Abstract
Opiates are among the most important drugs for treatment of moderate to severe pain and prolonged opiate administration is often required to treat chronic pain states. We investigated the neurobiological actions of sustained opiate administration revealing paradoxical pronociceptive adaptations associated with NK-1 receptor function. Sustained morphine delivered over 6 days elicited hyperalgesia in rats and mice during the period of opiate delivery. Sustained morphine administration increased substance P (SP) and NK-1 receptor expression in the spinal dorsal horn. Sustained morphine treatment also enhanced capsaicin-evoked SP release in vitro, and increased internalization of NK-1 receptors in response to noxious stimulation. While NK-1 receptor internalization was observed primarily in the superficial laminae of placebo-treated rats, NK-1 receptor internalization was seen in both superficial and deep lamina of the dorsal horn in morphine-treated animals. Morphine-induced hyperalgesia was reversed by spinal administration of an NK-1 receptor antagonist in rats and mice, and was observed in wildtype (NK-1+/+), but not NK-1 receptor knockout (NK-1−/−), mice. These data support a critical role for the NK-1 receptor in the expression of sustained morphine-induced hyperalgesia. Additionally, these data indicate that sustained opiate administration induces changes reminiscent of those associated with inflammatory pain. These opiate-induced changes might produce unintended deleterious actions in the course of pain treatment in patients. Understanding of sustained morphine-induced neurochemical changes will help identify approaches that limit the deleterious actions of opiates.
Keywords: Substance P, NK-1 receptor, Opioid-induced hyperalgesia, Inflammatory pain
1. Introduction
Opiate analgesics are the mainstay of pain management in conditions ranging from acute pain to chronic pain, including cancer pain, and are used as substitution treatment for opioid dependence. These clinical uses of opiates often require opiate treatment for extended periods. A potential problem which has been noted with sustained opiate administration is the paradoxical expression of pain in regions unaffected by the initial pain complaint (Compton et al., 2001; De Conno et al., 1991; Devulder, 1997; Doverty et al., 2001; Sjogren et al., 1994). Opioid-induced ‘pain’, i.e. hyperalgesia, has been reliably observed in preclinical models (Celerier et al., 2000; Gardell et al., 2002; Larcher et al., 1998; Mao et al., 1994; Vanderah et al., 2000, 2001a,b; Yaksh and Harty, 1988; Yaksh et al., 1986). Recent research has begun to uncover the neurobiological consequences of sustained opioid administration and reveal several neuroplastic adaptations that likely underlie opioid-induced hyperalgesia (Gardell et al., 2002; Ma et al., 2000; Vanderah et al., 2000, 2001a,b). This study explored the role of substance P and the NK-1 receptor in mediating these opiate-induced effects.
Substance P (SP) is an excitatory neurotransmitter synthesized by primary afferent nociceptors and released into the spinal cord after noxious stimulation (Duggan et al., 1987; McNally, 1999; Radhakrishnan and Henry, 1991; Schaible et al., 1990). SP preferentially binds to NK-1 receptors located in the spinal cord dorsal horn (Mantyh et al., 1995). Although blocking SP activity does not alter responses to acute nociceptive stimuli (De Felipe et al., 1998; Mantyh et al., 1997; Nichols et al., 1999), SP is known to contribute to chronic inflammatory pain and participate in central sensitization and associated hyper-algesia (Khasabov et al., 2002; Mantyh et al., 1997; Moochhala and Sawynok, 1984; Nichols et al., 1999). Once activated by SP, NK-1 receptors internalize rapidly in cells located in the superficial regions of the spinal dorsal horn and recycle to the plasma membrane (Mantyh, 2002; Mantyh et al., 1995). In circumstances of pathological pain, including chronic inflammation, there is increased SP expression in the sensory primary afferent accompanied by increased release of SP upon noxious stimulation, and consequent internalization of the NK-1 receptor, not only in the superficial regions, but in deep laminae of the spinal cord (Abbadie et al., 1997). These features have been suggested to represent the ‘neurochemical signature’ of inflammatory pain (Honore et al., 1999).
Our findings indicate that sustained opiate administration activates mechanisms that promote pain, in part, through the NK-1 receptor. These mechanisms are reminiscent of the plasticity associated with inflammatory pain and raise concerns about the neurobiological effects of opioid exposure in patients receiving opiates for extended periods.
2. Methods
2.1. Experimental animals
Male Sprague–Dawley rats (Harlan; Indianapolis, IN) weighed 200–300 g at time of testing. NK-1 receptor knockout mice were C57BL/6×129sv crossed with MF1 outbred mice. Male NK-1 receptor knockout and wildtype littermates were from heterozygous breeding pairs. NK-1 receptor knockout and wildtype mice weighed 20–30 g at time of testing. Male C57BL/6×129Sv (Taconic) weighing 20–30 g were used to test the effects of the NK-1 receptor antagonist in mice. Rats and mice were maintained in separate climate-controlled rooms on a 12-h light/dark cycle (lights on at 06:00 h) with food and water available ad libitum. All testing was performed in accordance with the policies and recommendations of the International Association for the Study of Pain and the National Institutes of Health guidelines for the handling and use of laboratory animals and received approval from the Institutional Care and Animal Use Committee at the University of Arizona.
2.2. Sustained morphine administration
Sustained systemic administration of morphine was accomplished by subcutaneous implantation of one or two morphine (75 mg, free base) pellets in mice or rats, respectively. Control groups receive the same number of placebo pellets. The morphine and placebo pellets were a generous gift of the National Institute on Drug Abuse (NIDA) and their use and doses in rats (two pellets) and mice (one pellet) were carried out according to standard methods (Gardell et al., 2002; Gold et al., 1994; Riba et al., 2002; Schmidt et al., 2002; Smith et al., 1999, 2002; Vanderah et al., 2001b; Yoburn et al., 1985). Previous studies have shown that implantation of two morphine pellets (75 mg, free base) results in a steady state of plasma morphine levels by 3 days post-pellet implant, ranging between 86.8±13.1 and 156±45 ng/ml, that was maintained through 12 days post-pellet implant (Gold et al., 1994; Yoburn et al., 1985). Mice were visually evaluated for signs of morphine-induced agonist actions by assessing the presence or absence of the Straub tail response and by observing the characteristic stereotypic locomotor actions in the home cage (Hasegawa et al., 1990; Nath et al., 1994). No signs of withdrawal (i.e. no diarrhea, wet-dog shaking, jumping, lacrimation, or piloerection) were observed in the animals throughout the study. The ability of the morphine pellet to induce thermal antinociception was verified in the C57BL/6×129Sv mice (n=6), NK-1 receptor knockout mice (N=3) and their wildtype littermates (N=4). Thermal antinociception to radiant heat was verified 2 and 6 h following pellet implant using the tail-flick test, since the cage-circling behavior prevented use of the paw-flick test.
2.3. Cannulation and drug administration
While under halothane anesthesia, rats were implanted with intrathecal catheters (polyethelene-10 tubing; 7.5 cm) as described previously (Yaksh and Rudy, 1976) for drug administration at the level of the lumbar spinal cord. After a 5-day recovery period all animals were tested for baseline paw-withdrawal responses prior to pellet implant. Rats were given a bolus injection containing 50 nmol (25 μg in 5 μl DMSO) of the NK-1 receptor antagonist, L-732,138 (Tocris, Balwin, Montana), or 5 ml DMSO alone was administered followed by a 9 μl saline flush. Mice received spinal administration of the NK-1 receptor antagonist, L-732,138 (25 μg in 5 μl DMSO) or 5 μl DMSO alone by lumbar puncture as described previously (Hylden and Wilcox, 1980). Species differences between non-peptide antagonists for the NK-1 receptors has been reported, with notable differences in affinity in human and guinea pig brain compared to rat and mouse brain (Cascieri et al., 1994). Although L-732,138 has been shown to have higher affinity for the cloned human receptor compared to the cloned rat receptor (200-fold difference), L-732,138 has been shown to have specificity for the NK-1 receptor, showing greater than 1000-fold higher affinity to the NK-1 receptor compared to the NK-2 or NK-3 receptors (Cascieri et al., 1994). This antagonist has the additional advantage of not antagonizing calcium channels, as has been reported with some other NK-1 receptor antagonists (Cahill and Coderre, 2002). The dose used in this study was based on previous studies done with this antagonist in rats (Cahill and Coderre, 2002).
2.4. Nociceptive testing
2.4.1. Thermal hypersensitivity
The method of Hargreaves et al. (1988) was used to assess paw-withdrawal latency to a thermal nociceptive stimulus. Rats and mice were allowed to acclimate within a Plexiglas enclosure on a clear glass plate in a quiet testing room. A radiant heat source was focused onto the plantar surface of the hindpaw, and paw-withdrawal latency was determined by a motion detector that halted both the lamp and timer when the paw was withdrawn. A maximal cut-off time of 32 s was used to prevent tissue damage.
