Abstract
The uptake and accumulation of the potent osmolytes glycine betaine and carnitine enable the food-borne pathogen Listeria monocytogenes to proliferate in environments of elevated osmotic stress, often rendering salt-based food preservation inadequate. To date, three osmolyte transport systems are known to operate in L. monocytogenes: glycine betaine porter I (BetL), glycine betaine porter II (Gbu), and a carnitine transporter OpuC. We investigated the specificity of each transporter towards each osmolyte by creating mutant derivatives of L. monocytogenes 10403S that possess each of the transporters in isolation. Kinetic and steady-state osmolyte accumulation data together with growth rate experiments demonstrated that osmotically activated glycine betaine transport is readily and effectively mediated by Gbu and BetL and to a lesser extent by OpuC. Osmotically stimulated carnitine transport was demonstrated for OpuC and Gbu regardless of the nature of stressing salt. BetL can mediate weak carnitine uptake in response to NaCl stress but not KCl stress. No other transporter in L. monocytogenes 10403S appears to be involved in osmotically stimulated transport of either osmolyte, since a triple mutant strain yielded neither transport nor accumulation of glycine betaine or carnitine and could not be rescued by either osmolyte when grown under elevated osmotic stress.
Listeriosis is a food-borne disease that mainly affects immunocompromised individuals and is approximately 25% fatal. The causative agent, Listeria monocytogenes, is a ubiquitous gram-positive organism that is often isolated from foods of plant or animal origin (21). The infectious dose for humans remains unknown and most likely varies depending on the immune status of the individual. Controlling the proliferation of L. monocytogenes in foods is often problematic, because the pathogen is markedly resistant to classical methods of food preservation such as acidification (grows at pH as low as 4.5 [4]), increased osmotic pressure (NaCl concentrations as high as 10%), and cold storage (temperatures as low as −0.5°C) (5).
Central to the pathogen's capacity to withstand and proliferate in environments of increased osmotic strength is its ability to accumulate compatible solutes intracellularly. Compatible solutes, sometimes called osmolytes, are low-molecular-weight, highly soluble compounds that bear no net charge at physiological pH and function as osmoprotectants in a variety of eukaryotic and prokaryotic organisms (31). The molecular basis of their action, although not completely understood, entails their accumulation to high levels inside the cell in response to increased external osmolarity, thus restoring turgor, without affecting cytoplasmic functions (31). Glycine betaine, carnitine, acetylcarnitine, γ-butyrobetaine, proline betaine, and 3-dimethylsulphoniopropionate have been shown to confer osmoprotection in L. monocytogenes in synthetic media (3). Neither glycine betaine nor carnitine can be synthesized by L. monocytogenes, and their accumulation is achieved by active transport from the environment. Two transport systems have been biochemically and genetically identified as glycine betaine transporters (9, 10, 13, 22), and one has been identified as a carnitine transporter (1, 7, 24, 29).
Glycine betaine porter II (Gbu) is an ATP binding cassette (ABC) transporter that is encoded by the gbu operon (13). Gbu, which mediates the uptake of glycine betaine, can be activated by increased osmotic pressure or decreased temperature (9, 13). Glycine betaine porter I (BetL) is a secondary transporter that is activated by an increase in medium osmolarity and mediates the cotransport of glycine betaine with a sodium ion (a glycine betaine-Na+ symporter) (10, 22). OpuC, the product of the opuC operon, is an ABC transporter that has been shown to transport carnitine in response to osmotic and cold stress (1, 7, 24).
The transport characteristics of Gbu, BetL, and OpuC have been studied by the isolation of strains with single mutations in genes encoding transport systems and comparing their ability to transport glycine betaine or carnitine to that of their respective wild-type isogens. Although this approach proved useful in isolating and identifying the three transporters, substrate specificity remains uncertain. Furthermore, whether additional glycine betaine and carnitine transporters operate in L. monocytogenes remains unknown. Therefore, the objectives of this work were to examine the transporters' specificities for glycine betaine and carnitine in detail, to investigate whether additional transporters for either osmolyte operate in L. monocytogenes under osmotic stress, and to compare the osmoprotective potentials of these transporters in environments where glycine betaine, carnitine, or both are present. In order to achieve these objectives, double and triple transporter mutants were constructed and characterized so that Gbu, BetL, and OpuC could be studied without mutual interference. A substantially similar study has appeared previously (30) but employed a different strain of L. monocytogenes and slightly different techniques, yielding different results.
MATERIALS AND METHODS
Bacterial strains, media, and chemicals.
L. monocytogenes 10403S was the wild-type strain used in this work (28). Cultures were kept on brain heart infusion (BHI) (Difco) agar plates at 4°C. Modified (lacking choline) Pine's medium (19) containing 0.5% glucose was used as the defined medium. Escherichia coli DH5α was maintained on Luria-Bertani agar (20). Glycine betaine, carnitine, NaCl, and KCl were purchased from Sigma Chemical Co. (St. Louis, Mo.). [methyl-14C]carnitine, and [methyl-14C]choline were purchased from Perkin-Elmer Life Sciences, Inc. (Boston, Mass.). [methyl-14C]glycine betaine was prepared by oxidation of [methyl-14C]choline (18). Ampicillin (50 μg/ml) and chloramphenicol (CHL) (10 μg/ml) were purchased from Sigma and used as appropriate. Standard methods were used for routine DNA manipulations (20). DNA polymerase was purchased from Eppendorf Scientific Inc. (Westbury, N.Y.), nucleotides and DNA-modifying enzymes were from Roche Molecular Biochemicals (Indianapolis, Ind.), and DNA purification was performed with cartridges from Qiagen Inc. (Valencia, Calif.).
Construction of L. monocytogenes transporter mutant strains. (i) Construction of single mutants.
