Abstract
SUN, YING AND JIANDE CHEN. Intestinal electric stimulation decreases fat absorption in rats: therapeutic potential for obesity.
Objective: Effective treatment of obesity is based on the restriction of food intake or reduction of absorption or both. The aim of this study was to study whether intestinal electric stimulation (IES) would reduce fat absorption and, thus, would be a potential therapy for obesity.
Research Methods and Procedures: Forty rats implanted with serosal electrodes and two jejunal cannulas were divided into 4 groups of 10 each: control (no stimulation), IES with long pulses, IES with trains of short pulses, and IES with trains of short pulses plus treatment with lidocaine. Jejunal transit and fat absorption of a 20-cm jejunal segment (between two cannulas) were investigated during a 45-minute period with or without IES.
Results: It was found that both methods of IES accelerated intestinal transit measured by recovery of phenol red and increased the percentage of triglycerides recovered from the distal cannula in comparison with the control group. IES with trains of short pulses was more effective than IES with long pulses in accelerating jejunal transit and reducing fat absorption. Neither of the two IES methods altered the output of fatty acids from the distal cannula. The effects of IES with trains of short pulses on the transit and fat absorption were partially abolished with the treatment of lidocaine.
Discussion: It was concluded that IES accelerates intestinal transit and reduces fat absorption, suggesting a therapeutic potential for obesity. IES with trains of short pulses is more effective than IES with long pulses, and its effects are partially mediated by enteric nerves, jejunum.
Keywords: electric stimulation, intestinal transit, fat absorption, enteric nerves, jejunum
Introduction
Obesity is a global problem, affecting an estimated 300 million people worldwide (1). Obesity is defined as an excess of total body fat that is documented by BMI >30 kg/m2. In addition to the increased morbidity and functional limitations associated with obesity, ∼325,000 deaths in the United States each year among nonsmokers are attributable to obesity (2). Therefore, prevention and treatment of obesity are paramount in the whole world. However, therapeutics for obesity are not satisfactory. Behavior modification and pharmacotherapies are effective for only a short time (3,4). Although surgical treatment results in substantial and sustained weight loss, its application is very limited due to its morbidity and complications (5).
The objective of the surgical therapy is to limit food intake, reduce absorption, or both. The small intestine plays a crucial role in maintaining absorption of nutrients and cholesterol homeostasis. It regulates the amount of dietary cholesterol that enters the body and has a high rate of cholesterol biosynthesis that, in some species and under certain physiological conditions, may exceed that of the liver (6,7). Moreover, intestinal mucosa is involved in cholesterol esterification (8) and synthesis of various apolipoproteins (9-12).
Absorption of nutrients in the small intestine has been reported to be affected by the alteration of intestinal motility in humans (13-15), rats (16), and pigs (17). After meals, an increase of stationary contractions, i.e., motility changed from a propulsive to a segmenting pattern, is associated with a linear increase in transit time; a delay in luminal transit is associated with a linear increase in the absorption of nutrients (17). It is reported that endotoxemia results in rapid intestinal transit and decreases jejunal absorption of water, electrolytes, and glucose (18). On the contrary, somatostatin delays the luminal transit and increases the absorption of carbohydrate, protein, and fat (19).
Therapeutic potentials of gastric or intestinal electric stimulation (IES)1 have been explored for various gastrointestinal motility disorders. A few studies have demonstrated that gastric or intestinal electric stimulation can delay or accelerate gastric or intestinal transit, depending on the location of electrodes. It seems that backward gastric or intestinal electric stimulation is able to delay gastric emptying or intestinal transit (20,21), whereas forward IES is able to accelerate intestinal transit (22-24). Recently, Chen and Lin (23) reported that jejunal forward electric stimulation accelerated intestinal transit slowed by fat-induced ileal brake in a canine model. Meanwhile, IES may also influence small bowel absorption independently of alterations in the pacesetter potential. It has been reported that backward jejunal electric stimulation slows or reverses the flow of liquid chyme through the paced segment and leads to enhanced absorption of water, nutrients, and electrolytes in canine (25,26) and in rat (27). Postprandial backward electric stimulation has been shown to induce an increase in body weight and a decrease in fecal fat and nitrogen losses during the test period in a canine model of short bowel syndrome (28). The enhanced enteric absorption with backward IES is mediated, in part, by an α-adrenergic mechanism (29). Forward IES slightly decreases the output of water, glucose, and sodium from the jejunal segment (30). However, the effects of forward IES on fat absorption and related mechanisms have never been reported, to the best of our knowledge; the therapeutic potential of IES for obesity has not been explored.
Therefore, the aim of this study was to explore the therapeutic potential of IES for obesity by investigating its effects on intestinal transit and fat absorption, as well as possible mechanisms involved with these effects in a rodent model.
Research Methods and Procedures
Subjects
Forty Male Sprague-Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) weighing 250 to 400 grams were housed under a temperature- and humidity-controlled condition in a 12-hour light/dark (6 am to 6 pm) cycle. Before the experiments, all rats were starved overnight with free access to water. The Institutional Animal Care and Use Committee at the Oklahoma City Veterans Administration Medical Center approved the surgical and experimental protocol.