2.4.2. Tail-flick test
Nociceptive testing was performed by placing the distal third of the tail of the mouse in a water bath maintained at 52 °C. The latency to tail-withdrawal was measured to 0.1 s, and a cut-off latency of 10 s was used to prevent tissue injury. The tail-flick test was used to verify morphine pellet-induced antinociception at 2 and 6 h post-pellet as the mice displayed cage-circling behaviors which prevented accurate assessment of antinociceptive responses using the paw-flick test. Data were converted to percent antinociception by the following formula: (response latency−baseline latency)/(cut-off−baseline latency)×100.
2.5. Tissue extraction and preparation
Two (2) or 6 days following morphine or saline pellet implant, rats were deeply anesthetized with halothane and decapitated. The rats received no nociceptive stimulation prior to tissue extraction. The spinal column was cut through at the pelvic girdle. Hydraulic extrusion was performed by inserting a 16-gauge needle into the sacral vertebral canal and expelling with ice-cold saline. The spinal cord was immediately placed on ice in a glass Petri dish, and the dorsal half of the lumbar cord was dissected. Tissue samples that were to be quantified for SP content were then immediately frozen in liquid nitrogen and stored at 70 °C until assayed. Tissues for use in the SP release assay were weighed and chopped into 0.2 mm cubes with a McIlwain tissue chopper (Mickle Laboratory Engineering, Gomshall, UK).
2.6. Release assay
Minced tissue samples were placed in a 1 cm3 chamber and continuously superfused with oxygenated modified Krebs' buffer (135 mM NaCl, 3.5 mM KCl, 1 mM MgCl2, 20 mM NaHCO3, 1 mM NaHPO4, 2.5 mM CaCl2, 3.3 mM dextrose, 0.1 mM ascorbic acid, 10 mM thiorphan, and 0.1% bovine serum albumin) maintained at 37 °C, pH 7.4, at a rate of 0.5 ml/min with a Brandel Superfusion Pump (Brandel, Gaithersburg, MD). The tissue was allowed to equilibrate for 45 min. Superfusate was collected in 3 min intervals into test tubes using a fraction collector (Gilson, Middleton, WI). A total of five fractions (12–15 min) were collected before adding capsaicin. Capsaicin was then added for a perfusion concentration of 3 μM for 6 min (two fractions). Superfusate was then collected for an additional 27–30 min (9–10 fractions).
2.7. Total SP content
Tissues were placed in 2 ml of 0.01 N HCl and homogenized using a Polytron, followed by centrifugation at 2500×g for 20 min. The supernatant was diluted 1:400 in modified Krebs' buffer and then assayed for total SP content using the radioimmunoassay described below.
2.8. Radioimmunoassay for substance P
Samples for the release and content assays were pre-incubated with 100 μl of a C-terminally directed anti-substance P antibody (Penninsula Laboratories, San Carlos, California). Samples were each mixed with 100 μl of [125I-Tyr8]-substance P (at 20,000–25,000 cpm per assay tube) and 50 μl of goat anti-rabbit antiserum coupled to ferric beads. The [125I-Tyr8]-substance P bound to the substance P antibody was separated from the free tracer through immunomagnetic separation (PerSeptive Diagnostics, Cambridge, MA). The immunoprecipitates were determined by gamma counting. Standard curves were generated and substance P content was determined through logit-log analysis. This assay has a minimal detection limit of 1–3 fmol/tube. Evoked release was calculated as the total amount of substance P released (i.e. substance P-immunoreactivity) during capsaicin infusion above the basal release of substance P.
2.9. Hindpaw stimulation for NK-1 receptor internalization
Thermal or mechanical stimulation was applied 10–15 min after rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) as previously described (Abbadie et al., 1997). Noxious mechanical or thermal stimuli were applied according to the methods of Abbadie et al. (1997). For noxious mechanical stimulation, the hindpaw was pinched by a hemostat that was applied to the distal aspect of the left hindpaw for 2 min. For the noxious thermal stimulation, the rats left hindpaw, to just below the ankle, was dipped into a water bath heated to 52 °C for 2 min. A separate group of morphine and placebo-pelleted animals that received no hindpaw stimulation was included as unstimulated controls.
2.10. Immunocytochemistry
Two (2) or 6 days following morphine or saline pellet implant, animals received an overdose of sodium pentobarbital (100 mg/kg, i.p.) and were perfused transcardially with 0.1 M PBS until the exudate ran clear followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Lumbar spinal cords were harvested and postfixed in 4% paraformaldehyde overnight and cryoprotected with 20% sucrose in 0.1 M PBS. Immunostaining for the NK-1 receptor and the substance P peptide was performed on 20 μm coronal lumbar spinal cord sections from tissues of rats that had received no nociceptive stimulation prior to tissue collection. For the NK-1 receptor internalization study, the time between the end of thermal or mechanical stimulation and the beginning of the fixative flow was ∼7–8 min. Immunostaining for NK-1 receptor internalization was performed on 30 μm lumbar spinal cord sections (from L2 to L6 segments) cut in the sagittal plane. NK-1 receptor primary antibody (generously donated by Dr Patrick Mantyh, Veterans Administration Medical Center, Minneapolis, Minnesota) was diluted 1:5000 for receptor internalization, and 1:10,000 for the coronal sections. Substance P receptor primary antibody was diluted 1:10,000. Sections were then incubated in Alexa Fluor 568 goat anti-rabbit IgG (diluted 1:1000).
2.11. Image analysis and quantification
Fluorescence images of spinal cord sections were acquired with a Nikon E800 fluorescence microscope outfitted with a filter set for Cy3 (excitation 540–580 nm/emission 560–620 nm) and a Hamamatsu C5810 color CCD camera and its proprietary Image Processor software (Hamamatsu Photonic System, Bridgewater, NJ, USA). Digital images were output using Adobe Photoshop 6.0 (Adobe System, Inc., San Jose, CA, USA).
2.12. Quantification of NK-1 receptor immunoreactivity
NK-1 receptor internalization in cell bodies was analyzed with 20× and 60× objectives on a Nikon E800 fluorescence microscope outfitted with a Hamamatsu C5810 color CCD camera connected to a computer. All NK-1 receptor-like immunoreactive (LI) cell bodies were counted in laminae I, III–IV, and V–VI of the dorsal horn ipsilateral to the side of stimulation in L4. In neurons that have internalized the NK-1 receptor, the cytoplasm contains bright, immunofluorescent NK-1 receptor-immunoreactive endosomes, defined as an intensely NK-1 receptor-immunoreactive intracellular organelle 0.1–0.7 μm in diameter that is clearly not part of the external plasma membrane (Abbadie et al., 1997). In the present study, we considered a cell to have internalized receptors if it contained >20 endosomes as previously described (Abbadie et al., 1997; Mantyh, 1995). Although our quantitative analysis was performed on tissue observed with illuminated fluorescence, to illustrate better the patterns of receptor internalization that were induced in the different treatment conditions, we examined some sections by confocal microscopy. The confocal images in this report were collected with an Zeiss confocal microscope (Arizona Research Laboratories Imaging Facilities, Tucson Arizona) and transferred in Zeiss LSM data server (version 5). Montages were created in Adobe Photoshop (version 6.0).
2.13. Data analysis
Pairwise comparisons were made using Student's t-test. In the case of multiple comparisons, such as time-course analysis, means were compared to baseline values by analysis of variance (ANOVA), followed by post hoc Fisher's Least Significant Difference test for multiple comparisons. A probability level of 0.05 indicates significance for all tests.
