Abstract
Medroxyprogesterone acetate (MPA) commonly is used in contraception and hormone replacement therapy. However, little is known about its effects within the central nervous system. Using ovariectomized pigtail macaques (Macaca nemestrina), we evaluated the potential for MPA to antagonize estradiol (E2) effects on female sociosexual behavior. Subjects (n = 6) were treated sequentially with placebo, E2 alone, E2 + progesterone (P4), and E2 + MPA. The order of treatments was balanced among subjects, and equimolar quantities of P4 and MPA were administered. During each treatment period, female sexual initiation rates, anxiety-related behavior, and aggression were recorded. Treatment with E2 alone induced a substantial rise in female sexual initiation rates. Although concurrent P4 treatment failed to significantly inhibit sexual behavior, MPA treatment markedly antagonized E2’s effects. Neither the E2-only nor the E2 + P4 treatment had an impact on aggression rates, but the E2 + MPA treatment induced a significant rise in this behavior. Both MPA and P4 counteracted the effect of E2 on measures of anxiety. These findings suggest that MPA antagonizes certain behavioral effects of E2 that may be beneficial to women, and that it does so more profoundly or in ways that endogenous P4 does not. The marked increase in aggression seen during MPA treatment suggests that production of negative affect may be a particularly serious side effect of MPA.
Abbreviations: CEE, Conjugated equine estrogens; DMSO, dimethylsulfoxoide; E2, estradiol; GABA, γ-aminobutyric acid; MPA, medroxyprogesterone acetate; P4, progesterone
Medroxyprogesterone acetate (MPA) commonly is used both in contraception and hormone replacement therapy. Like other progestogens, MPA counteracts the proliferative effects of estrogen treatment in the uterus and protects against endometrial hyperplasia (1, 2). By contrast, in the breast, there is a growing consensus that the addition of MPA and other progestogens increases mammographic density and breast cancer risk beyond that seen through unopposed estrogen use (3–7) (but see Ref. 8). However, because of the cell cycle and pathway-dependent effects of progestogens (4, 9), they may have apoptotic as well as proliferative actions in mammary tissue (3, 4, 7, 8). Moreover, these effects may vary with the type of progestogen used (10–13). Thus, although some in vitro studies have shown that MPA inhibits growth within certain cellular contexts (11, 13–16), in vivo studies in monkeys and humans suggest that, overall, MPA enhances proliferative estrogen effects in mammary tissue (17–19) and that it may do so to a greater extent than some other progestogens (20).
Compared with what is known about the effects of MPA in uterine and mammary tissue, very little is known about the impact of MPA on estrogen’s actions within the central nervous system. However, some initial studies suggest that, within certain neurochemical systems, MPA has antagonistic properties that differ from those of endogenous progesterone (P4). In the dorsal raphe of macaques, P4 has little impact on estradiol’s (E2’s) ability to augment levels of tryptophan hydroxylase, the rate limiting enzyme in serotonin synthesis, but MPA significantly antagonizes the effects of conjugated equine estrogens (CEE) (21). Similarly, in cultured hippocampal neurons, P4 only slightly diminishes E2’s ability to potentiate the glutamate-mediated rises in intracellular Ca2+ that have been implicated in the enhancement of learning and memory; moreover, P4 actually enhances E2’s ability to protect against glutamate toxicity. By contrast, MPA markedly inhibits both of these E2-mediated actions (22, 23). Finally, with respect to cholinergic function, long-term treatment of macaques with CEE + MPA, but not CEE alone, reduces choline acetyltransferase and acetylcholinesterase activity in the medial septum and the diagonal band of Broca, areas of major cholinergic innervation to the hippocampus and cerebral cortex (24).
In contrast to the molecular studies clearly illustrating the antagonistic properties of MPA within certain neurochemical systems, the psychological effects of MPA are poorly understood. Although data from several observational studies on the use of MPA in contraception have shown a tendency for women to complain of mood changes, depression, and loss of libido, other such studies have failed to find these effects, and the role of MPA in the etiology of these symptoms has been debated (25). For use in hormone replacement therapy, a number of double-blind studies using a placebo-controlled or crossover design have shown that MPA is associated with a decline in libido and a rise in anxiety, irritability, and depression during the progestogen phase of cyclic regimes (26–28). Nonetheless, other controlled studies have found that MPA does not antagonize the effects of estrogen treatment (29), and that its use in hormone replacement therapy may result in more favorable mood outcomes than seen through the use of formulations containing other progestogens (28, 30, 31).
To overcome the difficulties associated with studying changes in mood and libido in women, we have used pigtail macaques (Macaca nemestrina) in a monkey model for evaluating the effects of MPA on behavior. Through the use of an animal model, we eliminated the need for subjective assessments and retrospective reports. Instead, we quantified the spontaneous expression of relevant behaviors and used these measures as proxies for emotional states that women experience. Macaques were chosen because they share many aspects of reproductive biology with women, including an approximately 28-d cycle with a nearly identical pattern of hormonal fluctuation (32). Moreover, female macaques maintain complex social networks (33), and, as in other primates, including women, gonadal hormones modulate sexual motivation rather than the ability to copulate (34). Given these similarities, we used a macaque model to test the hypothesis that MPA would attenuate the effects of E2 replacement therapy on sociosexual behavior in ovariectomized females.
Subjects and Methods
Subjects and housing
Study subjects were six ovariectomized pigtail macaques (Macaca nemestrina) living in a stable social group consisting of one adult male and 22 adult females and their dependent offspring. The group was housed at the Field Station of the Yerkes National Primate Research Center, Emory University, Lawrenceville, GA. The enclosure for the group consisted of an outdoor compound (~230 m2) with an attached indoor area (~16.5 m2). To minimize dominance effects, the two top- and bottom-ranking individuals in this social group were avoided for use as subjects. All subjects were parous but had undergone bilateral ovariectomy 6 months before the start of this study. All research was conducted in accordance with the National Institutes of Health and United States Department of Agriculture guidelines and standards and was approved by the Emory University Institutional Animal Care and Use Committee.
Hormone treatments
Subjects were tested from October 2002 through July 2003 under the following conditions: 1) placebo; 2) 17β-E2 alone; 3) E2 + P4; and 4) E2 + MPA. All subjects were pretested initially under the placebo condition, but the order of all subsequent treatments was balanced among subjects. Each hormone treatment was administered for 1 wk and was separated by a minimum 3-wk washout period.
E2 was administered through the use of SILASTIC (Dow Corning Corp., Midland, MI) capsules (3 × 4.5 cm; inside diameter, 3.35 mm; outside diameter, 4.65 mm) containing crystalline 17β-E2 (Product E-8875, Sigma-Aldrich, St. Louis, MO). MPA (Product M-1629, Sigma-Aldrich) was dissolved in a vehicle of dimethylsulfoxide (DMSO) and infused via osmotic minipump (2ML/1, Alza Corp., Palo Alto, CA) at a rate of 160 μg/kg·d. This dose was selected to mimic circulating levels women experience when using MPA in contraception and hormone replacement therapy. The concentration of MPA delivered ranged from 4.7–6.7 mg/ml to compensate for differences in each subject’s body weight. P4 (Product P-3972, Sigma-Aldrich) was delivered similarly via osmotic minipump in a vehicle of DMSO. A dose of 130 μg/kg·d was used as the molar equivalent of the MPA treatment and was delivered at a concentration of 3.8–5.4 mg/ml. Both the SILASTIC capsules and the osmotic minipumps were implanted sc in the space between the scapulae while subjects were under general anesthesia (ketamine hydrochloride, 10 mg/kg, im).
Behavioral testing
Subjects were observed on d 3–7 of each treatment period. During behavioral testing, the social group was locked into the outdoor area of its enclosure and observed from a tower with an unobstructed view of the entire compound. Data were entered directly into a personal Data Assistant (III XE, Palm Corp., Milpitas, CA) running a custom software program (HandObs, Center for Behavioral Neuroscience, Atlanta, GA) that captured event-sequential data with an elapsed time in thousandths of a minute from the test start. Postprocessing programs allowed for the extraction of frequencies, durations, and sequences of behavior in real time.
