Abstract
A 3.4-kb cryptic plasmid was obtained from a new isolate of Rhodobacter blasticus. This plasmid, designated pMG160, was mobilizable by the conjugative strain Escherichia coli S17.1 into Rhodobacter sphaeroides, Rhodobacter capsulatus, and Rhodopseudomonas palustris. It replicated in the latter strains but not in Rhodospirillum rubrum, Rhodocyclus gelatinosus, or Bradyrhizobium species. Plasmid pMG160 was stably maintained in R. sphaeroides for more than 100 generations in the absence of selection but showed segregational instability in R. palustris. Instability in R. palustris correlated with a decrease in plasmid copy number compared to the copy number in R. sphaeroides. The complete nucleotide sequence of plasmid pMG160 contained three open reading frames (ORFs). The deduced amino acid sequences encoded by ORF1 and ORF2 showed high degrees of homology to the MobS and MobL proteins that are involved in plasmid mobilization of certain plasmids. Based on homology with the Rep protein of several other plasmids, ORF3 encodes a putative rep gene initiator of plasmid replication. The functions of these sequences were demonstrated by deletion mapping, frameshift analysis, and analysis of point mutations. Two 6.1-kb pMG160-based E. coli-R. sphaeroides shuttle cloning vectors were constructed and designated pMG170 and pMG171. These two novel shuttle vectors were segregationally stable in R. sphaeroides growing under nonselective conditions.
The purple non-sulfur bacteria (PNSB) are an assemblage of phenotypically diverse species. Under anaerobic conditions, in the light all species grow photoheterotrophically when they are supplied with various organic substrates or photoautotrophically when CO2 is the sole carbon source. Under microaerobic to aerobic conditions in the dark, many representatives can grow chemoheterotrophically, and some grow chemoautotrophically (34). PNSB provide useful tools for the study of carbon metabolism, including CO2 fixation.
We have been conducting biochemical and genetic studies of the intermediary metabolism underlying the complex modes of growth in the PNSB (4, 23, 24). To do this, development of a versatile host-vector system is essential. Despite the fact that several strains of PNSB, including Rhodobacter sphaeroides (15), Rhodobacter capsulatus (20, 45), and Rhodospirillum rubrum (31), harbor one or more large endogenous plasmids, until recently (25) no cloning vector had been designed for PNSB by using endogenous plasmids. Instead, to date, only broad-host-range vectors, such as relatively large (10- to 20-kb) derivatives of plasmid RK2 (7, 8, 28), have been used. The main shortcoming of this approach is the intrinsic segregational instability of these elements under nonselective conditions in R. sphaeroides (9), R. rubrum (36), and Rhodopseudomonas palustris (25). Other vectors derived from the broad-host-range plasmid RSF1010 have likewise been constructed to enable manipulation of PNSB; these vectors include the cloning vectors pNH2 (21) and pDSK519 (28), the cosmid vector pJRD215 (41), and a lacZ fusion vector (33). Nevertheless, the RSF1010 derivative pDSK519 has similarly been demonstrated to be unstable under nonselective conditions in R. sphaeroides (this study) and in R. palustris (25).
In order to establish a versatile vector system to facilitate genetic analysis of PNSB, we screened numerous PNSB strains for endogenous plasmids that are relatively small. Isolation of the 15-kb cryptic plasmid pMG101 and subsequent construction of the stable 5.7-kb cloning vectors pMG103 and pMG105 have been described previously (25). These plasmids can replicate in their original host, R. palustris, and in closely related strains of Bradyrhizobium species but not in other PNSB. To meet the need for a stable cloning system for R. sphaeroides, using an identical strategy, we identified an endogenous plasmid in a closely related microorganism and constructed novel mobilizable and stable R. sphaeroides-Escherichia coli shuttle vectors.
In this paper we describe the isolation, mobilization function, replication range, stability, and sequence of the 3.4-kb cryptic plasmid pMG160 and construction of novel genetic tools for manipulation of R. sphaeroides.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or sourcea |
|---|---|---|
| Strains | ||
| E. coli S17.1 | RF4 2-Tc::Mu-Km::Tn7 pro res mod+ | 9 |
| E. coli JM109 | recA1 endA1 gyrA96 thi hsdR17 supE44 relA1Δ(lac-proAB)/F′[traD36 proAB+ lacIqlacZΔM15] | Takara |
| R. blasticus TCRI-14 | Natural R. sphaeroides isolate harboring plasmid pMG160 | This study |
| R. sphaeroides ATCC 17023 | Type strain | ATCC |
| R. capsulatus ATCC 33303 | ATCC | |
| R. capsulatus ATCC 23782 | ATCC | |
| R. rubrum ATCC 11170 | Type strain | ATCC |
| R. gelatinosus ATCC 17011 | Type strain | ATCC |
| R. palustris ATCC 17001 | Type strain | ATCC |
| R. palustris No. 7 | Alcohol-assimilating PNSB | 17 |
| Bradyrhizobium sp. strain USDA 4362 | Phototrophic Bradyrhizobium species | USDA |
| Bradyrhizobium sp. strain USDA 4377 | Phototrophic Bradyrhizobium species | USDA |
| B. japonicum ATCC 10324 | Type strain | ATCC |
| Plasmids | ||
| pHSG298 | Kmr, α-lac multicloning site, M13 ori | Takara |
| pHSG298X | Kmr, α-lac multicloning site, M13 ori, additional XhoI site compared to pHSG298 | 25 |
| pHSG299 | Kmr, α-lac multicloning site (reverse direction of pHSG298X and pHSG298), M13 ori | Takara |
| pRK415 | Tetr, α-lac multicloning site, mobilizable (oriT), broad-host-range plasmid RK2 derivative | 28 |
| pDK519 | Kmr, α-lac multicloning site, broad-host-range plasmid RSF1010 derivative | 28 |
| pBBR1MCS2 | Kmr, α-lac multicloning site, broad-host-range | 30 |
| pMG103 | Kmr, α-lac multicloning site, E coli-R. palustris cloning shuttle vector | 25 |
| pMG160 | 3.4-kb natural plasmid (mobS+mobL+repA+) of R. sphaeroides TCRI-14 | This study |
| pMG162 | Kmr, pMG160 linearized with NaeI and ligated to HincII-linearized pHSG298 | This study |
| pMG162-1 | Kmr, pMG162 derivative, deletion of nucleotides 3210-3431 | This study |
| pMG162-2 | Kmr, pMG162 derivative, deletion of nucleotides 2974-3431 | This study |
| pMG162-3 | Kmr, pMG162 derivative, deletion of nucleotides 1-41 (the 4th base of the NaeI site is the coordinate origin) | This study |
| pMG162-4 | Kmr, pMG162 derivative, deletion of nucleotides 1-81 | This study |
| pMG162-5 | Kmr, pMG162 derivative, deletion of nucleotides 1-121 | This study |
