Abstract
Background
The development of microarray resources for the chicken is an important step in being able to profile gene expression changes occurring in birds in response to different challenges and stimuli. The creation of an immune-related array is highly valuable in determining the host immune response in relation to infection with a wide variety of bacterial and viral diseases.
Results
Here we report the development of chicken immune-related cDNA libraries and the subsequent construction of a microarray containing 5190 elements (in duplicate). Clones on the array originate from tissues known to contain high levels of cells related to the immune system, namely Bursa, Peyers patch, thymus and spleen. Represented on the array are genes that are known to cluster with existing chicken ESTs as well as genes that are unique to our libraries. Some of these genes have no known homologies and represent novel genes in the chicken collection. A series of reference genes (ie. genes of known immune function) are also present on the array. Functional annotation data is also provided for as many of the genes on the array as is possible.
Conclusion
Six new chicken immune cDNA libraries have been created and nearly 10,000 sequences submitted to GenBank [GenBank: AM063043-AM071350; AM071520-AM072286; AM075249-AM075607]. A 5 K immune-related array has been developed from these libraries. Individual clones and arrays are available from the ARK-Genomics resource centre.
Background
In recent years, the tools available to the field of chicken genomics have increased greatly. Detailed genetic and physical maps have been constructed [1], as well as BAC contig maps [2,3] and a radiation hybrid panel [4]. There is also a substantial EST collection [5], SNP database and many full-length cDNAs have been sequenced. The development of these resources has culminated with the recent publication of the chicken draft sequence [6]. The chicken can now be regarded as an important model organism for use in comparative genomics, residing in a potentially informative position in the evolutionary ladder. The chicken is also an extremely useful model for developmental biologists and geneticists as well as being a commercially important species.
The latest tools being developed for the chicken are microarrays. There are several small tissue-specific arrays being used by individual labs. These include an intestinal array (3,072 clones) [7], a macrophage-specific array (4,906 clones) [8], a lymphocyte array (3,011 clones) [9] and an 11 K array based on genes found in heart progenitor cells [10]. A 13 K genome-wide array is also available from ARK-Genomics [11] (Roslin, UK) and from the Fred Hutchinson Cancer Research Centre (Seattle, USA) [12]. We have designed a 5 K immune-related array created from libraries developed from tissue (Bursa, spleen, Peyers patch, thymus) from birds which were previously inoculated with a combination of different vaccines to various common avian diseases including bacterial, protozoa and virus disease-causing organisms (E. coli, Newcastle Disease Virus (NDV), Infectious Bursal Disease Virus (IBDV), coccidiosis, Marek's Disease (MD) and salmonella). The tissues we chose are highly representative of T and B cell populations and were used in order to optimise the numbers of immunologically – related genes that would be present in our libraries. Many known immune genes that have been recently identified in the chicken EST collections [13] have also been added to the array. This array provides a valuable, cost-effective resource for the investigation of immunological gene expression. It has been created from a pool of stimulated immune tissues and contains genes that represent a wide spectrum of immune functions as well as previously unidentified sequences. Each gene on the array is also functionally annotated as much as possible. Gene ontology [14] data and Blast [15] information is provided for each clone, where that information is available.
Results and discussion
Construction of the array
Six immune-related libraries were specifically developed for the construction of a 5 K chicken array. Immune tissue from birds inoculated with different vaccine regimes (see Methods) was used to develop two standard libraries. These both underwent two rounds of normalization, thus providing us with six libraries. Initially, 10,173 clones were randomly chosen from the libraries for sequencing. The number chosen from each library depended on the titre (colonies/microlitre) of that particular library. The 10,173 clones that were sequenced were searched for poor quality sequence (<100 bp after removal of vector, repeats etc.) and unwanted Blast homologies, as described in the Methods section. The numbers of high quality sequences (9,434 – which have been submitted to Genbank) from each library are shown in Table 1. Cluster analysis was then undertaken, which resulted in the grouping of clones from which we would choose the 5,000 that were to be represented on the array.
Table 1.
Clones sequenced from each library.
| Library | No. of clones |
| Chicken immune 1 ('B cell' standard) | 975 |
| Chicken immune 2 ('B cell' normalized 1) | 2,394 |
| Chicken immune 3 ('B cell' normalized 2) | 1,600 |
| Chicken immune 4 ('T cell' standard) | 2,563 |
| Chicken immune 5 ('T cell' normalized 1) | 918 |
| Chicken immune 6 ('T cell' normalized 2) | 984 |
Genes on the array
The clones on the array are derived from custom-made immune-related chicken cDNA libraries. Libraries developed from tissue from Bursa, spleen and Peyers patch were our representative 'B cell' libraries, and libraries developed from thymus were so-called 'T cell' libraries (the names 'B and T cell libraries' are used purely for ease of reference and in no way indicate that the libraries consist of pure cell populations). Clones from both standard and normalized libraries are present on the array. One clone representing each of the 3,811 clusters is included on the array, along with a random selection of singleton clones (1,067). The sequence of each of the clones was also subjected to a Blast search of the SwissProt and TREMBL databases and the highest hit to each sequence was reported. Searches were carried out at a stringency of 1e-10 (this relatively low stringency was to ensure that we identified as many immune homologies as possible). Chicken immune genes have a relatively low level of sequence conservation with mammals, hence the lower stringency used in these searches). We wanted to ensure that certain genes were also represented on the array as 'reference' genes. This included a range of known immune-related genes for which a clone was already available – either from the existing EST databases [12] or from our novel libraries. Various cytokines, chemokines, cell surface antigens, receptors and MHC molecules were all included (Table 2). The expression profile of genes of unknown function can thus be compared with the profiles of these genes whose roles are known. Standard array controls were also spotted on the array, including various spot report buffers (positive and negative controls for the Cy3 and Cy5 dyes), salmon sperm DNA, calf thymus DNA, bovine genomic DNA (negative controls), chicken genomic DNA, gamma actin and GAPDH (positive controls). Each clone is represented in duplicate.
Table 2.