Two different sets of observations were carried out each day: one set in which the subjects’ social group remained intact, and another in which the adult male was temporarily removed. Female sexual initiation rates were recorded during the observations with the adult male in the group, whereas other behaviors were monitored during the observations with the adult male separated. This separation was used to control for differences in behavior attributable to sexual competition rather than underlying differences in hormonal effects on behaviors related to anxiety states. The order of the two observation types was alternated each day so that the recording of sexual behavior was balanced, taking place before the male’s separation in some cases and following his return in others.
For the observations focusing on sexual behavior, the adult male was followed for 1 h. During this period, all sexual initiations that study subjects directed toward him were recorded. Copulations also were noted, but were not included in the data analysis because they were thought to reflect the interaction between male and female sexual interest, and we were interested specifically in hormonal effects on female sexual motivation. For the behavior sampling that took place while the male was out of the group, the study subjects were ordered randomly each day and followed for a half-hour focal observation period. During these observations, all cases of aggression, self-scratching, and the amount of time spent locomoting were recorded. Aggression was used as a measure of irritability and the propensity to engage in conflict due to an underlying state of anxiety. However, because changes in other motivational states could lead to an increase in aggressive behavior, we also recorded rates of self-scratching, an established measure of anxiety in nonhuman primates (35). This behavior pattern has been found to increase in situations of psychosocial stress (36–41), and it is selectively enhanced or reduced through the respective administration of anxiogenic and anxiolytic drugs (36, 42–45). Finally, because at high levels progestogens can have a sedative effect (46, 47), we monitored the amount of time subjects spent locomoting under each of the treatments. We chose this behavior because it is a good indicator of overall activity levels and has been used as a measure of general sedation in nonhuman primates (43, 48, 49).
Behavioral definitions for female sexual initiations and aggression followed those established in Short et al. (50), whereas the definitions for self-scratching and locomotion followed those established in Schino et al. (43). Sexual initiating behaviors included approach to within 1 m of the male (proximity), presentation of hindquarters (present), and individualized stereotyped behaviors for gaining the male’s attention (solicitations). Aggressive behaviors included open-mouth stares and non-contact lunges (threats), slapping of other individuals (hit), vigorous swaying movements and shaking of physical substrates (display), the rapid antagonistic pursuit of other individuals (chase), and aggressive contact, including biting, grabbing, pulling hair, and restraining others (attack). Self-scratching was defined as commonly observed in humans with a rapid repetitive movement of the hand or foot. Locomotion included walking, running, and climbing. All behaviors were recorded by a single observer, and thus interrater reliability measures have not been reported.
Hormone analyses
Blood samples were collected from each subject between 0830 and 1330 h on d 3, 5, and 7 of each treatment period. Samples were obtained by saphenous venipuncture while subjects were unanesthetized and held under light hand restraint. For this procedure, subjects were trained to go into the indoor area of their compound and enter a transfer box. The transfer box then was used to place individuals in a cage with a small opening through which they could extend a leg for blood collection. Subjects were well accustomed to this procedure, which has no adverse effects on the reproduction or behavior of habituated animals (51)—handling time before collection of the blood sample typically was under 1 min for each animal, and subjects were released quickly into their group after blood collection. Subsequently, the blood samples were centrifuged, and the plasma was frozen at −20 C for later analysis.
Commercially available RIA kits (Diagnostic Products Corp., Los Angeles, CA) were used for the analysis of E2 and P4, with the addition of an extraction step (diethyl ether with 2% ethanol, J. T. Baker Pharmaceuticals, Phillipsburg, NJ). Use of the P4 assay kit without extraction has been established previously (52), but examination of the E2 kit has indicated that the addition of the extraction step markedly improves assay performance (53). Using 200-μl aliquots, the E2 assay has a sensitivity of 2.5 pg/ml and an upper limit of 500 pg/ml. The intraassay CV was 7.95%, and the interassay CV was 12.30% at 11.50 pg/ml and 4.50% at 96.23 pg/ml. Using 100-μl aliquots, the P4 assay has a sensitivity of 0.1 ng/ml and an upper limit of 40 ng/ml. The intraassay CV was 8.44%, and the interassay CV was 14.7% at 1.58 ng/ml and 11.67% at 12.01 ng/ml. MPA was analyzed using a commercially available kit (Immunometrics Ltd., London, UK). Using 200-μl aliquots, this assay has a sensitivity of 0.08 ng/ml and an upper limit of 2.5 ng/ml. The intraassay CV was 7.79%, and the interassay CV was 9.54% at 0.61 ng/ml and 2.47% at 2.27 ng/ml.
Statistical analyses
Differences in behavior and hormone concentration across treatments were analyzed with ANOVA models for repeated measures using a log transformation for the analysis of behavioral data to control for non-homogeniety of variance. Post hoc analyses, using the Bonferroni test to adjust the observed significance level for multiple comparisons, were conducted in comparing each mean to the other means in a series and identifying significant main effects of categorical variables. Statistical analyses were performed using SPSS software (version 11.5; SPSS, Inc., Chicago, IL), and tests having a P value of 0.05 or less were considered significant.
In addition to determining whether differences between means were statistically significant, we evaluated the magnitude of difference between means by computing Cohen’s d as an indicator of effect size (41). Cohen’s d is a measure of explained variance that describes the magnitude of a difference between means in sd units. It is calculated as the difference between means divided by the sd of the means. By convention, d = 0.2 indicates a small effect, d = 0.5 indicates a moderate effect, and d > 0.8 indicates a large effect (54). A measure of effect size provides an important complement to traditional data testing because of the possibility for finding a statistically significant difference that represents only a small degree of variance, which is of less theoretical interest than a significant finding that reflects a large magnitude of change (d > 0.8).
To confirm the absence of order effects, we used a one-way ANOVA to determine whether overall rates of behavior remained constant across the first, second, and third treatment periods; in the absence of treatment carryover effects, rates of behavior were expected to remain constant because the same number of subjects was placed on each of the treatments during all of the test periods. For ease of interpretation, means ± sem for our behavioral measures have been reported and presented graphically using the original rather than the log-transformed data. Individual data points have been presented in tabular form.
Results
Serum hormone levels varied across the four treatment conditions for E2 (F3,15 = 57.8, P < 0.001) and P4 (F3,15 = 29.3, P < 0.001). MPA was below the limit of detection except when it was administered during the E2 + MPA condition (Table 1). Post hoc analyses for serum E2 levels revealed that placebo values differed from those obtained during the other treatment periods (placebo vs. all other treatments, P ≤0.001). Serum P4 values from the E2 + P4 treatment condition were significantly higher than those obtained during all other treatment periods (E2 + P4 vs. all other treatments, P < 0.03), with effect size analyses revealing the greatest elevation relative to the placebo condition (E2 + P4 vs. placebo, Cohen’s d = 3.88; E2 + P4 vs. E2-only, Cohen’s d = 1.74; E2 + P4 vs. E2 + MPA, Cohen’s d = 1.56).
TABLE 1.
Serum concentrations of 17β-E2, P4, and MPA by treatment period
| Treatment period
|
||||
|---|---|---|---|---|
| Hormone measured | E2 only | E2 + P4 | E2 + MPA | Placebo |
| E2 (pg/ml) | 114 ± 10a | 107 ±3.4a | 103 ± 8.4a | 8.4 ± 1.1 |
| P4 (ng/ml) | 1.0 ±0.9b | 1.9 ± 0.2a | 1.1 ± 0.1b | 0.7 ± 0.1 |
| MPA (ng/ml) | <0.08 | <0.08 | 0.9 ± 0.5 | <0.08 |
Exceeds the placebo condition at the level of P < 0.001.
Exceeds the placebo condition at the level of P < 0.05.
Across successive test periods, there was no difference in overall rates of sexual initiations (F2,15 = 0.098; P = 0.91), aggression (F2,15 = 1.06, P = 0.37), or self-scratching (F2,15 = 0.14, P = 0.88), suggesting a lack of carryover effects. Moreover, the hormone treatments had no influence on locomotion rates (F3,18 = 1.415; P = 0.27), indicating that a general sedation effect could not account for changes in our measures intended as proxies for underlying emotional states.