3. Results
3.1. NK-1 receptor antagonist in rats
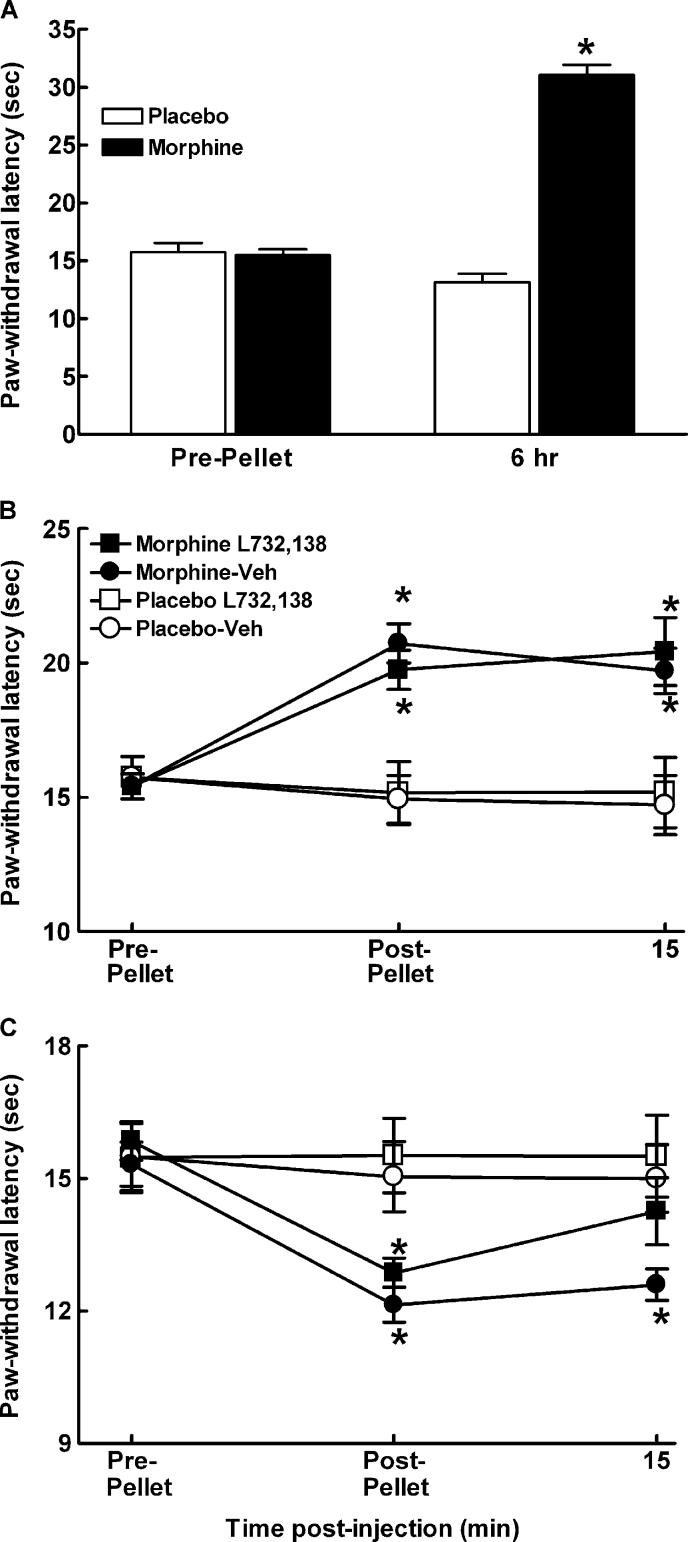
Sustained exposure to morphine has previously been shown to reliably produce increased sensitivity to noxious thermal stimuli (Gardell et al., 2002; Vanderah et al., 2001a, b). In order to determine whether the NK-1 receptor plays a role in sustained morphine-induced thermal hypersensitivity, a selective NK-1 receptor antagonist L-732,138 (Cahill and Coderre, 2002), was evaluated for possible reversal of morphine-induced thermal hypersensitivity in rats. Paw-withdrawal latencies were determined in rats prior to implantation of morphine or placebo pellets and again 6 h, 2 and 6 days after pellet implantation. Sustained morphine exposure produced thermal antinociception within 6 h after pellet implantation (P<0.05, Fig. 1A). Diminished residual antinociception was observed 2 days after pellet implantation (P<0.05, Fig. 1B, post-pellet). The initial morphine-induced antinociception was followed by the time-dependent expression of thermal hypersensitivity that was fully evident by post-pellet day 6 (P<0.05, Fig. 1C). Paw-withdrawal responses were next determined after spinal administration of L-732,138 at 2 and 6 days after morphine or placebo pellets were implanted (Fig. 1B,C). While L-732,138 did not alter the responses of the morphine or placebo-treated animals to thermal stimulation at the day 2 time-point (Fig. 1B), the thermal hypersensitivity seen in the morphine-treated animals at post-pellet day 6 was fully reversed (Fig. 1C, P<0.05).
Fig. 1.
Male Sprague–Dawley rats were implanted with two placebo or morphine pellets. (A) Morphine induced a robust antinociception within 6 h of pellet implant. (B) Rats were tested 2 days post-pellet implantation. Paw-withdrawal latencies of morphine-pelleted animals were longer than placebo-pelleted rats, indicating hypoalgesia, P≤0.05. Intrathecal administration of the NK-1 receptor antagonist, L-732,138 (25 μg/5 μl) or the vehicle control (DMSO) did not alter the responses of placebo or morphine-pelleted rats to the thermal stimulus at 15 min post-injection. (C) Rats were tested 6 days post-pellet implantation. Paw-withdrawal latencies of morphine-pelleted animals were lower than the placebo-pelleted rats, indicating that thermal hyperalgesia emerges by 6 days post-morphine pellet. Paw-withdrawal latencies were measured 15 min after intrathecal administration of the NK-1 receptor antagonist, L-732,138 (25 μg/5 μl). Intrathecal administration of L-732,138 reversed thermal hyperalgesia within 15 min post-administration in morphine-pelleted rats, but did not alter the response of placebo-pelleted animals. Intrathecal administration of the vehicle did not alter responses of the morphine or placebo-pelleted animals. Graphs represent mean±SEM. Each treatment group consisted of 12 rats. * indicates significant difference from pre-pellet baselines.
To control for the possibility that the prolonged exposure of the rats' hindpaw to the radiant heat source at 6 h post-morphine pellet implant might result in increased thermal sensitivity on day 6 following pellet implant, the three following experiments were performed (data not shown). One group of rats received baseline tests on the Hargreaves test, had two morphine pellets implanted subcutaneously, and were tested 6 days after pellet implant with no additional exposure to the thermal stimulus (n=4). These rats showed a significant drop in paw-flick latencies compared to pre-pellet baselines, from 17.3±0.8 to 13.4±0.6 s, P<0.05. A separate group had baseline pawflick latencies measured, followed by an acute injection of morphine (10 mg/kg, s.c.) and paw-flick latencies were determined 30 min later (n=5). This dose of morphine significantly increased paw-flick latencies to the cut-off of 32 s. Notably, these elevated paw-flick latencies were not different from those observed 6 h following morphine pellet implant (32.0±0.0 and 31.1±1.0 s, respectively). The ipsilateral hindpaw was again tested on the Hargreaves apparatus 6 days later with no additional morphine exposure. The paw-flick latencies 6 days following morphine injection were not different from pre-injection baseline values (18.1±0.7 and 17.4±0.7 s, respectively). A third group of rats (n=5) had baseline paw-flick latencies measured, and were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). The animals were placed on the Hargreaves apparatus and their hindpaw was exposed to the radiant heat source for 32 s, the cut-off time used to prevent tissue damage in our studies. The ipsilateral hindpaw was tested for thermal hypersensitivity on the Hargreaves test 6 days later. Paw-flick latencies in these animals did not differ from baseline values (19.7±0.9 and 21.2±1.4 s, respectively). Together, these controls provide strong evidence that the sustained exposure to morphine through subcutaneous implant of morphine pellets elicits thermal hypersensitivity, and that this thermal hypersensitivity is not due to repeated testing of the animals
3.2. NK-1 receptor antagonist in mice
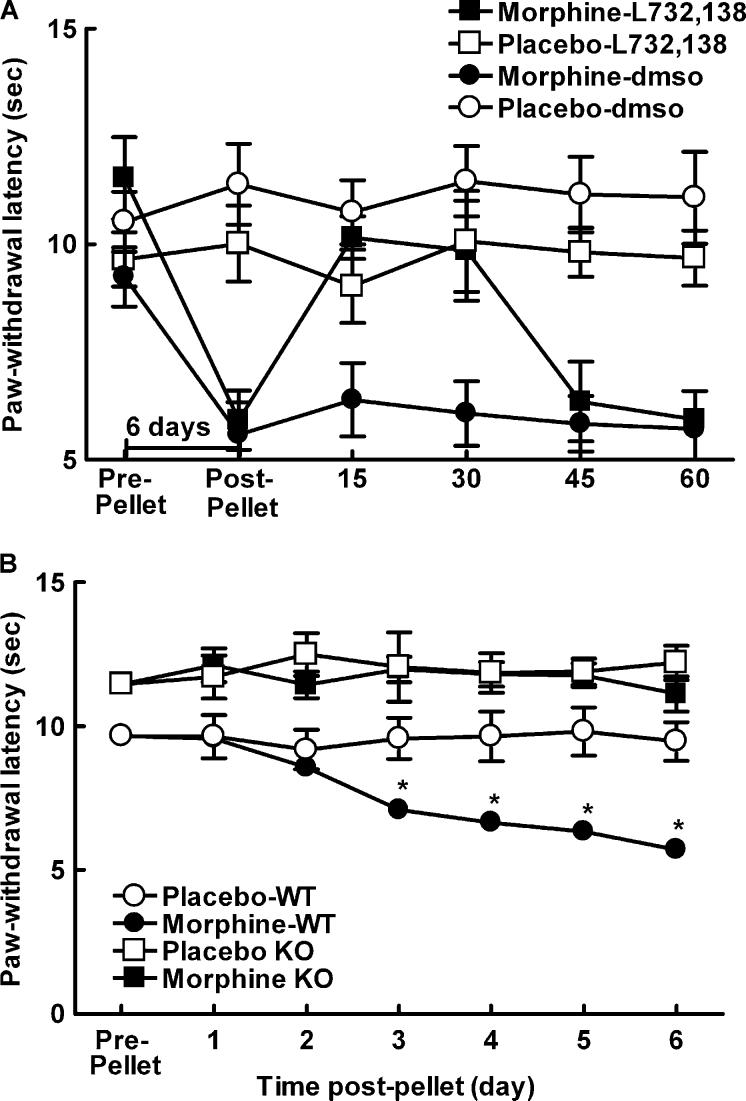
The ability of the NK-1 receptor antagonist L-732,138 to reverse sustained morphine-induced thermal hypersensitivity was also assessed in C57BL/6×129Sv mice. All mice that received morphine pellets demonstrated clear Straub tail behavior and exhibited characteristic cage-circling behavior within 30 min of morphine pellet implantation; these effects are typical of morphine agonist actions (Hasegawa et al., 1990; Nath et al., 1994). Paw-withdrawal latencies were determined in mice prior to implantation of morphine or placebo pellets and again 6 days following pellet implant. Thermal hypersensitivity emerged by 6 days following morphine pellet implant, with paw-flick latencies significantly below pre-pellet baselines, P<0.05 (Fig. 2A, post-pellet). Spinal administration of the NK-1 receptor antagonist, L-732,138, at 6 days following morphine pellet implant reversed the morphine-induced thermal hypersensitivity within 15 min post-administration, P<0.05 (Fig. 2A). This reversal lasted through 30 min post-drug administration, with the paw-flick latencies returning to pre-drug values by 45 min post-drug. The ability of the morphine pellet to induce thermal antinociception was verified in a separate group of mice 2 and 6 h following pellet implant using the tail-flick test, since the cage-circling behavior prevented use of the paw-flick test. Morphine pellets induced a robust thermal antinociception 2 and 6 h following pellet implant, increasing tail-flick latencies from 4.9±0.2 to 10±0.0 s 2 h, and 10±0.0 s 6 h post-pellet implant (data not shown).