The splicing by overlap extension (SOE) PCR approach (11) was used to create internal deletion constructs for gbu, betL, and opuC. For the gbu operon, two ∼400-bp fragments were amplified: one in the 5′ region of the operon, inside the gbuA gene (nucleotides 612 to 1012 of the operon) was amplified using primers gSOE-A and gSOE-B (Table 1). The second fragment was at the 3′ region of the operon, inside the gbuC gene (nucleotides 2849 to 3213), amplified using primers gSOE-C and gSOE-D. Following purification, the two PCR products were used as templates in a third PCR with primers gSOE-A and gSOE-D, which generated a 766-bp gbu hybrid product bearing a 1,836-bp deletion. Purified product was digested with EcoRI and XbaI, cloned into the E. coli-L. monocytogenes shuttle vector pKSV7 (25), and transformed into E. coli DH5α to yield plasmid pMIA. pMIA was electroporated (17) into L. monocytogenes 10403S, and transformants were selected on BHI plates containing CHL (10 μg/ml). Several colonies were picked from the transformation plates and individually propagated at 40°C (a nonpermissive temperature for pKSV7 replication) in BHI broth containing CHL (10 μg/ml) for approximately 30 generations, and colonies with chromosomally integrated plasmid were isolated in BHI-CHL plates at 40°C. A single colony from the BHI-CHL plates was picked up and propagated for approximately 50 generations in BHI broth at 30°C to select for excision of the plasmid from the chromosome. Curing of the excised plasmid was achieved by sequential passage of the culture (approximately 30 generations) in BHI broth at 40°C, after which the culture was colony purified on BHI plates. Five hundred colonies were screened for loss of CHL resistance by replica plating (patching) on BHI-CHL and BHI plates, and among those showing CHL sensitivity, those having a mutant allele in the chromosome were identified using PCR (primers gSOE-F and gSOE-R). One of these mutants, L. monocytogenes ASA1 (SOEgbu10), was used for further genetic manipulations (construction of double mutants).
TABLE 1.
PCR primers used in this study
| Primer | Sequence (5′→3′) |
|---|---|
| gSOE-Aa | CGT CGA ATT CAG CCT ACG AGC |
| gSOE-B | TCG AGC AAT TGA TCT TGC AT |
| gSOE-Cb | ATG CAA GAT CAA TTG CTC GAC CTT CCG CGT ACC AAG TGT TAG |
| gSOE-Da | TAA ATT CTA GAA GTA TTT GGA AGC |
| gSOE-F | CTT TGG TTT ATT CCC GAA CAG AAC |
| gSOE-R | TTT GTC ATG TAT TTT GGT ACT GC |
| oSOE-Aa | GTA ACG AAG CTT TAT AAA GGG G |
| oSOE-B | AAT CAA GTT TTG CTC TGC |
| oSOE-Cb | GCA GAG CAA AAC TTG ATT CAA ACA ATT CCG GCA CTA GCA ATG T |
| oSOE-Da | GCG TTG GAT CCA ACC AAG AAG T |
| oSOE-F | TGG ACA GCA GCA ACG TAT AG |
| oSOE-R | CAT TTA TGA TAA AAA GTT TAC TAC |
| bSOE-Aa | TTT CTA GAA AGT AAT TTT GGT TGG TAT |
| bSOE-B | TCC CCA GTG GAA GAA TGA |
| bSOE-Cb | TCA TTC TTC CAC TGG GGA ATT TTT GTC GAA CAA CAT GGT AAT |
| bSOE-Da | AAT CGA AGC TTT TTG AAG CGC TGT |
| bSOE-F | AGT CCG ATT GGC TCG ATT CGA C |
| bSOE-R | TCG CGA AAT AGT CGC GGC AAA GC |
Nucleotide substitutions to create restriction sites are underlined.
Overhangs complementary to SOE-B primers are underlined.
Similarly, for the opuC operon, two fragments were amplified: one in the 5′ region of the operon, inside the opuCA gene (nucleotides 200 to 652 of the operon) was amplified using primers oSOE-A and oSOE-B (Table 1). The second fragment was at the 3′ region of the operon, inside the opuCD gene (nucleotides 3170 to 3598), amplified using primers oSOE-C and oSOE-D. Following purification, the two PCR products were used as templates in a third PCR with primers oSOE-A and oSOE-D, which generated an 882-bp opuC hybrid product bearing an internal 2,517-bp deletion. Purified product was digested with HindIII and BamHI, cloned into vector pKSV7 and transformed into E. coli DH5α to yield plasmid pZWH. pZWH was electroporated into L. monocytogenes 10403S, and transformants were selected on BHI-CHL plates. The remaining integration, excision, and curing steps as well as the screening for opuC deletion mutants were analogous to those used to construct mutant SOEgbu10. CHL-sensitive mutants were examined for chromosomal deletion using PCR (primers oSOE-F and oSOE-R). The resulting mutant with an in-frame deletion in the opuC operon (SOEopuC15) was designated ASA2.
For creation of a betL mutant, the SOE (bSOE-A, bSOE-B, bSOE-C, and bSOE-D) and forward and reverse (bSOE-F and bSOE-R) primers used were of the same sequence as those used by Sleator et al. (23). The hybrid 600-bp betL construct (comprised of two regions in the 5′ and 3′ end of betL) was digested with XbaI and HindIII, cloned into pKSV7, and transformed into E. coli DH5α to yield plasmid pAEK. pAEK was similarly electroporated into L. monocytogenes 10403S. The remaining steps were identical to those described above for isolation of the other single mutants. The resulting L. monocytogenes betL mutant (SOEbetL25) with an internal 681-bp deletion was designated ASA3.
(ii) Construction of double mutants and triple mutant.
Double transporter mutant strains (SOEgbu10-betL25 and SOEopuC15-betL25) were constructed by electroporating the pKSV7 plasmid derivatives pMIA and pZWH, respectively, into L. monocytogenes ASA3 (the betL deletion mutant SOEbetL25), and this was followed by the integration, excision, curing and, screening steps described above. These strains were designated ASA4 and ASA5, respectively. Finally, constructions of the third double mutant, ASA6 (SOEgbu10-opuC15), and the triple transporter mutant, ASA7 (SOEgbu10-opuC15-betL25), were initiated by electroporating pMIA into ASA2 and ASA5, respectively. Allelic exchange events were confirmed by PCR amplification using the SOE-F and SOE-R primer sets.
Measurement of generation time under osmotic stress in the presence and absence of osmolytes.