Female sexual initiation rates varied significantly across the treatment conditions (F3,15 = 20.4, P < 0.001; Fig. 1 and Table 2). Post hoc analyses revealed that the E2-only treatment induced a rise in female sexual initiation rates relative to the placebo condition (P = 0.016). The addition of P4 failed to significantly attenuate the effect of E2 on female sexual initiations (P = 0.276), although rates of behavior during the E2 + P4 condition also failed to rise above those observed under the placebo condition (P = 0.181). By contrast, the MPA + E2 treatment significantly attenuated rates of female sexual behavior, both in comparison with the E2-only (P = 0.001) and the E2 + P4 condition (P = 0.038). The magnitude of the inhibitory effect on female sexual initiations also was much greater for MPA compared with P4; the effect size was moderate for the difference in initiation rates between the E2-only vs. the E2 +P4 condition (Cohen’s d = 0.62), whereas it was quite large for the difference in initiation rates between the E2-only vs. the E2 + MPA condition (Cohen’s d = 2.56).
Fig. 1.
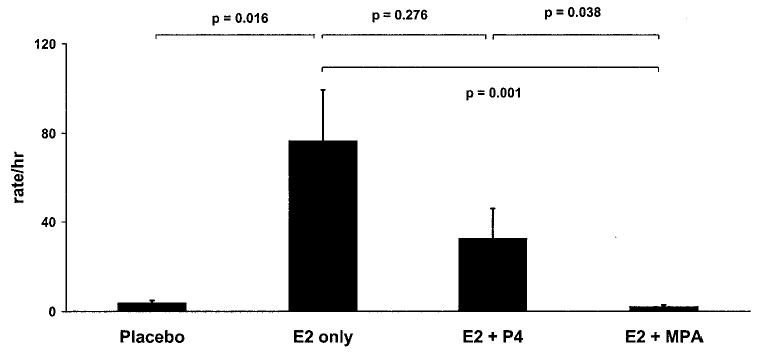
Female sexual initiation rates. Treatment with E2 alone increased female sexual initiation rates. Cotreatment with P4 diminished this effect somewhat, whereas a molar equivalent dose of MPA markedly antagonized E2’s actions.
TABLE 2.
Individual subject scores for behavioral measuress
| Treatment period
|
|||||
|---|---|---|---|---|---|
| Behavior (rate/h) | Subject | E2 only | E2 + P4 | E2 + MPA | Placebo |
| Sexual initiations | 1 | 61.9 | 16.8 | 4.0 | 2.0 |
| 2 | 166.4 | 11.5 | 4.8 | 6.0 | |
| 3 | 44.0 | 34.1 | 1.0 | 7.2 | |
| 4 | 2.0 | 0.8 | 0.0 | 1.1 | |
| 5 | 89.5 | 40.5 | 2.3 | 4.3 | |
| 6 | 96.8 | 92.0 | 0.5 | 3.2 | |
| Aggression | 1 | 0.6 | 5.3 | 2.5 | 1.5 |
| 2 | 1.2 | 3.2 | 8.7 | 5.3 | |
| 3 | 1.3 | 1.2 | 1.3 | 1.0 | |
| 4 | 2.0 | 2.5 | 4.8 | 2.0 | |
| 5 | 5.3 | 2.4 | 9.5 | 5.0 | |
| 6 | 0.6 | 2.0 | 2.4 | 1.0 | |
| Self-scratching | 1 | 8.4 | 51.3 | 32.2 | 14.5 |
| 2 | 6.6 | 18.3 | 13.3 | 21.8 | |
| 3 | 2.5 | 7.8 | 21.3 | 15.0 | |
| 4 | 2.0 | 3.8 | 12.6 | 11.0 | |
| 5 | 10.0 | 16.2 | 13.3 | 10.0 | |
| 6 | 13.3 | 16.7 | 34.2 | 34.4 | |
The significant variation in aggression rates across conditions (F3,15 = 4.2, P = 0.023; Fig. 2 and Table 2) reflected the large increase in behavior induced through MPA treatment. Rates of aggression failed to differ from the placebo condition during the E2-only (P = 1.00) or the E2 + P4 treatment period (P = 1.00). By contrast, the addition of MPA induced a significant rise in behavior relative to the placebo condition (P = 0.007), with an effect size indicating a large magnitude of change (Cohen’s d = 0.80). Aggression rates did not differ significantly between the E2 + MPA and the E2 + P4 treatment (P = 1.0), and the effect size indicated a moderate difference (Cohen’s d = 0.66).
Fig. 2.
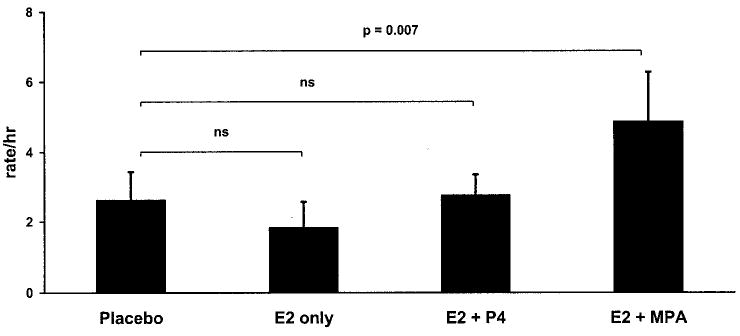
Aggression rates. Neither treatment with E2 alone nor E2 + MPA impacted aggression rates, but the addition of MPA to E2 induced a significant rise in behavior. ns, Not significant.
The hormonal treatments also had a significant effect on rates of self-scratching behavior (F3,15 = 7.51, P = 0.003; Fig 3 and Table 2). The E2-only treatment induced a downward trend in self-scratching relative to the placebo condition, but this difference failed to reach significance (P = 0.086). This effect was countered by both progestogen treatments, although the increase relative to the E2-only treatment reached significance under the MPA + E2 (P = 0.050) but not the P4 + E2 condition (P = 0.072). Moreover, the effect size for the E2-only vs. the E2 + MPA treatment (Cohen’s d = 1.91) was more than 75% larger than the effect size for the E2-only vs. the E2 + P4 treatment (Cohen’s d = 1.07).
Fig. 3.
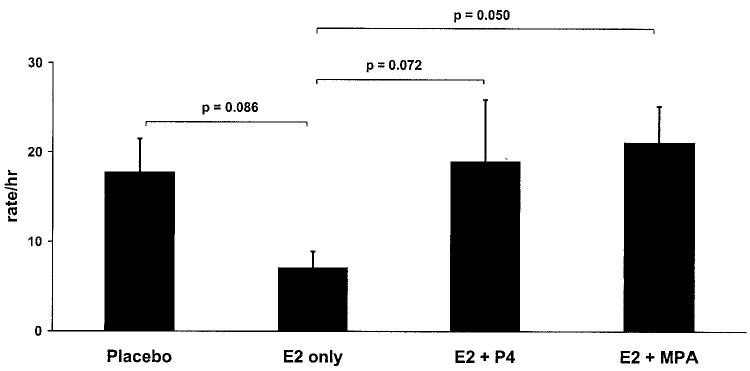
Self-scratching rates. Both P4 and MPA countered the ameliorative effect of E2 on self-scratching, an established measure of anxiety in non-human primates.
Discussion
In this study using a monkey model, MPA dramatically altered female sociosexual behavior, inducing certain undesirable effects and offsetting several beneficial behavioral actions of E2. Treatment with E2 alone induced a substantial rise in female sexual initiation rates. Although cotreatment with P4 did not significantly reduce the effects of E2, MPA markedly antagonized its actions. Both P4 and MPA counteracted the effects of E2 on self-scratching behavior as a measure of anxiety. By contrast, although neither E2 treatment alone or in combination with P4 impacted aggression rates, the addition of MPA induced a significant rise in this behavior. These behavioral changes suggest that MPA may disrupt the mood-elevating effects of estrogen treatment in women (3, 55), and that it may do so more profoundly or in ways that natural P4 does not.