Fig. 2.
(A) Male C57BL/6×129Sv mice were implanted with one placebo or morphine pellet. All mice showed Straub tail and characteristic stereotypic circling behavior within 30 min of morphine pellet administration. Morphine-treated mice showed decreased paw-withdrawal latencies compared to the placebo-treated animals 6 days following pellet implant, P<0.05, indicating morphine-induced hypersensitivity in these animals. Intrathecal administration of the NK-1 receptor antagonist 6 days post-morphine pellet reversed morphine-induced thermal hypersensitivity within 15 min post-administration, P<0.05, and this reversal lasted approximately 30 min, with paw-flick latencies back to pre-administration baselines 45 min post-administration of L-732,138. Intrathecal administration of L-732,138 did not alter paw-flick latencies of placebo-treated animals. (B) Male NK-1−/− or NK-1+/+ mice were implanted with one placebo or morphine pellet. NK-1−/− mice had longer paw-withdrawal latencies compared to NK-1+/+ mice throughout the testing period, P≤0.05. NK-1+/+ mice treated with morphine showed decreased paw-withdrawal latencies compared to the NK-1+/+ placebo-treated animals starting 3 days post-pellet implant, P≤0.05, indicating morphine-induced hypersensitivity in these animals. Morphine-pelleted NK-1−/− mice showed paw-withdrawal latencies that were equivalent to placebo-pelleted NK-1−/− mice across the entire 6-day testing period, indicating no morphine-induced hypersensitivity in these animals. * indicates significant difference from pre-pellet baselines.
3.3. NK-1 receptor knockout mice
The possible role of the NK-1 receptor in the mediation of morphine-induced thermal hypersensitivity was also explored using mice lacking the NK-1 receptor (NK-1−/−) and wildtype controls (NK-1+/+). The latency to withdraw the hindpaw from a noxious heat source applied to the plantar aspect of the hindpaw was tested prior to (at baseline) and at 24 h intervals following implantation of a placebo or morphine pellet (Fig. 2B). The NK-1−/− mice showed mild baseline hypoalgesia when compared to placebo controls (P<0.05). Morphine treatment induced a time-dependent reduction in heat-induced paw-withdrawal latency in NK-1+/+ mice, showing development of sustained thermal hypersensitivity by 3 days after pellet implant, P<0.05. Thermal hypersensitivity was not observed in the placebo-treated mice, indicating that the thermal hypersensitivity observed in the morphine-pelleted animals is due to the sustained morphine exposure, and not due to repeated testing. In contrast, morphine-treated NK-1−/− mice showed paw-withdrawal thresholds that were constant across time, indicating the importance of the NK-1 receptor in morphine-induced thermal hypersensitivity.
The ability of the morphine pellet to induce thermal antinociception was verified in separate groups of NK-1−/− (N=3) and NK-1+/+ (N=4) mice. The antinociceptive effects of the morphine pellets were determined 2 and 6 h following pellet implant using the tail-flick test (data not shown). The morphine pellets produced a robust antinociception within 2 h of morphine pellet implant that was also observed 6 h following morphine pellet implant. Morphine produced 95% MPE in KO and 100% MPE in WT mice 2 h after pellet implant, and 99% MPE in KO and 98% MPE in WT mice 6 h after pellet implant. There were no differences in baseline tail-flick latencies between the KO and WT mice (2.6±0.1 and 2.4±0.3 s, respectively). Tail-flick latencies did not differ between KO and WT mice 2 h following pellet implant (9.6±0.7 and 9.5±0.4 s, respectively) or 6 h following pellet implant (9.9±0.2 and 10.0±0.1 s, respectively).
3.4. Spinal SP immunoreactivity and content
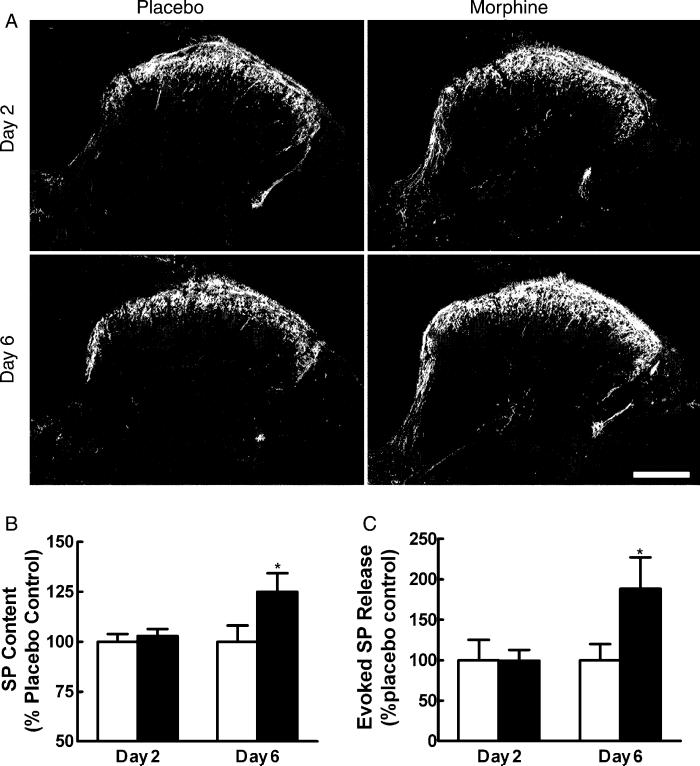
Within the spinal cord dorsal horn, SP-like immunoreactivity in placebo-treated rats was particularly intense in lamina I and the outer region of lamina II (Fig. 3A). In agreement with previous observations (Donnerer et al., 1992), little or no SP-like immunoreactivity was observed in the inner lamina II and in lamina III of the spinal dorsal horn, except for what was seen in fibers crossing towards deeper laminae. In laminae IV–V, clusters of SP-like immunoreactive areas separated by regions of sparse staining were observed. SP immunoreactivity was also noted in the lateral spinal nucleus and around the central canal, extending dorsally along the medial edge of the dorsal horn. Sustained morphine exposure produced an up-regulation of immunoreactivity for SP in the lumbar dorsal horn of the spinal cord in a time-dependent manner. Enhanced fluorescence labeling for SP was observed in the dorsal horn 6 days, but not 2 days after morphine pellet implantation when compared to immunostaining in tissues from placebo-pelleted rats (Fig. 3A) (preabsorbed control not shown). The increased intensity of SP immunoreactivity was observed in the superficial laminae of the dorsal horn. Quantification of SP content in the dorsal horn by radioimmunoassay 2 and 6 days after pellet implantation confirmed that morphine treatment increased SP content in the dorsal horn 6 days, but not 2 days after pellet implant (Fig. 3B).