List of known immune genes added as reference genes to the array.
| Gene | EST | Clone ID | Accession no. |
| AH221 | CTN2_C0000858f10.q1kT7SCF | C0000858F10 | AM064266 |
| AH294/RANTES | 603404971F1 | C0000737M17 | BU397782 |
| β2 microglobulin | CTst_C0000869a17.q1kT7SCF | C0000869A17 | AM068376 |
| BAFF | CBN1_C0000466j11.q1kT7SCF | C0000467J11 | AM066201 |
| BMP10 | 604156553F1 | C0003869J14 | BU210183 |
| BMP2 | 603213309F1 | C0003763A3 | BU444424 |
| BMP4 | 603363891F1 | C0000429F23 | BU473912 |
| BMP6 | 603604307F1 | C0003811M1 | BU287807 |
| BMP7 | 603500540F1 | C0000717O9 | BU333004 |
| BMP8A | 603956728F1 | 603956728F1 | BU425800 |
| CC chemokine receptor 6 | 603508559F1 | C0002794E15 | BU267610 |
| CC CKR 11 | 603367511F1 | C0000439E3 | BU465158 |
| CC LARC/MIP-3A | 603534015F1 | C0002806N3 | BU398190 |
| cCAF | CBN1_C0000465h11.q1kT7SCF | C0000465H11 | AM065832 |
| CD135 antigen | 603812446F1 | C0001334K11 | BU376898 |
| CD137 | pat.pk0038.d7.f | C0004737E4 | AI980851 |
| CD153 | pat.pk0072.b5.f | C0004738K22 | AI982035 |
| CD154 | 603535227F1 | C0001006F7 | BU398104 |
| CD18 | CBN1_C0000468e11.q1kT7SCF | C0000468E11 | AM066422 |
| CD2 | pgn1c.pk014.i9 | pk014I9 | CB017050 |
| CD200 | pat.pk0062.c8.f | C0004738C17 | AI981679 |
| CD226 | pat.pk0020.d12 | C0004739G22 | AI980296 |
| CD28 | CTst_C0000892d20.q1kT7SCF | C0000892D20 | AM070143 |
| CD3 | C0001679M3_G02_008.AB1 | C0001679M3 | AM070515 |
| CD36 | 603543789F1 | C0001028A23 | BU311037 |
| CD4 | pk017a12 | pk017a12 | CB017654 |
| CD40L | pgm2n.pk009.d11 | pk009d11 | BM488880 |
| CD44-like | 603745662F1 | C0003894K15 | BU253134 |
| CD45 | 603767294F1 | C0003827K23 | BU446679 |
| CD59 | 603212850F1 | C0000363D13 | BU447971 |
| CD63 antigen | 603783352F1 | C0001268G14 | BU243877 |
| CD7 | pat.pk0040.d6.f | C0004737K4 | AI981043 |
| CD79A | CBst_CHK02000039f07.q1kT7SCF | CHK0200003F7 | AM071949 |
| CD8 | CTst_C0000877k01.q1kT7SCF | C0000877K1 | AM069615 |
| CD82 | CTst_C0000892l24.q1kT7SCF | C0000892L24 | AM070329 |
| CD83 antigen | 603771889F1 | C0001238B24 | BU457418 |
| CD84 | CTN1_C0000798o19.q1kT7SCF | C0000798O19 | AM070961 |
| CD98 light chain | CBN1_C0000465c24.q1kT7SCF | C0000465C24 | AM065739 |
| Chemokine receptor like 2 | 603764351F1 | C0001219F13 | BU444213 |
| Chicken cytokine | pat.pk0050.e9.f | C0004737J11 | AI981311 |
| CHIR-A | 603478533F1 | C0003884A9 | BU359209 |
| CHIR-B | CBst_CHK02000039l03.q1kT7SCF | CHK0200003L3 | AM072078 |
| cMGF | pat.pk0060.h1.f | C0004737P22 | AI981598 |
| Complement C3 | CBN1_C0000468j22.q1kT7SCF | C0000468J22 | AM066546 |
| Complement C4 | 603811612F1 | C0001332G3 | BU376477 |
| Complement C7 | 603668434F1 | C0001140D9 | BU416108 |
| Complement C8α | 603782386F1 | C000164L21 | BU242118 |
| complement H | 603735023F1 | C0001154N6 | BU295434 |
| complement receptor 1 | 603819479F1 | C0001351N20 | BU268132 |
| Complement1 | CBN2_C0000485f23.q1kT7SCF | C0000485F23 | AM068133 |
| Cremp | 603114782F1 | C0003743C21 | BU126768 |
| C-type lectin | HFU603551466C18 | C0004763C18 | AM063354 |
| CX 3C chemokine receptor 1 | 603949695F1 | C0003852N23 | BU204148 |
| CXC-R4 | CTst_C0000878f17.q1kT7SCF | C0000878F17 | AM069849 |
| Cytokine like protein 17 | 603773283F1 | C0001242E21 | BU459791 |
| Cytokine receptor like 9 | 603472805F1 | C0000591J1 | BU477689 |
| Death receptor 6 | CBN1_C0000466l11.q1kT7SCF | C0000467L11 | AM066244 |
| DSL-1 | 603321647F1 | C0000418M11 | BU239031 |
| EMAP II | 603364164F1 | C0003776P16 | BU475067 |
| ephrin type A receptor 2 | 603121949F1 | C0000241A13 | BU133519 |
| FAS antigen | 603737578F1 | C0001181L19 | BU300974 |
| FASL decoy receptor 3 | CTN2_C0000856k13.q1kT7SCF | C0000856K13 | AM064070 |
| GATA-3 | CTN2_C0000858a24.q1kT7SCF | C0000858A24 | AM064179 |
| GDF10 | 603530236F1 | C0000994G10 | BU351257 |
| GDF8 | 603775823F1 | C0001248N6 | BU458566 |
| GDF9 | 603741256F1 | C0001166O13 | BU300398 |
| glycoprotein 130 | 603369157F1 | C0002739A15 | BU460413 |
| GMCSF | CF258055 | CF258055 | CF258055 |
| HCC1 | pat.pk0059.g4.f | pk059g4 | AW061438 |
| ICSBP | 603568552F1 | C0001037G14 | BU383423 |
| IFNα | 603486811F1 | C0000622F3 | BU319434 |
| IFNα/β-R2 | 603783234F1 | C0001268C9 | BU243612 |
| IFNγ | 603766180F1 | C0003825O20 | BU444142 |
| IFNγR2 | 603606133F1 | C0001120B10 | BU294744 |
| IFP35 | 603123028F1 | C0000243H7 | BU135331 |
| Ig light chain VJC region | C0000914E7_C03_018.AB1 | C0000914E7 | AM064528 |
| Ig heavy chain VDJ region | 603534767F1 | C0002807F18 | BU398082 |
| IK cytokine | 603368212F1 | C0000440H3 | BU460192 |
| IL-10 | CF258071 | CF258071 | CF258071 |
| IL11 receptor | 603402722F1 | C0000518K5 | BU250398 |
| IL-12β | 603603708F1 | 603603708F1 | BU291084 |
| IL12-p35 | 603761859F1 | C0002846F16 | BU474924 |