To inoculate Pine's medium for growth rate determination, L. monocytogenes cultures were grown overnight at 30°C in BHI broth, and 1-ml aliquots were centrifuged at 11,750 × g for 10 min. The pellets were washed twice with 1-ml portions of Pine's medium and used to inoculate (0.5%) 125-ml Pyrex nephelo flasks containing Pine's medium. These flask cultures were grown at 30°C to cell densities of ca. 2 × 109 CFU/ml, diluted 10-fold in Pine's medium, and used to inoculate (1%) eight sets of 125-ml Pyrex nephelo flasks containing 15 ml of Pine's medium. These sets were incubated with mild shaking (40 rpm) at 30°C. Cultures were stressed with either 0.7 M NaCl or an isosmotic equivalent of KCl (0.72 M) and incubated in the absence of osmolytes or in the presence of 1 mM glycine betaine, 1 mM carnitine, or both osmolytes at a concentration of 0.5 mM each. Unstressed cultures with and without osmolytes were used as controls. Growth was monitored with a Klett-Summerson photoelectric colorimeter with a green (no. 54) filter. Each combination of strain, salt stress, and osmolyte was tested in triplicate. Specific growth rate constants (κ) were calculated by plotting the natural logarithm of the number of Klett units versus time and converted to their respective generation time values (g).
Transport and steady-state cytoplasmic levels of glycine betaine and carnitine.
Transport of glycine betaine and carnitine was examined for the wild-type L. monocytogenes 10403S, all three double mutant strains (ASA4, ASA5, and ASA6), and the triple mutant strain, ASA7. Transport of these osmolytes was examined at 30°C under osmotic stress provided by 0.7 M NaCl or 0.72 M KCl and under baseline conditions (no salt added). For each combination of strain and osmotic stress, transport assays were done in duplicate using 100 μM [methyl-14C]glycine betaine and [methyl-14C]carnitine as described previously (14). Uptake rates were normalized to total cellular protein, which was determined using the bicinchoninic acid method (27) (Pierce Chemical, Rockford, Ill.) and are reported as nanomoles of osmolyte per minute per milligram of cellular protein.
L. monocytogenes 10403S, ASA4, ASA5 ASA6, and ASA7 were grown aerobically in modified Pine's medium containing 0.5 mM (each) glycine betaine and carnitine and 0.7 M NaCl. Unstressed cultures grown at 30°C with added osmolytes served as controls. Cultures were harvested at late log phase by centrifugation (4,080 × g for 10 min at 4°C). The pelleted cells were immediately washed with ice-cold 0.775 M NaCl solution. Cytoplasmic contents were extracted with ice-cold 7% perchloric acid as described elsewhere (26). Extracts were analyzed by natural-abundance 13C nuclear magnetic resonance (13C-NMR) as previously described (1). Total cellular protein at the time of harvest was used to normalize compatible solute concentrations.
RESULTS
Single, double, and triple transporter mutant construction.
Allelic exchange mutagenesis was used to create deletions in the gbu and opuC operons and the betL gene, which encode the ATP-driven glycine betaine transporter Gbu, the ATP-driven carnitine transporter OpuC, and the Na+-glycine betaine symporter BetL, respectively. In each case, DNA fragments near the 5′ and 3′ ends were independently amplified, spliced, and cloned into the E. coli-L. monocytogenes shuttle vector pKSV7 to generate the recombinant-pKSV7 vector derivatives pMIA, pZWH, and pAEK that carry the spliced regions of the gbu and opuC operons and betL gene, respectively. Each recombinant pKSV7 derivative was independently electroporated into the wild-type L. monocytogenes 10403S. Chromosomal integration of plasmid vectors was selected for by growth of transformed L. monocytogenes cells for several generations in BHI broth at 40°C in the presence of CHL (10 μg/ml). Excision of chromosomally integrated plasmids was facilitated by repeated growing of cells in BHI at 30°C in the absence of antibiotic pressure, and curing of excised plasmids was carried out by subsequent shift of the growth temperature at 40°C for several generations, after which bacteria were colony isolated on BHI plates and individual colonies were screened for loss of CHL resistance. Several CHL-sensitive colonies were screened by PCR to find bacteria in which the allelic exchange event resulted in a mutant phenotype (chromosomal replacement of wild-type copy of the gene with the mutant allele). This first round of allelic exchange mutagenesis resulted in three mutant derivatives: L. monocytogenes ASA1, a mutant with a 1,836-bp deletion in the gbu operon (from nucleotide 1013 in gbuA to nucleotide 2848 in gbuC); L. monocytogenes ASA2, a mutant with a 2,518-bp deletion in the opuC operon (from nucleotide 653 in opuCA to nucleotide 3169 in opuCD); and L. monocytogenes ASA3, a mutant with a 681-bp deletion in the betL gene (from nucleotide 623 to 1303).
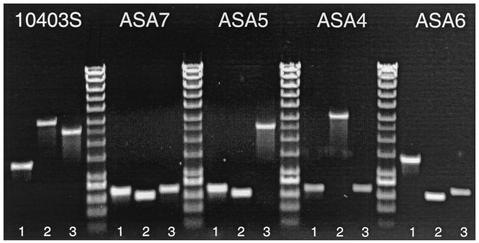
L. monocytogenes single transporter mutant strains ASA2 and ASA3 were used as starting strains for a second round of two-step allele replacement mutagenesis which began with the transformation of ASA3 with the recombinant vectors pMIA or pZWH and with the transformation of ASA2 with pMIA. The three resulting double-mutant strains were ASA4, a strain with deletions in the gbu operon and betL gene; ASA5, a double betL and opuC mutant; and ASA6, a double mutant in gbu and opuC (Table 2). Construction of a mutant in all three genes (ASA7) was initiated by transforming pMIA into ASA5 and proceeding in the same manner as described above. Figure 1 shows the sizes of DNA fragments resulting from amplification of chromosomal DNA (extracted from the wild-type L. monocytogenes 10403S, the three double mutants and the triple mutant) with the three SOE-F and SOE-R gene-specific primer sets. Table 3 presents the expected wild-type and mutant DNA sizes after PCR amplification of chromosomal DNA by the respective pair of SOE-F and SOE-R primer for each gene.
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant property or genotype | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | ||
| L. monocytogenes | ||
| 10403S | Wild type | 28 |
| ASA1 | Δgbu10 | This study |
| ASA2 | ΔopuC15 | This study |
| ASA3 | ΔbetL25 | This study |
| ASA4 | Δgbu10 ΔbetL25 | This study |
| ASA5 | ΔopuC15 ΔbetL25 | This study |
| ASA6 | Δgbu10 ΔopuC15 | This study |
| ASA7 | Δgbu10 ΔopuC15 ΔbetL25 | This study |
| Plasmids | ||
| pKSV7 | 25 | |
| pMIA | pKSV7 carrying spliced DNA from gbu | This study |
| pZWH | pKSV7 carrying spliced DNA from opuC | This study |
| pAEK | pKSV7 carrying spliced DNA from betL | This study |
FIG. 1.