Fig. 3.
(A) Sustained morphine up-regulates SP immunofluorescence in the spinal cord. Tissue from placebo-treated rats is shown in the left column, and that from morphine-treated rats is shown in the right column. No apparent differences in SP immunoreactivity are seen between the spinal sections obtained from placebo-treated and morphine-treated rats 2 days after pellet implant. The staining intensity of SP in tissues from morphine-treated rats on day 6 is enhanced compared to placebo-treated rats on day 6. The enhanced staining is apparent in laminae I and II. Scale bar, 200 μm. (B) Radioimmunoassay shows no differences in SP content between placebo animals in the 2 and 6 days post-pellet implant. Morphine did not alter SP content in rats 2 days post-pellet implant. SP content was elevated 6 days post-morphine pellet implant (*P<0.05). (C) The capsaicin-evoked release of SP from spinal tissues in vitro 2 and 6 days after placebo or morphine pellet implant is represented as the amount of SP above the baseline release for each individual group. Basal levels of SP release did not differ among the treatment groups. Evoked SP release was not different between tissues from placebo- and morphine-treated groups 2 days after pellet implant (P>0.05). However, tissues from the 6-day morphine-treated group showed a significantly greater level of capsaicin-evoked release of SP (*P<0.05). Graphs represent mean±SEM. Each treatment group consisted of 12 rats.
3.5. Evoked release of SP
Unstimulated (basal) as well as capsaicin-evoked release of SP was measured in isolated spinal cords 2 and 6 days after morphine or placebo pellet implant. No differences were observed in the unstimulated release in these groups (data not shown). Capsaicin-stimulated release of SP in tissues taken from rats 2 days after placebo pellets did not differ significantly from that observed in tissues taken from rats 6 days after placebo pellets. In contrast, capsaicin-evoked release of SP in tissues taken from rats 6 days after morphine pellets was significantly greater than in tissues taken from rats 2 days after either the placebo- or morphine pellet implants (Fig. 3C).
3.6. NK-1 receptor immunoreactivity
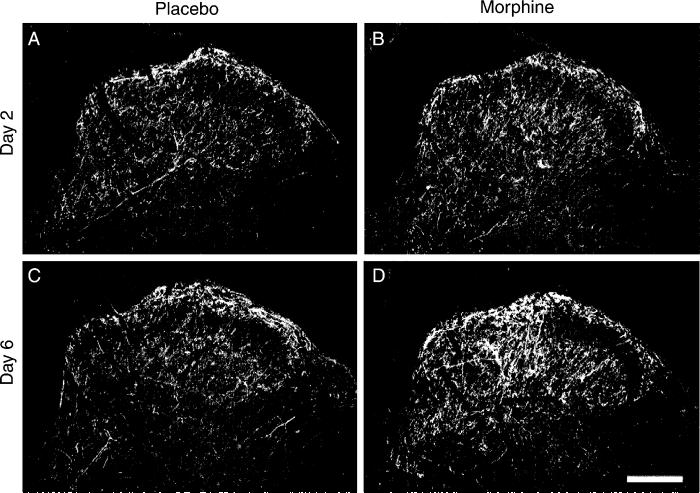
To determine whether sustained exposure to morphine alters spinal NK-1 receptors in parallel with the increase observed in SP-ir, NK-1 receptor immunoreactivity was measured in the spinal cord (Fig. 4). As previously reported, the densest staining for NK-1 receptor immunoreactivity is found on cell bodies and dendrites in lamina I (Abbadie et al., 1997). Cell bodies containing NK-1 receptor immunoreactivity were almost completely absent within lamina II, although there was a dense plexus of NK-1 receptor positive dendrites in both laminae I and II. In the deeper laminae, NK-1 receptor positive neuronal cell bodies were located in laminae III–V, with many of these deep NK-1 receptor positive neurons having dorsally directed dendritic arbors that crossed into the substantia gelatinosa. Sustained morphine exposure produced an up-regulation of immunoreactivity for the NK-1 receptor in the lumbar dorsal horn of the spinal cord in a time-dependent manner. Enhanced fluorescence labeling for the NK-1 receptor was observed in the dorsal horn 6 days (Fig. 4D) but not 2 days (Fig. 4B) after morphine pellet implantation when compared to immunostaining in tissues taken from placebo-pelleted rats at post-implantation days 2 and 6 (Fig. 4A,C, respectively) (preabsorbed control not shown).
Fig. 4.
Sustained morphine up-regulates spinal NK-1 receptors. Tissue from placebo-treated rats is shown in the left column, and that from morphine-treated rats is shown in the right column. No apparent differences in NK-1 receptor immunoreactivity is seen between the spinal sections obtained from placebo-treated and morphine-treated rats 2 days after pellet implantation (A, B). The staining intensity of NK-1 receptors in tissues from morphine-treated rats on day 6 is enhanced compared to placebo-treated rats on day 6 (C, D). The enhanced staining is apparent in both laminae I/II and in deeper laminae. Scale bar, 200 μm.
3.7. NK-1 receptor internalization
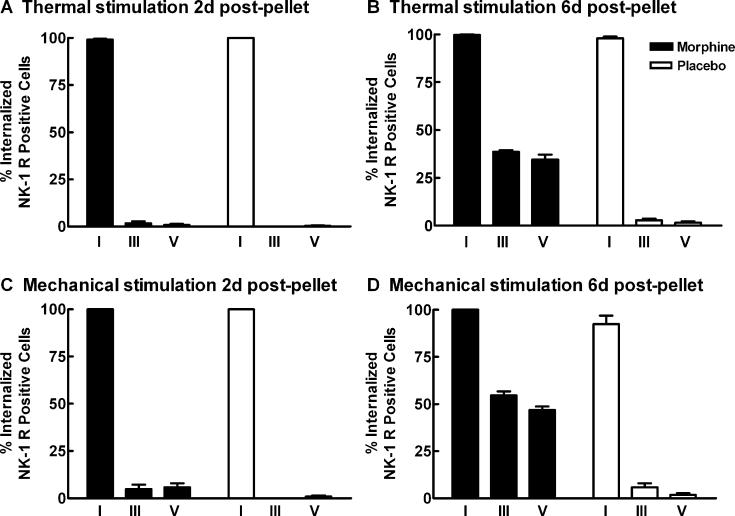
Previous studies have used NK-1 receptor internalization as a marker for SP release in vivo (Abbadie et al., 1997; Honore et al., 1999). For this reason, we determined whether the increased evoked release of SP in morphine-treated rats resulted in increased distribution of NK-1 receptors internalized within the dorsal horn of the spinal cord after noxious thermal or mechanical stimulation. NK-1 receptor internalization in cell bodies in the spinal dorsal horn was quantified in sagittal sections of the spinal cords as previously described (Abbadie et al., 1997; Honore et al., 1999). Patterns of thermal and mechanical stimulation-induced NK-1 receptor internalization in rats with placebo pellets were similar to those previously reported in naive rats. Thermal or mechanical stimulation-induced NK-1 receptor internalization was restricted to cell bodies contained in lamina I. Although NK-1 receptor labeled cell bodies were observed in laminae III–VI, the labeling was only found on the outer membrane of these cells (Fig. 5A,C). The patterns of thermal or mechanical stimulation-induced NK-1 receptor internalization in animals treated with morphine across 2 days were not different from that seen in the placebo controls, with no NK-1 receptor internalization observed in laminae III–V. However, in animals treated with morphine across 6 days, both thermal and mechanical stimulation induced NK-1 receptor internalization in lamina I, and in deeper laminae III–V (Fig. 5B,D). NK-1 receptor internalization was quantified as the percent of NK-1 receptor-immunoreactive cell bodies showing receptor internalization in lamina I, laminae III–IV, and lamina V in the L4 segment of the spinal cord. Sustained exposure to morphine across 6 days increased NK-1 receptor internalization in laminae III–V in response to thermal and mechanical stimulation (Fig. 6). To determine whether the morphine-induced increase in NK-1 receptors induced an increase in NK-1 receptor-immunofluorescence within the cell in the absence of any stimulation, NK-1 receptor internalization was determined in morphine and placebo-pelleted animals that did not receive hindpaw stimulation (unstimulated controls). Placebo or morphine-treated animals that received no stimulation showed very low levels of NK-1 receptor internalization throughout the dorsal horn of the spinal cord 6 days following pellet implant (data not shown). Less than 5% of all NK-1 receptor-immunoreactive cell bodies in the dorsal horn showed NK-1 receptor internalization in either treatment group. These data suggest that the NK-1 receptor internalization observed in deep dorsal horn neurons in rats treated with morphine across 6 days is due to the noxious stimulation rather than due to increased vesicular transport of the NK-1 receptors to the neural membrane. Further, the lack of an increase in NK-1 receptor internalization in superficial dorsal horn laminae in the absence of noxious stimulation suggests that there is not an increased activation of NK-1 receptors through a basal increase in SP release. However, since the quantification of NK-1 receptor internalization is based on an ‘all or none’ criterion (>20 endosomes within the cell), subtle differences in NK-1 receptor internalization may not be reflected in the data.