The P4 levels we produced in this study were somewhat elevated under all of our experimental treatment as compared to our placebo condition. This rise in P4 among our ovariectomized subjects may be attributable to E2 stimulation of adrenal P4 output stemming from decreased P4 metabolism. Indeed, E2 treatment curtails the conversion of P4 to its reduced metabolite, allopregnanolone (56, 57). Therefore, under our experimental conditions, all of which included E2 treatment, P4 may have accumulated in its unchanged form; by contrast under our placebo condition, it may have been converted to a form that was easily eliminated or not detected with our assay. Nonetheless, irrespective of the mechanism behind this difference, the small rise in endogenous P4 was outweighed by the change we produced through our P4 treatments. Moreover, this relatively small increase induced through E2 treatment was present in all of our experimental conditions, yet it failed to prevent our E2-only treatment from inducing behavioral changes that were attenuated by the addition of exogenous P4, and especially MPA.
The serum levels of MPA and P4 that we did produce under the respective treatment periods are of clear biological relevance because they are less than, or equivalent to, the blood levels women experience on contraception and hormone replacement therapy. The mean circulating level of MPA for the females in our study was 0.92 ± 0.06 ng/ml. By comparison, long-acting injectable contraceptives routinely produce blood levels that reach 1 ng/ml and may even approach 2 ng/ml during the initial weeks after administration (58–60). Similarly, hormone replacement formulations containing a 5-mg oral dose of MPA commonly produce peak blood levels exceeding 1.5 ng/ml that may approach 2 ng/ml (61). For P4, the mean circulating concentration we produced during our E2 + P4 treatment was 1.9 ± 0.06 ng/ml. This level is again similar or somewhat lower than the serum concentrations women commonly experience when using P4 in hormone replacement—whereas the use of a 45-mg dose of transvaginal gel produces somewhat higher blood concentrations, approaching 4 ng/ml (62), a 100-mg oral dose of micronized P4 has been found to produce peak blood levels of 1.0–2.5 ng/ml, when measured through assay protocols that eliminate cross-reactivity with the substantial quantity of conjugated metabolites produced by oral administration (63, 64). Hence, the MPA and P4 levels we produced among our subjects clearly were within the range that woman routinely experience on contraception and hormone replacement and may have generated similar effects on behavior and neurochemical function.
Although we did generate analogous conditions by closely approximating serum hormone levels, other aspects of our study limit the extent to which our findings can be used to infer that the use of MPA will have negative mood consequences for women. In particular, our subjects were treated over a limited period, and their behavior was monitored after they had only 3–7 d of hormone exposure. This period is far shorter than women experience through the use of continuous combined hormone replacement regimes and long-acting injectable contraceptives. Even monthly contraceptive pills and cyclic hormone replacement regimes expose women to progestogens for a more prolonged period. Hence, in the future, it will be important to evaluate the effects of MPA over the course of weeks and months to determine whether the changes it induces are temporary or chronic. Moreover, our findings must be interpreted carefully because of our need to rely on behavioral measures that are proxies rather than direct measures of emotional states. As such, these measures potentially can be influenced by factors other than changes in mood. In particular, both E2 and P4 receptors are present in the skin (65, 66), and although many women experience a decline in collagen synthesis at menopause, resulting in a thinning and drying out of the skin (67–69), estrogen replacement can restore skin quality and alleviate these symptoms (70–72). Thus it is possible that the reduction in scratching we observed during our E2-only treatment was due to the alleviation of dryness and itchiness of the skin, which was then countered through our progestogen treatments. However, estrogen treatment has been shown to improve skin quality over the course months (71–73), in contrast to the acute time period over which our observations were conducted. Moreover, self-scratching is a well-established measure of anxiety among nonhuman primates (35–45), and the increase in aggression we observed suggests that MPA, in fact, exerts effects within certain neurochemical systems.
Having shown that MPA can have a pronounced impact on behavior, the molecular mechanisms through which MPA exerts its effects must now be determined. Potentially, MPA simply could act as a potent agonist at progesterone receptors. The inhibition of sexual behavior we observed through progestogen treatment, in fact, was expected on the basis of prior research. Rodent studies have shown that acutely P4 stimulates sexual behavior through its action at E2-induced hypothalamic progesterone receptors; however through prolonged exposure, P4 exerts inhibitory effects by blocking the continued induction of its own receptor (74). Although this mechanism of inhibition has not been demonstrated in primates, research on monkeys has shown that the inhibitory effects of P4 are most pronounced when levels rise relative to E2 (75, 76). Given the potential for P4 to inhibit sexual behavior through its own receptor, MPA could exert even more pronounced effects by binding more strongly to relevant P4 receptors within the brain. Relative binding studies, in fact, have shown that, in comparison with P4, MPA has an equal or greater affinity for P4 receptors (77, 78). Moreover, the potential for MPA to bind to P4 receptors within relevant neurochemical systems is suggested by the finding in male macaques that both MPA and P4 accumulate in their unchanged form in cell nuclei of the hypothalamus and preoptic area, regions of the brain critical for the regulation of sexual behavior (79, 80). Nonetheless, most studies examining the biological potency of MPA once bound to P4 receptors have been conducted in peripheral tissues of the reproductive system (e.g. Refs. 81–83). Because the relative strength of action among various progestogens can differ across organ systems (81), the results of these studies focusing on MPA’s potency in peripheral tissues cannot be extrapolated with confidence to predict its central effects.
In addition to actions mediated through progesterone receptors, MPA may induce changes in behavior through its effects at other steroid receptors. Indeed, at androgen receptors, some studies have shown MPA to have pronounced agonistic properties (e.g. Refs. 84–86). However, other studies have found MPA to antagonize androgen receptor mediated effects (e.g. Ref. 87), and MPA’s relative binding affinity for androgen receptors is considerably weaker than has been found for the 19-nortestosterone derived progestogens (77, 78). By contrast, at glucocorticoid receptors, MPA has a relative binding affinity intermediate to dexamethasone and the natural ligand cortisol (77, 78, 88). Additionally, MPA has been shown to induce pronounced glucocorticoid-like effects (88–93). Indeed, Gibbs et al. (24) speculate that MPA impairs cholinergic function, whereas P4 may not, because of its substantial glucocorticoid properties. Nonetheless, because the relative effects of MPA have the potential to vary across tissue types, its actions at glucocorticoid receptors within relevant neurochemical systems must be evaluated to determine whether the glucocorticoid properties of MPA underlie the behavioral changes we observed.
In addition to countering the effects of E2 through its actions at glucocorticoid receptors, MPA also may offset E2’s actions by blocking the conversion of endogenous P4 to its neuroactive metabolite, allopregnanolone. Synthesized peripherally and within the brain through the sequential actions of 5α-reductase and 3α-hydroxysteroid dehydrogenase, allopregnanolone acts as a positive allosteric modulator of γ-aminobutyric acid (GABA)A receptors (94–96). The importance of allopregnanolone in the regulation of behavior has been illustrated clearly in rodent studies, which have shown that allopregnanolone has anxiolytic and antidepressive effects on behavior that can be potentiated or inhibited through the respective administration of a GABAA agonist or antagonist (97–102). In women, some studies have shown that, during the luteal phase, individuals diagnosed with premenstrual syndrome or dysphoric disorder have lower allopregnanolone levels (103, 104), whereas others have found the opposite or no effect (105–107). Nonetheless, women who have been diagnosed with premenstrual mood disorders report greater increases in anxiety and irritability during cycles in which they have lower allopregnanolone levels (104, 106, 107). Similarly, individuals suffering from depression also have been shown to have reduced allopregnanolone levels that are normalized through successful treatment with antidepressants (108–110). Hence, because alterations in allopregnanolone levels have been linked to mood disorders, MPA could have induced the changes we observed through its effects on the metabolism of this reduced P4 metabolite. MPA, in fact, acts as a competitive inhibitor of 3α-hydroxysteroid dehydrogenase activity, and therefore blocks the conversion and accumulation of allopregnanolone (111–113). Moreover, the parallel metabolite of MPA (tetrahydroMPA) has no effect on GABAA receptors, and MPA itself acts as a negative modulator (114). Hence, although estrogens are thought to elevate mood (3, 55), MPA may offset this effect by blocking the anxiolytic and antidepressive actions of allopregnanolone.