| IL-13R2 | 603519773F1 | C0000972A19 | BU341330 |
| IL-15 | 603102514F1 | C0002655L4 | BU202444 |
| IL-16 | 603130176F1 | C0002702H20 | BU114872 |
| IL17 receptor | 603211483F1 | C0000350E22 | BU448712 |
| IL-18 | 603508766F1 | C0002794D18 | BU271029 |
| IL-1β | 603217760F1 | C0003766G15 | BU455380 |
| IL-2 | pat.pk022.e2 | pk022e2 | AI980311 |
| IL20 receptor | 603591538F1 | C0001088K9 | BU241765 |
| IL-2Rα | pat.pk0012.h3 | pk0012h3 | AI980106 |
| IL-2Rγ | CBN1_C0000360j15.q1kT7SCF | C0000360J15 | AM064841 |
| IL-4 | 603772775F1 | ChEST708f19 | BU450270 |
| Il-4R | 603490820F1 | 603490820F1 | BU324362 |
| IL-6 | pat.pk0076.f2.f | C0004739G21 | AI982185 |
| Interleukin enhancer binding factor 3 | 603322645F1 | C0000420J12 | BU239448 |
| IRAK2 | 603831145F1 | ChEST821j11 | BU435261 |
| IRAK4 | 603208981F1 | ChEST185a21 | BU441365 |
| IRF1 | 603960463F1 | C0002900N15 | BU418343 |
| IRF10 | CBN1_C0000360l15.q1kT7SCF | C0000360L15 | AM064884 |
| IRF2 | 604146465F1 | C0003862A18 | BU438609 |
| IRF3 | CTst_C0000892j09.q1kT7SCF | C0000892J9 | AM070266 |
| IRF4 | 6O4_B10_077 | C0000885O4 | AM072251 |
| IRF5 | pat.pk0067.c5.f | pk067c5 | AI981854 |
| IRF6 | 603111427F1 | C0000188F7 | BU109331 |
| JSC | CTst_C0000878m23.q1kT7SCF | C0000878M23 | AM069996 |
| K60 | 603470605F1 | C0002774I2 | BU479398 |
| lymphotactin | 603733847F1 | C0001151N12 | BU300469 |
| MCSF1-receptor | 603220574F1 | C0000383C19 | BU432910 |
| MDV vIL-8 | CBst_C0000222p17.q1kT7SCF | C0000222P17 | AM071831 |
| MHC class I | CBst_CHK02000039o05.q1kT7SCF | CHK0200003O5 | AM072147 |
| MHC class I minor | CTst_C0000873a15.q1kT7SCF | C0000873A15 | AM068728 |
| MHC class II beta | HFU603551341A11 | C0004763A11 | AM063247 |
| MIF | 604141521F1 | C0001517I5 | BU438017 |
| MX | 603775783F1 | C0001248E22 | BU457953 |
| NKL | 603539011F1 | C0001016E4 | BU309556 |
| N-pac | 604157079F1 | 604157079F1 | BU210048 |
| NRAMP1 | pk013p5 | pk013p5 | BI394251 |
| NRAMP2 | 603953027F1 | C0001451E8 | BU203948 |
| OCIF | 603157972F1 | C0000333O17 | BU410189 |
| opioid receptor sigma 1 | 603341826F1 | C0003775C8 | BU254440 |
| Orphan chemokine receptor | 603234142F1 | C0000403C12 | BU418544 |
| PIT54 | 603150061F1 | C0000313I16 | BU126277 |
| platelet activating receptor | pat.pk0002.b12 | C0004739A2 | AI979750 |
| PRC1 | 603475588F1 | 603475588F1 | BU355757 |
| prostaglandin synthase | CBN1_C0000466j02.q1kT7SCF | C0000467J2 | AM066193 |
| Regulator of cytokinesis 1 | 603475588F1 | C0000598P22 | BU355757 |
| RING3 | CBst_C0000222p04.q1kT7SCF | C0000222P4 | AM071819 |
| SCA-2 | CTN1_C0000853f13.q1kT7SCF | C0000853F13 | AM071100 |
| SCYa13 | 603534566F1 | 603534566F1 | BU397023 |
| SCYA4 | 603742061F1 | C0001168I15 | BU299262 |
| SIGIRR | 603321436F1 | C0002934H10 | BU240159 |
| SOCS1 | 603758706F1 | C0003823K20/L1 | BU218362 |
| SOCS2 | 603322984F1 | 603322984F1 | BU239208 |
| SOCS5 | 603492126F1 | C0000636C7 | BU326390 |
| STAT1 | 603957345F1 | ChEST927i11 | BU425993 |
| STAT2 | pat.pk0027.a6.f | C0004737C3 | AI980571 |
| STAT5b | CBst_C0000222j02.q1kT7SCF | C0000222J2 | AM071688 |
| TAP2 | 603732809F1 | C0000758A3 | BU298074 |
| Tapasin | CTst_C0000873d22.q1kT7SCF | C0000873D22 | AM068799 |
| TARC | pat.pk0031.f10.f | C0004737K13 | AI980713 |
| T-bet | pgn1c.pk013.h8 | pgn1cpk013.h8 | CB016768 |
| Tcell receptor α | CBst_CHK02000039p10.q1kT7SCF | CHK0200003P10 | AM072172 |
| Tcell receptor β | UEB603581072O18 | C0004765O18 | AM063532 |
| Tcell receptor γ | CTst_C0000878m08.q1kT7SCF | C0000878M8 | AM069981 |
| Tcell receptor ζ | CTst_C0000874j17.q1kT7SCF | C0000874J17 | AM069278 |
| TGFβ | 603758578F1 | C0003823K20 | BU215243 |
| Thymosin beta 4 | ODP603945810C04 | C0004766C4 | AM063804 |
| TLR1/6/10 | 603760940F1 | C0001211O10 | BU471724 |
| TLR2 | 603588755F1 | C0001081M13 | BU374739 |
| TLR3 | 603781018F1 | C0001261D6 | BU242827 |
| TLR4 | 603470778F1 | C0002774L20 | BU475859 |
| TLR5 | 603230983F1 | C0000395E22 | BU420247 |
| TLR7/8/9 | 603160284F2 | C0002711G22 | BU435893 |
| TRAF1 | pat.pk0072.d3.f | C0004738M6 | AI982046 |
| TRAF2 | 603217872F1 | C0003766O18 | BU455745 |
| TRAF5 | CTst_C0000877n08.q1kT7SCF | C0000877N8 | AM069687 |
The genes in bold come from the immune libraries described in this paper
Analysis of the immune clones
All the sequences of the clones on the array were subject to Blast homology searches against the SwissProt and TREMBL databases using a cut-off value of 1e-10. Using this means of detection, many known immune-related molecules were identified, including cytokines, interferons, interleukins, transcription factors, receptors, cell differentiation antigens, MHC molecules and genes for proteins belonging to the TOLL receptor pathway. Proteins homologous to hypothetical human proteins and mouse cDNAs were also identified.
