Abstract
Dendritic cells (DCs) are involved in the pathogenesis of measles virus (MV) infection by inducing immune suppression and possibly spreading the virus from the respiratory tract to lymphatic tissues. It is becoming evident that DC function can be modulated by the involvement of different receptors in pathogen interaction. Therefore, we have investigated the relative contributions of different MV-specific receptors on DCs to MV uptake into and infection of these cells. DCs express the MV receptors CD46 and CD150, and we demonstrate that the C-type lectin DC-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN) is a novel receptor for laboratory-adapted and wild-type MV strains. The ligands for DC-SIGN are both MV glycoproteins F and H. In contrast to CD46 and CD150, DC-SIGN does not support MV entry, since DC-SIGN does not confer susceptibility when stably expressed in CHO cells. However, DC-SIGN is important for the infection of immature DCs with MV, since both attachment and infection of immature DCs with MV are blocked in the presence of DC-SIGN inhibitors. Our data demonstrate that DC-SIGN is crucial as an attachment receptor to enhance CD46/CD150-mediated infection of DCs in cis. Moreover, MV might not only target DC-SIGN to infect DCs but may also use DC-SIGN for viral transmission and immune suppression.
Measles virus (MV) is a highly contagious human pathogen and remains a leading cause of childhood mortality in developing countries (28). The high mortality rate is associated with transient immune suppression during and after acute MV infection (5, 7). Dendritic cells (DCs) are thought to play a pivotal role in the pathogenesis of MV infection and to be involved in the immunosuppressive effects during and after acute MV infection (35, 39). Different mechanisms for MV interference with DC function have been described. In transgenic mice, MV infection inhibits DC development and expansion through type I interferon/STAT2-specific signaling (20). In vitro, MV inhibits CD40 ligand-dependent DC maturation (38), and MV-infected DCs fail to stimulate proliferation and actively suppress T-cell proliferation (13, 19, 21, 36, 40). MV infection of plasmacytoid DCs interferes with Toll-like receptor-dependent IFN production (33). Moreover, MV infection of DC-T-cell cocultures induces Fas-mediated DC apoptosis (37).
DCs are abundantly present in the tracheal epithelium, where MV enters the body, and therefore can be responsible for the transport of MV to lymphoid organs and subsequent transmission to T cells (13), similar to the role of DCs in human immunodeficiency virus type 1 (HIV-1) transmission (6). Since the interaction of MV with DCs can be important for both viral dissemination and modulation of T-cell responses, it is important to identify the different MV-specific receptors on DCs, their viral ligands, and the relative contributions of these interactions to MV uptake and spread.
The known receptors for the MV hemagglutinin (H) protein are the complement receptor CD46 and the signaling lymphocyte activation molecule (SLAM/CD150). CD46 is present on all human nucleated cells; however, it is almost exclusively used by attenuated MV strains (2, 10, 24, 29). CD150 is expressed by thymocytes, activated B cells, T cells, monocytes, and DCs and mediates the entry of all MV strains (12, 42). Recent studies have demonstrated that several pathogens target the DC-specific C-type lectin DC-SIGN for their dissemination or to modulate DC functions to facilitate their survival (15). DC-SIGN has a high affinity for mannose-containing carbohydrates expressed by viral glycoproteins (25). DC-SIGN binds various viruses, including the HIV envelope glycoprotein gp120, and can thereby enhance HIV-1 infection of T cells in trans (17). Here we have identified DC-SIGN as a novel receptor for MV. Immature DCs interact strongly with MV through both DC-SIGN and CD150. Both viral attachment and infection of immature DCs with MV strongly depend on DC-SIGN. However, DC-SIGN is not an entry receptor, since it does not mediate the entry of MV in DC-SIGN-transfected cell lines. Our data demonstrate that MV uses a two-step mechanism to infect immature DCs: MV targets DC-SIGN to interact with the MV receptors on immature DCs, which mediate the direct infection of DCs. Thus, DC-SIGN functions as an attachment receptor for MV, which is crucial for DC infection.
MATERIALS AND METHODS
Antibodies.
The following antibodies were used: DC-SIGN-specific mouse antibodies AZN-D1 and AZN-D2 (18), the CD46-specific mouse antibody 13/42, the SLAM-specific mouse antibody 5C6, the MV H-specific mouse antibody L77, the MV F-specific mouse antibody A5047, the LFA-1-specific mouse antibody L7 (all generated in our laboratory), goat anti-human immunoglobulin G (IgG), goat anti-mouse IgG conjugated with peroxidase (Jackson Immunoresearch, West Grove, PA), and goat anti-mouse IgG antibody conjugated with fluorescein isothiocyanate (FITC; Zymed Laboratories Inc., South San Fransisco, Calif.).