Surgical Procedures
Under general anesthesia with ketamine (60 mg/kg) and xylazine (7 mg/kg), the abdominal midline incision of the rats was performed, and a 20-cm length of middle-distal jejunum was chosen as the testing segment. Two fistula were made: one located in the proximal segment, ∼20 cm from the pylorus, and the other in the distal segment, ∼40 cm from the pylorus. A polyethylene tube (PE-90) was inserted from the proximal fistula into the testing segment for perfusing fat or saline solution, and another polyethylene tube (PE-240) was connected to the distal fistula for collecting effluents from the testing segment. Two pairs of 28-gauge cardiac pacing wires (A&E Medical, Farming-dale, NJ) were implanted on the serosal surface of the testing segment, 1 and 2 cm distal to the proximal fistula, respectively. The abdomen was irrigated with normal saline and covered with plastic wrap to prevent fluid loss by evaporation. Body temperature was maintained at ≈36 to 38 °C with a lamp throughout the experiment.
Experimental Design
The rats were randomized into four groups (10 each): control without electric stimulation, IES with long pulses, IES with trains of short pulses, and lidocaine plus IES with trains of short pulses. After a 30-minute saline perfusion for cleaning the luminal contents and recording of jejunal myoelectrical activity, a bolus injection of 1 mL of 5% phenol red (0.5 mg/mL) was given as a nonabsorbable marker, and then triolein (3 mM) emulsion was continuously perfused for 45 minutes with a Reglo Digital roller pump (0.18 mL/min; Ismatec SA, Glattbrugg, Switzerland). The perfusion rate was chosen to ensure that fat in the solution had sufficient contact time with intestinal mucosa without introducing intestinal stasis. In the IES groups, electric stimulations were performed for 45 minutes simultaneously with the perfusion. In the lidocaine group, 2% lidocaine was dropped onto the serosal surface of the test jejunum (0.5 mg/mL per 10 cm) every 10 minutes for inhibiting the activity of the myoenteric plexus of the jejunum (31,32) during the 45-minute perfusion period. Distal jejunal contents were collected every 15 minutes for 45 minutes. At the end of the perfusion, the solution remaining in the testing jejunal lumen was collected with 2-mL air injection. The phenol red, triglycerides (TGs), and fatty acids in each of the samples were analyzed and calculated.
Preparation of TG Emulsion
Lipase (0.3 grams, 50 mL; Sigma-Aldrich, Milwaukee, WI) and bile salts (sodium taurocholate, 10 mM; Sigma-Aldrich) with triolein (95% of TGs, 3 mM; Sigma-Aldrich) were added into the Krebs' solution (111 mM NaCl, 6 mM KCl, 45 mM NaHCO3, and 0.2 mM CaCl2) to facilitate the absorption of fat and to ensure its physiological condition. The mixed TG solution was then emulsified by sonication.
Analysis and Calculation of Phenol Red, Fat, and Fatty Acids
The effluents from the distal testing segment were centrifuged for 10 minutes at 1560g and the supernatant stored at −20 °C until analysis. To 2 mL of supernatant, 3 mL of 0.3 N Ba(OH)2, 1.5 mL of 6.6% lead acetate 3H2O, 1.5 mL of 5% ZnSO4 7 H2O, 1.5 mL of 0.3 N Ba(OH)2, and 2 mL of 2.7 MgCl2 6 H2O were added. The mixture was mixed thoroughly after each addition and then finally filtered (Whatman type 541; Whatman, Clifton, NJ). Phenol red in the filtrate was determined by measuring the absorption at a wavelength of 540 nm using a spectrophotometer (Turner Spectrophotometer, SP-830, Apogent Technologies Company, Dubuque, IA). The tested jejunal transit time was expressed as the percentage of phenol red recovery. The percentage of phenol red recovery was calculated as the ratio between the recovered phenol red and the total amount of phenol red.
TGs require digestion to fatty acids and monoglycerides for micellar solubilization and efficient absorption in the lumen of small intestine. Therefore, we measured TG and fatty acids separately. TG in the collected samples was measured using enzymatic reagents (DADE Behring Inc., Newark, DE) by a DADE Dimension RXL chemistry analyzer (DADE). The percentage of TG recovery is defined as the ratio between the recovered TG and the total amount of TG perfused during the 45-minute study period.
Fatty acids in each of the collected samples were analyzed using the method of VandeKamer (33). The amount of fatty acids was expressed as 5.907 (A/Q), in which A equals the amount (milliliters) of 0.1 N alkali used in titration, and Q represents the weight (grams) of each sample. Fat absorption was assessed as inversely proportional to the recovered TG and fatty acids from the distal cannula.
IES
IES was applied using the proximal pair of electrodes. The IES with long pulses was performed using a frequency 10% higher than the frequency of the jejunal myoelectrical activity recorded at baseline, a pulse width of 200 ms, and amplitude of 4 mA (constant current output). The IES with trains of short pulses was conducted using a train on-time of 2 seconds and off-time of 3 seconds, a pulse frequency of 20 Hz, a pulse width of 2 ms, and pulse amplitude of 4 mA.