In conclusion, we have demonstrated that MPA has a pronounced impact on social and sexual behavior in female macaques. These changes in behavior likely reflect alterations in neurochemical function that underlie the changes in mood and loss of libido that women may experience when using MPA for contraception or hormone replacement therapy. Our results suggest that investigations of the sexual and mood consequences of MPA administration in humans would be an important addition to the literature on hormone therapy. Having determined that MPA can profoundly affect behavior, future research must focus on the molecular mechanisms through which MPA exerts these actions, and how these are different from or similar to the actions of other progestogens.
Acknowledgments
We appreciate the technical assistance of Kathy Chikazawa, Jennie Crosby, Jeff Fisher, Susie Lackie, Ariande Legendre, Jessica Raper, and Ruth Yoda. All assays were performed in the Endocrine Core Lab at the Yerkes National Primate Research Center, which center is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Footnotes
This work was supported, in part, by the Center for Behavioral Neuroscience, a Science and Technology Center Program of the National Science Foundation, Agreement no. IBN-9876754, and by the National Institute of Health Grants RR00165, HD38917, and HD044161
References
- 1.The Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275:370–375. doi: 10.1001/jama.1996.03530290040035. [DOI] [PubMed] [Google Scholar]
- 2.Sitruk-Ware R 2000 Progestins in hormonal replacement therapy and prevention of endometrial disease. In: Sitruk-Ware R, Mischell Jr DR, eds. Progestins and antiprogestins in clinical practice. New York: Marcel Dekker, Inc.; 269–277
- 3.The North American Menopause Society Position Statement. Role of progestogen in hormone therapy for postmenopausal women: position statement of The North American Menopause Society. Menopause. 2003;10:113–132. doi: 10.1097/00042192-200310020-00003. [DOI] [PubMed] [Google Scholar]
- 4.Kenemans P, Bosman A. Breast cancer and post-menopausal hormone therapy. Best Pract Res Clin Endocrinol Metab. 2003;17:123–137. doi: 10.1016/s1521-690x(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 5.Stahlberg C, Pedersen AT, Lynge E, Ottesen B. Hormone replacement therapy and risk of breast cancer: the role of progestins. Acta Obstet Gynecol Scand. 2003;82:335–344. [PubMed] [Google Scholar]
- 6.Greendale GA, Reboussin BA, Sie A, Singh HR, Olson LK, Gatewood O, Bassett LW, Wasilauskas C, Bush T, Barrett-Connor E. Effects of estrogen and estrogen-progestin on mammographic parenchymal density. Ann Intern Med. 1999;130:262–269. doi: 10.7326/0003-4819-130-4_part_1-199902160-00003. [DOI] [PubMed] [Google Scholar]
- 7.Santen RJ, Pinkerton J, McCartney C, Petroni GR. Risk of breast cancer with progestins in combination with estrogen as hormone replacement therapy. J Clin Endocrinol Metab. 2001;86:16–23. doi: 10.1210/jcem.86.1.7269. [DOI] [PubMed] [Google Scholar]
- 8.Eden J. Progestins and breast cancer. Am J Obstet Gynecol. 2003;188:1123–1131. doi: 10.1067/mob.2003.201. [DOI] [PubMed] [Google Scholar]
- 9.Musgrove EA, Sutherland RL 2000 Effects of progestin on the breast: regulation of cell cycle progression by progestins. In: Sitruk-Ware R, Mischell Jr DR, eds. Progestins and antiprogestins in clinical practice. New York: Marcel Dekker, Inc.; 59–75
- 10.Jordan VC, Jeng MH, Catherino WH, Parker CJ. The estrogenic activity of synthetic progestins used in oral contraceptives. Cancer. 1993;71:1501–1505. doi: 10.1002/cncr.2820710415. [DOI] [PubMed] [Google Scholar]
- 11.Lippert C, Seeger H, Wallwiener D, Mueck AO. The effect of medroxyprogesterone acetate and norethisterone on the estradiol stimulated proliferation in MCF-7 cells: comparison of continuous combined versus sequential combined estradiol/progestin treatment. Eur J Gynaecol Oncol. 2001;22:331–335. [PubMed] [Google Scholar]
- 12.Milewicz T, Kolodziejczyk J, Krzysiek J, Basta A, Sztefko K, Kurek S, Stachura J, Gregoraszczuk EL. Cyproterone, norethindrone, medroxyprogesterone and levonorgestrel are less potent local human growth hormone and insulin-like growth factor I secretion stimulators than progesterone in human breast cancer explants expressing the estrogen receptor. Gynecol Endocrinol. 2002;16:319–329. [PubMed] [Google Scholar]
- 13.Seeger H, Wallwiener D, Mueck AO. The effect of progesterone and synthetic progestins on serum- and estradiol-stimulated proliferation of human breast cancer cells. Horm Metab Res. 2003;35:76–80. doi: 10.1055/s-2003-39061. [DOI] [PubMed] [Google Scholar]
- 14.Thuneke I, Schulte HM, Bamberger AM. Biphasic effect of medroxyprogesterone-acetate (MPA) treatment on proliferation and cyclin D1 gene transcription in T47D breast cancer cells. Breast Cancer Res Treat. 2000;63:243–248. doi: 10.1023/a:1006432600478. [DOI] [PubMed] [Google Scholar]
- 15.Ahola TM, Alkio N, Manninen T, Ylikomi T. Progestin and G protein-coupled receptor 30 inhibit mitogen-activated protein kinase activity in MCF-7 breast cancer cells. Endocrinology. 2002;143:4620–4626. doi: 10.1210/en.2002-220492. [DOI] [PubMed] [Google Scholar]
- 16.Ahola TM, Purmonen S, Pennanen P, Zhuang YH, Tuohimaa P, Ylikomi T. Progestin up-regulates G-protein-coupled receptor 30 in breast cancer cells. Eur J Biochem. 2002;269:2485–2490. doi: 10.1046/j.1432-1033.2002.02912.x. [DOI] [PubMed] [Google Scholar]
- 17.Cline JM, Soderqvist G, von Schoultz E, Skoog L, von Schoultz B. Effects of hormone replacement therapy on the mammary gland of surgically postmenopausal cynomolgus macaques. Am J Obstet Gynecol. 1996;174:93–100. doi: 10.1016/s0002-9378(96)70379-4. [DOI] [PubMed] [Google Scholar]
- 18.Cline JM, Soderqvist G, von Schoultz E, Skoog L, von Schoultz B. Effects of conjugated estrogens, medroxyprogesterone acetate, and tamoxifen on the mammary glands of macaques. Breast Cancer Res Treat. 1998;48:221–229. doi: 10.1023/a:1005984932268. [DOI] [PubMed] [Google Scholar]
- 19.Hofseth LJ, Raafat AM, Osuch JR, Pathak DR, Slomski CA, Haslam SZ. Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast. J Clin Endocrinol Metab. 1999;84:4559–4565. doi: 10.1210/jcem.84.12.6194. [DOI] [PubMed] [Google Scholar]
- 20.Suparto IH, Williams JK, Cline JM, Anthony MS, Fox JL. Contrasting effects of two hormone replacement therapies on the cardiovascular and mammary gland outcomes in surgically postmenopausal monkeys. Am J Obstet Gynecol. 2003;188:1132–1140. doi: 10.1067/mob.2003.237. [DOI] [PubMed] [Google Scholar]
- 21.Bethea CL, Mirkes SJ, Shively CA, Adams MR. Steroid regulation of tryptophan hydroxylase protein in the dorsal raphe of macaques. Biol Psychiatry. 2000;47:562–576. doi: 10.1016/s0006-3223(99)00156-0. [DOI] [PubMed] [Google Scholar]
- 22.Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- 23.Nilsen J, Brinton RDCA. Impact of progestins on estradiol potentiation of the glutamate calcium response. Neuroreport. 2002;13:825–830. doi: 10.1097/00001756-200205070-00018. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs RB, Nelson D, Anthony MS, Clarkson TB. Effects of long-term hormone replacement and of tibolone on choline acetyltransferase and acetylcholinesterase activities in the brains of ovariectomized, cynomologous monkeys. Neuroscience. 2002;113:907–914. doi: 10.1016/s0306-4522(02)00239-7. [DOI] [PubMed] [Google Scholar]