Cells and viruses.
Immature DCs were cultured as described before (32). In short, human blood monocytes were isolated from buffy coats by use of a Ficoll gradient and a subsequent CD14 selection step using a MACS system (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Purified monocytes were differentiated into immature DCs in the presence of interleukin-4 (IL-4) and granulocyte-macrophage colony-stimulating factor (500 and 800 U/ml, respectively; Schering-Plough, Brussels, Belgium). On day 6, the phenotype of the cultured DCs was confirmed by flow cytometry and expressed high levels of major histocompatibility complex class I and II molecules, CD11b, CD11c, and ICAM-1 and low levels of CD80 and CD86. Mature DCs were generated by culturing immature DCs (day 4) in the presence of lipopolysaccharide (10 ng/ml) for 48 h. Stable K562, CHO, and Raji (formerly referred to as THP-B) (44) transfectants expressing wild-type DC-SIGN, CD46, or CD150 were generated as previously described (17). Meljuso cells stably expressing MV F or MV H were previously generated and described (8). The MV vaccine strain Edmonston was grown on Vero cells (African green monkey kidney cells; maintained in minimum essential medium with 5% fetal calf serum), whereas the MV wild-type strain WTF was grown on BJAB cells (human lymphoblastoid cell line; maintained in RPMI with 10% fetal calf serum); both strains were titrated on B95a cells (marmoset B-cell line; maintained in RPMI with 5% fetal calf serum). Viruses were purified by discontinuous sucrose gradient ultracentrifugation and then UV inactivated (1.5 J/cm2). Mock controls were purified cell lysates from mock-infected cells obtained using a similar purification method. Amounts used in individual experiments are expressed as PFU of the former live virus. A recombinant MV strain expressing green fluorescent protein (EDeGFP) was grown on Vero cells and titrated on B95a cells (11).
Fluorescent bead adhesion assay.
The fluorescent bead adhesion assay was performed as described before (17). In short, streptavidin was covalently coupled to carboxylate-modified TransFluorSpheres (488/645 nm by 1.0 μm; Molecular Probes, Eugene, OR). The streptavidin-coated beads were incubated with biotinylated-F(ab′)2-fragment goat anti-mouse IgG (6 μg/ml; Jackson Immunoresearch), followed by an overnight incubation with a mouse monoclonal anti-MV antibody (L77 or A5047; 10 μg/ml) at 4°C. The beads were washed and incubated with purified UV-inactivated MV overnight at 4°C. The MV-coated beads were added to cells at a ratio of 20:1. Cells (50,000) were incubated with beads for 45 min at 37°C. Mannan (1 mg/ml), EGTA (10 mM), and blocking antibodies against DC-SIGN, CD46, and CD150 (20 μg/ml) were used to determine the specificity of the adhesion. Binding was measured by fluorescence-activated cell sorting analysis and depicted as the percentage of cells that bound fluorescent beads.
DC-SIGN-Fc binding ELISA.
Recombinant DC-SIGN consists of the extracellular portion of DC-SIGN (amino acid residues 64 to 404) fused at the C terminus to the human IgG1 Fc domain. DC-SIGN-Fc was produced in Chinese hamster ovary K1 cells after transfection with the DC-SIGN-Sig-pIgG1-Fc vector (5 μg/1 × 106 cells). The soluble DC-SIGN-Fc binding assay was performed as previously described (14). In short, goat anti-mouse IgG antibodies (4 μg/ml; Jackson Immunoresearch Laboratory, West Grove, PA) were used to coat enzyme-linked immunosorbent assay (ELISA) plates for 1 h at 37°C. After blocking of the plates, murine antibodies against MV (1 μg/ml) were added for 1 h at 37°C and incubated for 18 h at 4°C with different concentrations of purified UV-inactivated virions (10 PFU/ml to 1 × 106 PFU/ml) for 1 h at 4°C. DC-SIGN-Fc (1 μg/ml) was added for 2 h at room temperature. DC-SIGN-Fc binding was determined using a peroxidase-conjugated goat anti-human Fc antibody.
The DC-SIGN-Fc capture ELISA was performed as follows. UV-inactivated MV virions (15 × 106 PFU/ml) were lysed in lysis buffer (1% Triton X-100, 10 mM Tris-acetate-EDTA, pH 8.2, 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2) for 1 h at 4°C. Goat anti-human IgG antibodies (4 μg/ml; Jackson Immunoresearch Laboratory, West Grove, PA) were used to coat plates for 1 h at 37°C. After blocking of the plates, DC-SIGN-Fc was used to coat the ELISA plates for 1 h at 37°C and incubated with virus lysates for 18 h at 4°C to allow the capture of MV proteins. Captured viral glycoproteins were detected by incubation with MV F- or H-specific antibody (1 μg/ml) for 2 h at room temperature. Binding was determined using a peroxidase-conjugated goat anti-mouse Fc antibody.