Jejunal Myoelectrical Activity Recording
Jejunal myoelectrical activity was recorded using the distal pair of electrodes at baseline for 30 minutes using a multichannel recorder (Acqknowledge III, model EOG 100 A; Biopac Systems, Inc., Santa Barbara, CA). Recorded signals were displayed on a computer monitor and saved on the hard disk of an IBM-compatible 486 personal computer. The high cut-off frequency of the amplifier was 35 Hz with an initial sampling frequency of 100 Hz. The frequency of the jejunal myoelectrical activity was analyzed visually and used to determine the stimulation frequency of IES with long pulses. Spectral analysis was performed to compute the percentage of normal slow waves (34-36).
Statistical Analysis
ANOVA was applied to assess the differences in phenol red recovery, TG recovery, and fatty acids among the four groups. Unpaired Student's t test was applied to investigate the difference between the paired data. Linear regression was utilized for analyzing the relationship between phenol red recovery and TG recovery. Statistical significance was assigned at p < 0.05. All data were presented as means ± SE.
Results
Jejunal Myoelectrical Activity
Regular slow waves were observed in the jejunal myoelectrical recording at baseline. The frequency of the jejunal slow waves was 25.3 ± 0.33 cycles per minute (range: 20.5 to 29). The percentage of slow waves within a frequency range of 22 to 28 cpm was 96.6 ± 2.6%.
Effects of IES on Jejunal Transit and Possible Mechanism Involving Enteric Nerves
A significant acceleration in jejunal transit was observed with IES. As shown in Figures 1 and 2, IES with either long pulses or trains of short pulses significantly increased the percentage of recovered phenol red (ANOVA, p < 0.001). The percentage of recovered phenol red was 36.8 ± 7.2% in the control group and 71.3 ± 3.2% in the group of IES with long pulses (p < 0.001) and 82.7 ± 3.8% in the group of IES with trains of short pulses (p < 0.001) at 15 minutes; 55.4 ± 7.3% (control), 83.5 ± 4.3% (IES with long pulses, p < 0.01) and 92.1 ± 2.7% (IES with trains of short pulses, p < 0.001) at 30 minutes; and 62.3 ± 7.1% (control), 86.9 ± 4.0% (IES with long pulse, p < 0.005), and 94.6 ± 2.2% (IES with trains of short pulses, p < 0.001) at 45 minutes. It was also noted that the percentage of phenol red recovery in the group of IES with trains of short pulses was significantly higher than that in the group of IES with long pulses at 15 minutes (p < 0.02).
Figure 1.
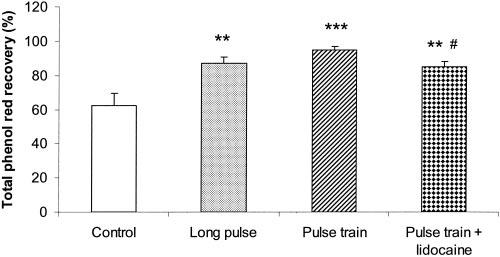
Effects of IES on intestinal transit. Intestinal transit is represented by the percentage of recovered phenol red at 45 minutes. Values are the mean ± SE. **, p < 0.01; ***, p < 0.001 (vs. the control group, n = 10). #, p < 0.05 (vs. the trains of short pulses group, n = 10).
Figure 2.
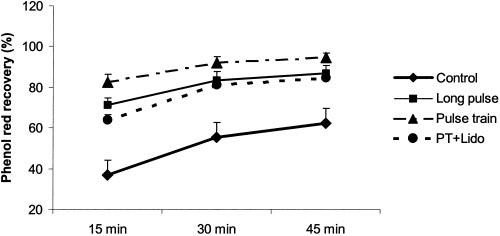
Effects of IES on intestinal transit at 15, 30, and 45 minutes. Intestinal transit is represented by the percentage of recovered phenol red. The percentage of recovered phenol red in the group of IES with trains of short pulses and lidocaine was significantly lower than that in the group of IES with trains of short pulses but without lidocaine at each of the corresponding times, but was still higher than that in the control groups at each of the corresponding times. Values are the mean ± SE.
The acceleration of jejunal transit by IES with trains of short pulses was found to be partially mediated by the enteric nerves. The percentage of recovered phenol red from the distal fistula in the groups of IES with both trains of short pulses and lidocaine was significantly lower (64.0 ± 2.5% at 30 minutes, 81.0 ± 3.1% at 30 minutes, 84.4 ± 3.4% at 45 minutes) than that in the group of IES with trains of short pulses but without lidocaine at each of the corresponding times, but was still higher than that in the control group at each of the corresponding times (see Figure 2).
Effects of IES on TG Absorption
IES with either long pulses or trains of short pulses significantly increased the percentage of TG recovery (ANOVA, p < 0.001). The percentage of TG recovery was 8.4 ± 2.2% in the control group and 15.5 ± 1.9% in the IES group with long pulses (p < 0.02, vs. control) and 19.5 ± 1.9% in the IES Group with trains of short pulse (p < 0.001, vs. control) at 15 minutes; 29.6 ± 4.2% (control), 41.0 ± 1.6% (long pulses, p < 0.02), and 47.7 ± 3.2% (trains of short pulses, p < 0.005) at 30 minutes; and 63.2 ± 5.2% (control), 78.3 ± 1.7% (long pulse, p < 0.02), and 94.1 ± 2.3% (trains of short pulses, p < 0.001) at 45 minutes (Figure 3).