Fig. 5.
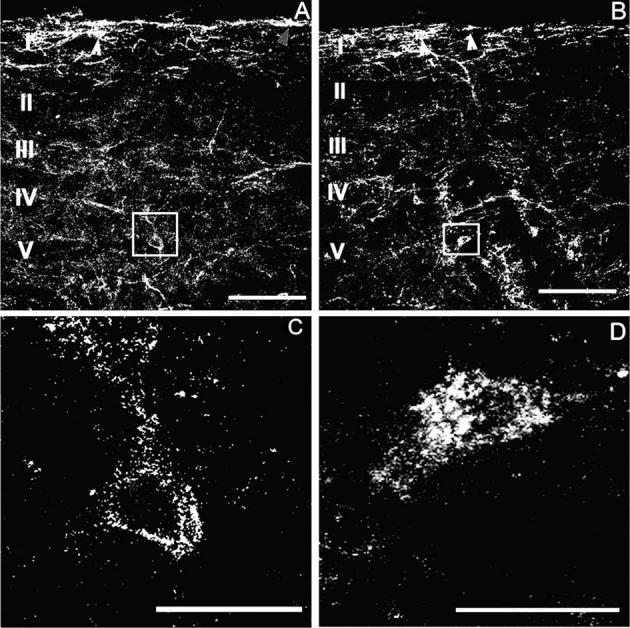
Sustained morphine-induced NK-1 receptor internalization in deep dorsal horn neurons. Confocal images show that mechanical stimulation (2 min pinch) induced NK-1 receptor internalization in the spinal cord dorsal horn of rats 6 days after placebo (A, C) or morphine (B, D) pellets were implanted subcutaneously. NK-1 receptor internalization after mechanical stimulation is confined to lamina I spinal neurons in placebo-pelleted animals (A). Arrowheads indicate neurons with NK-1 receptor internalization in lamina I spinal neurons. In contrast, sustained exposure to morphine induces NK-1 receptor internalization in deeper laminae III–V neurons as well as in lamina I neurons (B). Arrows indicate lamina I spinal neurons with NK-1 receptor internalization. High power magnification illustrates the difference between deep dorsal horn neurons with and without NK-1 receptor internalization (C, D). Rats that were treated with morphine pellets across 6 days show neurons that have internalized the NK-1 receptor (D). The cytoplasm of these neurons contains bright, immunofluorescent NK-1 receptor-immunoreactive endosomes, defined as an intensely NK-1 receptor-immunoreactive intracellular organelle 0.1–0.7 μm in diameter that are clearly not part of the external plasma membrane (D). In placebo-treated rats, deep dorsal horn neurons contained less than five endosomes per cell (C). In the present study, we considered a cell to have internalized receptors if it contained >20 endosomes. (A) and (C) were produced by superimposition of three optical sections taken at 2.4 μm in sagittal sections of L4, the scale bar is 100 μm; (B) and (D) were produced by superimposition of seven optical sections taken at 0.6 μm at L4, the scale bar is 20 mm.
Fig. 6.
Sustained morphine increases noxious stimulation induced NK-1 receptor internalization in deep dorsal horn neurons. Male Sprague–Dawley rats were implanted with two placebo or morphine pellets. Separate groups were exposed to mechanical (2 min pinch) or thermal (2 min at 52 °C) stimulation 2 and 6 days after pellet implantation; the animals were perfused 5 min post-stimulation, and lumbar spinal cord tissues were collected and processed for NK-1 receptor immunoreactivity. The percentage of NK-1 receptor labeled neurons at the L4 segment that contained internalized NK-1 receptors after thermal or mechanical stimulation were counted in laminae I, III, and V of the spinal cord. The thermal and mechanical stimulation induced internalization of the NK-1 receptor in approximately 100% of the NK-1 receptor labeled lamina I cells across all conditions. (A) and (C) show that under 5% of NK-1 receptor labeled neurons in laminae III–V showed NK-1 receptor internalization after the thermal or mechanical stimulation 2 days after placebo or morphine pellet implantation. (B) and (D) show that 6 days after morphine pellet implants, both the thermal and mechanical stimuli induced an increase in the percentage of NK-1 receptor labeled cells that internalized the NK-1 receptor in laminae III and V compared to the placebo-pelleted animals. Graphs represent mean±SEM. Each treatment group consisted of 3–5 rats.
4. Discussion
Data from these studies indicate that sustained morphine treatment (a) induces NK-1 receptor dependent hyperalgesia; (b) does not induce hyperalgesia in NK-1−/− mice; (c) increases spinal SP content; (d) enhances evoked SP release within the spinal cord; (e) increases spinal NK-1 receptor expression; and (f) elicits stimulus-induced NK-1 receptor internalization in both superficial and deep spinal dorsal horn neurons. These data support the concept that sustained morphine administration induces neuroadaptive changes that result in enhancement of nociceptive input. Moreover, the changes induced by sustained morphine exposure are similar to those observed in states of inflammatory injury, suggesting that sustained morphine-induced hyperalgesia and inflammatory hyperalgesia are associated with some common neuroplastic changes within the spinal cord and likely share some common underlying mechanisms.
Subcutaneous implantation of the morphine pellets produced expected antinociception within the first 2–6 h in both rats and in all strains of mice tested, including the NK-1 receptor knockout mice. Hyperalgesia was clearly established by day 6 of continuous morphine exposure in all animals except the NK-1 receptor knockout mice. As previously reported, sustained morphine-induced thermal hypersensitivity was evident during continuous release of morphine by the pellets and at times during which no signs of opiate withdrawal could be observed (Gardell et al., 2002; present study). The possibility that the thermal hypersensitivity observed in rats resulted from the suprathreshold exposure to the thermal stimulus (i.e. approximately 30 s) at the 6 h time-point was addressed with three control experiments. One experiment demonstrated that sustained exposure to morphine across 6 days elicited thermal hypersensitivity in rats that did not receive suprathreshold exposure to noxious thermal stimulation prior to day 6 post-pellet implantation. Two other experiments demonstrated that naive rats that received suprathreshold exposure to thermal stimulation (∼30 s) following anesthesia or an acute administration of morphine did not show thermal hypersensitivity 6 days later. These data indicate that the thermal hypersensitivity observed in rats treated with morphine pellets is specifically due to the sustained morphine exposure.
The findings of the present study add to the growing body of evidence, suggesting that morphine-induced hyperalgesia is likely mediated by changes in excitatory neurotransmission. When evaluated by radioimmunoassay, SP content was increased at day 6 but not day 2 after morphine administration indicating a time-dependent regulation of SP expression. The increase in content was seen to be functionally relevant to the observed hyperalgesia as enhanced capsaicin-evoked release of SP was seen at day 6 but not day 2 after morphine administration. The relevance of the increased SP release was validated by the effectiveness of the NK-1 receptor antagonist in blocking opiate-induced hyperalgesia at the same time-course, as well as by the changes in the pattern of NK-1 receptor internalization following evoked stimuli (see below). Additionally, the physiological significance of increased SP release is underscored by the failure of the NK-1 receptor knockout mice to develop thermal hyperalgesia. Thus, increased content and capsaicin-evoked release of SP and other excitatory transmitters such as CGRP (Gardell et al., 2002) are observed at the same time as the thermal hypersensitivity, days 6 and 7, respectively, but not prior to the development of the thermal hypersensitivity, days 2 and 1, respectively. The relative contributions of SP and CGRP to opiate-induced hyperalgesia are unknown, but the present data emphasize the contribution of SP signaling at the NK-1 receptor.