- 25.Kaunitz AM. Injectable long-acting contraceptives. Clin Obstet Gynecol. 2001;44:73–91. doi: 10.1097/00003081-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Sherwin BB. The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal women. J Clin Endocrinol Metab. 1991;72:336–343. doi: 10.1210/jcem-72-2-336. [DOI] [PubMed] [Google Scholar]
- 27.Bjorn I, Bixo M, Nojd KS, Collberg P, Nyberg S, Sundstrom-Poromaa I, Backstrom T. The impact of different doses of medroxyprogesterone acetate on mood symptoms in sequential hormonal therapy. Gynecol Endocrinol. 2002;16:1–8. [PubMed] [Google Scholar]
- 28.Bjorn I, Bixo M, Nojd KS, Nyberg S, Backstrom T. Negative mood changes during hormone replacement therapy: a comparison between two progestogens. Am J Obstet Gynecol. 2000;183:1419–1426. doi: 10.1067/mob.2000.107781. [DOI] [PubMed] [Google Scholar]
- 29.Kirkham C, Hahn PM, Van Vugt DA, Carmichael JA, Reid RL. A randomized, double-blind, placebo-controlled, crossover trial to assess the side effects of medroxyprogesterone acetate in hormone replacement therapy. Obstet Gynecol. 1991;78:93–97. [PubMed] [Google Scholar]
- 30.Wolfe BM, Koval JJ, Nisker JA. Impact on postmenopausal symptoms of adding continuous C-21 versus C-19 progestin to estrogen. Maturitas. 1999;33:153–161. doi: 10.1016/s0378-5122(99)00053-5. [DOI] [PubMed] [Google Scholar]
- 31.Saure A, Planellas J, Poulsen HK, Jaszczak P. A double-blind, randomized, comparative study evaluating clinical effects of two sequential estradiol-progestogen combinations containing either desogestrel or medroxyprogesterone acetate in climacteric women. Maturitas. 2000;34:133–142. doi: 10.1016/s0378-5122(99)00103-6. [DOI] [PubMed] [Google Scholar]
- 32.Mahoney CJ. A study of the menstrual cycle in Macaca irus with special reference to the detection of ovulation. J Reprod Fertil. 1970;21:153–163. doi: 10.1530/jrf.0.0210153. [DOI] [PubMed] [Google Scholar]
- 33.Melnick DJ, Perl MC 1987 Cercopithecines in multimale groups: genetic diversity and population structure. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, eds. Primate societies. Chicago: University of Chicago Press; 121–134
- 34.Wallen K. Desire and ability: hormones and the regulation of female sexual behavior. Neurosci Biobehav Rev. 1990;14:233–241. doi: 10.1016/s0149-7634(05)80223-4. [DOI] [PubMed] [Google Scholar]
- 35.Maestripieri D, Schino G, Aureli F, Troisi A. A modest proposal: displacement activities as an indicator of emotions in primates. Anim Behav. 1992;44:967–979. [Google Scholar]
- 36.Maestripieri D, Martel FL, Nevison CM, Simpson MJ, Keverne EB. Anxiety in rhesus monkey infants in relation to interactions with their mother and other social companions. Dev Psychobiol. 1991;24:571–581. doi: 10.1002/dev.420240805. [DOI] [PubMed] [Google Scholar]
- 37.Baker KC, Aureli F. Behavioural indicators of anxiety: an empirical test in chimpanzees. Behaviour. 1997;134:1031–1050. [Google Scholar]
- 38.Aureli F. Post-conflict anxiety in nonhuman primates: the mediating role of emotion in conflict resolution. Aggress Behav. 1997;23:315–328. [Google Scholar]
- 39.Aureli F, de Waal FBM. Inhibition of social behavior in chimpanzees under high-density conditions. Am J Primatol. 1997;41:213–228. doi: 10.1002/(SICI)1098-2345(1997)41:3<213::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 40.Diezinger F, Anderson JR. Starting from scratch a first look at a displacement activity in group-living rhesus monkeys. Am J Primatol. 1986;11:117–124. doi: 10.1002/ajp.1350110204. [DOI] [PubMed] [Google Scholar]
- 41.Manson JH, Perry S. Correlates of self-directed behaviour in wild whitefaced capuchins. Ethology. 2000;106:301–317. [Google Scholar]
- 42.Crawley J, Ninan P, Pickar D, Chrousos GP, Linnoila M, Skolnick P, Paul SM. Neuropharmacological antagonism of the β-carboline-induced “anxiety” response in rhesus monkeys. J Neurosci. 1985;5:477–485. doi: 10.1523/JNEUROSCI.05-02-00477.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schino G, Troisi A, Perretta G, Monaco V. Measuring anxiety in nonhuman primates: effect of lorazepam on macaque scratching. Pharmacol Biochem Behav. 1991;38:889–891. doi: 10.1016/0091-3057(91)90258-4. [DOI] [PubMed] [Google Scholar]
- 44.Martin JR, Bos M, Jenck F, Moreau JL, Mutel V, Sleight AJ, Wichmann J, Andrews SJ, Berendsen HHG, Broekkamp CLE, Ruigt GSF, Kohler C, van Delft ALF. 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J Pharmacol Exp Ther. 1998;286:913–924. [PubMed] [Google Scholar]
- 45.Moreau JL, Griebel G, Jenck F, Martin JR, Widmer U, Haefely WE. Behavioral profile of the 5HT1A receptor antagonist (S)-UH-301 in rodents and monkeys. Brain Res Bull. 1992;29:901–904. doi: 10.1016/0361-9230(92)90163-r. [DOI] [PubMed] [Google Scholar]
- 46.Friess E, Tagaya H, Trachsel L, Holsboer F, Rupprecht R. Progesterone-induced changes in sleep in male subjects. Am J Physiol Endocrinol Metab. 1997;272:E885–E891. doi: 10.1152/ajpendo.1997.272.5.E885. [DOI] [PubMed] [Google Scholar]
- 47.Lancel M, Faulhaber J, Holsboer F, Rupprecht R. Progesterone induces changes in sleep comparable to those of agonistic GABAA receptor modulators. Am J Physiol Endocrinol Metab. 1996;271:E763–E772. doi: 10.1152/ajpendo.1996.271.4.E763. [DOI] [PubMed] [Google Scholar]
- 48.Vellucci SV, Herbert J, Keverne EB. The effect of midazolam and β-carboline carboxylic acid ethyl ester on behaviour, steroid hormones and central monoamine metabolites in social groups of talapoin monkeys. Psychopharmacology. 1986;90:367–372. doi: 10.1007/BF00179193. [DOI] [PubMed] [Google Scholar]
- 49.Casey DE. Dopamine D1 (SCH 23390) and D2 (haloperidol) antagonists in drugnaive monkeys. Psychopharmacology 107:18–22 Dopamine D-sub-1 (SCH 23390) and D-sub-2 (haloperidol) antagonists in drug-naive monkeys. Psychopharmacology. 1992;107:18–22. doi: 10.1007/BF02244960. [DOI] [PubMed] [Google Scholar]
- 50.Short R, England N, Bridson WE, Bowden DM. Ovarian cyclicity, hormones, and behavior as markers of aging in female pigtailed macaques (Macaca nemestrina) Gerontology. 1989;44:B131–B138. doi: 10.1093/geronj/44.5.b131. [DOI] [PubMed] [Google Scholar]
- 51.Walker ML, Gordon TP, Wilson ME. Reproductive performance in capture-acclimated female rhesus monkeys (Macaca mulatta) J Med Primatol. 1982;11:291–302. [PubMed] [Google Scholar]
- 52.Wilson ME, Fisher J, Chikazawa K, Yoda R, Legendre A, Mook D, Gould KG. Leptin administration increases nocturnal concentrations of LH and GH in juvenile female rhesus monkeys. J Clin Endocrinol Metab. 88:4874–4883. doi: 10.1210/jc.2003-030782. [DOI] [PubMed] [Google Scholar]
- 53.Pazol K, Kaplan JR, Abbott D, Appt S, Wilson ME. Practical measurement of total and bioavailable estradiol in female macaques. Clin Chim Acta. 2004;340:117–126. doi: 10.1016/j.cccn.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 54.Cohen J 1988 Statistical power analysis for the behavioral sciences. Some issues in power analysis. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 531–542