| pMG162-6 | Kmr, pMG162 derivative, deletion of nucleotides 1-196 | This study |
| pMG162-7 | Kmr, pMG162 derivative, deletion of nucleotides 1-1165 | This study |
| pMG162-8 | Kmr, pMG162 derivative, deletion of nucleotides 1-1950 | This study |
| pMG162-9 | Kmr, pMG162 derivative, deletion of nucleotides 1-2082 | This study |
| pMG162-10 | Kmr, pMG162 derivative, deletion of nucleotides 1-2227 | This study |
| pMG164 | Kmr, pMG162 derivative, deletion of nucleotides 3210-1950 | This study |
| pMG162-11 | Kmr, pMG162 derivative, 2-bp frameshift at position 161 | This study |
| pMG162-12 | Kmr, pMG162 derivative, 4-bp frameshift at position 992 | This study |
| pMG162-13 | Kmr, pMG162 derivative, 2-bp frameshift at position 2218 | This study |
| pMG162-14 | Kmr, pMG162 derivative, point mutation at positions 117 (G→A) and 119 (G→A) | This study |
| pMG170 | Kmr, 6.1-kb, α-lac multicloning site, pHSG298X ligated to pMG160 | This study |
| pMG171 | Kmr, 6.1-kb, α-lac multicloning site, pHSG299X ligated to pMG160 | This study |
| pMG170-prr | Kmr, pMG170 with a 2.6-kb BamHI-KpnI fragment encoding R. sphaeroides prr genes | This study |
| pRK415-prr | Kmr, pRK415 with a 2.6 kb BamHI-KpnI fragment encoding R. sphaeroides prr genes | This study |
| pBBR1MCS2-prr | Kmr, pBBR1MCS2 with a 2.6-kb BamHI-KpnI fragment encoding R. sphaeroides prr genes | This study |
| pMG170-pckA | Kmr, pMG170 with a 2.3-kb BamHI-PstI fragment encoding R. palustris pckA gene | This study |
| pBBRIMCS2-pckA | Kmr, pBBRIMCS2 with a 2.6-kb BamHI-KpnI fragment encoding R. palustris No. 7 pckA gene | This study |
ATCC, American Type Culture Collection; USDA, U. S. Department of Agriculture.
Culture conditions.
E. coli strains were grown aerobically at 37°C in Luria-Bertani medium (38). PNSB were cultivated aerobically at 30°C in van Niel's complex medium (42). Bradyrhizobium species were grown aerobically at 30°C in 805 medium (25). When appropriate, media were supplemented with the following antibiotics. For E. coli, ampicillin and kanamycin were each used at a final concentration of 50 μg ml−1, and tetracycline was used at a final concentration of 25 μg ml−1; for R. palustris, kanamycin was used at a final concentration of 200 μg ml−1; and for other PNSB and Bradyrhizobium species, kanamycin was used at a final concentration of 50 μg ml−1 and tetracycline was used at a final concentration of 10 μg ml−1.
DNA techniques.
Plasmid DNA was isolated by the alkaline lysis procedure (38). Restriction endonucleases, the Klenow fragment, and T4 ligase were obtained from Takara (Kyoto, Japan) and were used according to the manufacturer's instructions. E. coli strains were transformed by the CaCl2 method (38). PNSB and Bradyrhizobium species were transformed by conjugation from E. coli S17.1 (9) or by electroporation (25). Restriction fragments were isolated, when required, from agarose (1%, wt/vol) gels with a GENECLEAN III kit (Bio 101, Vista, Calif.) used as recommended by the manufacturer.
Isolation of an endogenous plasmid from PNSB.
PNSB were isolated from enrichment cultures under anaerobic light conditions with bubbling nitrogen by using a Rhodospirillaceae enrichment medium (2) containing succinate (0.05%), malate (0.05%), and acetate (0.05%) as carbon sources. Isolates were grown aerobically, and plasmid DNA was isolated as described above.
DNA sequencing.
To determine the complete nucleotide sequence of plasmid pMG160, a series of pMG162 deletion derivatives were constructed by using a Kilo-sequence deletion kit (Takara) as recommended by the manufacturer. DNA was sequenced on both strands by using an automated 373A DNA sequencer (Applied Biosystems/Perkin-Elmer, Foster City, Calif.). DNA sequence data were analyzed by using the INHERIT (Applied Biosystems/Perkin-Elmer) and Genetyx (Software Development, Tokyo, Japan) programs.
Isolation and analysis of 16S rDNA.
Full-length 16S ribosomal DNA (rDNA) of Rhodobacter blasticus strain TCRI-14 was amplified by PCR, as previously described (25). The DNA sequence of the amplified 16S rDNA was determined as described above. The rDNA sequences of strain TCRI-14 and reference strains covering a total of 1,229 nucleotide positions were aligned by using the CLUSTAL V program (19). A phylogeny was reconstructed by the neighbor-joining method (37) from Knuc values (29). Variable domains and unidentified base positions were not considered in the analysis.
Segregational plasmid stability.
Stability in R. sphaeroides and in R. palustris of pMG160 derivatives and other broad-host-range plasmids was estimated as previously described (25).
Construction of E. coli-R. sphaeroides shuttle cloning vectors.
Plasmid pMG170 was constructed by ligating pMG160 linearized by using NaeI with pHSG298X linearized by using XhoI. The XhoI site of plasmid pHSG298X is located between the replication origin and the kanamycin resistance gene (25). The XhoI cohesive ends were blunted by using the Klenow fragment. The ligation event created two XhoI sites. Similarly, plasmid pMG171 was constructed by ligating the 4.2-kb ApaLI-ClaI fragment from pMG170 containing the pMG160 replication origin to the 1.8-kb ApaLI-ClaI fragment from pHSG299 (25) containing the same polylinker in the β-galactosidase (lacZ) gene as pHSG298X but in the opposite orientation.
Plasmid copy number determination.
Plasmid copy numbers were determined as previously described (25). The 2.6-kb BamHI-KpnI fragment containing the prrA, prrB, and prrC genes from R. sphaeroides (12, 13) was amplified by PCR by using R. sphaeroides chromosomal DNA as the template and oligonucleotide primers P1 (ATATGGATCCAGGCTCTCGCCCTTGGCCAG) and P2 (CTCTGGTACCAAGGTCGGACGCGGCATCCG). The PCR product was subsequently cloned into the BamHI-KpnI sites of plasmids pMG170, pRK415 (28), and pBBR1MCS2 (30). The resulting plasmids were designated pMG170-prr, pRK415-prr, and pBBR1MCS2-prr, respectively. Each chimeric plasmid was used to transform R. sphaeroides. Southern hybridization of total DNA from recombinant R. sphaeroides strains harboring plasmid pMG170-prr, pRK415-prr, or pBBR1MCS2-prr was performed by using as a probe the same 2.6-kb BamHI-KpnI fragment containing the R. sphaeroides prrA, prrB, and prrC genes that was used in construction of the plasmids. The probe was labeled with the DIG system (Boehringer, Mannheim, Germany). Chromosomal DNA was digested with BamHI, KpnI, and SmaI. Signal detection was performed with a Fujifilm LAS-1000 image analyzer system (Fuji, Tokyo, Japan).