Sequences, which gave no Blast homology to anything in the nucleotide or protein databases, accounted for about 38% of the clones. Either the search parameters were too stringent to identify these genes or the chicken sequence was sufficiently divergent to be undetectable in a standard Blast search. This is a common feature of immune-related genes, and it is often very difficult to identify such genes by sequence homology to mammalian homologues. Some of these sequences may also represent non-conserved 3' UTR regions of genes. This set of clones may also include genes that have never been identified before and are not represented in the sequence databases. Further, more detailed analysis of these sequences can sometimes help elucidate the nature of the gene in question. Protein sequences can be predicted from the EST nucleotide sequence using programs such as ESTscan [[16] and [17]], which takes in to account sequencing errors and thus potential frame-shift mutations which are often present when there is only one EST sequence available for study. Conserved motifs and domains can then sometimes be identified for example, using the Pfam database [18], which is a large collection of multiple sequence alignments and hidden Markov models covering many common protein domains and families. PSI-Blast searches can also help identify to which type of family a gene will belong.
During clustering analysis, our 10,000 immune sequences were aligned with 398,000 existing chicken ESTs. This highlighted 3,845 clusters that contained one or more sequence from our immune libraries and 1,959 singleton clones. This analysis also identified 40 novel clusters that only contained sequences from our new libraries. Upon Blast analysis, 7 of these clusters were found to represent known chicken genes (initially appearing unique as they aligned to a different part of the gene sequence from existing ESTs), 18 showed homology to genes in other species and 15 clusters proved to have no known homology to anything currently in the databases. At the time, we searched against 398,000 existing chicken ESTs. Now however, there are currently 550,510 chicken ESTs in the databases (dbEST release 080505). A current search has shown that 9 of our sequences are indeed still unique to our libraries and have no known identifiable homologue, although two of the sequences do show some similarity to two predicted chicken sequences (AM065333 and the hypothetical protein XP_429359; AM065802 and the predicted P114-RHO-GEF protein XP_418249). Eight of these sequences are identifiable in the whole genome sequence, as shown in Table 3.
Table 3.
Genomic location of unique chicken ESTs as identified by the University of Santa Cruz Blat site http://genome.ucsc.edu/cgi-bin/hgBlat?command=start
| Clone | GenBank Accession No. | Genomic location |
| CBN1_C0000465p01.q1kT7 | AM065989 | chr15: 5562143–5562781 |
| CBN1_C0000464a07.q1kT7 | AM065333 | chr11_random: 637442–638117 |
| CBN1_C0000465g01.q1kT7 | AM065802 | chr28: 3396525–3399444 |
| CBN2_C0000485f09.q1kT7SCF | AM068122 | chr7: 1319368–1319989 |
| CBst_C0000222i16.q1kT7SCF | AM071684 | no hit |
| CTN2_C0000856o09.q1kT7 | AM064132 | chr17: 9342673–9343272 |
| CBN1_C0000360j04.q1kT7 | AM064831 | chr21: 2494538–2494813 |
| CBN1_C0000360n18.q1kT7 | AM064932 | chr1: 172893324–172893990 |
| CBN1_C0000463a02.q1kT7 | AM064982 | chr4: 50741781–50742197 |
Gene ontology (GO) annotations
In order to try and elucidate the function of the genes on the array further, we tried to assign as much annotation to the sequences as possible. GO annotations were assigned to some sequences after searching the GGI and UMIST databases [19], while other annotation was derived from hits to orthologous human sequences from the ENSEMBL [20] and GENSCAN [21] databases, as described in the 'methods' section. Having annotation derived from orthologous human genes means that cross-species comparisons between chicken and human array data may be possible. A search of the ENSEMBL database provided information on 2,292 GO-term associations, the GGGI database 1,542 and GENSCAN 566, while the UMIST full-length cDNA database provided a further 365 annotations. The sequences on the array cover a total of 227 GO terms, with 73% of all the sequences having at least one GO entry assigned to it. The available annotation for the array sequences is broken down as follows: 52% of genes have a 'cellular component' term assigned, 60% have 'molecular function' and 56% of sequences have the 'biological process' described. 83% of all the genes on the array have some kind of gene description and after searching each sequence against the sequences in the Ensembl chicken genome collection (July 2005 genebuild [22]), 78% of sequences were found to have a known chromosomal location. Now that all these sequences have been added to GenBank and thus have an accession number which can be directly linked into the ENSEMBL databases (work currently underway), obtaining comprehensive, up-to-date annotation data will become much easier.
A file showing the complete annotation for all the sequences on the array is available as supplementary material (Additional file 1). However, Additional file 2 provides an overview of the broad functional classes that are represented by the genes on the array. These are based on more general GO annotations derived from the GO-slims database at EBI, and allow us an insight into the different classes of genes present on the array without having to look at detailed functional annotation for each individual gene.
Annotation is also available for some (9,137) of the ESTs in the UMIST collection. By comparing the relevant GO slims [23] terms for the sequences in this collection with those present on our array, we are able to see which types of genes appear to be enriched in our set, compared with a larger, more general collection of EST sequences. As can be seen (shown in bold) in Additional file 2, certain classes of gene appear to be more highly represented. For instance, genes involved in protein transport are more abundant in our set of clones, as are those involved in the response to stimulus. This is consistent with our attempts to pre-select for higher numbers of genes involved in the immune system.
Quality of the array
To assess the quality of the array, various hybridization comparisons were undertaken. Three different conditions were addressed: 1). self v self 2). biological replicate A v biological replicate B and 3). Control sample v activated sample. Dye swap experiments were also carried out for conditions 2 and 3. The 'self' sample was a reference RNA consisting of a pool of various chicken lung samples. The biological replicates were lung samples from two 6-week-old chickens that had not been treated or challenged in any way. In the third group of hybridisations, the 'control' sample was from a similarly, untreated bird and the 'activated' sample was obtained from the lungs of a bird that had been challenged with the avian influenza strain H9N2 five days previously. The graphs in Fig 1 show the tight correlation between self/self (R2 = 0.9273) and between replicates (R2 = 0.8766), whereas a much higher level of variance is seen when an activated sample is compared against a control (R2 = 0.7601).
