Marrec, C., B. Hyronimus, P. Bressollier, B. Verneuil, and M. C. Urdaci. 2000. Biochemical and genetic characterization of coagulin, a new antilisterial bacteriocin in the pediocin family of bacteriocins, produced by Bacillus coagulans I4. Appl. Environ. Microbiol. 66:5213-5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mann, M., R. C. Hendrickson, and A. Pandey. 2001. Analysis of proteins and proteomes by mass spectrometry. Annu. Rev. Biochem. 70:437-473. [DOI] [PubMed] [Google Scholar]
- 16.McAuliffe, O., R. P. Ross, and C. Hill. 2001. Lantibiotics: structure, biosynthesis and mode of action. FEMS Microbiol. Rev. 25:285-308. [DOI] [PubMed] [Google Scholar]
- 17.Nes, I. F., and H. Holo. 2000. Class II antimicrobial peptides from lactic acid bacteria. Biopolymers 55:50-61. [DOI] [PubMed] [Google Scholar]
- 18.Piard, J.-C., O. P. Kuipers, H. S. Rollema, M. J. Desmazeaud, and W. M. de Vos. 1993. Structure, organization, and expression of the lct gene for lacticin 481, a novel lantibiotic produced by Lactococcus lactis. J. Biol. Chem. 268:16361-16368. [PubMed] [Google Scholar]
- 19.Pridmore, D., N. Rekhif, A.-C. Pittet, B. Suri, and B. Mollet. 1996. Variacin, a new lanthionine-containing bacteriocin produced by Micrococcus varians: comparison to lacticin 481 of Lactococcus lactis. Appl. Environ. Microbiol. 62:1799-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rincé, A., A. Dufour, S. Le Pogam, D. Thuault, C. M. Bourgeois, and J.-P. Le Pennec. 1994. Cloning, expression, and nucleotide sequence of genes involved in production of lactococcin DR, a bacteriocin from Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 60:1652-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rincé, A., A. Dufour, P. Uguen, J.-P. Le Pennec, and D. Haras. 1997. Characterization of the lacticin 481 operon: the Lactococcus lactis genes lctF, lctE, and lctG encode a putative ABC transporter involved in bacteriocin immunity. Appl. Environ. Microbiol. 63:4252-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryzhov, V., and C. Fenselau. 2001. Characterization of the protein subset desorbed by MALDI from whole bacterial cells. Anal. Chem. 73:746-750. [DOI] [PubMed] [Google Scholar]
- 23.Sahl, H.-G., R. W. Jack, and G. Bierbaum. 1995. Biosynthesis and biological activities of lantibiotics with unique post-translational modifications. Eur. J. Biochem. 230:827-853. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Simon, D., and A. Chopin. 1988. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie 70:559-566. [DOI] [PubMed] [Google Scholar]
- 26.Stein, T., S. Borchert, B. Conrad, J. Feesche, B. Hofemeister, J. Hofemeister, and K.-D. Entian. 2002. Two different lantibiotic-like peptides originate from the ericin gene cluster of Bacillus subtilis A1/3. J. Bacteriol. 184:1703-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein, T., and K.-D. Entian. 2002. Maturation of the lantibiotic subtilin: matrix-assisted laser desorption/ionization time-of-flight mass spectrometry to monitor precursors and their proteolytic processing in crude bacterial cultures. Rapid Commun. Mass Spectrom. 16:103-110. [DOI] [PubMed] [Google Scholar]
- 28.Thuault, D., E. Béliard, J. Le Guern, and C. M. Bourgeois. 1991. Inhibition of Clostridium tyrobutyricum by bacteriocin-like substances produced by lactic acid bacteria. J. Dairy Sci. 74:1145-1150. [DOI] [PubMed] [Google Scholar]