The prr probe hybridized to a 3.0-kb BamHI-SmaI fragment originating from the chromosome and to a 2.6-kb BamHI-KpnI fragment originating from plasmids pMG170-prr, pRK415-prr, and pBBR1MCS2-prr. Since only one copy of the prr gene is present in each genome, plasmid copy numbers were calculated by determining the ratio of plasmid-derived hybridization signals to chromosome-derived hybridization signals by using the Fujifilm Image Gauge program (version 3.1; Fuji).
In a similar manner, the copy numbers of plasmids pMG170 and pBBR1MCS2 in R. palustris were estimated by using the R. palustris pckA gene (25). The 2.2-kb BamHI-PstI fragment containing the phosphoenolpyruvate carboxykinase (pckA) gene from R. palustris No. 7 (24) was amplified by PCR performed with R. palustris No. 7 chromosomal DNA as the template and primers P3 (ATATGGATCCCGCCGGCGAAGCCGACTGGC) and P4 (CTCTCTGCAGGCGCAGATGAATCA), and it was subcloned into the BamHI-PstI sites of plasmid pMG170. The resulting plasmid was designated pMG170-pckA and was used to transform R. palustris No. 7. Likewise, the 2.2-kb BamHI-SpeI fragment containing the R. palustris No. 7 pckA gene was amplified by PCR performed with R. palustris No. 7 chromosomal DNA as the template and primers P3 and P5 (CTCTACTAGTGCGCAGATGAATCA). The resulting PCR product was subcloned into the BamHI-SpeI sites of plasmid pBBR1MCS2. The resulting plasmid was designated pBBR1MCS2-pckA and was used to transform R. palustris No. 7.
Southern hybridization of total DNA from R. palustris No. 7 harboring plasmid pMG160-pckA restricted with SmaI, BamHI, and PstI and Southern hybridization of total DNA from R. palustris No. 7 harboring plasmid pBBR1MCS2-pckA restricted with SmaI, BamHI, PstI, and SpeI were performed by using as a probe the same 2.2-kb BamHI-PstI fragment containing the R. palustris No. 7 pckA gene labeled with the DIG system (Boehringer). Hybridization of this probe resulted in detection of two fragments in each sample. One of these fragments corresponded to the 2.5-kb SmaI-PstI fragment of the chromosomally encoded pckA gene. The second fragment corresponded either to the internal 2.2-kb BamHI-PstI fragment originating from plasmid pMG160-pckA or to the internal 2.2-kb BamHI-SpeI fragment originating from plasmid pBBR1MCS2. Hybridization signals were detected with a Fujifilm LAS-1000 image analyzer system (Fuji). As described above, plasmid copy numbers were calculated by determining the ratio of the two hybridization signals from each sample by using the Fujifilm Image Gauge program (version 3.1; Fuji).
Nucleotide sequence accession numbers.
The DDBJ/EMBL/GenBank accession numbers for the sequences described in this paper are AB082959 (plasmid pMG160), AB017799 (strain TCRI-14 rDNA), AB082960 (plasmid pMG170), and AB082961 (plasmid pMG171).
RESULTS
Isolation of plasmid pMG160 and main morphological characteristics of its host strain.
The screening method which was previously employed to isolate novel PNSB strains is biased towards isolation of R. palustris that can utilize alcohol (25). As a result, with the aim of isolating cryptic plasmids that replicate in other genera of the PNSB group, we repeated the screening procedure using enrichment media based on succinate, malate, and acetate as carbon sources and on bubbling nitrogen gas during anaerobic cultivation. In particular, this approach takes advantage of the nitrogen fixation properties of PNSB. We isolated various PNSB strains by searching for small plasmids (<20 kb). Approximately 100 strains were isolated from sewage, rice fields, and other places. One of these strains, TCRI-14, harbored an endogenous plasmid that was approximately 3.4-kb long. This plasmid was designated pMG160.
Strain TCRI-14 is a gram-negative, nonsporulating rod with rounded ends. The cells divide by binary fission. Cultures are red to brown-red under anaerobic conditions in the light and pale pink to white under aerobic conditions in the dark. The in vivo spectrum of cell suspensions grown anaerobically in the light had absorption maxima at 375, 590, 805, and 860 nm, which is characteristic of the presence of bacteriochlorophyll a. Likewise, the spectrum had absorption maxima at 450, 480, and 510 nm, demonstrating that carotenoids of the normal spheroidene series were present (34). These results indicate that strain TCRI-14 is a member of the genus Rhodobacter.
In order to confirm the phylogenetic position of strain TCRI-14, we determined the sequence of 1,439 nucleotides of the rDNA of this strain. The resulting sequence was compared with known 16S rDNA sequences, as described previously (25) (Fig. 1). Strain TCRI-14 formed a tight phylogenetic cluster with R. blasticus, R. sphaeroides, and R. capsulatus and was less closely related to other members of the PNSB or to Bradyrhizobium and Rhizobium species. A bootstrap analysis confirmed the monophyly of the strain TCRI-14 cluster in 100% of the trees generated (14). These results confirm that strain TCRI-14 belongs to the genus Rhodobacter and that it is phylogenetically closely related to R. sphaeroides and R. capsulatus. The near identity (98%) of its 16S rDNA sequence with that of R. blasticus ATCC 33485 (GenBank accession number D16429) indicates that strain TCRI-14 is a strain of R. blasticus (synonyms, Rhodopseudomonas blastica and Rhodobacter blastica [27, 35]).
FIG. 1.
Unrooted distance matrix tree showing phylogenetic relationships of selected strains of proteobacteria based on 16S rDNA sequences. The topology of the phylogenetic tree was evaluated by performing a bootstrap analysis with 1,000 replicates. The numbers at the nodes indicate bootstrap percentages based on 1,000 replicates. The accession numbers for the sequences used are as follows: strain TCRI-14, AB017799; Rhodobacter sphaeroides, D16425; Rhodobacter capsulatus, D16428; Rhodobacter blasticus, D16429; Rhodopseudomonas palustris, D25312; Rhodoplanes roseus, D25313; Rhodopseudomonas acidophila, M34128; Rhodopseudomonas viridis, D25314; Rhodospirillum photometricum, D30777; Rhodophila globiformis, D86513; Rhodobacter sulfidophilus, D16430; Rhodospirillum rubrum, D30778; Rhodospirillum salexigens, M59070; Rhodocyclus tenuis, D16208; Rhodocyclus purpureus, M34132; and Rhodocyclus gelatinosus, D16214.
Replication range, mobilization, and stability of plasmid pMG160.