Figure 3.
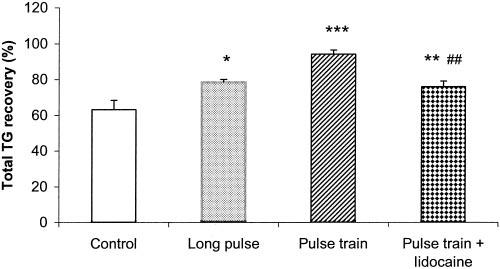
Effects of IES on the fat absorption. Fat absorption is represented by the percentage of TG recovery at 45 minutes. Values are the mean ± SE. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (vs. the control group, n = 10). ##, p < 0.01 (vs. the trains of short pulses group, n = 10).
The percentage of TG recovery in the group of IES with trains of short pulses was significantly higher than that in the group of IES with long pulse at 45 minutes (78.3 ± 1.7% vs. 94.1 ± 2.3%, p < 0.01).
The increase of the percentage of TG recovery by IES with trains of short pulses was also found to be partially mediated by the enteric nerves. The percentage of TG recovery from the distal fistula in the IES group with both trains of short pulses and lidocaine was significantly lower than that in the IES group with trains of short pulses but without lidocaine at 45 minutes (75.9 ± 3.2% vs. 94.1 ± 2.3%, p < 0.005), but was still higher than that in the control groups (63.2 ± 5.2%, p < 0.005) (see Figure 3).
Effects of IES on Fatty Acid Absorption
IES had no significant effects on the output of fatty acids from the distal cannula (Figure 4). The amount of fatty acids collected from the distal cannula was 18.1 ± 8.5 mg/dl in the control group and 31.0 ± 12.4 mg/dl in the IES group with long pulses (p = 0.48) and 44.6 ± 14.2 mg/dl in the IES group with trains of short pulses (p = 0.53) at 45 minutes. Nevertheless, the total amount of fatty acids collected from the distal cannula in the 45-minute period was significantly lower in the IES group with trains of short pulses plus lidocaine than in the IES group with trains of short pulses but without lidocaine (11.2 ± 2.2 vs. 44.6 ± 14.2 mg/dl, p < 0.05), indicating the involvement of enteric nerves.
Figure 4.
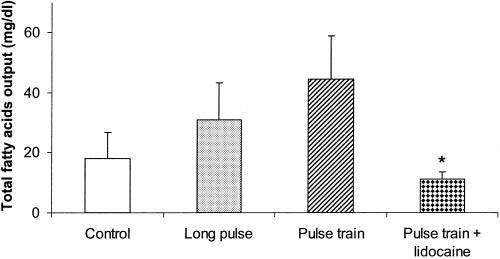
Effects of IES on the fatty acids. Values are the mean ± SE. *, p < 0.05 (vs. the trains of short pulses group, n = 10).
Relationship between Intestinal Transit and Fat Absorption
No correlation was noted between the percentage of phenol red recovery and TG recovery at 45 minutes in any of the three groups (control, IES with long pulses, and IES with trains of short pulses) (r = 0.036, p = 0.92; r = 0.21, p = 0.6; r = 0.42, p = 0.23, respectively). However, there was a significant correlation between the percentage of phenol red recovery and TG recovery at 45 minutes in the group of IES with trains of short pulses and lidocaine (r = 0.64, p < 0.05) (Figure 5).
Figure 5.
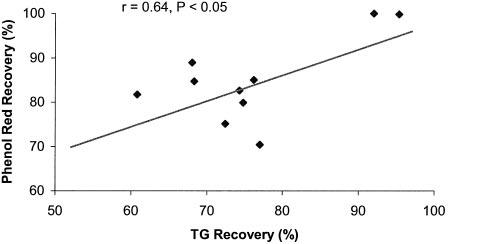
Relationship between fat absorption and jejunal transit in the group of IES with lidocaine. Fat absorption is represented by the percentage of TG recovery at 45 minutes. Intestinal transit is represented by the percentage of recovered phenol red at 45 minutes (n = 10). Plotted values are mean of the percentage of TG recovery and the percentage of recovered phenol red. r = 0.64, p < 0.05.
Discussion
Our results showed that IES significantly increased the percentages of recovered phenol red and TG in the tested jejunum. IES with trains of short pulses had a stronger effect on the transit and fat absorption than IES with long pulses. Lidocaine significantly inhibited the effect of IES with trains of short pulses on the transit and fat absorption, suggesting the involvement of enteric nerves.
The middle-distal jejunum was chosen as the testing segment. Although some nutrient absorption occurs in the stomach and colon, only absorption from the small intestine is of clinical importance. In addition, the absorption of dietary cholesterol occurs predominantly in the jejunum (37). Moreover, most dietary lipid is absorbed by the middle one-third of the jejunum (38). Therefore, the investigation of fat absorption of the middle-distal jejunum is of specific importance.
Electric stimulation at different locations leads to different results. Most previous studies with IES have applied backward stimulation and have aimed at delaying transit or increasing absorption (21,24). A few studies have used forward stimulation and have reported an acceleration of intestinal transit (22-24,39). Forward electric stimulation was used in this study because our aim was to accelerate intestinal transit and decrease fat absorption.