- 55.Freedman MA. Quality of life and menopause: the role of estrogen. J Womens Health. 2002;11:703–718. doi: 10.1089/15409990260363661. [DOI] [PubMed] [Google Scholar]
- 56.Genazzani AR, Bernardi F, Stomati M, Monteleone P, Luisi S, Rubino S, Farzati A, Casarosa E, Luisi M, Petraglia F. Effects of estradiol and raloxifene analog on brain, adrenal and serum allopregnanolone content in fertile and ovariectomized female rats. Neuroendocrinology. 2000;72:162–170. doi: 10.1159/000054583. [DOI] [PubMed] [Google Scholar]
- 57.Stomati M, Bernardi F, Luisi S, Puccetti S, Casarosa E, Liut M, Quirici B, Pieri M, Genazzani AD, Luisi M, Genazzani AR. Conjugated equine estrogens, estrone sulphate and estradiol valerate oral administration in ovariectomized rats: effects on central and peripheral allopregnanolone and β-endorphin. Maturitas. 2002;43:195–206. doi: 10.1016/s0378-5122(02)00205-0. [DOI] [PubMed] [Google Scholar]
- 58.Rahimy MH, Ryan KK, Hopkins NK. Lunelle monthly contraceptive injection (medroxyprogesterone acetate and estradiol cypionate injectable suspension): steady-state pharmacokinetics of MPA and E2 in surgically sterile women. Contraception. 1999;60:209–214. doi: 10.1016/s0010-7824(99)00086-4. [DOI] [PubMed] [Google Scholar]
- 59.Rahimy MH, Cromie MA, Hopkins NK, Tong DM. Lunelle monthly contraceptive injection (medroxyprogesterone acetate and estradiol cypionate injectable suspension): effects of body weight and injection sites on pharmacokinetics. Contraception. 1999;60:201–208. doi: 10.1016/s0010-7824(99)00085-2. [DOI] [PubMed] [Google Scholar]
- 60.Ortiz A, Hirol M, Stanczyk FZ, Goebelsmann U, Mishell DR. Serum medroxyprogesterone acetate (MPA) concentrations and ovarian function following intramuscular injection of depo-MPA. J Clin Endocrinol Metab. 1977;44:32–38. doi: 10.1210/jcem-44-1-32. [DOI] [PubMed] [Google Scholar]
- 61.Svensson LO, Johnson SH, Olsson SE. Plasma concentrations of medroxyprogesterone acetate, estradiol and estrone following oral administration of Klimaxil, Trisequence/Provera and Divina. A randomized, single-blind, triple crossover bioavailability study in menopausal women. Maturitas. 1994;18:229–238. doi: 10.1016/0378-5122(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 62.Fanchin R, De Ziegler D, Bergeron C, Righini C, Torrisi C, Frydman R. Transvaginal administration of progesterone. Obstet Gynecol. 1997;90:396–401. doi: 10.1016/s0029-7844(97)00270-6. [DOI] [PubMed] [Google Scholar]
- 63.Levine H, Watson N. Comparison of the pharmacokinetics of Crinone 8% administered vaginally versus Prometrium administered orally in postmenopausal women. Fertil Steril. 2000;73:516–521. doi: 10.1016/s0015-0282(99)00553-1. [DOI] [PubMed] [Google Scholar]
- 64.Nahoul K, Dehennin L, Jondet M, Roger M. Profiles of plasma estrogens, progesterone and their metabolites after oral or vaginal administration of estradiol or progesterone. Maturitas. 1993;16:185–202. doi: 10.1016/0378-5122(93)90064-o. [DOI] [PubMed] [Google Scholar]
- 65.Jemec GB, Wojnarowska F. The distribution of p29 protein in normal human skin. Br J Dermatol. 1987;117:217–224. doi: 10.1111/j.1365-2133.1987.tb04119.x. [DOI] [PubMed] [Google Scholar]
- 66.Hodgins MB, Spike RC, Mackie RM, MacLean AB. An immunohistochemical study of androgen, oestrogen and progesterone receptors in the vulva and vagina. Br J Obstet Gynaecol. 1998;105:216–222. doi: 10.1111/j.1471-0528.1998.tb10056.x. [DOI] [PubMed] [Google Scholar]
- 67.Brincat M, Kabalan S, Studd JW, Moniz CF, de Trafford J, Montgomery J. A study of the decrease of skin collagen content, skin thickness, and bone mass in the postmenopausal woman. Obstet Gynecol. 1987;70:840–845. [PubMed] [Google Scholar]
- 68.Chow SN, Huang CC, Lee YT. Demographic characteristics and medical aspects of menopausal women in Taiwan. J Formos Med Assoc. 1997;96:806–811. [PubMed] [Google Scholar]
- 69.Palacios S. Current perspectives on the benefits of HRT in menopausal women. Maturitas. 1999;33:1–13. [PubMed] [Google Scholar]
- 70.Dunn LB, Damesyn M, Moore AA, Reuben DB, Greendale GA. Does estrogen prevent skin aging? Results from the First National Health and Nutrition Examination Survey (NHANES I) Arch Dermatol. 1997;133:339–342. doi: 10.1001/archderm.133.3.339. [DOI] [PubMed] [Google Scholar]
- 71.Brincat M, Yuen AW, Studd JW, Montgomery J, Magos AL, Savvas M. Response of skin thickness and metacarpal index to estradiol therapy in postmenopausal women. Obstet Gynecol. 1987;70:538–541. [PubMed] [Google Scholar]
- 72.Brincat M, Versi E, Moniz CF, Magos A, de Trafford J, Studd JW. Skin collagen changes in postmenopausal women receiving different regimens of estrogen therapy. Obstet Gynecol. 1987;70:123–127. [PubMed] [Google Scholar]
- 73.Brincat M, Versi E, O’Dowd T, Moniz CF, Magos A, Kabalan S, Studd JW. Skin collagen changes in post-menopausal women receiving oestradiol gel. Maturitas. 1987;9:1–5. doi: 10.1016/0378-5122(87)90045-4. [DOI] [PubMed] [Google Scholar]
- 74.Blaustein JD, Erskine MS 2002 Feminine sexual behavior: cellular integration of hormonal and afferent information in the rodent forebrain. In: Pfaff DW, ed. Hormones, brain and behavior. Amsterdam: Academic Press; 139–213
- 75.Wilson ME, Gordon TP, Collins DC. Variation in ovarian steroids associated with the annual mating period in female rhesus monkeys (Macaca mulatta) Biol Reprod. 1982;27:530–539. doi: 10.1095/biolreprod27.3.530. [DOI] [PubMed] [Google Scholar]
- 76.Zehr JL, Maestripieri D, Wallen K. Estradiol increases female sexual initiation independent of male responsiveness in rhesus monkeys. Horm Behav. 1998;33:95–103. doi: 10.1006/hbeh.1998.1440. [DOI] [PubMed] [Google Scholar]
- 77.Rebar RW, Zeserson K. Characteristics of the new progestogens in combination oral contraceptives. Contraception. 1991;44:1–10. doi: 10.1016/0010-7824(91)90101-k. [DOI] [PubMed] [Google Scholar]
- 78.Kuhl H. Pharmacokinetics of oestrogens and progestogens. Maturitas. 1990;12:171–197. doi: 10.1016/0378-5122(90)90003-o. [DOI] [PubMed] [Google Scholar]
- 79.Bonsall RW, Rees HD, Michael RP. Uptake of medroxyprogesterone acetate by progestin and androgen target neurons in the brain and pituitary gland of male cynomolgus monkeys. Neuroendocrinology. 1990;52:573–580. doi: 10.1159/000125646. [DOI] [PubMed] [Google Scholar]
- 80.Rees HD, Bonsall RW, Michael RP. Pre-optic and hypothalamic neurons accumulate [3H]medroxyprogesterone acetate in male cynomolgus monkeys. Life Sci. 1986;39:1353–1359. doi: 10.1016/0024-3205(86)90333-4. [DOI] [PubMed] [Google Scholar]
- 81.Schoonen WG, Dijkema R, de Ries RJ, Wagenaars JL, Joosten JW, de Gooyer ME, Deckers GH, Kloosterboer HJ. Human progesterone receptor A and B isoforms in CHO cells. II. Comparison of binding, transactivation and ED50 values of several synthetic (anti)progestagens in vitro in CHO and MCF-7 cells and in vivo in rabbits and rats. J Steroid Biochem Mol Biol. 1998;64:157–170. doi: 10.1016/s0960-0760(97)00161-1. [DOI] [PubMed] [Google Scholar]