Figure 1.
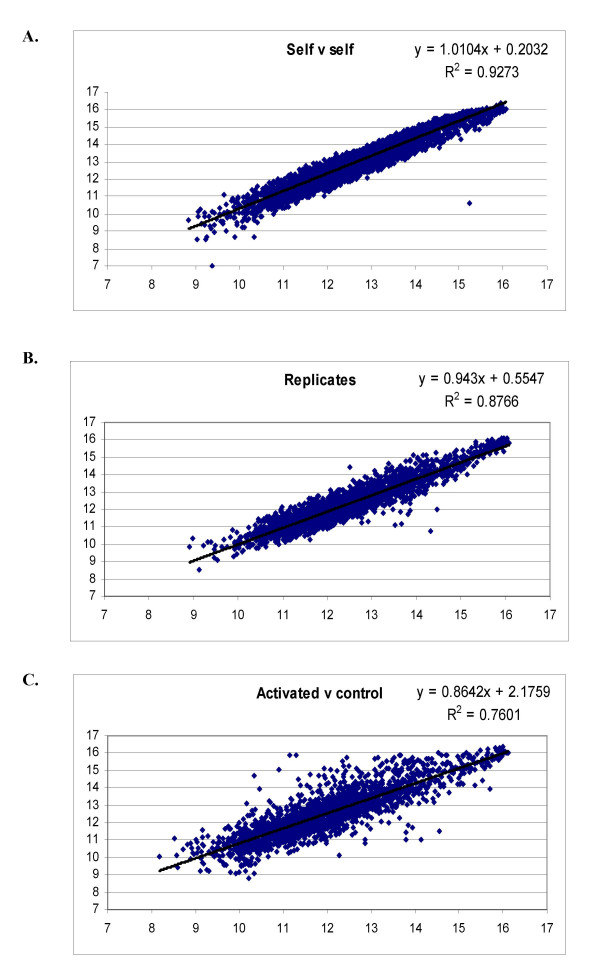
Scatter plots showing the variance between A). self/self hybridisation B). two biological replicates and C). a control sample compared with an activated sample. Very little spread is seen with the self/self hybridisation and between the two replicates, as would be expected. However, differences in gene expression can be seen between the activated and control samples.
The boxplots in Fig 2 also demonstrate the differing variances between the comparisons. The greatest variance is shown for the activated animals compared with the controls as would be expected. Regression analysis for each of the data sets confirm the increased variance with correlation coefficients of r = 0.872 for activated samples, r = 0.936 for replicate samples and r = 0.963 for self/self sample data sets.
Figure 2.
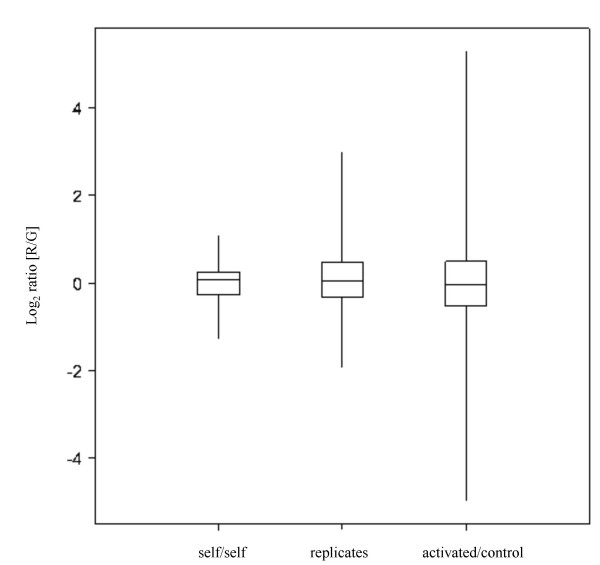
Box plots showing the variance between self/self hybridisation, two biological replicates and a control sample compared with an activated sample. Boxes represent the interquartile range from 25–75%, with the median marked. Outliers to this range are also shown.
Using the array
This array is available from the Ark-Genomics resource facility at Roslin Institute, providing an immune-focused array which, for anyone interested in immune-research, offers a much more cost-effective and time-saving platform for gene expression experiments, instead of using the large oligo arrays which have thousands more genes, many of which will be of no interest. Analysis of data is also thus much easier and far less time-consuming. Information on the array has been deposited in ArrayExpress (Accession: A-MEXP-307) [[24] and [25]] (Additional file 1) and very soon all the sequences will be submitted to the Ensembl database with links to all the GO annotation information in the GOA database [26].
Conclusion
We have constructed a 5 K chicken cDNA microarray, which is highly selected for genes expressed in tissues which have an immune function. This targeted array contains enough widely-expressed genes (whose expression won't be changing) to enable good normalization, as well as containing numerous known immune genes (from our novel libraries and from existing EST collections). The array also contains many genes with as yet unknown homology and function as well as a few novel genes which are specific to the libraries from which the array was created. These genes of unknown function could well have a role in either the adaptive or innate immune response, and thus provide a valuable resource for analysis of gene expression changes occurring in birds that have been subject to immune challenge. The array has been proven to provide highly reproducible results and is now available to the chicken/microarray community as a whole.
Methods
Sample collection
Eight groups of 38 chickens (3-week-old) were vaccinated with two different vaccine regimes. The eight groups were males and females of a commercial line of hybrid broiler (Ross 306, Aviagen, Newbridge, Midlothian, UK) and layer (Lohman Brown, Lohmann Tierzucht, Cuxhaven Germany) chicks given one of the two vaccination schemes. Group 1 were given vaccines for E. coli (0.5 ml in left breast muscle), ND and IBDV (0.5 ml in right breast muscle) formulated in alum-gel and oil-based immuno-potentiators. Intramuscular injections were given to ensure that all the birds were given an equal dose. Group 2 vaccines consisted of Paracox 8 [Eimeria sp.] (0.1 ml in drinking water), Nobilis Rismavac-CA126 [MD] (0.2 ml intramuscularly in leg) and Salenovac [S. enteritidis] (0.5 ml intramuscularly in leg). Tissue samples were obtained (unvaccinated); 5 hr, 24 hr, 72 hr and 7 days post vaccination. Samples from groups of 5 birds were pooled. Tissues collected were Bursa, spleen, Peyers patch and thymus. Tissue from Bursa, spleen and Peyers patch were pooled to make the 'B-cell' libraries and the thymus tissue was used to construct the 'T-cell' libraries. The tissues and time points chosen were in order to try and maximise the number of immune-related transcripts, including those which may only be expressed transiently. All experimental protocols were authorized under the UK Animals (Scientific Procedures) Act, 1986.