DNA sizes after PCR amplification of chromosomal DNA extracted from wild-type L. monocytogenes 10403S; the double-mutant derivatives ASA4 (ΔbetS Δgbu), ASA5 (ΔbetL ΔopuC), and ASA6 (Δgbu ΔopuC); and the triple mutant ASA7 (ΔbetL Δgbu ΔopuC) with the gene specific SOE-F and SOE-R primers. Lanes 1, PCR with betL primers; lanes 2, PCR with opuC primers; lanes 3, PCR with gbu primers. DNA ladder positions (in base pairs) from bottom to top: 200, 400, 600, 800, 1,000, 1,500, 2,000, 2,500, 3,000, 4,000, 5,000, 6,000, 8,000, and 10,000.
TABLE 3.
Expected DNA size after PCR amplification of wild-type and mutant chromosomal DNA with primers SOE-F and SOE-Ra
| L. monocytogenes gene | Size (bp) in:
|
|
|---|---|---|
| 10403S | SOE mutant | |
| gbu | 2,575 | 738 |
| opuC | 3,149 | 631 |
| betL | 1,411 | 730 |
gSOE-F and gSOE-R for gbu, oSOE-F and oSOE-R for opuC, and bSOE-F and bSOE-R for betL.
Uptake of glycine betaine and carnitine by L. monocytogenes under osmotic stress.
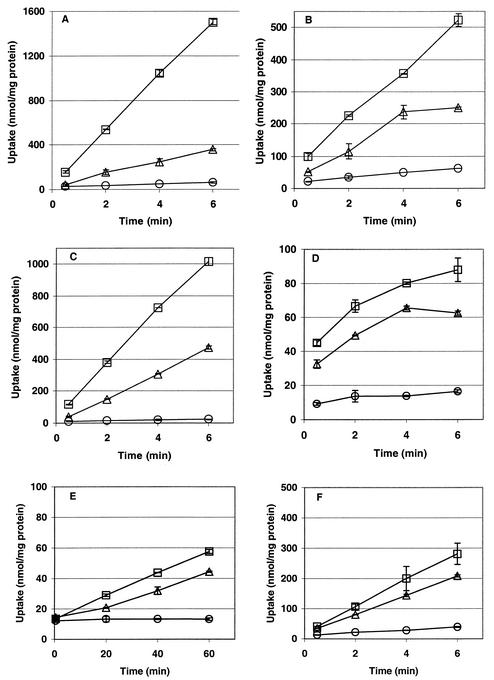
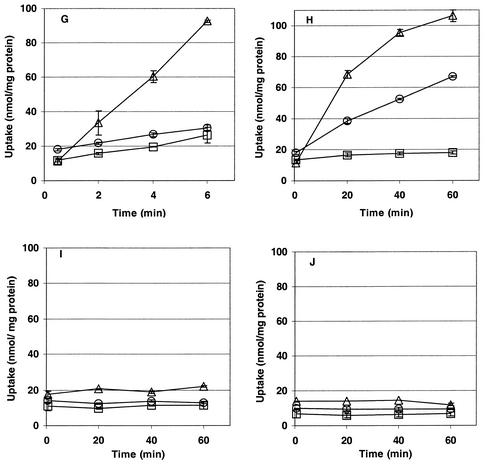
L. monocytogenes 10403S, ASA4, ASA5, ASA6, and ASA7 were grown at 30°C in modified Pine's medium in the presence of 0.7 M NaCl or 0.72 M KCl or without added salt. Exponentially growing cultures were centrifuged and resuspended in buffer of the same osmolality as that of the growth medium, and uptake of glycine betaine or carnitine was measured over time after addition of [methyl-14C]glycine betaine or [methyl-14C]carnitine to the cultures (Fig. 2).
FIG. 2.
Osmolyte transport activity of L. monocytogenes 10403S and mutant derivatives ASA4, ASA5, ASA6, and ASA7. Uptake of 100 μM [14C]glycine betaine (A, C, E, G, and I) or carnitine (B, D, F, H, and J) was measured in strain 10403S (A and B), ASA5 (C and D), ASA4 (E and F), ASA6 (G and H), and ASA7 (I and J) grown to late log phase in modified Pine's medium at 30°C with 0.7 M NaCl (triangles), 0.72 M KCl (squares), or no added salt (circles). Transport was assayed as described in Materials and Methods. Error bars indicate the range of duplicate values. Note that the scales on the time axes vary.
Transport of glycine betaine.
Glycine betaine transport in L. monocytogenes is osmotically activated (14) (Fig. 2A). Under osmotic stress, the rate of transport through the ABC transporter Gbu (Fig. 2C) was orders of magnitude higher than that of the secondary Na+ symporter BetL (Fig. 2G). BetL efficiently transported glycine betaine only under NaCl-mediated stress. The nonzero rate of transport under KCl-mediated stress was equal to the baseline level of transport (observed in unstressed cells) and was probably due to the baseline osmolality of the assay buffer and the presence of sodium ion from the growth medium and assay buffer (Fig. 2G). Weak glycine betaine transport from OpuC was observed under both stresses (Fig. 2E).
Transport of carnitine.
Carnitine transport in L. monocytogenes is also osmotically regulated (Fig. 2B). Our results show that Gbu (Fig. 2D) and OpuC (Fig. 2F) can readily mediate carnitine uptake in response to osmotic stress. Weak, transient carnitine uptake was also observed by BetL when Na+ was present in the assay medium (Fig. 2H). As expected, the strongest carnitine uptake was observed by the dedicated carnitine transporter OpuC (Fig. 2F).
No uptake of either osmolyte was observed by the triple mutant L. monocytogenes ASA7, even under conditions of osmotic activation (Fig. 2I and J).
Compatible solute accumulation under osmotic stress.
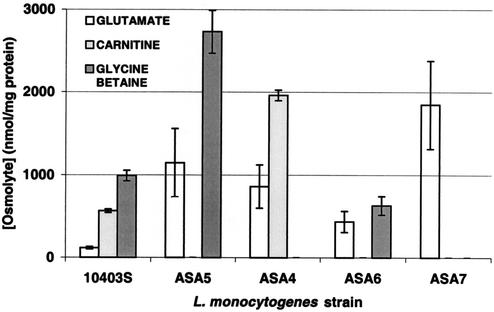
L. monocytogenes strains (10403S, ASA4, ASA5, ASA6, and ASA7) were grown in defined medium at 30°C with a 0.5 mM concentration (each) of glycine betaine and carnitine, and with or without 0.7 M NaCl. 13C-NMR analysis was performed on perchloric acid extracts of cells from cultures in balanced growth. With the exception of low levels of glutamate, no other osmolyte was detected in cell extracts of any strain in the absence of stress (data not shown).