Likewise, different parameters of electric stimulation may have different effects on gastrointestinal functions. For example, gastric electric stimulation (GES) with long pulses (in the order of milliseconds) improves gastric motility (40-42). GES with short pulses (in the order of microseconds) improves symptoms of nausea and vomiting (43,44). Stimulation with trains of short pulses has been frequently used in electroacupuncture (45), and a recent report has indicated that electroacupuncture with trains of short pulses prevents vomiting and behaviors suggestive of nausea induced by vasopressin, and the antiemetic effect is vagally mediated (46). Recently, Chen and Lin (23) reported that forward IES with long pulses accelerated intestinal transit slowed by fat-induced ileal brake in dogs. They used isotopes as the testing markers and found that the percentage of marker recovery increased from 19.2% to 84.6%. Mintchev et al. found that electric stimulation with trains of short pulses accelerated the movement of gastric (47) and colonic (48) solid contents. Accordingly, in this study, we chose long pulses (pulse width of 200 ms) and trains of short pulses. The results obtained from this study were in agreement with the previous findings (22-24,39), i.e., intestinal transit was accelerated with IES with either long pulses or trains of short pulses. In addition, we found that IES with trains of short pulses was more effective than IES with long pulses in the acceleration of intestinal transit. Comparing the energy used for stimulation between the two methods of IES, we further found that IES with trains of short pulses was more efficient (consumed less energy) than IES with long pulses.
Most importantly, our results showed, for the first time, that forward IES with either long pulses or trains of short pulses reduced fat absorption, i.e., more TG was recovered from the distal jejunum with IES. Similar to its effect on intestinal transit, IES with trains of short pulses was found to be more effective than that with long pulses in reducing fat absorption. In a previous study, Layzell and Collin (28) reported that postprandial backward IES induced an increase in body weight and a decrease in fecal fat and nitrogen losses during the test period in a canine model of short bowel syndrome. The enhanced enteric absorption with backward IES was mediated, in part, by an α-adrenergic mechanism (29). Forward IES was previously reported to slightly decrease the output of water, glucose, and sodium from the jejunal segment (30). However, the effect of forward IES on fat absorption has never been reported. Accelerated transit induced by IES shortened the time of contact between jejunal epithelial cells and fat solution; this might be partially responsible for the reduction of fat absorption. However, there was a lack of one-to-one relationship between jejunal transit and TG recovery. This phenomenon seems to suggest that transit and absorption are associated but not correlated one-to-one with each other and that different mechanisms may be involved with the accelerated transit and reduced fat absorption. Intestinal transit is closely related to intestinal motility that is integrated and modulated by intrinsic (in the gut wall) or extrinsic nerves and muscle contraction, whereas fat absorption is more dependent on the function of the intestinal absorption cells. For example, the small intestine contains three distinct proteins related to the intracellular lipid binding protein family: the liver-type fatty acid binding protein, the intestinal fatty acid binding protein, and the ileal lipid binding protein (49). Recently, a novel monoacylglycerol acyltransferase (MGAT), designated MGAT3, was identified (50). MGAT catalyzes the synthesis of diacylglycerol using 2-monoacylglycerol and fatty acylcoenzyme A. This enzymatic reaction is believed to be an essential and rate-limiting step for the absorption of fat in the small intestine. How electric stimulation affects the activity of enteric nerves, intestinal proteins, and enzymes would be the subjects of further research.
Our results also showed that the total amount of fatty acids was significantly lower in the IES group with trains of short pulses plus lidocaine than in the IES group with trains of short pulses but without lidocaine, suggesting the involvement of enteric nerves. Nevertheless, we found that IES with either long pulses or trains of short pulses had no significant effects on the output of fatty acids. Once TG was perfused into the testing segment, part of it would be broken down into monoglycerides and fatty acids, and the remaining part would be expelled out of the segment. The accelerated intestinal transit with IES was expected to increase the output of fatty acids from the testing segment. On the other hand, however, the accelerated transit with IES reduced the time for TG to be broken down as fatty acids, i.e., fewer fatty acids were produced with IES. Obviously, these two effects were opposite and, thus, could lead to a nonsignificant or inconsistent result regarding the percentage recovery of fatty acids from the distal cannula.
The mechanisms behind the effects of electric stimulation on transit and fat absorption are unclear. Some studies have suggested that the effect of GES on gastric motility is myogenic, whereas its effect on symptoms is neurogenic and is possibly mediated through the vagal afferent pathway (51,52). One study showed that α-blockade with phentolamine or celiac and superior mesenteric ganglionectomy, but not β-blockade or vagotomy, mediated the enhanced absorption with backward IES (29). The role of enteric nerves has not been examined. To elucidate whether enteric nerves are involved with IES we dropped lidocaine, a local anesthesia, on the surface of the jejunum in the group with trains of short pulses. Previous morphological studies with 14C-labeled lidocaine have demonstrated that lidocaine dropped onto the small intestine of the rat penetrated into the muscle layer of the intestinal wall and inhibited activity of the myoenteric nerve plexus (31). In this study, we applied lidocaine in the same manner and found that lidocaine partially abolished the effect of IES on jejunal transit and fat absorption. This suggests, for the first time, the involvement of enteric nerves with IES with trains of short pulses.