Library construction
Six libraries were constructed at Incyte Genomics (Palo Alto, CA): a standard and 2 normalized Bursa/spleen/Peyers patch libraries and a standard and 2 normalized thymus libraries. cDNA synthesis was initiated using an oligo (dT) primer, using methylated C in the first strand synthesis reaction. Following this first strand reaction, double-stranded cDNA was blunted, ligated to NotI adapters, digested with EcoRI, size-selected, and cloned into the NotI and EcoRI compatible sites of a custom modified MCS of the pBluescript (KS+) vector. Normalization was done in two rounds using conditions adapted from [[27] and [28]] except that a significantly longer re-annealing hybridization was used. Around 10,000 clones were then sequenced at the Sanger Institute according to their protocols. Using the T7 primer, sequence was generated from the 5' end of each clone by the dideoxy chain termination method using an ABI 3700 sequence analyser (Applied Biosystems, Foster City, CA).
EST sequence analysis
Bioinformatic analysis commenced with 10,173 sequences. After eliminating poor quality sequence and repeats, 9,434 of these sequences remained after screening with phred [29], RepeatMasker [30], Crossmatch [31] and XNUN [32]. Certain unwanted sequences were then identified after using the Blast algorithm [[33] and [34]] and screening the results for specific keywords. These included 'ribosomal', 'mitochondrial', 'Newcastle', 'Mareks', 'Eimeria', 'Salmonella' and 'E. coli'. 8,154 sequences passed these criteria. These sequences were then clustered against the existing UMIST and EMBL chicken EST sequences using TIGR's clustering tool, tgicl [35]. This resulted in 3,845 clusters which contained one or more sequence from our libraries and 1,959 singletons. The following clones were chosen for inclusion on the array: 3,770 cluster representatives, 1,067 singletons and 157 reference immune genes: 93 clones from the UMIST collection, 41 from our immune libraries, 21 clones from the Delaware set [36] and 2 clones courtesy of R. Zoorob (CNRS, France) (Table 2).
Construction of the array
The immune array was constructed from 4994 chicken EST clones plus 196 control elements (landing lights (positional controls), GAPDH, gamma actin, salmon sperm DNA, calf thymus DNA, chicken and bovine genomic DNA and a variety of spotting buffers). Plasmid DNA was prepared using MagAttract 96 Miniprep chemistry on a Biorobot 8000 platform (Qiagen Ltd., Crawley, UK), and the cDNA inserts were amplified using CGATTAAGTTGGGTAACGC (fwd) and CAATTTCACACAGGAAACAG (rev) in 50 ul reactions using 1 ul of DNA as a template. Amplified DNA was purified by Multiscreen 384 well PCR purification plates (Millipore, Watford, UK) on a Multiprobe II liquid handling platform (Perkin Elmer, Beaconsfield, UK) and the reactions confirmed by agarose gel electrophoresis and quantified by Picogreen assay (Molecular Probes, Invitrogen, Paisley, UK) on a Flouroskan Ascent flourescent plate reader (Thermo Life Science, Basingstoke, UK). DNA was resuspended to 150 ng/ul in spot buffer (150 mM Sodium phosphate, 0.01% SDS) before being spotted in duplicate on to amino-silane coated slides (CMT-GAPSII, Corning, Schiphol-Rijk, The Netherlands) using a Biorobotics MicroGrid II spotter (Genomic Solutions, Huntingdon, UK). Slides were then treated using succinic anhydride and 1-methyl-2-pyrrolidinone (Sigma, Poole, UK) to block unbound amino groups, followed by a wash in 95°C MilliQ water before hybridisation.
RNA preparation and labelling
Total RNA was isolated from lung tissue using a Trizol extraction according to the manufacturer's protocol (Invitrogen, Paisley, UK) and subsequently purified using the RNeasy Midi RNA Purification kit (Qiagen Ltd., Crawley, UK). RNA concentration was determined spectrophotometrically and RNA quality was determined using an Agilent 2100 Bioanalyser (Agilent Technologies, Waldbronn, Germany). Cy3 or Cy5 was incorporated into each sample using the Fairplay labelling kit (Stratagene, La Jolla, CA) and the labelled cDNA cleaned-up after passage through DyeEx columns (Qiagen Ltd., Crawley, UK). Labelling efficiency was determined by running 0.5 μl of each sample on a 1% agarose gel and measuring the intensity of fluorescence on a GeneTac LS IV scanner (Genomic Solutions, Huntingdon, UK).
Hybridizations
Microarray hybridizations were carried out overnight using a GeneTAC automated hybridization system [37] (Genomic Solutions, Huntingdon, UK). Hybridizations (125 μl) were carried out in Genomic Solutions hybridization solution (Cat. no. RP#0025) in a stepped hybridization: 55°C for 3 hr, 50°C for 3 hr and then 45°C for 12 hr. Slides were then washed in Genomic Solutions wash buffers (Cat. nos. CS#0038, CS#0039 and CS#0040). Upon removal from the hybridization stations, slides were washed for 1 min in Post-Wash buffer (CS#0040) and a further minute in isopropanol, followed by centrifugation at 1000 rpm for 6 min. Dried slides were scanned in a Scanarray 5000 scanner (GSI Lumonics, Rugby, UK) fitted with Cy3 and Cy5 filters.
Data analysis
To indicate the suitability of the new array to discriminate the differences in the experimental treatments, hybridizations comparing samples with controls and controls with controls were performed. Control (vehicle treated) animals were compared with immunologically challenged animals (activated slides) and control animals were also compared with other control individuals (replicate slides). The same animal was also compared with itself (self/self). Each comparison was completed in duplicate and with a dye flip. Dye-swaps are carried out in order to deal with any residual dye-bias remaining after labelling. However, this is generally not a problem, due to the indirect labelling method employed. Data was extracted from the slide using Bluefuse software (BlueGnome, Cambridge, UK). Features with poor confidence information (confidence <0.30, flagged D and E) were eliminated from the analysis. M v A plots [where M = log2 (Cy5/Cy3) and A = 1/2*(log2(Cy5) + log2(Cy3)] of the data for each slide (data not shown) were suitably linear to require only a simple global normalisation of the data. Data from slides of similar treatments was pooled and a boxplot produced for each comparison (Genstat v8.1, VSN International Ltd., Hemel Hempstead, Herts, UK).