When grown under osmotic stress (0.7 M NaCl), wild-type L. monocytogenes accumulated glycine betaine, carnitine, and glutamate (Fig. 3). Cell extracts of mutant ASA5, which only possesses the Gbu transporter, had increased levels of glycine betaine and glutamate, but carnitine accumulation was not detected. Extracts of ASA4 (only OpuC transporter present) contained increased levels of carnitine and glutamate but no detectable glycine betaine. Extracts of mutant ASA6 (only BetL porter present) contained increased levels of glutamate and a level of glycine betaine that was comparable to, albeit somewhat lower than, that of the wild type and contained no detectable carnitine. Glutamate was the sole detectable osmolyte in extracts of the triple mutant ASA7. However glutamate accumulation by ASA7 was the highest among those measured in extracts of all other (wild-type or double-mutant) strains. It is therefore evident that, when transport of glycine betaine or carnitine is impaired, osmotically stressed L. monocytogenes increases the accumulation of glutamate and that of the other potent osmolyte (carnitine or glycine betaine). We have also observed this phenomenon in chilled, stressed cells (2). When osmotically stressed L. monocytogenes is impaired in transport of both glycine betaine and carnitine, as is the case with the triple mutant ASA7, glutamate was the only solute that accumulated appreciably. Whether the accumulation of glutamate is the result of active transport, increased biosynthesis, or reduced turnover remains unknown.
FIG. 3.
Compatible solute accumulation by L. monocytogenes 10403S and mutant L. monocytogenes ASA5, ASA4, ASA6, and ASA7 during balanced growth in modified Pine's medium with 0.5 mM glycine betaine and carnitine and under 0.7 M NaCl stress. Exponentially growing cultures were harvested and washed, and cytoplasmic contents were extracted with perchloric acid. Alanine (50 mM) was added to each extract as an internal standard, and compatible solutes were quantitated by using natural-abundance 13C-NMR spectroscopy.
Growth under osmotic stress in the presence or absence of glycine betaine and carnitine.
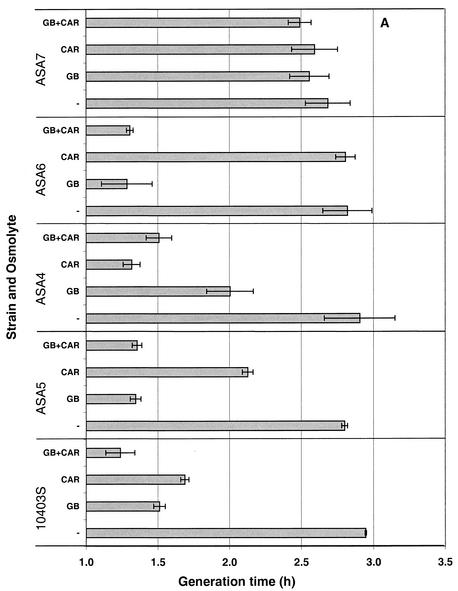
The effectiveness of each transport system in alleviating osmotic stress in media with different compatible solute composition was determined by measuring the growth of the five strains in Pine's medium with 0.7 M NaCl (Fig. 4A), with 0.72 M KCl (Fig. 4B), or without added salt (data not shown), in the absence or presence of 1 mM glycine betaine or carnitine or a 0.5 mM concentration of each osmolyte combined. Regardless of the osmolyte present, unstressed cultures grew with a generation time (mean ± standard deviation) of 1.33 ± 0.09 h, and no significant differences in growth were observed among stressed cultures in the absence of osmolytes (Fig. 4).
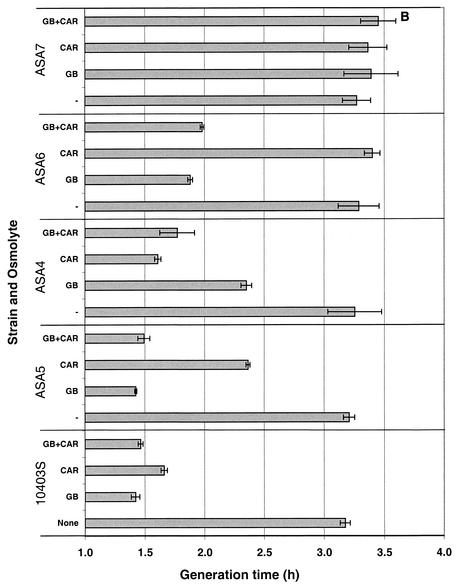
FIG. 4.
Growth characteristics of L. monocytogenes 10403S (wild type), ASA5 (containing only Gbu), ASA4 (containing only OpuC), ASA6 (containing only BetL), and ASA7 (triple deletion). Cultures were grown in BHI medium, washed, and used to inoculate (1%) modified Pine's medium. These cultures were grown to late log phase, 10-fold diluted, and used to inoculate (1%) modified Pine's medium containing 0.7 M NaCl (A) or 0.72 M KCl (B), in the presence of 1 mM glycine betaine (GB), 1 mM carnitine (CAR), or a 0.5 mM concentration of each osmolyte (GB+CAR) or in the absence of osmolytes (−). Error bars indicate ±1 standard deviation of triplicate values.
The following comparisons can be made in the growth of the five strains under osmotic stress conferred by 0.7 M NaCl. Compared to the growth rates observed in the absence of osmolytes, wild-type L. monocytogenes grew twice as fast in the presence of either osmolyte in the growth medium, with glycine betaine being slightly more osmoprotective than carnitine. Mutant ASA5, which possesses only the Gbu porter, also showed a 50% reduction in the generation time when glycine betaine was present; the presence of carnitine did confer some osmoprotection (24% reduction in generation time), but distinctly less than that offered by glycine betaine. An analogous response was exhibited by strain ASA4, which possesses only the carnitine transporter OpuC. Carnitine was very effective (generation time reduced by 55%), whereas glycine betaine was less effective (30% reduction). For strain ASA6, which only carries the glycine betaine symporter BetL, glycine betaine provided an osmoremedial effect that was equal in magnitude to that in strain ASA5, whereas the inclusion of carnitine did not result in any reduction in generation time. Although carnitine transport was observed by BetL in the presence of sodium (Fig. 2H), the transport was weak, reaching approximately 100 nmol of carnitine/mg of cell protein after 1 h; this level of intracellular carnitine may be insufficient to alleviate osmotic stress. Finally, the growth rate of the triple mutant ASA7 could not be enhanced by either osmolyte, as the resulting growth rates were indistinguishable from those of cultures without added osmolytes.