Plasmid pMG160 is relatively small compared to known endogenous plasmids originating from bacteria belonging to the PNSB group. A restriction map of plasmid pMG160 was generated, and the shuttle vector pMG162 was constructed by linearizing pMG160 plasmid DNA with NaeI and ligating it to HincII-linearized and blunt-ended plasmid pHSG298 DNA. Plasmid pHSG298 does not replicate in PNSB. The ligation mixture was used to transform E. coli. PNSB strains were transformed both by electroporation and by conjugation with E. coli S17.1. Plasmid pMG162 could replicate in R. sphaeroides ATCC 17023, in R. capsulatus ATCC 33303 and ATCC 23782, and in R. palustris No. 7 (17) and ATCC 17001. On the other hand, no kanamycin-resistant transformants or transconjugants were recovered from R. rubrum ATCC 11170, R. gelatinosus ATCC 17011, Bradyrhizobium japonicum ATCC 10324, or phototrophic Bradyrhizobium strains USDA 4362 and USDA 4377. Transformability of all of these strains except R. gelatinosus ATCC 17011 (which we did not previously test) with a kanamycin resistance-conferring replicative plasmid has been reported elsewhere (9, 25, 36), corroborating the view that pMG162 does not replicate in these organisms. In addition, while plasmid pMG162 was shown to be mobilizable by the conjugative strain E. coli S17.1, no mobilization was observed from E. coli strain JM109, demonstrating that the tra gene products present in E. coli S17.1 are necessary for the mobilization process to occur.
In order to assess the stability of plasmid pMG160, R. sphaeroides and R. palustris cultures containing pMG162 were grown under nonselective conditions. The broad-host-range plasmids pDK519 (28), pRK415 (28), and pBBR1MCS2 (30) in R. sphaeroides and in R. palustris were used as controls for segregational instability. Plasmid pMG103 in R. palustris was used as a positive control for plasmid stability (25). We observed that pMG162 was stably maintained in R. sphaeroides for more than 150 generations but was unstable (0% maintenance) in R. palustris after 30 generations (Fig. 2). Although pBBR1MCS2 showed moderate stability in R. sphaeroides (56% maintenance after 100 generations), pBBR1MCS2 was unstable in R. palustris and all the other broad-host-range plasmids tested in this study were unstable in both organisms (Fig. 2). On the other hand, as previously observed, pMG103 was segregationally stable in R. palustris for more than 100 generations (25). Nevertheless, pMG162 in R. palustris and all the broad-host-range plasmids discussed above were stably maintained in both hosts when selective pressure (kanamycin or tetracycline challenge) was applied. The observed differences in stability could perhaps be ascribed to differences in copy number and in host factors in these strains, since interactions between host- and plasmid-encoded factors intervene in the plasmid replication process (43).
FIG. 2.
Segregational stability in R. sphaeroides of plasmids pMG162, pBBR1MCS2, pDK519, and pRK415. The stabilities of plasmid pMG162 (○) and the broad-host-range plasmids pBBR1MCS2 (□), pDK519 (•), and pRK415 (▪) in R. sphaeroides (A) and in R. palustris (B) under nonselective conditions were determined. Plasmid pMG103 (▴) was used as a stable plasmid control in R. palustris (25).
DNA sequence of 3.4-kb plasmid pMG160.
The complete nucleotide sequence (3.4 kb) of pMG160 was determined in order to define the plasmid-partitioning, replication, and mobilization functions. The G+C content of plasmid pMG160 was 67.0%, which is similar to the values previously reported for the genomes of R. sphaeroides (68.4 to 69.9%) and R. capsulatus (65.5 to 66.8%) (22). Computer analysis revealed the presence of three open reading frames (ORFs), ORF1, ORF2, and ORF3.
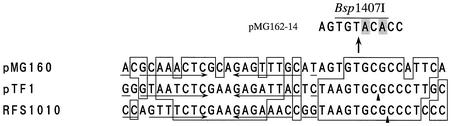
ORF1 (positions 136 to 438) consists of 303 nucleotides, corresponding to a gene product 100 amino acids long with a predicted molecular weight of 10,636. The deduced amino acid sequence shows low but significant levels of similarity to the sequences of the mobS gene product of plasmid pTF1 from Thiobacillus ferrooxidans (31.2%) (11) (accession no. X52699) and the mobC gene product of the broad-host-range plasmid RSF1010 (27.9%) (40) (accession no. M28829). ORF1 was therefore designated the mobS gene. Upstream of the pMG160 mobS gene, there is a 38-bp sequence (positions 89 to 126) homologous to the minimal 38-bp transfer origin (oriT) of plasmids pTF1 (sequence similarity, 67.6%) (11) and RSF1010 (60.0%) (3). The 38-bp pMG160 DNA has a palindromic sequence, as observed in the pTF1 and RSF1010 oriT sequences (11). Furthermore, there is significant conservation in pMG160, pTF1, and RSF1010 of the sequences in the immediate vicinity of the specific nick sites reported for pTF1 and RSF1010 by Scherzinger et al. (39) (Fig. 3).
FIG. 3.
Comparison of the putative oriT sequence of pMG160 with the 38-bp minimal oriT sequences of pTF1 and RSF1010. The putative oriT sequence of pMG160 was aligned with the 38-bp minimal oriT region of pTF1 and RSF1010. Nucleotides that were identical in at least two of the three sequences are enclosed in boxes. The possible palindromic sequence is indicated by the converging arrows. The arrowheads indicate the specific nick sites of the plasmids. The 2-bp point mutation in the putative oriT sequence in plasmid pMG162-14 is indicated above the sequence alignment. The nucleotides that were replaced are indicated by shading. A line above the mutated sequence indicates the location of the newly created Bsp1407I site.
ORF2 (positions 431 to 1,855) has an 8-bp overlap with the 3′ end of the putative mobS gene. It is transcribed in the same direction, and it comprises 1,425 nucleotides corresponding to 474 amino acids with an estimated molecular weight of 51,209. The deduced amino acid sequence indicates that there are low but significant levels of similarity along the length of the protein to the sequences of the mobL gene product of plasmid pTF1 from T. ferrooxidans (27.8%) (11) (accession no. X52699) and the mobA (or repB) gene product of the broad-host-range plasmid RSF1010 (22.4%) (40) (accession no. M28829). In addition, approximately 200 amino acids at the amino terminus of the ORF2 product also showed significant levels of similarity to the amino terminus of the traA gene product of plasmid pMRC01 from Lactococcus lactis (33.3%) (10) (accession no. AE001272), to the nes gene products of plasmid pSK41 (32.0%) (1) (accession no. AF051917) and plasmid pGO1 (32.0%) (5) (accession no. U50629) from Staphylococcus aureus, to the traA gene product of plasmid pNGR234a from Rhizobium sp. strain NGR234 (30.3%) (16) (accession no. P55418), and to the orf1 gene product of plasmid pIP501 from Streptococcus agalactiae (30.2%) (44) (accession no. L39769). All these proteins are putative oriT nickases that facilitate strand transfer of conjugative plasmids to recipient cells, and all the corresponding genes are neighbors of the oriT sequences in the corresponding plasmids. ORF2 was designated the mobL gene. However, for the three Mob proteins and the five oriT nickases, specific conserved regions were not observed (data not shown). Downstream of the putative mobL gene, there is an imperfect inverted repeat (positions 1,877 to 1,907) which has a GC-rich stem-loop structure.