Databases and sequence sources
Ensembl and Genscan predicted genes/peptide sequences for the chicken genome assembly (March 2004) were downloaded from the Ensembl database using Ensmart or the UCSC table browser [38]. Chicken EST sequences were downloaded from the TIGR Gallus gallus gene index (GGGI) [release 10.0] [[39] and [40]]. Chicken full-length cDNA sequences were downloaded from the UMIST www site (Sept 2004). Ensembl predicted peptide sequences for the human genome assembly (May 2004) were downloaded from the Ensembl database using Ensmart or the UCSC table browser.
Mapping array probes to chicken ESTs, cDNAs, genes and genome
Unique ESTs used to create the immune array were mapped to chicken cDNAs, ESTs, genes or the chicken genome assembly using NCBI Blastn (version 2.2.11). Identity was defined with > 95% sequence identity over 100-bp and then taking the top-scoring match to each EST to provide a unique sequence assignment. All repeats and low-complexity sequences were masked using RepeatMasker (version 3.1.0).
Definition of Gene Ontology terms and Gene Descriptions for array probes
Gene Ontology (GO) annotations [41] were all based on database hits in sequence similarity searches using Blastn. GO annotations were automatically transferred from these database records to the array probe entries. GO annotations were available for GGGI and UMIST EST/cDNA sequences. For chicken Ensembl or Genscan gene predictions, GO annotations were based on orthologous human peptide sequences. Orthologues were defined based on two cycles of Blastp between human and chicken proteins. An E_value cut off of less than 10-4, with the subject and query databases swapped between runs. By comparing E_values mutually best proteins pairs were selected as orthologues. When E_values were equal, bits score and sequence coverage were used as tiebreakers to select the top hit. For each array probe associated GO terms and a unique gene description was transferred from the orthologous database record. Finally a Perl script was used to create a non-redundant set of probe to GO records.
Frequency of GO and GO-Slim terms
GO terms (version 3.2.16) were downloaded from the Gene Ontology www site. More general GO terms were assigned using GoaSlim_map (June 2005) available from the GOA www site at EBI. The GO-Slim terms allowed us to estimate e.g. the frequency of array probes associated with the biological process Metabolism (GO:0008152).
Data processing
Perl scripts (version 5.8.5) and SQL were used throughout to manipulate and filter data sets.
Authors' contributions
JS contributed to the design of the array, carried out the microarray experiments and drafted the manuscript. DS carried out quality control, clustering and BLAST searches of the DNA sequences. PH was responsible for tissue sample collection. RTT statistically analysed the microarray data. WGJD and VEJCS were involved in experimental design. EJG contributed to the array design. DWB carried out all the bioinformatics involved in establishing GO annotations. All authors read and approved the final manuscript.
Supplementary Material
The file supp_mat.xls is an excel file which contains annotation information on the sequences present on the immune array and is available as supplementary material. An ArrayExpress http://www.ebi.ac.uk/arrayexpress file is available under accession number A-MEXP-307.
The file supp_mat_2.xls is an excel file and contains a summary of functional groups present on the array (GO slims). Percentages are calculated as a fraction of the total number of classes represented within one functional description. For example, 42.4% of all the genes are involved in some kind of physiological process. Of these, 14.3% are involved in transport, with 10.2% of these genes being specifically involved in electron transport. This breakdown of functional classes is compared to those represented by 9,137 of the ESTs in the UMIST collection (data available from http://chick.umist.ac.uk). Entries shown in bold define the GO classifications that appear to be enriched in the sequences represented on the immune array compared with this subset of the UMIST chicken ESTs.
Acknowledgments
Acknowledgements
The authors would like to thank Incyte Genomics (|Palo Alto, CA) for construction of the normalized cDNA libraries and The Wellcome Trust Sanger Institute (Hinxton, UK) for sequencing 10,000 cDNA clones from the libraries. Thanks also to Frazer Murray of ARK-Genomics (Roslin) for invaluable technical assistance and to Theo Jansen (Intervet International B.V., Boxmeer, The Netherlands) for the preparation of the vaccine formulations. This project was funded by Intervet International B.V, Boxmeer, The Netherlands, the Biotechnology and Biological Science Research Council (BBSRC) and partly by a BSIK VIRGO consortium grant, the Netherlands (grant nr. 03012).
Contributor Information
Jacqueline Smith, Email: Jacqueline.smith@bbsrc.ac.uk.
David Speed, Email: David.speed@bbsrc.ac.uk.
Paul M Hocking, Email: Paul.hocking@bbsrc.ac.uk.
Richard T Talbot, Email: Richard.Talbot@bbsrc.ac.uk.
Winfried GJ Degen, Email: Winfried.Degen@intervet.com.
Virgil EJC Schijns, Email: Virgil.Schijns@intervet.com.
Elizabeth J Glass, Email: Liz.glass@bbsrc.ac.uk.
David W Burt, Email: Dave.burt@bbsrc.ac.uk.