The stress alleviation by osmolytes in Pine's medium with 0.72 M KCl for each of the five strains was parallel to that described above for NaCl. The only noticeable difference was the somewhat-weaker alleviating effect of glycine betaine in strain ASA6, a result that can be explained by the need of the symporter for sodium ions for efficient glycine betaine uptake.
DISCUSSION
A key feature enabling L. monocytogenes to tolerate environments of elevated osmotic stress is the pathogen's ability to accumulate the compatible solutes glycine betaine and carnitine from its environment. Recent work has led to the biochemical and genetic identification of three transport systems. BetL (10) was first identified biochemically in membrane vesicles as a glycine betaine-Na+ symporter that responds to hyperosmotic gradients having a strict transport requirement for sodium ion and was later identified at the genetic level as a secondary transport system that was designated BetL (22). Gbu (9), the product of the gbu operon (13), was identified as an osmotically activated and cold-activated glycine betaine ABC transporter. OpuC, an ABC transporter, has been identified in four L. monocytogenes strains by three laboratories as an osmotically regulated (1, 7, 24) and cold-activated (1) carnitine transporter.
Given the number, complexity, and varying range of substrate specificity exhibited by the compatible solute transporters in Bacillus subtilis (12) and the lack of such detailed knowledge in L. monocytogenes we sought to study the three currently known compatible solute transporters in L. monocytogenes in terms of their substrate specificity for the two most important osmolytes, glycine betaine and carnitine, and determine whether the transport of either or both osmolytes is mediated through yet another unknown system.
Glycine betaine transport in L. monocytogenes in response to osmotic stress.
Sleator et al. (22) compared glycine betaine uptake of wild-type L. monocytogenes L028 and that of L028B (a BetL− insertionally inactivated mutant derivative) in potassium phosphate buffer at 30°C, under osmotic stress imposed by 0.3 M NaCl; they found that the ability of the mutant to transport glycine betaine was significantly impaired compared to that of the wild-type strain, but not completely abolished. More recently, Mendum and Smith (15) used a transposon-inactivated mutant derivative of strain 10403S (LTG59) which lacks Gbu to investigate transport of glycine betaine through BetL. The rates of glycine betaine uptake for the two strains were compared in the range of 0 to 8% NaCl, and it was demonstrated that the mutant accumulated the osmolyte much more slowly, particularly at NaCl concentrations above 3% NaCl. The maximum uptake for both strains occurs at 4% NaCl. Whereas the difference in uptake between the wild type and mutant was moderate at 1 to 3% NaCl, the preponderance of the wild-type glycine betaine transport activity was absent in the mutant at or above 4% NaCl, indicating that Gbu is the predominant salt-activated glycine betaine uptake system at high salt concentrations.
Uptake data presented here reinforce the fact (10) that porter I is an osmotically activated glycine betaine porter that requires Na+ for efficient uptake of the osmolyte (Fig. 2G). Ko and Smith (13) demonstrated that an L. monocytogenes 10403S mutant (LTG59) having the gbu operon insertionally inactivated displayed a fourfold-lower rate of glycine betaine uptake relative to the wild-type in response to osmotic stress conferred by 8% NaCl and that the growth rate of the mutant at 8% NaCl and 100 μM glycine betaine was about one-half that of the parent strain. The authors further determined that Na+ was required for the residual uptake activity in LTG59 by assaying LTG59 in ACES [N-(carbamoylmethyl)-2-aminoethanesulfonic acid] buffer (13) containing 4% KCl as the stressing salt. They showed that a deficiency in Na+ further reduced the rate of uptake in LTG59 to about 1% that of the parent strain, indicating that nearly all of the residual activity obtained in LTG59 is dependent on Na+.
Glycine betaine transport in L. monocytogenes in response to osmotic stress has also been studied in mutant strains lacking the carnitine transporter OpuC. Fraser et al. (7) reported no differences in glycine betaine uptake between L. monocytogenes EGD and the opuCA::pAULA mutant derivative in potassium phosphate buffer with 0.5 M NaCl. In the same study, the concentration of glycine betaine in steady-state solute pools of the mutant under NaCl stress (0.5 M) was 35% lower than that in wild type, but no difference was observed in solute pools under KCl stress (0.5 M). In fact, in the latter case, betaine pools of the mutant were slightly higher than those of the wild type. The authors concluded that OpuC does not play an important role in betaine accumulation in L. monocytogenes. In a subsequent study Sleator et al. (24) studied glycine betaine uptake in two different L. monocytogenes strains (ScottA and L028) and ScottAC and L028C, the respective mutant derivatives in which the opuC operon had been insertionally inactivated. Interestingly, the authors showed that glycine betaine transport at 3% NaCl was weaker in both mutant strains compared to their wild-type levels but, whereas the reduction in the ScottAC mutant (relative to the level of transport by ScottA) was pronounced, the corresponding reduction in strain L028C was minor. These results demonstrate that in these two strains OpuC is involved, although to a different extent, in osmotically stimulated betaine transport.
Uptake data presented in this study demonstrate that in L. monocytogenes 10403S, osmotically activated transport of glycine betaine is mediated primarily by two transporters, Gbu and BetL (Fig. 2A, C, E, G, and I). In both ASA5 and ASA6, which only possess betaine porters Gbu or BetL, respectively, addition of glycine betaine drastically improves growth under stress. The weak uptake of betaine displayed by strain ASA4 (Fig. 2E) may be sufficient over time to provide some osmoprotection, as evidenced by the fact that under osmotic stress, strain ASA4 grows approximately 30% faster in the presence than in the absence of betaine. Thus, as in the case of L. monocytogenes EGD, the involvement of OpuC in glycine betaine transport under stress in L. monocytogenes 10403S seems to be minor. It is nonetheless able to confer a measurable degree of osmotolerance.
Carnitine transport in L. monocytogenes in response to osmotic stress.