The reduced fat absorption with IES strongly suggests that IES may have a great potential for the treatment of obesity, which affects >60% of the population in the United States (53). Obesity has raised both national and international attention because of its detrimental impact on health and the enormous economic burden it imposes (54). Although there are multiple therapeutic regimens for obesity, including reduced-energy diets, physical activity/exercise, behavior modification (55), pharmacotherapy (3,4), and surgery (5), none of them is completely satisfactory. Diets, behavior modification, and pharmacotherapies are effective only in the short term. Although surgical therapies are effective in reducing weight in the long term, their applications are limited due to their morbidity, mortality, and complications. There is a great need to explore new therapeutic options. Electric stimulation may play a role in the future treatment of obesity. The efficacy of GES for obesity has been under intensive clinical investigation. Promising data have been reported in a number of clinical trials (56,57). The data in this current study indicates that electric stimulation of the small intestine is also of great potential for the treatment of obesity.
In conclusion, IES accelerates intestinal transit and reduces fat absorption, suggesting a therapeutic potential for obesity. IES with trains of short pulses is more effective than IES with long pulses, and its effects on intestinal transit and fat absorption are partially mediated by enteric nerves.
Acknowledgment
This work was partially supported by NIH Grant 1R43-DK063733-01.
Footnotes
According to U.S. code, all journals requesting payment of author page charges in order to defray the cost of publication are required to publish a disclaimer. This article must, therefore, be marked “advertisement” in compliance with U.S.C. Section 1734 solely to indicate this fact.
Nonstandard abbreviations: IES, intestinal electric stimulation; TG, triglyceride; GES, gastric electric stimulation; MGAT, monoacylglycerol acyltransferase.
References
- 1.World Health Organization http://www.who.int/nut/obs.htm. Controlling the Global Obesity Epidemic: 2002. accessed September 3, 2003.
- 2.Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530–8. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 3.Bray GA, Tartaglia LA. Medicinal strategies in the treatment of obesity. Nature. 2000;404:672–7. doi: 10.1038/35007544. [DOI] [PubMed] [Google Scholar]
- 4.Klein S. The war against obesity: attacking a new front. Am J Clin Nutr. 1999;69:1061–3. doi: 10.1093/ajcn/69.6.1061. [DOI] [PubMed] [Google Scholar]
- 5.NIH conference Gastrointestinal surgery for severe obesity: Consensus Development Conference Panel. Ann Intern Med. 1991;115:956–61. [PubMed] [Google Scholar]
- 6.Field FJ, Kam NT, Mathur SN. Regulation of cholesterol metabolism in the intestine. Gastroenterology. 1990;99:539–51. doi: 10.1016/0016-5085(90)91040-d. [DOI] [PubMed] [Google Scholar]
- 7.Lutton C, Ferezou J, Serougne C, et al. Critical analysis of the use of 14C-acetate for measuring in vivo rat cholesterol synthesis. Reprod Nutr Dev. 1990;30:71–84. doi: 10.1051/rnd:19900107. [DOI] [PubMed] [Google Scholar]
- 8.Helgerud P, Saarem K, Norum KR. Acyl-CoA-cholesterol acyltransferase in human small intestine: its activity and some properties of the enzymatic reaction. J Lipid Res. 1981;22:271–7. [PubMed] [Google Scholar]
- 9.Wu A, Windmueller HG. Identification of circulating apolipoproteins synthesized by rat small intestine in vivo. J Biol Chem. 1978;253:2525–8. [PubMed] [Google Scholar]
- 10.Glickman RM, Green PHR. The intestine as a source of apolipoprotein A-I. Proc Natl Acad Sci USA. 1977;74:2569–73. doi: 10.1073/pnas.74.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green PHR, Glickman RM, Saudek CD, Blum CB, Tall AR. Human intestinal lipoproteins: studies in chyluric subjects. J Clin Invest. 1979;64:233–42. doi: 10.1172/JCI109444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rachmilewitz D, Albers JJ, Saunders DR, Fainaru M. Apoprotein synthesis by human duodenojejunal mucosa. Gastroenterology. 1978;75:677–82. [PubMed] [Google Scholar]
- 13.Fordtran JS, Soergel KH, Ingelfinger FJ. Intestinal absorption of D-xylose in man. N Engl J Med. 1962;267:274–9. doi: 10.1056/NEJM196208092670602. [DOI] [PubMed] [Google Scholar]