References
- Schmid M, Nanda I, Guttenbach M, Steinlein C, Hoehn M, Schartl M, Haaf T, Weigend S, Fries R, Buerstedde JM, Wimmers K, Burt DW, Smith J, A'Hara S, Law A, Griffin DK, Bumstead N, Kaufman J, Thomson PA, Burke T, Groenen MA, Crooijmans RP, Vignal A, Fillon V, Morisson M, Pitel F, Tixier-Boichard M, Ladjali-Mohammedi K, Hillel J, Maki-Tanila A, Cheng HH, Delany ME, Burnside J, Mizuno S. First report on chicken genes and chromosomes 2000. Cytogenet Cell Genet. 2000;90:169–218. doi: 10.1159/000056772. [DOI] [PubMed] [Google Scholar]
- Aerts J, Crooijmans R, Cornelissen S, Hemmatian K, Veenendaal T, Jaadar A, van der Poel J, Fillon V, Vignal A, Groenen M. Integration of chicken genomic resources to enable whole-genome sequencing. Cytogenet Genome Res. 2003;1024:297–303. doi: 10.1159/000075766. [DOI] [PubMed] [Google Scholar]
- Ren C, Lee MK, Yan B, Ding K, Cox B, Romanov MN, Price JA, Dodgson JB, Zhang HB. A BAC-based physical map of the chicken genome. Genome Res. 2003;13:2754–2758. doi: 10.1101/gr.1499303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisson M, Lemiere A, Bosc S, Galan M, Plisson-Petit F, Pinton P, Delcros C, Feve K, Pitel F, Fillon V, Yerle M, Vignal A. ChickRH6: a chicken whole-genome radiation hybrid panel. Genet Sel Evol. 2002;34:521–533. doi: 10.1051/gse:2002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman PE, Sanz-Ezquerro J, Overton IM, Burt DW, Bosch E, Fong WT, Tickle C, Brown WR, Wilson SA, Hubbard SJ. A comprehensive collection of chicken cDNAs. Curr Biol. 2002;12:1965–1969. doi: 10.1016/S0960-9822(02)01296-4. [DOI] [PubMed] [Google Scholar]
- International Chicken Genome Sequencing Consortium (ICGSC) Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- van Hemert S, Ebbelaar BH, Smits MA, Rebel JM. Generation of EST and microarray resources for functional genomic studies on chicken intestinal health. Anim Biotechnol. 2003;13:133–143. doi: 10.1081/ABIO-120026483. [DOI] [PubMed] [Google Scholar]
- Bliss TW, Dohms JE, Emara MG, Keeler CL., Jr Gene expression profiling of avian macrophage activation. Vet Immunol Immunopath. 2005;105:289–299. doi: 10.1016/j.vetimm.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Neiman PE, Ruddell A, Jasoni C, Loring G, Thomas SJ, Brandvold KA, Lee Rm, Burnside J, Delrow J. Analysis of gene expression during myc oncogene-induced lymphomagenesis in the bursa of Fabricius. Proc Natl Acad Sci (USA) 2001;98:6378–6383. doi: 10.1073/pnas.111144898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrakhte M, Schultheiss TM. Construction and analysis of a subtracted library and microarray of cDNAs expressed specifically in chicken heart progenitor cells. Dev Dynam. 2004;230:290–298. doi: 10.1002/dvdy.20059. [DOI] [PubMed] [Google Scholar]
- ARKGenomics http://www.ark-genomics.org
- Burnside J, Neiman P, Tang J, Bascom R, Aronszajn M, Talbot R, Burt DW, Delrow J. Development of a cDNA array for chicken gene expression analysis. BMC Genomics. 2005;6:13. doi: 10.1186/1471-2164-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Speed D, Law AS, Glass EJ, Burt DW. In silico identification of chicken immune-related genes. Immunogenetics. 2004;56:122–133. doi: 10.1007/s00251-004-0669-y. [DOI] [PubMed] [Google Scholar]
- Gene ontology www site http://www.geneontology.org/
- Blast at NCBI http://www.ncbi.nlm.nih.gov/BLAST/
- Iseli C, Jongeneel CV, Bucher P. ESTScan: a program for detecting, evaluating, and reconstructing potential coding regions in EST sequences. Proc Int Conf Intell Syst Mol Biol. 1999:138–148. [PubMed] [Google Scholar]
- ESTscan http://www.ch.embnet.org/software/ESTScan.html
- Pfam http://www.sanger.ac.uk/Software/Pfam/
- BBSRC chicken EST database http://chick.umist.ac.uk/
- Ensembl genome databases http://www.ensembl.org/
- Genscan http://genes.mit.edu/GENSCAN.html
- Ensemble 2005 chicken genebuild ftp://ftp.ensembl.org/pub/chicken-32.1h/data/fasta/dna/
- GO slims http://www.geneontology.org/GO.slims.shtml
- Brazma A, Parkinson H, Sarkans U, Shojatalab M, Vilo J, Abeygunawardena N, Holloway E, Kapushesky M, Kemmeren P, Lara GG, Oezcimen A, Rocca-Serra P, Sansone S. ArrayExpress – a public repository for microarray gene expression data at the EBI. Nucleic Acids Res. 2003;31:68–71. doi: 10.1093/nar/gkg091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ArrayExpress http://www.ebi.ac.uk/arrayexpress
- Gene ontology annotation at EBI http://www.ebi.ac.uk/GOA/
- Soares MB, Bonaldo MF, Jelene P, Su L, Lawton L, Efstratiadis A. Construction and characterization of a normalized cDNA library. Proc Natl Acad Sci U S A. 1994;91:9228–9232. doi: 10.1073/pnas.91.20.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaldo MF, Lennon G, Soares MB. Normalization and subtraction: two approaches to facilitate gene discovery. Genome Res. 1996;6:791. doi: 10.1101/gr.6.9.791. [DOI] [PubMed] [Google Scholar]
- Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998;8:175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- RepeatMasker http://www.repeatmasker.org/
- Crossmatch http://www.genome.washington.edu/UWGC/analysistools/Swat.cfm
- Claverie JM, States D. Information enhancement methods for large scale sequence analysis. Computers Chem. 1993;17:191–201. doi: 10.1016/0097-8485(93)85010-A. [DOI] [Google Scholar]
- Blast at NCBI http://www.ncbi.nlm.nih.gov/BLAST/
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1006/jmbi.1990.9999. [DOI] [PubMed] [Google Scholar]
- Pertea G, Huang X, Liang F, Antonescu V, Sultana R, Karamycheva S, Lee Y, White J, Cheung F, Parvizi B, Tsai J, Quackenbush J. TIGR gene indices clustering tools (TGICL): a software system for fast clustering of large EST datasets. Bioinformatics. 2003;19:651–652. doi: 10.1093/bioinformatics/btg034. [DOI] [PubMed] [Google Scholar]
- University of Delaware EST collection http://www.chickest.udel.edu/
- GenomicSolutions http://www.genomicsolutions.com/showPage.php?title=GeneMachines%20HybStation
- UCSC genome bioinformatics site http://genome.ucsc.edu/
- TIGR gene indices http://www.tigr.org/tdb/tgi/
- Quackenbush J, Cho J, Lee D, Liang F, Holt I, Karamycheva S, Parvizi B, Pertea G, Sultana R, White J. The TIGR Gene Indices: analysis of gene transcript sequences in highly sampled eukaryotic species. Nucleic Acids Res. 2001;29:159–64. doi: 10.1093/nar/29.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gene Ontology Consortium Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The file supp_mat.xls is an excel file which contains annotation information on the sequences present on the immune array and is available as supplementary material. An ArrayExpress http://www.ebi.ac.uk/arrayexpress file is available under accession number A-MEXP-307.
The file supp_mat_2.xls is an excel file and contains a summary of functional groups present on the array (GO slims). Percentages are calculated as a fraction of the total number of classes represented within one functional description. For example, 42.4% of all the genes are involved in some kind of physiological process. Of these, 14.3% are involved in transport, with 10.2% of these genes being specifically involved in electron transport. This breakdown of functional classes is compared to those represented by 9,137 of the ESTs in the UMIST collection (data available from http://chick.umist.ac.uk). Entries shown in bold define the GO classifications that appear to be enriched in the sequences represented on the immune array compared with this subset of the UMIST chicken ESTs.