ORF3 is located downstream of the putative mobL gene (positions 2,136 to 3,155). It is transcribed in the same direction as the mob genes, and it consists of 1,020 nucleotides, corresponding to 339 amino acids with a predicted molecular weight of 37,588. The deduced amino acid sequence exhibits low but significant levels of similarity to the sequences of the rep gene products of plasmid pMO1 from Francisella tularensis (23.6%) (accession no. AF055345), plasmid pEA29 from Erwinia amylovora (23.2%) (32) (accession no. AF264948), plasmid pFA3 from Neisseria gonorrhoeae (20.5%) (18) (accession no. M31727), and plasmid pYC from Yersinia pestis (18.1%) (accession no. AF152 923). ORF3 was therefore designated the repA gene. However, comparative amino acid sequence alignment revealed that there are no specific conserved regions in the five Rep proteins (data not shown). Upstream of the pMG160 repA gene, there are three 16-bp perfect direct repeats (GGGAGAAATGGGGCCG; positions 2,031 to 2, 088), which are separated by 5-bp spacers (CAAAA). Downstream of the pMG160 repA gene, we observed an imperfect inverted repeat (positions 3,345 to 3,378) constituting a GC-rich stem-loop structure.
Localization of the replication, stability, and mobilization regions.
A series of deletion mutants, frameshift mutants, and point mutants of plasmid pMG162 were constructed (Fig. 4) in an attempt to identify the replication, stability, and mobilization regions of pMG160. These plasmids were used to transform R. sphaeroides by electroporation as a way to determine the functionality of the replication regions. The mobilization properties of the mutants were estimated by conjugation by using the conjugative strain E. coli S17.1. Plasmid pHSG298, which contains an M13 replication origin, was not able to propagate in R. sphaeroides and thus was used in these experiments as a negative control. Plasmid pMG162 was used as a positive control for plasmid replication.
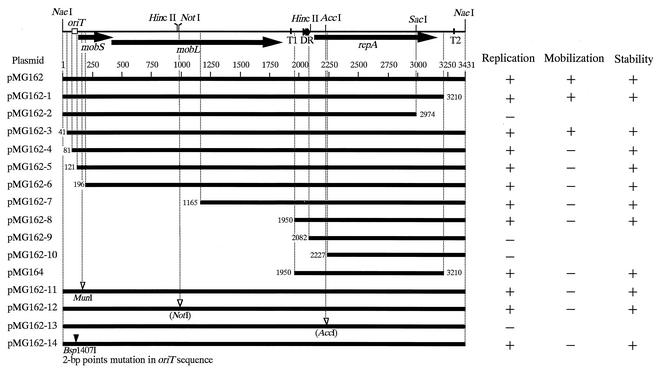
FIG. 4.
Deletion mapping and gene disruption analysis of plasmid pMG160. Deletion mapping and gene disruption analysis of plasmid pMG160 were carried out in order to identify the mobilization, stability, and replication regions. A partial restriction cleavage map of pMG160 is shown at the top. The arrows indicate the mobS, mobL, and repA genes. The solid arrowheads indicate direct repeats. The putative oriT sequence is indicated by an open box. Two GC-rich stem-loop structures (T1 and T2) are indicated by vertical lines. The scale is graduated (in base pairs) from the left (NaeI site) to the right (NaeI site), corresponding to the nucleotide sequence of the 3,431-bp NaeI-NaeI fragment. Plasmid pMG162 derivatives comprising deletion and frameshift mutants are listed on the left. A horizontal line represents each fragment. The coordinates (in base pairs) of the deletion mutations are shown at the ends of the lines. The open arrowheads indicate the positions (in base pairs) of frameshift mutations, and the solid arrowheads indicate the positions (in base pairs) of point mutations. Construction of these plasmids is described in Table 1. Replication was considered positive when kanamycin-resistant transformants grew following electrotransformation and when the presence of episomic DNA was confirmed by alkaline lysis extraction of plasmid DNA followed by gel electrophoresis. Replication was considered negative when no transformants were obtained in three repeat experiments. Mobilization experiments were carried out as described in Materials and Methods. Plasmid mobilization was confirmed by using a scheme similar to that used to demonstrate replication. Plasmid stability in R. sphaeroides was evaluated as described in Materials and Methods. Stability represents 100% plasmid maintenance.
Plasmid pMG162-1, containing a 3,210-bp fragment (positions 1 to 3,210) but lacking the region downstream of the repA gene that includes a GC-rich stem-loop structure, can replicate in R. sphaeroides like the original plasmid, pMG162. Moreover, it is also mobilizable from E. coli S17.1 into R. sphaeroides. On the other hand, plasmid pMG162-2, containing a 2,974-bp fragment (positions 1 to 2,974) that constitutes a deletion mutation in the repA carboxy terminus, was not able to replicate in R. sphaeroides. A series of additional deletion plasmids were constructed (Fig. 4). Plasmids pMG162-3 to pMG162-8, all of which included an intact repA gene and the three-direct-repeat structure, could replicate in R. sphaeroides. However, plasmid pMG162-9, which contains a 1,350-bp fragment (positions 2,082 to 3,431) with an intact repA gene but lacks the three-direct-repeat structure, as well as plasmid pMG162-10, which contains a 1,205-bp fragment (positions 2,227 to 3,431) comprising repA gene sequences except the amino terminus, could not replicate in R. sphaeroides. In addition, a frameshift mutation created by insertion of 2 bp into the AccI site in the repA sequence in plasmid pMG162 prevented replication in R. sphaeroides. The resulting frameshift plasmid, pMG162-13, was constructed by digestion of pMG162 with AccI, followed by blunting with the Klenow fragment and ligation. The data obtained suggest that the repA gene product and its upstream region, including the three-direct-repeat structure, are essential for pMG160 replication. Moreover, the observation that plasmid pMG164 containing a 1,261-bp fragment (positions 1,950 to 3,210) replicates in R. sphaeroides but pMG162-10 does not replicate in R. sphaeroides precisely mapped the replication origin and the minimum region necessary for plasmid replication. In these experiments, all the plasmids that were able to replicate in R. sphaeroides also showed segregational stability (100% maintenance) under nonselective conditions. This observation suggests that the replication and stability functions of this plasmid are linked.