- 14.Schiller LR, Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Studies of the mechanism of the antidiarrheal effect of codeine. J Clin Invest. 1982;70:999–1008. doi: 10.1172/JCI110711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Read NW. Diarrhee motrice. Clin Gastroenterol. 1986;15:657–86. [PubMed] [Google Scholar]
- 16.Sababi M, Bengtsson UH. Enhanced intestinal motility influences absorption in anaesthetized rat. Acta Physiol Scand. 2001;172:115–22. doi: 10.1046/j.1365-201X.2001.00849.x. [DOI] [PubMed] [Google Scholar]
- 17.Huge A, Weber E, Ehrlein HJ. Effects of enteral feedback inhibition on motility, luminal flow, and absorption of nutrients in proximal gut of minipigs. Dig Dis Sci. 1995;40:1024–34. doi: 10.1007/BF02064192. [DOI] [PubMed] [Google Scholar]
- 18.Cullen JJ, Doty RC, Ephgrave KS, Hinkhouse MM, Broadhurst K. Changes in intestinal transit and absorption during endotoxemia are dose dependent. J Surg Res. 1999;81:81–6. doi: 10.1006/jsre.1998.5452. [DOI] [PubMed] [Google Scholar]
- 19.Eisenbraun J, Ehrlein HJ. Effects of somatostatin on luminal transit and absorption of nutrients in the proximal gut of minipigs. Dig Dis Sci. 1996;41:894–901. doi: 10.1007/BF02091528. [DOI] [PubMed] [Google Scholar]
- 20.Kelly KA, Code CF. Duodenal-gastric reflux and slowed gastric emptying by electrical pacing of the canine duodenal pacesetter potentials. Gastroenterology. 1977;72:429–33. [PubMed] [Google Scholar]
- 21.O'Connell PR, Kelly KA. Enteric transit and absorption after canine ileostomy: effect of pacing. Arch Surg. 1987;122:1011–7. doi: 10.1001/archsurg.1987.01400210049007. [DOI] [PubMed] [Google Scholar]
- 22.Miedema BM, Kelly KA. The Roux stasis syndrome: Treatment by pacing and prevention by use of an “uncut” Roux limb. Arch Surg. 1992;127:295–300. doi: 10.1001/archsurg.1992.01420030057011. [DOI] [PubMed] [Google Scholar]
- 23.Chen JD, Lin HC. Electrical pacing accelerates intestinal transit slowed by fat-induced ileal brake. Dig Dis Sci. 2003;48:251–6. doi: 10.1023/a:1021911023155. [DOI] [PubMed] [Google Scholar]
- 24.Soper NJ, Geisler KL, Sarr MG, Kelly KA, Zinsmeister AR. Regulation of canine jejunal transit. Am J Physiol. 1990;259:G928–33. doi: 10.1152/ajpgi.1990.259.6.G928. [DOI] [PubMed] [Google Scholar]
- 25.Sarr MG, Kelly KA, Gladen HE. Electrical control of canine jejunal propulsion. Am J Physiol. 1981;240:G355–60. doi: 10.1152/ajpgi.1981.240.5.G355. [DOI] [PubMed] [Google Scholar]
- 26.Collin J, Kelly KA, Phillips SF. Increased canine jejunal absorption of water, glucose, and sodium with intestinal pacing. Am J Dig Dis. 1978;23:1121–4. doi: 10.1007/BF01072888. [DOI] [PubMed] [Google Scholar]
- 27.Sawchuk A, Nogami W, Goto S, et al. Reverse electrical pacing improves intestinal absorption and transit time. Surgery. 1986;100:454–60. [PubMed] [Google Scholar]
- 28.Layzell T, Collin J. Retrograde electrical pacing of the small intestine-a new treatment for the short bowel syndrome? Br J Surg. 1981;68:711–3. doi: 10.1002/bjs.1800681012. [DOI] [PubMed] [Google Scholar]
- 29.Bjorck S, Kelly KA, Philips SF. Mechanisms of enhanced canine enteric absorption with intestinal pacing. Am J Physiol. 1987;252:G548–53. doi: 10.1152/ajpgi.1987.252.4.G548. [DOI] [PubMed] [Google Scholar]
- 30.Collin J, Kelly KA, Phillips SF. Absorption from the jejunum is increased by forward and backward pacing. Br J Surg. 1979;66:489–92. doi: 10.1002/bjs.1800660712. [DOI] [PubMed] [Google Scholar]
- 31.Cassuto J, Siewert A, Jodal M, Lundgren O. The involvement of intramural nerves in cholera toxin induced intestinal secretion. Acta Physiol Scand. 1983;117:195–202. doi: 10.1111/j.1748-1716.1983.tb07197.x. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Fihn B-M, Jodal M, Sjovall H. Effects of neural blocking agents on motor activity and secretion in the proximal and distal rat colon: evidence of marked segmental differences in nicotinic receptor activity. Scand J Gastroenterol. 2000;35:380–8. doi: 10.1080/003655200750023949. [DOI] [PubMed] [Google Scholar]
- 33.Van De Kamer JH, Bokkel Huinink HT, Weyers HA. Rapid method for the determination of fat in feces. J Biol Chem. 1949;177:347–55. [PubMed] [Google Scholar]
- 34.Abo M, Liang J, Qian L, Chen JD. Distension-induced myoelectrical dysrhythmia and effect of intestinal pacing in dogs. Dig Dis Sci. 2000;45:129–35. doi: 10.1023/a:1005425814046. [DOI] [PubMed] [Google Scholar]
- 35.Abo M, Kono T, Lu CL, Chen JD. Effects of caffeine on gastrointestinal myoelectric activity and colonic spike activity in dogs. Scand J Gastroenterol. 2000;35:368–74. doi: 10.1080/003655200750023921. [DOI] [PubMed] [Google Scholar]