Previous work in three laboratories has demonstrated that carnitine transport, although reduced, is still present to various extents in L. monocytogenes OpuC− mutants, indicating that an alternate route(s) for carnitine uptake must exist in Listeria. Fraser et al. (7) reported that both basal transport and osmotically (0.5 M NaCl) stimulated carnitine transport were abolished in an L. monocytogenes EGD OpuC− mutant, whereas only a small fraction of the wild-type carnitine levels could be detected in mutant solute pools under stress. Sleator et al. (24) examined carnitine transport in L. monocytogenes L028 and ScottA strains and their OpuC− mutant derivatives L028C and ScottAC. Under identical transport conditions (3% NaCl) the results showed that, whereas a large reduction was exhibited in the rate of carnitine uptake for ScottAC both in the presence and absence of salt stress relative to ScottA, the L028 parent strain had very low levels of carnitine uptake (10-fold lower than that for ScottA). This experiment demonstrated that differences in carnitine transport between different L. monocytogenes strains exist. Recent work in our laboratory using L. monocytogenes 10403S demonstrated a large decrease in osmotically stimulated (4 and 8% KCl) carnitine transport by an OpuC− mutant (1). The residual transport activity of the mutant was also shown to be osmotically activated (1). In the present communication we show that the primary alternate carnitine pathway is the Gbu (Fig. 2D). Strain ASA5, containing only gbu, showed osmotically activated carnitine transport (Fig. 2D), and when grown under osmotic stress in the presence of carnitine, it grew significantly faster than in the absence of carnitine. The further deletion of gbu from the ΔopuC ΔbetL mutant ASA5 to produce ASA7 abolished carnitine transport altogether (Fig. 2J), indicating that carnitine transport in ASA5 occurred via Gbu. Mendum and Smith also arrived at this conclusion and reported a Km of Gbu for carnitine of 4 mM (16). Very recently, Fraser and O'Byrne reported evidence for a low-affinity carnitine transporter (8) in strain EGD. Our data suggest that this low-affinity transporter is Gbu.
Weak and transient osmotically activated carnitine uptake by BetL was observed only under NaCl-mediated osmotic stress (Fig. 2H). However, steady-state carnitine accumulation by ASA6 growing in the presence of 0.7 M NaCl in defined medium supplemented by glycine betaine and carnitine could not be detected by NMR, and given the inability of added carnitine to promote growth of ASA6 under NaCl stress (Fig. 4) it is most likely that BetL-mediated carnitine uptake is of little if any osmoprotective value in vivo.
The triple mutant strain ASA7 showed no uptake of either glycine betaine or carnitine in the presence (isosmotic concentrations of NaCl and KCl) or absence of added salt. The NMR spectra of ASA7 grown under stress in the presence of both glycine betaine and carnitine were devoid of both osmolytes. Finally, addition of these osmolytes individually or in combination in ASA7 cultures grown under stress (NaCl or KCl) had no osmoprotective effect. Therefore, osmolyte uptake and accumulation data and growth rate data presented here provide evidence that no other transporter for either glycine betaine or carnitine operates in L. monocytogenes 10403S at 30°C in the presence or absence of elevated osmolarity. Furthermore, the absence of observable transport of either osmolyte by this mutant shows that the low levels of transport observed in the other mutants are real and proceed through one or more of the three transporters.
While this work was under review, Wemekamp-Kamphuis and coworkers (30) reported experiments similar to those described herein, but they used a different strain, LO28. Their results differed from ours in three respects. Their ΔbetL ΔopuC mutant (LO28BC) did not grow well and was excluded from the analysis, transport via OpuC did not appear to be osmotically activated, and residual carnitine transport by their triple mutant (LO28BCG) led them to propose the existence of a fourth transport system. We note that the parent strain LO28 exhibits very low carnitine transport compared to that observed in ScottA (8) or 10403S (1). Our mutant ASA4 shows osmotic stimulation of carnitine transport by either KCl or NaCl (Fig. 2F), suggesting that this difference represents variation in either activity or regulation between strains. The existence of a fourth transporter was previously suggested by Fraser and O'Byrne (8) because an opuCA insertional mutant exhibited residual carnitine transport activity, an activity which we attribute to Gbu (above). The triple mutant LO28BCG reported by Wemekamp-Kamphuis et al. (30) also exhibited residual carnitine transport activity; our triple deletion mutant ASA7 did not. Strain LO28BCG was produced using SOE methodology to delete 1-kb portions of the gbu operon and 681 bp of the betL gene. opuC on the other hand, was interrupted by plasmid insertion, using a 1.1-kb fragment of the opuCB gene carried on pCPL5 to target the insertion to opuCB (24). Neither the sequence of the 1.1-kb fragment nor the exact site of insertion was reported, and we cannot assess the likelihood that a functional fusion protein might be produced. Indeed, the same laboratory has described plasmid insertion as “a technique which can lead to phenotypic reversion” (23). Indeed, Table 1 of their work (30) shows that the growth rates of their Δgbu ΔbetL and Δgbu ΔbetL ΔopuC mutants under salt stress and in the presence of carnitine are indistinguishable. Similarly, their Fig. 5 shows that the osmolyte accumulation profiles of these two strains are essentially identical. The simplest interpretation of the data is that there are only three transporters for glycine betaine and carnitine and that the residual carnitine transport in LO28BCG arises from the production of functional OpuC despite the insertion. An alternate and less likely explanation is that another transporter (or transporter component) exists (e.g., lmo1421 or lmo1422 [8]), but must function in concert with one of the genes or systems that have been deleted in strain ASA7.
Recent work has revealed variation in transport and utilization of osmolytes among different L. monocytogenes strains (6, 24). In order to quantitate the extent of strain variation in osmolyte transport, transport studies and comparisons among strains should be conducted by controlling influencing variables (growth conditions, composition of growth and assay media, type and level of osmotic stress, transport and accumulation assay protocols, and types of transporter inactivation mutants), as such variables may differentially regulate the expression and/or activity levels of these transport systems.
Understanding the details of compatible solute uptake by bacteria can lead to the identification or design of effective inhibitors that could retard or even block the uptake of these osmolytes and therefore extend the shelf life and safety of foods. Because such studies in the case of L. monocytogenes are just recently beginning to emerge (15), for an immediate and definite contribution to safety, focus should be given on inclusion of osmolyte concentrations in foods as variables in bacterial growth modeling studies.
Acknowledgments
This work was supported by grant 98-TSL-01 from Dairy Management, Inc., and by NSF grant NSF OSTI 97-24412 to the UCD NMR Facility.