Although plasmid pMG162-3, which contains a 3,391-bp fragment (positions 41 to 3,431) but lacks 40 bp from the NaeI site upstream of the putative oriT sequence, can be mobilized from the conjugative strain E. coli S17.1 into R. sphaeroides, plasmid pMG162-4, which contains a 3,351-bp fragment (positions 81 to 3,431) with a similar (from the NaeI site) but longer 80-bp deletion, did not exhibit any mobilization. Likewise, plasmid pMG162-5 bearing a 3,311-bp fragment (positions 121 to 3,431) which lacked a portion of the putative oriT sequence and plasmid pMG162-6 carrying a 3,236-bp fragment (positions 196 to 3,341) with a deletion in both the putative oriT sequence and the amino terminus of the mobS gene could not be mobilized. The view that both the mobS and mobL gene products are essential for pMG160 mobilization was supported by the results of frameshift mutation experiments. Using PCR, we inserted into plasmid pMG162 a 2-bp frameshift mutation that generated a new and unique MunI site within the mobS gene (positions 161 to 170; CTGTCATGGT was changed to CTGTCAATTGGT). Likewise, we created another frameshift mutation in this plasmid by inserting 4 bp into the NotI site of the mobL sequence by digestion of pMG162 with NotI, followed by blunting with the Klenow fragment and ligation. The mutations in the resulting plasmids, pMG162-11 and pMG162-12 (Fig. 4), respectively, eliminated the mobilization property. Moreover, using PCR, we introduced a 2-bp point mutation into the putative oriT sequence of pMG162 by replacing two guanines with two adenines. The latter mutation could be detected by the presence of a new and unique Bsp1407I site (Fig. 3 and 4). As expected, the resulting pMG162-14 plasmid could not be mobilized. Plasmids pMG162-11, pMG162-12, and pMG162-14 replicated in R. sphaeroides, as demonstrated by the appearance of transformants following electroporation and cultivation on selective medium. These data suggest that the mobS and mobL gene products, as well as the putative oriT sequence, play important roles in the mobilization function of plasmid pMG160.
Construction of E. coli-R. sphaeroides shuttle cloning vectors.
To develop a versatile host-vector system, we constructed the E. coli-R. sphaeroides shuttle cloning vectors pMG170 and pMG171. Both of these plasmids are 6,068 bp long and contain a kanamycin resistance marker. They were constructed as described in Materials and Methods. These shuttle vectors have a polylinker containing unique EcoRI, KpnI, BamHI, XbaI, SalI, PstI, Sse8387I, and SphI sites in the β-galactosidase (lacZα) gene. By using a host such as E. coli JM109 plated on isopropyl-β-d-thiogalactopyranoside (IPTG)-5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) agar plates, insertional inactivation could be used to identify recombinant plasmids (Fig. 5). We verified that both pMG170 and pMG171 were able to replicate in R. sphaeroides, that they could be mobilized from E. coli S17.1 into R. sphaeroides, and that they were maintained in R. sphaeroides for more than 100 generations without selective pressure.
FIG. 5.
Physical and genetic maps of pMG170 and pMG171. lacZα, β-galactosidase α-peptide gene from pHSG298 or pHSG299; Kmr, kanamycin resistance gene; pMG160 ori, the 3.4-kb NaeI-NaeI fragment of whole pMG160 DNA including the mobS, mobL, and repA genes; pHSG298 ori and pHSG299 ori, replication origins of pHSG298 and pHSG299, respectively. The arrows indicate the directions of transcription. Unique restriction sites are shown. There are two XhoI sites in these plasmids. Compared to pMG170, in plasmid pMG171 the lacZα multicloning site is inverted.
In order to estimate the copy number of pMG170 in R. sphaeroides and R. palustris, plasmid pMG170-prr containing the R. sphaeroides prr genes and plasmid pMG170-pckA including the R. palustris pckA gene were constructed. Only single copies of both the prr and pckA genes are present in the corresponding original genomes (25). As control plasmids we used pBBR1MCS2-prr and pRK415-prr for copy number estimation in R. sphaeroides and pBBR1MCS2-pckA for copy number estimation in R. palustris. Southern blotting of R. sphaeroides total DNA harboring the various plasmids was conducting by using the 2.6-kb BamHI-KpnI PCR product encoding the prrABC genes as a probe (Fig. 6). The plasmid-derived and chromosome-derived hybridization signals were determined. The corresponding ratios suggested that the number of pMG170 copies per chromosome is in the range from 18 to 23 in R. sphaeroides. In this species, the number of copies of the broad-host-range plasmid pBBR1MCS2 was found to be in the range from 9 to 14, while the number of copies of the RK2 derivative pRK415 was in the range from 4 to 7, suggesting that there is a strong correlation between copy number and stability. Similarly, by using the pckA probe, the numbers of copies of plasmids pMG170 and pBBR1MCS2 were determined in R. palustris to be two or three and one, respectively.
FIG. 6.
Numbers of copies of plasmid pMG170 in R. sphaeroides and R. palustris. Plasmid copy numbers were determined by Southern blot hybridization analysis. Copy numbers were determined for pMG170 in R. sphaeroides (A) and in R. palutris (D), for pBBR1MCS2 in R. sphaeroides (B) and in R. palutris (E), and for pRK415 in R. sphaeroides (C). Total DNA from the R. sphaeroides wild type and R. sphaeroides transformants harboring plasmid pMG170-prr, pBBR1MCS2-prr, or pRK415-prr was digested with BamHI, KpnI, and SmaI. Similarly, total DNA from wild-type R. palutris and R. palutris transformants containing pMG170-pckA or pBBR1MCS2-pckA was digested with BamHI, PstI, and SmaI. Lanes 1, 2.6-kb PCR fragment containing the prrA, prrB, and prrC genes from R. sphaeroides (A, B, and C) and the 2.2-kb PCR fragment containing the pckA gene from R. palustris (D and E); lanes 2, BamHI-SmaI-digested R. sphaeroides wild-type chromosomal DNA (A, B, and C) and SmaI-PstI-digested R. palustris wild-type chromosomal DNA (D and E); lanes 3 to 8, 1, 0.75, 0.5, 0.25, 0.1, and 0.05 μg of BamHI-SmaI-KpnI-digested total DNA, respectively, from R. sphaeroides harboring plasmid pMG170-prr (A), pBBR1MCS2-prr (B), or pRK415-prr (C) and from R. palustris harboring plasmid pMG170-pckA (D) or pBBR1MCS2-pckA (E). In each gel, the upper band corresponds to chromosomal DNA fragments, whereas the lower bands correspond to episomic DNA fragments.
DISCUSSION
We isolated a previously undiscovered strain of R. blasticus, strain TCRI-14, that harbors a 3.4-kb cryptic plasmid. This plasmid, designated pMG160, encodes replication, mobilization, and stability functions. Each of the genes and the origin of transfer were precisely mapped by deletion analysis, by analysis of frameshift and point mutations, and by gene sequencing. Plasmid pMG160 is a narrow-range genetic element that replicates and is stable in R. sphaeroides but shows 96.5% stability after 100 generations in R. capsulatus. On the other hand, it is highly segregationally unstable in R. palustris, and it does not propagate in R. rubrum, R. gelatinosus, B. japonicum, or phototrophic Bradyrhizobium strains. The whole episome was used to construct two E. coli-R. sphaeroides cloning shuttle vectors, pMG170 and pMG171, that are segregationally stable in R. sphaeroides without selective pressure. Isolation of plasmid pMG160 thus yielded a versatile host-vector system that can be used to facilitate genetic manipulation of Rhodobacter species, combining stability and mobilization properties.