- 36.Abo M, Kono T, Wang Z, Chen JD. Intestinal inhibitory reflexes: effect of distension on intestinal slow waves. Dig Dis Sci. 2001;46:1177–85. doi: 10.1023/a:1010642708258. [DOI] [PubMed] [Google Scholar]
- 37.Heinemann T, Axtmann G, Van Bergmann K. Comparison of intestinal absorption of cholesterol with different plant sterols in man. Eur J Clin Invest. 1993;23:827–31. doi: 10.1111/j.1365-2362.1993.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 38.Turnberg LA, Riley SA. Digestion and absorption of nutrients and vitamins. In: Sleisenger MH, editor. Gastrointestinal Disease. 5th W.B. Saunders Company; Philadelphia, PA: 1993. pp. 977–1008. [Google Scholar]
- 39.Karlstrom L, Kelly KA. Ectopic jejunal pacemakers and gastric emptying after Roux gastrectomy: effect of intestinal pacing. Surgery. 1989;106:867–71. [PubMed] [Google Scholar]
- 40.Lin ZY, McCallum RW, Schirmer BD, Chen JDZ. Effects of pacing parameters in the entrainment of gastric slow waves in patients with gastroparesis. Am J Physiol. 1998;274:G186–91. doi: 10.1152/ajpgi.1998.274.1.G186. [DOI] [PubMed] [Google Scholar]
- 41.Lin XM, Peters LJ, Hayes J, Chen JDZ. Entrainment of segmental small intestinal slow waves with electrical stimulation in dogs. Dig Dis Sci. 2000;45:652–6. doi: 10.1023/a:1005466904380. [DOI] [PubMed] [Google Scholar]
- 42.McCallum RW, Chen JDZ, Lin ZY, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114:456–61. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- 43.McCallum RW, Hocking M, Koch KL, et al. Efficacy of chronic GES in the treatment of symptomatic gastroparesis: results at 6 and 12 months from the VAWESS study. Am J Gastroenterol. 2000;95:2470. [Google Scholar]
- 44.Qian LW, Peters LJ, Chen JDZ. Effects of different electric stimulations on vasopressin-induced motion sickness-like symptoms and gastric slow wave abnormalities in dogs. Dig Dis Sci. 1999;44:2154. [Google Scholar]
- 45.Qian LW, Peters LI, Chen JDZ. Effects of electroacupuncture on gastric migrating myoelectrical complex in dogs. Dig Dis Sci. 1999;44:56–62. doi: 10.1023/a:1026645931867. [DOI] [PubMed] [Google Scholar]
- 46.Chen JD, Qian L, Ouyang H, Yin J. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124:401–9. doi: 10.1053/gast.2003.50048. [DOI] [PubMed] [Google Scholar]
- 47.Mintchev MP, Sanmiguel CP, Amaris M, Bowes KL. Microprocessor-controlled movement of solid gastric content using sequential neural electrical stimulation. Gastroenterology. 2000;118:258–63. doi: 10.1016/s0016-5085(00)70207-1. [DOI] [PubMed] [Google Scholar]
- 48.Amaris MA, Rashev PZ, Mintchev MP, Bowes KL. Microprocessor controlled movement of solid colonic content using sequential neural electrical stimulation. Gut. 2002;50:475–9. doi: 10.1136/gut.50.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agellon LB, Toth MJ, Thomson AB. Intracellular lipid binding proteins of the small intestine. Mol Cell Biochem. 2002;239:79–82. [PubMed] [Google Scholar]
- 50.Cheng D, Nelson TC, Chen J, et al. Identification of acylcoenzyme A: monoacylglycerol acyltransferase 3, an intestinal specific enzyme implicated in dietary fat absorption. J Biol Chem. 2003;18(278):13611–4. doi: 10.1074/jbc.C300042200. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Qian L, Ueno T, Chen J. Mechanisms of various gastric electrical stimulations. Gastroenterology. 2000;118:A669. abstr. [Google Scholar]
- 52.Tack J, Coulie B, Van Cutsem E, Ryden J, Janssens J. The influence of gastric electric stimulation on proximal gastric motor and sensory function in severe idiopathic gastroparesis. Gastroenterology. 1999;116:A1090. [Google Scholar]
- 53.Hatahet MA, Dhurandhar NV. Antiobesity drugs: current and future issues. Curr Diab Rep. 2002;2:409–15. doi: 10.1007/s11892-002-0105-3. [DOI] [PubMed] [Google Scholar]
- 54.Wolf AM, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998;6:97–106. doi: 10.1002/j.1550-8528.1998.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 55.Wadden TA, Foster GD. Behavioral treatment of obesity. Med Clin North Am. 2000;84:441–61. doi: 10.1016/s0025-7125(05)70230-3. [DOI] [PubMed] [Google Scholar]
- 56.Cigaina V. Gastric pacing as therapy for morbid obesity: preliminary results. Obes Surg. 2002;12:12–6s. doi: 10.1007/BF03342141. [DOI] [PubMed] [Google Scholar]
- 57.D'argent J. Gastric electrical stimulation as therapy of morbid obesity: preliminary results from the French study. Obes Surg. 2002;12:21–5s. doi: 10.1381/096089202762552638. [DOI] [PubMed] [Google Scholar]