The hyperalgesic effects elicited by the opiates were readily reversed by an NK-1 receptor antagonist and were not observed in NK-1 receptor knockout mice suggesting a critical role for this receptor, and its endogenous excitatory transmitter, in the mediation of opiate-induced hyperalgesia. In parallel to these observations, an increase in spinal NK-1 receptor immunoreactivity was observed 6 days, but not 2 days following pellet implant. Although the increased immunofluorescence is not quantitative and may not reflect an increase in functional NK-1 receptors, evaluation of NK-1 receptor internalization suggests that sustained morphine exposure enhances stimulus-evoked SP release and results in increased activation of NK-1 receptors within the deep dorsal horn neurons of the spinal cord. Noxious thermal and mechanical stimulation-induced NK-1 receptor internalization occurred in both superficial and deep dorsal horn neurons following morphine treatment, while NK-1 receptor internalization was seen only in the superficial lamina of the dorsal horn with placebo treatment. Again, the time course of internalization of NK-1 receptors in the deep dorsal horn matched the time-related increase in spinal SP content and enhanced evoked release, being seen at day 6, but not day 2, after morphine treatment. Critically, very low levels of NK-1 receptor internalization were observed in the superficial or deep dorsal horn in unstimulated animals treated with morphine across 6 days. These data suggest that the increased immunofluorescence observed within the spinal cord is not due to increased intracellular localization of NK-1 receptors. These observations support the concept that sustained morphine exposure results in enhanced stimulus-evoked SP transmission that parallels NK-1 receptor dependent thermal hypersensitivity. It should be noted that enhanced NK-1 receptor internalization was also observed with noxious mechanical stimulation, suggesting that this mechanism also is an important part of other noxious modalities.
The data from the present study indicate that sustained morphine-induced changes mirror, to a large degree, the neuronal plasticity resulting from inflammatory pain states. Inflammatory pain is characterized by up-regulation of spinal dynorphin (Nahin et al., 1989; Ruda et al., 1988), CGRP and SP in the DRG and spinal cord (Donnerer et al., 1992, 1993), and the NK-1 receptor within the spinal cord (Abbadie et al., 1996, 1997; Trafton et al., 1999). Moreover, increased distribution of neurons showing NK-1 receptor internalization was observed following noxious stimulation, with noxious stimulation induced NK-1 receptor internalization in deep dorsal horn neurons, instead of only in lamina I neurons as observed in untreated controls (Abbadie et al., 1997). Moreover, intrathecal administration of the NK-1 antagonist L-732,138 reverses inflammation-induced hyperalgesia (Birch et al., 1992; Gao et al., 2003; Ren et al., 1996; Traub, 1996). NK-1 receptor knockout mice showed decreased sensitivity to inflammation-induced pain, showing decreased nociceptive behaviors during the second phase of the formalin response and reduced FOS-positive neurons within the spinal cord following formalin injection into the hindpaw (De Felipe et al., 1998) as well as reduced mechanical hypersensitivity following intraplantar Mycobacterium tuberculosus (Kidd et al., 2003). These data suggest that the neuroadaptive changes observed following prolonged exposure to sustained morphine are similar to those observed in inflammatory pain states, and that SP and the NK-1 receptor play a critical role in the mediation of hyperalgesia in both cases.
The hypothesis that some common mechanisms may underlie morphine-induced hyperalgesia and inflammation-induced hyperalgesia is also supported by the demonstration that descending facilitatory pathways play a critical role in both states (Gardell et al., 2002; Porreca et al., 2002; Urban and Gebhart, 1999; Vanderah et al., 2001a). While the precise relationship of descending pain facilitation to spinal plasticity remains unknown, some studies strongly implicate NK-1 receptor expressing cells in the establishment of this descending system in states of chronic pain. Selective ablation of NK-1 receptor expressing neurons in laminae I–III with a SP-saporin conjugate (SP-SAP) effectively reduces pain sensitivity after inflammation and nerve injury (Mantyh et al., 1995, 1997; Nichols et al., 1999; Suzuki et al., 2002), as well as central sensitization and windup (Suzuki et al., 2002). NK-1 receptor expressing lamina I neurons are predominantly nociceptive-specific projection neurons (Todd et al., 2002) that have been suggested to indirectly activate descending brainstem facilitatory pathways that regulate nociceptive processing (Suzuki et al., 2002). It is tempting to speculate that NK-1 receptor expressing neurons in lamina I might ultimately activate descending facilitatory pathways to elicit spinal plasticity, providing a possible point of intersection between NK-1 receptor expressing ascending projections, descending pain facilitation mechanisms and changes in primary afferents as characterized by increased SP content and evoked release (present observations) and increased CGRP content and evoked release (Gardell et al., 2002).
How changes produced by sustained morphine might interact or influence the neurochemical and physiological features associated with chronic pain is unknown. Three important consequences may result from sustained opioid-induced neuroplasticity in the presence of chronic pain. First, the neurobiological changes associated with morphine exposure alone reveal features that promote pain, suggesting the possibility of synergistic negative interactions between opioids and chronic pain states such as neuropathic or cancer pain. Second, prolonged opiate therapy increases the efficiency of noxious afferent traffic resulting from the disease or normal patient activity (e.g. coughing, movement) thus reducing the expected therapeutic efficacy of the opiate analgesia. Third, prolonged opiate treatment could elicit new areas of opiate-precipitated pain that is different from the original pain being treated. Thus, prolonged treatment of pain with opiates may cause unintentional harm to patients. Ultimately, knowledge of the mechanisms of possible deleterious actions of opiates may allow the development of new chemical approaches which can prevent these effects as well as change the way in which these drugs are used clinically.
Acknowledgements
This research was supported by Grants DA16431 and DA12656.
References
- Abbadie C, Brown JL, Mantyh PW, Basbaum AI. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–9. doi: 10.1016/0306-4522(95)00343-h. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Trafton J, Liu H, Mantyh PW, Basbaum AI. Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation. J Neurosci. 1997;17:8049–60. doi: 10.1523/JNEUROSCI.17-20-08049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch PJ, Harrison SM, Hayes AG, Rogers H, Tyers MB. The non-peptide NK-1 receptor antagonist, (+/−)-CP-96,345, produces antinociceptive and anti-oedema effects in the rat. Br J Pharmacol. 1992;105:508–10. doi: 10.1111/j.1476-5381.1992.tb09008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Coderre TJ. Attenuation of hyperalgesia in a rat model of neuropathic pain after intrathecal pre- or post-treatment with a neurokinin-1 antagonist. Pain. 2002;95:277–85. doi: 10.1016/S0304-3959(01)00410-9. [DOI] [PubMed] [Google Scholar]
- Cascieri MA, Macleod AM, Underwood D, Shiao LL, Ber E, Sadowski S, Yu H, Merchant KJ, Swain CJ, Strader CD, Fong TM. Characterization of the interaction of N-acyl-L-tryptophan benzyl ester neurokinin antagonists with the human neurokinin-1 receptor. J Biol Chem. 1994;269:6587–91. [PubMed] [Google Scholar]
- Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92:465–72. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001;63:139–46. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- De Conno F, Caraceni A, Martini C, Spoldi E, Salvetti M, Ventafridda V. Hyperalgesia and myoclonus with intrathecal infusion of high-dose morphine. Pain. 1991;47:337–9. doi: 10.1016/0304-3959(91)90225-M. [DOI] [PubMed] [Google Scholar]
- De Felipe C, Herrero JF, O'Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F, Hunt SP. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–7. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- Devulder J. Hyperalgesia induced by high-dose intrathecal sufentanil in neuropathic pain. J Neurosurg Anesthesiol. 1997;9:146–8. doi: 10.1097/00008506-199704000-00007. [DOI] [PubMed] [Google Scholar]
- Donnerer J, Schuligoi R, Stein C. Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: evidence for a regulatory function of nerve growth factor in vivo. Neuroscience. 1992;49:693–8. doi: 10.1016/0306-4522(92)90237-v. [DOI] [PubMed] [Google Scholar]
- Donnerer J, Schuligoi R, Stein C, Amann R. Upregulation, release and axonal transport of substance P and calcitonin gene-related peptide in adjuvant inflammation and regulatory function of nerve growth factor. Regul Pept. 1993;46:150–4. [PubMed] [Google Scholar]