The sequence analysis results suggested that pMG160 is a novel plasmid belonging to a new plasmid family since there are only low levels of similarity between its mobS, mobL, and repA genes and those of other plasmids, including Rhodobacter plasmids. Alternatively, it may be worthwhile to refine our understanding of the roles that these various mob genes may have in plasmid activity. Given the conservation of the 38-bp oriT with a palindromic sequence, the mechanisms underlying the mobilization process of pMG160 appear to be akin to the mechanisms for transfer of plasmid pTF1 originating from T. ferrooxidans (11) and of broad-host-range plasmid RSF1010 (40).
The three ORFs encoded by pMG160 are transcribed in the same direction, and mobS and mobL are putatively cotranscribed. A putative transcription terminator consisting of a GC-rich stem-loop structure is present downstream of mobL. Both mobS and mobL, as well as the origin of transfer oriT, are necessary for mobilization to occur. On the other hand, replication and stability functions are linked. The repA gene comprises 1,020 bp with a putative terminator consisting of a GC-rich stem-loop structure downstream of its stop codon. The repA gene product exhibits low but significant levels of similarity with its counterparts in a variety of plasmids. Upstream of the pMG160 repA gene are three typical iterons (16-bp direct repeats) necessary for pMG160 replication. Iterons have been shown to represent binding sites for Rep proteins and to play a role in plasmid copy number control. In addition, upstream of the iterons is an AT-rich region (63% A+T, coordinates 1932 to 2018). A genetic organization consisting of replicons controlled by iterons has been observed for numerous broad-host-range vectors that are stably maintained. AT-rich regions are genetic elements that are associated with strand opening and assembly of host initiation factors. These features are general features of replication via a theta mechanism (6). Moreover, plasmids pEA29 and pFA3 (whose RepA proteins exhibit low but significant levels of homology with the RepA protein of pMG160) have a similar organization in their replicons, as exemplified by the presence of a cluster of direct repeats and the presence of an AT-rich region located upstream of the iterons (18, 32). In addition, plasmid pMG160 and plasmid pEA29 lack the typical domains displayed by plasmids replicating via a rolling-circle mechanism often characterized by structural and segregational instability problems (26). These observations are consistent with a theta structure-mediated replication mechanism for both pEA29 (32) and pMG160. The numbers of copies of the pMG160 derivatives pMG170 and pMG171 are different in R. sphaeroides and in R. palustris. Differences in the numbers of copies, in addition to host factors, may account for the observed differences in stability in these two closely related organisms.
Despite the drawback that the host range is relatively narrow, because of their intrinsic properties pMG170 and pMG171 are ideal cloning vectors for genetic manipulation of Rhodobacter species. These two novel E. coli-Rhodobacter shuttle vectors can be mobilized from a conjugative strain. They bear the ColE1 replication origin for high-copy-number replication in E. coli and the pMG160 replication origin for replication in R. sphaeroides and R. palustris, and the numbers of copies in these species are 18 to 23 and 2 or 3, respectively. Moreover, these two vectors contain a polylinker that enables insertional inactivation of the lacZ gene. Importantly, in the absence of selective pressure they exhibit 100% segregational stability in R. sphaeroides and thus are ideal plasmids for fundamental research on R. sphaeroides and its industrial microbiology.
Acknowledgments
We are especially grateful to Samuel Kaplan (The University of Texas) for the gift of plasmid pBBR1MCS2, for critical reading of the manuscript, and for helpful comments. We thank Yasuyoshi Nakagawa (Institute for Fermentation, Osaka, Japan) for his help with phylogenetic analysis.
This work was supported by a grant from the New Energy and Industrial Technology Development Organization.
REFERENCES
- 1.Berg, T., N. Firth, S. Apisiridej, A. Hettiaratchi, A. Leelaporn, and R. A. Skurray. 1998. Complete nucleotide sequence of pSK41: evolution of staphylococcal conjugative multiresistance plasmids. J. Bacteriol. 180:4350-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biebl, H., and N. Pfenning. 1981. Isolation of members of the family Rhodospirillaceae, p. 267-273. In M. P. Starr, H. Stolp, H. G. Truper, A. Balows, and H. G. Schlegel (ed.), The prokaryotes. A handbook on habitats, isolation, and identification of bacteria, vol. 1. Springer-Verlag, Berlin, Germany.
- 3.Brasch, M. A., and R. J. Meyer. 1987. A 38 base-pair segment of DNA is required in cis for conjugative mobilization of broad host-range plasmid R1162. J. Mol. Biol. 198:361-369. [DOI] [PubMed] [Google Scholar]
- 4.Brenner, V., M. Inui, N. Nunoura, K. Momma, and H. Yukawa. 1998. Studies on CO2 fixation in PNSB: utilization of waste as the additional source of carbon for CO2 fixation by PNSB, p. 593-596. In T. Inui, M. Anpo, K. Izui, S. Yanagida, and T. Yamaguchi (ed.), Advances in chemical conversions for mitigating carbon dioxide. Studies in Surface Science and Catalysis, vol. 114. Elsevier Science B. V., Amsterdam, The Netherlands.