REFERENCES
- 1.Angelidis, A. S., L. T. Smith, L. M. Hoffman, and G. M. Smith. 2002. Identification of OpuC as a chill-activated and osmotically activated carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 68:2644-2650. [DOI] [PMC free article] [PubMed]
- 2.Angelidis, A. S., L. T. Smith, and G. M. Smith. 2002. Elevated carnitine accumulation by Listeria monocytogenes impaired in glycine betaine transport is insufficient to restore wild-type cryotolerance in milk whey. Int. J. Food Microbiol. 75:1-9. [DOI] [PubMed] [Google Scholar]
- 3.Bayles, D. O., and B. J. Wilkinson. 2000. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett. Appl. Microbiol. 30:23-27. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan, R. L., and L. A. Klawitter. 1990. Effects of temperature and oxygen on the growth of Listeria monocytogenes at pH 4.5. J. Food Sci. 55:1754-1756. [Google Scholar]
- 5.Cole, M. B., M. V. Jones, and C. Holyoak. 1990. The effect of pH, salt concentration and temperature on the survival and growth of Listeria monocytogenes. J. Appl. Bacteriol. 69:63-72. [DOI] [PubMed] [Google Scholar]
- 6.Dykes, G. A., and S. M. Moorhead. 2000. Survival of osmotic and acid stress by Listeria monocytogenes strains of clinical or meat origin. Int. J. Food Microbiol. 56:161-166. [DOI] [PubMed] [Google Scholar]
- 7.Fraser, K. R., D. Harvie, P. J. Coote, and C. P. O'Byrne. 2000. Identification and characterization of an ATP binding cassette L-carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 66:4696-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser, K. R., and C. P. O'Byrne. 2002. Osmoprotection by carnitine in a Listeria monocytogenes mutant lacking the OpuC transporter: evidence for a low affinity carnitine uptake system. FEMS Mircobiol. Lett. 211:189-194. [DOI] [PubMed] [Google Scholar]
- 9.Gerhardt, P. N. M., L. T. Smith, and G. M. Smith. 2000. Osmotic and chill activation of glycine betaine porter II in Listeria monocytogenes membrane vesicles. J. Bacteriol. 182:2544-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerhardt, P. N. M., L. T. Smith, and G. M. Smith. 1996. Sodium-driven, osmotically activated glycine betaine transport in Listeria monocytogenes membrane vesicles. J. Bacteriol. 178:6105-6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horton, R. M., Z. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-530, 532, 534-535. [PubMed]
- 12.Kempf, B., and E. Bremer. 1998. Stress responses of Bacillus subtilis to high osmolarity environments: uptake and synthesis of osmoprotectants. J. Biosci. (Bangalore) 23:447-455. [Google Scholar]
- 13.Ko, R., and L. T. Smith. 1999. Identification of an ATP-driven, osmoregulated glycine betaine transport system in Listeria monocytogenes. Appl. Environ. Microbiol. 65:4040-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko, R., L. T. Smith, and G. M. Smith. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bacteriol. 176:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendum, M. L., and L. T. Smith. 2002. Characterization of glycine betaine porter I from Listeria monocytogenes and its roles in salt and chill tolerance. Appl. Environ. Microbiol. 68:813-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendum, M. L., and L. T. Smith. 2002. The Gbu glycine betaine porter and carnitine uptake in osmotically stressed Listeria monocytogenes cells. Appl. Environ. Microbiol. 68:5647-5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park, S. F., and G. S. A. B. Stewart. 1990. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene 94:129-132. [DOI] [PubMed] [Google Scholar]
- 18.Perroud, B., and D. Le Redoulier. 1985. Glycine betaine transport in E. coli: osmotic modulation. J. Bacteriol. 161:393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pine, L., M. J. Franzus, and G. B. Malcolm. 1986. Guanine is a growth factor for Legionella species. J. Clin. Microbiol. 23:163-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Schuchat, A., K. A. Deaver, J. D. Wenger, B. D. Plikaytis, L. Mascola, R. W. Pinner, A. L. Reingold, C. V. Broome, et al. 1992. Role of foods in sporadic listeriosis. I. Case-control study of dietary risk factors. J. Am. Med. Assoc. 267:2041-2045. [PubMed] [Google Scholar]
- 22.Sleator, R. D., C. G. M. Gahan, T. Abee, and C. Hill. 1999. Identification and disruption of betL, a secondary glycine betaine transport system linked to the salt tolerance of Listeria monocytogenes LO28. Appl. Environ. Microbiol. 65:2078-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sleator, R. D., C. G. M. Gahan, B. O'Driscoll, and C. Hill. 2000. Analysis of the role of betL in contributing to the growth and survival of Listeria monocytogenes LO28. Int. J. Food Microbiol. 60:261-268. [DOI] [PubMed] [Google Scholar]
- 24.Sleator, R. D., J. Wouters, C. G. M. Gahan, T. Abee, and C. Hill. 2001. Analysis of the role of OpuC, an osmolyte transport system, in salt tolerance and virulence potential of Listeria monocytogenes. Appl. Environ. Microbiol. 67:2692-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith, K., and P. Youngman. 1992. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis SpoIIM Gene. Biochimie 74:705-711. [DOI] [PubMed] [Google Scholar]
- 26.Smith, L. T., and G. M. Smith. 1989. An osmoregulated dipeptide in stressed Rhizobium meliloti. J. Bacteriol. 171:4714-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 28.Sun, A. N., A. Camilli, and D. A. Portnoy. 1990. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect. Immun. 58:3770-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verheul, A., F. M. Rombouts, R. R. Beumer, and T. Abee. 1995. An ATP-dependent L-carnitine transporter in Listeria monocytogenes Scott A is involved in osmoprotection. J. Bacteriol. 177:3205-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wemekamp-Kamphuis, H. H., J. A. Wouters, R. D. Sleator, C. G. M. Gahan, C. Hill, and T. Abee. 2002. Multiple deletions of the osmolyte transporters BetL Gbu and OpuC of Listeria monocytogenes affect virulence and growth at high osmolarity. Appl. Environ. Microbiol. 68:4710-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yancey, P. H., M. E. Clark, S. C. Hand, R. D. Bowlus, and G. N. Somero. 1982. Living with water stress: evolution of osmolyte systems. Science 217:1214-1222. [DOI] [PubMed] [Google Scholar]