- Doverty M, White JM, Somogyi AA, Bochner F, Ali R, Ling W. Hyperalgesic responses in methadone maintenance patients. Pain. 2001;90:91–6. doi: 10.1016/s0304-3959(00)00391-2. [DOI] [PubMed] [Google Scholar]
- Duggan AW, Morton CR, Zhao ZQ, Hendry IA. Noxious heating of the skin releases immunoreactive substance P in the substantia gelatinosa of the cat: a study with antibody microprobes. Brain Res. 1987;403:345–9. doi: 10.1016/0006-8993(87)90073-4. [DOI] [PubMed] [Google Scholar]
- Gao Y-J, Zhang Y-Q, Zhao Z-Q. Involvement of spinal neurokinin-1 receptors in the maintenance but not induction of carrageenan-induced thermal hyperalgesia in the rat. Brain Res Bull. 2003;61:587–93. doi: 10.1016/s0361-9230(03)00215-6. [DOI] [PubMed] [Google Scholar]
- Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP, Jr, Lai J, Porreca F. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci. 2002;22:6747–55. doi: 10.1523/JNEUROSCI.22-15-06747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Stinus L, Inturrisi CE, Koob GF. Prolonged tolerance, dependence and abstinence following subcutaneous morphine pellet implantation in the rat. Eur J Pharmacol. 1994;253:45–51. doi: 10.1016/0014-2999(94)90755-2. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Kurachi M, Okuyama S, Araki H, Otomo S. 5-HT3 receptor antagonists inhibit the response of [kappa] oploid receptors in the morphine-reduced straub tail. Eur J Pharmacol. 1990;190:399–401. doi: 10.1016/0014-2999(90)94205-c. [DOI] [PubMed] [Google Scholar]
- Honore P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, Mantyh PW. Spinal substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. J Neurosci. 1999;19:7670–8. doi: 10.1523/JNEUROSCI.19-17-07670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden JLK, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–6. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Khasabov SG, Rogers SD, Ghilardi JR, Peters CM, Mantyh PW, Simone DA. Spinal neurons that possess the substance P receptor are required for the development of central sensitization. J Neurosci. 2002;22:9086–98. doi: 10.1523/JNEUROSCI.22-20-09086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd BL, Inglis JJ, Vetsika K, Hood VC, De Felipe C, Bester H, Hunt SP, Cruwys SC. Inhibition of inflammation and hyperalgesia in NK-1 receptor knock-out mice. Neuroreport. 2003;14:2189–92. doi: 10.1097/00001756-200312020-00011. [DOI] [PubMed] [Google Scholar]
- Larcher A, Laulin JP, Celerier E, Le Moal M, Simonnet G. Acute tolerance associated with a single opiate administration: involvement of N-methyl-d-aspartate-dependent pain facilitatory systems. Neuroscience. 1998;84:583–9. doi: 10.1016/s0306-4522(97)00556-3. [DOI] [PubMed] [Google Scholar]
- Ma W, Zheng WH, Kar S, Quirion R. Morphine treatment induced calcitonin gene-related peptide and substance P increases in cultured dorsal root ganglion neurons. Neuroscience. 2000;99:529–39. doi: 10.1016/s0306-4522(00)00226-8. [DOI] [PubMed] [Google Scholar]
- Mantyh PW. Neurobiology of substance P and the NK-1 receptor. J Clin Psychiatry. 2002;63(Suppl 11):6–10. [PubMed] [Google Scholar]
- Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Rapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci USA. 1995;92:2622–6. doi: 10.1073/pnas.92.7.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–9. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;14:2301–12. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP. Pain facilitatory circuits in the mammalian central nervous system: their behavioral significance and role in morphine analgesic tolerance. Neurosci Biobehav Rev. 1999;23:1059–78. doi: 10.1016/s0149-7634(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Moochhala SM, Sawynok J. Hyperalgesia produced by intrathecal substance P and related peptides: desensitization and cross desensitization. Br J Pharmacol. 1984;82:381–8. doi: 10.1111/j.1476-5381.1984.tb10773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahin RL, Hylden JLK, Iadarola MJ, Dubner R. Peripheral inflammation is associated with increased dynorphin immunoreactivity in both projection and local circuit neurons in the superficial dorsal horn of the rat lumbar spinal cord. Neurosci Lett. 1989;96:247–52. doi: 10.1016/0304-3940(89)90386-8. [DOI] [PubMed] [Google Scholar]
- Nath C, Gupta MB, Patnaik GK, Dhawan KN. Morphine-induced straub tail response: mediated by central [mu]2-opioid receptor. Eur J Pharmacol. 1994;263:203–5. doi: 10.1016/0014-2999(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–61. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–25. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan V, Henry JL. Novel substance P antagonist, CP-96,345, blocks responses of cat spinal dorsal horn neurons to noxious cutaneous stimulation and to substance P. Neurosci Lett. 1991;132:39–43. doi: 10.1016/0304-3940(91)90428-v. [DOI] [PubMed] [Google Scholar]
- Ren K, Iadarola MJ, Dubner R. An isobolographic analysis of the effects of N-methyl-d-aspartate and NK-1 tachykinin receptor antagonists on inflammatory hyperalgesia in the rat. Br J Pharmacol. 1996;117:196–202. doi: 10.1111/j.1476-5381.1996.tb15174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba P, Ben Y, Smith AP, Furst S, Lee NM. Morphine tolerance in spinal cord is due to interaction between {micro}- and delta-receptors. J Pharmacol Exp Ther. 2002;300:265–72. doi: 10.1124/jpet.300.1.265. [DOI] [PubMed] [Google Scholar]
- Ruda MA, Iadarola MJ, Cohen LV, Young WS., 3rd In situ hybridization histochemistry and immunocytochemistry reveal an increase in spinal dynorphin biosynthesis in a rat model of peripheral inflammation and hyperalgesia. Proc Natl Acad Sci USA. 1988;85:622–6. doi: 10.1073/pnas.85.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible HG, Jarrott B, Hope PJ, Duggan AW. Release of immunoreactive substance P in the spinal cord during development of acute arthritis in the knee joint of the cat: a study with antibody microprobes. Brain Res. 1990;529:214–23. doi: 10.1016/0006-8993(90)90830-5. [DOI] [PubMed] [Google Scholar]
- Schmidt BL, Tambeli CH, Barletta J, Luo L, Green P, Levine JD, Gear RW. Altered nucleus accumbens circuitry mediates pain-induced antinociception in morphine-tolerant rats. J Neurosci. 2002;22:6773–80. doi: 10.1523/JNEUROSCI.22-15-06773.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren P, Jensen NH, Jensen TS. Disappearance of morphine-induced hyperalgesia after discontinuing or substituting morphine with other opioid agonists. Pain. 1994;59:313–6. doi: 10.1016/0304-3959(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Smith FL, Dombrowski DS, Dewey WL. Involvement of intracellular calcium in morphine tolerance in mice. Pharmacol Biochem Behav. 1999;62:381–8. doi: 10.1016/s0091-3057(98)00168-3. [DOI] [PubMed] [Google Scholar]
- Smith FL, Javed R, Elzey MJ, Welch SP, Selley D, Sim-Selley L, Dewey WL. Prolonged reversal of morphine tolerance with no reversal of dependence by protein kinase C inhibitors. Brain Res. 2002;958:28–35. doi: 10.1016/s0006-8993(02)03394-2. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK-1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–26. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Puskar Z, Spike RC, Hughes C, Watt C, Forrest L. Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance p-containing afferents and respond to noxious stimulation. J Neurosci. 2002;22:4103–13. doi: 10.1523/JNEUROSCI.22-10-04103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Marchand S, Mantyh PW, Basbaum AI. Spinal opioid analgesia: how critical is the regulation of substance P signaling? J Neurosci. 1999;19:9642–53. doi: 10.1523/JNEUROSCI.19-21-09642.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RJ. The spinal contribution of substance P to the generation and maintenance of inflammatory hyperalgesia in the rat. Pain. 1996;67:151–61. doi: 10.1016/0304-3959(96)03076-X. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci USA. 1999;96:7687–92. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW, Gardell LR, Burgess SE, Ibrahim M, Dogrul A, Zhong CM, Zhang ET, Malan TP, Jr, Ossipov MH, Lai J, Porreca F. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci. 2000;20:7074–9. doi: 10.1523/JNEUROSCI.20-18-07074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW, Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 2001a;92:5–9. doi: 10.1016/s0304-3959(01)00311-6. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Suenaga NM, Ossipov MH, Malan TP, Jr, Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci. 2001b;21:279–86. doi: 10.1523/JNEUROSCI.21-01-00279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Harty GJ. Pharmacology of the allodynia in rats evoked by high dose intrathecal morphine. J Pharmacol Exp Ther. 1988;244:501–7. [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357–8. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Harty GJ, Onofrio BM. High dose of spinal morphine produce a nonopiate receptor-mediated hyperesthesia: clinical and theoretic implications. Anesthesiology. 1986;64:590–7. doi: 10.1097/00000542-198605000-00008. [DOI] [PubMed] [Google Scholar]
- Yoburn BC, Chen J, Huang T, Inturrisi CE. Pharmacokinetics and pharmacodynamics of subcutaneous morphine pellets in the rat. J Pharmacol Exp Ther. 1985;235:282–6. [PubMed] [Google Scholar]