- 82.Pridjian G, Schmit V, Schreiber J. Medroxyprogesterone acetate: receptor binding and correlated effects on steroidogenesis in rat granulosa cells. J Steroid Biochem. 1987;26:313–319. doi: 10.1016/0022-4731(87)90095-1. [DOI] [PubMed] [Google Scholar]
- 83.Di Carlo F, Racca S, Conti G, Gallo E, Muccioli G, Sapino A, Bussolati G. Effects of long-term administration of high doses of medroxyprogesterone acetate on hormone receptors and target organs in the female rat. J Endocrinol. 1984;103:287–293. doi: 10.1677/joe.0.1030287. [DOI] [PubMed] [Google Scholar]
- 84.Lin YC, Bullock LP, Bardin CW, Jacob ST. Effect of medroxyprogesterone acetate and testosterone on solubilized RNA polymerases and chromatin template activity in kidney from normal and androgen-insensitive (Tfm/Y) mice. Biochemistry. 1978;17:4833–4838. doi: 10.1021/bi00615a034. [DOI] [PubMed] [Google Scholar]
- 85.Labrie C, Cusan L, Plante M, Lapointe S, Labrie F. Analysis of the androgenic activity of synthetic “progestins” currently used for the treatment of prostate cancer. J Steroid Biochem. 1987;28:379–384. doi: 10.1016/0022-4731(87)91054-5. [DOI] [PubMed] [Google Scholar]
- 86.Bardin CW, Brown T, Isomaa VV, Janne OA. Progestins can mimic, inhibit and potentiate the actions of androgens. Pharmacol Ther. 1983;23:443–459. doi: 10.1016/0163-7258(83)90023-2. [DOI] [PubMed] [Google Scholar]
- 87.Kemppainen JA, Langley E, Wong CI, Bobseine K, Kelce WR, Wilson EM. Distinguishing androgen receptor agonists and antagonists: distinct mechanisms of activation by medroxyprogesterone acetate and dihydrotestosterone. Mol Endocrinol. 1999;13:440–454. doi: 10.1210/mend.13.3.0255. [DOI] [PubMed] [Google Scholar]
- 88.Kontula K, Paavonen T, Luukkainen T, Andersson LC. Binding of progestins to the glucocorticoid receptor. Correlation to their glucocorticoid-like effects on in vitro functions of human mononuclear leukocytes. Biochem Pharmacol. 1983;32:1511–1518. doi: 10.1016/0006-2952(83)90474-4. [DOI] [PubMed] [Google Scholar]
- 89.Selman PJ, Mol JA, Rutteman GR, van Garderen E, van den Ingh TS, Rijnberk A. Effects of progestin administration on the hypothalamic-pituitary-adrenal axis and glucose homeostasis in dogs. J Reprod Fertil Suppl. 1997;51:345–354. [PubMed] [Google Scholar]
- 90.Selman PJ, Wolfswinkel J, Mol JA. Binding specificity of medroxyprogesterone acetate and proligestone for the progesterone and glucocorticoid receptor in the dog. Steroids. 1996;61:133–137. doi: 10.1016/0039-128x(95)00216-d. [DOI] [PubMed] [Google Scholar]
- 91.Winneker R, Parsons J. Glucocorticoid-like actions of medroxyprogeterone acetate upon MtTW15 rat mammosomatotropic pituitary tumors. Endocrinology. 1981;109:99–105. doi: 10.1210/endo-109-1-99. [DOI] [PubMed] [Google Scholar]
- 92.Bamberger CM, Else T, Bamberger AM, Beil FU, Schulte HM. Dissociative glucocorticoid activity of medroxyprogesterone acetate in normal human lymphocytes. J Clin Endocrinol Metab. 1999;84:4055–4061. doi: 10.1210/jcem.84.11.6091. [DOI] [PubMed] [Google Scholar]
- 93.Ishida Y, Heersche JN. Pharmacologic doses of medroxyprogesterone may cause bone loss through glucocorticoid activity: an hypothesis. Osteoporos Int. 2002;13:601–605. doi: 10.1007/s001980200080. [DOI] [PubMed] [Google Scholar]
- 94.Laidlaw IJ, Clarke RB, Howell A, Owen AWMC, Potten CS, Anderson E. The proliferation of normal human breast tissue implanted into athymic nude mice is stimulated by estrogen but not progesterone. Endocrinology. 1995;136:164–171. doi: 10.1210/endo.136.1.7828527. [DOI] [PubMed] [Google Scholar]
- 95.Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- 96.Paul S, Purdy R. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- 97.Akwa Y, Purdy RH, Koob GF, Britton KT. The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–125. doi: 10.1016/s0166-4328(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 98.Bitran D, Purdy RH, Kellog CK. Anxiolytic effect of progesterone is associated with increases in cortical alloprenanolone and GABAA receptor function. Pharmacol Biochem Behav. 1993;45:423–428. doi: 10.1016/0091-3057(93)90260-z. [DOI] [PubMed] [Google Scholar]
- 99.Khisti RT, Chopde CT. Serotonergic agents modulate antidepressant-like effect of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in mice. Brain Res. 2000;865:291–300. doi: 10.1016/s0006-8993(00)02373-8. [DOI] [PubMed] [Google Scholar]
- 100.Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in mice forced swim test. Pharmacol Biochem Behav. 2000;67:137–143. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 101.Rodgers RJ, Johnson NJT. Behaviorally selective effects of neuroactive steroids on plusmaze anxiety in mice. Pharmacol Biochem Behav. 1998;59:221–232. doi: 10.1016/s0091-3057(97)00339-0. [DOI] [PubMed] [Google Scholar]
- 102.Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–315. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- 103.Monteleone P, Luisi S, Tonetti A, Bernardi F, Genazzani AD, Luisi M, Petraglia F, Genazzani AR. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142:269–273. doi: 10.1530/eje.0.1420269. [DOI] [PubMed] [Google Scholar]
- 104.Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90:709–714. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- 105.Schmidt P, Purdy R, Moore P, Jr, Paul S, Rubinow D. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab. 1994;79:1256–1260. doi: 10.1210/jcem.79.5.7962316. [DOI] [PubMed] [Google Scholar]
- 106.Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- 107.Wang M, Seippel L, Purdy R, Backstrom T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one. J Clin Endocrinol Metab. 1996;81:1076–1082. doi: 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- 108.Romeo E, Strohle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- 109.Strohle A, Romeo E, Hermann B, Pasini A, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Concentrations of 3α-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol Psychiatry. 1999;45:274–277. doi: 10.1016/s0006-3223(98)00328-x. [DOI] [PubMed] [Google Scholar]
- 110.Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jarrell J. Studies on the developmental pattern of rat ovarian 3α-hydroxysteroid dehydrogenase: inhibition of the postpubertal activity with medroxyprogesterone acetate in vivo. J Steroid Biochem. 1984;21:151–156. doi: 10.1016/0022-4731(84)90376-5. [DOI] [PubMed] [Google Scholar]
- 112.Lee TC, Miller WL, Auchus RJ. Medroxyprogesterone acetate and dexamethasone are competitive inhibitors of different human steroidogenic enzymes. J Clin Endocrinol Metab. 1999;84:2104–2110. doi: 10.1210/jcem.84.6.5646. [DOI] [PubMed] [Google Scholar]
- 113.Penning TM, Sharp RB, Krieger NR. Purification and properties of 3α-hydroxysteroid dehydrogenase from rat brain cytosol. Inhibition by nonsteroidal anti-inflammatory drugs and progestins. J Biol Chem. 1985;260:15266–15272. [PubMed] [Google Scholar]
- 114.McAuley JW, Kroboth PD, Stiff DD, Reynolds IJ. Modulation of [3H]flunitrazepam binding by natural and synthetic progestational agents. Pharmacol Biochem Behav. 1993;45:77–83. doi: 10.1016/0091-3057(93)90089-c. [DOI] [PubMed] [Google Scholar]