- 5.Climo, M. W., V. K. Sharma, and G. L. Archer. 1996. Identification and characterization of the origin of conjugative transfer (oriT) and a gene (nes) encoding a single-stranded endonuclease on the staphylococcal plasmid pGO1. J. Bacteriol. 178:4975-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.del Solar, G., R. Giraldo, M. J. Ruiz-Echevarria, M. Espinosa, and R. Diaz-Orejas. 1998. Replication and control of circular bacterial plasmids. Microbiol. Mol. Biol. Rev. 62:434-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ditta, G., T. Schmidhauser, E. Yakobson, P. Lu, X. W. Liang, D. R. Finlay, D. Guiney, and D. R. Helinski. 1985. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid 13:149-153. [DOI] [PubMed] [Google Scholar]
- 8.Ditta, G., S. Stanfield, D. Corbin, and D. R. Helinski. 1980. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 77:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donohue, T. J., and S. Kaplan. 1991. Genetic techniques in Rhodospirillaceae. Methods Enzymol. 204:459-485. [DOI] [PubMed] [Google Scholar]
- 10.Dougherty, B. A., C. Hill, J. F. Weidman, D. R. Richardson, J. C. Venter, and R. P. Ross. 1998. Sequence and analysis of the 60 kb conjugative, bacteriocin-producing plasmid pMRC01 from Lactococcus lactis DPC3147. Mol. Microbiol. 29:1029-1038. [DOI] [PubMed] [Google Scholar]
- 11.Drolet, M., P. Zanga, and P. C. Lau. 1990. The mobilization and origin of transfer regions of a Thiobacillus ferrooxidans plasmid: relatedness to plasmids RSF1010 and pSC101. Mol. Microbiol. 4:1381-1391. [DOI] [PubMed] [Google Scholar]
- 12.Eraso, J. M., and S. Kaplan. 1995. Oxygen-insensitive synthesis of the photosynthetic membranes of Rhodobacter sphaeroides: a mutant histidine kinase. J. Bacteriol. 177:2695-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eraso, J. M., and S. Kaplan. 1994. prrA, a putative response regulator involved in oxygen regulation of photosynthesis gene expression in Rhodobacter sphaeroides. J. Bacteriol. 176:32-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 15.Fornari, C. S., M. Watkins, and S. Kaplan. 1984. Plasmid distribution and analyses in Rhodopseudomonas sphaeroides. Plasmid 11:39-47. [DOI] [PubMed] [Google Scholar]
- 16.Freiberg, C., R. Fellay, A. Bairoch, W. J. Broughton, A. Rosenthal, and X. Perret. 1997. Molecular basis of symbiosis between Rhizobium and legumes. Nature 387:394-401. [DOI] [PubMed] [Google Scholar]
- 17.Fujii, T., A. Nakazawa, N. Sumi, H. Tani, A. Ando, and M. Yabuki. 1983. Utilization of alcohols by Rhodopseudomonas sp. No.7 isolated from n-propanol-enrichment cultures. Agric. Biol. Chem. 47:2747-2753. [Google Scholar]
- 18.Gilbride, K. A., and J. L. Brunton. 1990. Identification and characterization of a new replication region in the Neisseria gonorrhoeae beta-lactamase plasmid pFA3. J. Bacteriol. 172:2439-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins, D. G., A. J. Bleasby, and R. Fuchs. 1992. CLUSTAL V: improved software for multiple sequence alignment. Comput. Appl. Biosci. 8:189-191. [DOI] [PubMed] [Google Scholar]
- 20.Hu, N. T., and B. L. Marrs. 1979. Characterization of the plasmid DNAs of Rhodopseudomonas capsulata. Arch. Microbiol. 121:61-69. [Google Scholar]
- 21.Hunter, C. N., and G. Turner. 1988. Transfer of genes coding for apoproteins of reaction center and light-harvesting LH1 complexes to Rhodobacter sphaeroides. J. Gen. Microbiol. 134:1471-1480. [Google Scholar]
- 22.Imhoff, J. F. 1995. Taxonomy and physiology of phototrophic purple bacteria and green sulfur bacteria, p. 1-15. In R. E. Blankenship, M. T. Madigan, and C. E. Bauer (ed.), Anoxygenic photosynthetic bacteria. Advances in Photosynthesis, vol. 2. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 23.Inui, M., V. Dumay, K. Zahn, H. Yamagata, and H. Yukawa. 1997. Structural and functional analysis of the phosphoenolpyruvate carboxylase gene from the purple nonsulfur bacterium Rhodopseudomonas palustris No. 7. J. Bacteriol. 179:4942-4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inui, M., K. Nakata, J. H. Roh, K. Zahn, and H. Yukawa. 1999. Molecular and functional characterization of the Rhodopseudomonas palustris No. 7 phosphoenolpyruvate carboxykinase gene. J. Bacteriol. 181:2689-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inui, M., J. H. Roh, K. Zahn, and H. Yukawa. 2000. Sequence analysis of the cryptic plasmid pMG101 from Rhodopseudomonas palustris and construction of stable cloning vectors. Appl. Environ. Microbiol. 66:54-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahn, S. A. 1997. Rolling-circle replication of bacterial plasmids. Microbiol. Mol. Biol. Rev. 61:442-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawasaki, H., Y. Hoshino, A. Hirata, and K. Yamasato. 1993. Is intracytoplasmic membrane structure a generic criterion? It does not coincide with phylogenetic interrelationships among phototrophic purple nonsulfur bacteria. Arch. Microbiol. 160:358-362. [DOI] [PubMed] [Google Scholar]
- 28.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 29.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 30.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop, and K. M. Peterson. 1994. pBBR1MCS: a broad-host-range cloning vector. BioTechniques 16:800-802. [PubMed] [Google Scholar]
- 31.Kuhl, S. A., D. W. Nix, and D. C. Yoch. 1983. Characterization of a Rhodospirillum rubrum plasmid: loss of photosynthetic growth in plasmidless strains. J. Bacteriol. 156:737-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGhee, G. C., and A. L. Jones. 2000. Complete nucleotide sequence of ubiquitous plasmid pEA29 from Erwinia amylovora strain Ea88: gene organization and intraspecies variation. Appl. Environ. Microbiol. 66:4897-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nano, F. E., W. D. Shepherd, M. M. Watkins, S. A. Kuhl, and S. Kaplan. 1984. Broad-host-range plasmid vector for the in vitro construction of transcriptional/translational lac fusions. Gene 34:219-226. [DOI] [PubMed] [Google Scholar]
- 34.Pfennig, N., and H. G. Trüper. 1989. Anoxygenic phototrophic bacteria, p. 1635-1709. In J. T. Staley, M. P. Bryant, N. Pfennig, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 3. Williams and Wilkins, Baltimore, Md.
- 35.Rossello-Mora, R., and R. Amann. 2001. The species concept for prokaryotes. FEMS Microbiol. Rev. 25:39-67. [DOI] [PubMed] [Google Scholar]
- 36.Saegesser, R., R. Ghosh, and R. Bachofen. 1992. Stability of broad host range cloning vector in the phototrophic bacterium Rhodospirillum rubrum. FEMS Microbiol. Lett. 95:7-12. [Google Scholar]
- 37.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Scherzinger, E., V. Kruft, and S. Otto. 1993. Purification of the large mobilization protein of plasmid RSF1010 and characterization of its site-specific DNA-cleaving/DNA-joining activity. Eur. J. Biochem. 217:929-938. [DOI] [PubMed] [Google Scholar]
- 40.Scholz, P., V. Haring, B. Wittmann-Liebold, K. Ashman, M. Bagdasarian, and E. Scherzinger. 1989. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene 75:271-288. [DOI] [PubMed] [Google Scholar]
- 41.Sganga, M. W., and C. E. Bauer. 1992. Regulatory factors controlling photosynthetic reaction center and light-harvesting gene expression in Rhodobacter capsulatus. Cell 68:945-954. [DOI] [PubMed] [Google Scholar]
- 42.van Niel, C. B. 1944. The culture, general physiology, and classification of the non-sulfur purple and brown bacteria. Bacteriol. Rev. 8:1-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wada, C., and T. Yura. 1979. Escherichia coli mutants incapable of supporting replication of F-like plasmids at high temperature: isolation and characterization of mafA and mafB mutants. J. Bacteriol. 140:864-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, A., and F. L. Macrina. 1995. Streptococcal plasmid pIP501 has a functional oriT site. J. Bacteriol. 177:4199-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willison, J. C., J. P. Magnin, and P. M. Vignais. 1987. Isolation and characterization of Rhodobacter capsulatus strains lacking endogenous plasmids. Arch. Microbiol. 147:134-142. [Google Scholar]