MV infection.
CHO, CHO-DC-SIGN, CHO-CD46, and CHO-SLAM cells (5 × 104 cells) were plated in 24-well plates. Cells were infected with ED, WTF (multiplicity of infection [MOI], 0.5), or a mock control at 37°C. After 2 h, cells were washed with medium and grown in a medium containing the fusion inhibitory peptide z-d-Phe-l-Phe-l-Gly (0.2 mM; Bachem, Heidelberg, Germany) for 48 h. To measure infection, cells were stained with an anti-MV H antibody (L77), followed by staining with the goat anti-mouse IgG-FITC antibody; dead cells were stained with 7-amino-actinomycin D and excluded from the analysis. Expression was measured by flow cytometry. To determine syncytium formation of the cell cultures, representative phase-contrast photos were taken. DCs or Raji or Raji-DC-SIGN cells (2 × 106 cells/ml) were seeded in a round-bottom 96-well plate. Cells were incubated with blocking antibodies (20 μg/ml), mannan (50 μg/ml), or the carbohydrate control glucitol (50 μg/ml) for 45 min at 37°C. Cells were infected with MV WTF (MOI, 0.3 or 1), EDeGFP (MOI, 0.25), or a mock control for 2 h at 37°C. Cells were washed in medium and replated for 48 h in medium containing the fusion inhibitory peptide. WTF infection was measured by staining the cells with anti-MV H (L77), followed by staining with the goat anti-mouse IgG-FITC antibody. Afterwards, cells were fixed with 2% paraformaldehyde in phosphate-buffered saline, and FITC (WTF) or GFP (EDeGFP) expression was measured by flow cytometry.
Statistical analysis.
Statistical analysis was performed by using Student's t test, and significance levels were determined based on the respective controls.
RESULTS
Recombinant DC-SIGN interacts with different measles virus strains.
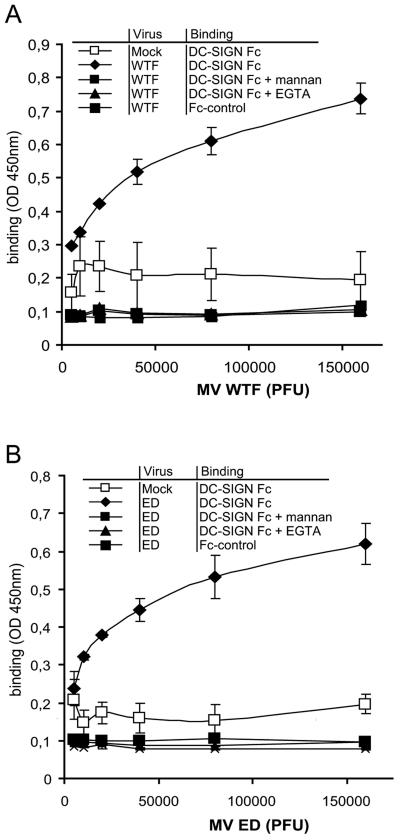
The interactions of recombinant DC-SIGN with the wild-type MV strain WTF and the MV vaccine strain Edmonston (ED) were investigated using recombinant DC-SIGN-Fc. Viruses were coated with an anti-MV F (A5047) antibody, and the interaction with DC-SIGN was measured using the DC-SIGN binding ELISA. DC-SIGN-Fc interacted with both WTF and ED in a dose-dependent way (Fig. 1A and B), demonstrating that recombinant DC-SIGN binds both MV strains. The binding of DC-SIGN-Fc was not significantly different between WTF and ED, suggesting that both strains bind with similar affinities. The interaction of DC-SIGN with MV is specific and mediated by the C-type lectin domain, since the polycarbohydrate mannan and the calcium chelator EGTA inhibited the interaction, even at high virus doses (Fig. 1A and B). Altogether, these findings indicate that whole MV virions from both MV strains bind to DC-SIGN.
FIG. 1.
Recombinant DC-SIGN interacts with MV strains ED and WTF. DC-SIGN-Fc interactions with MV ED and WTF were determined by a DC-SIGN-Fc binding ELISA. Viruses were coated with an anti-F antibody. DC-SIGN-Fc binding to different concentrations of MV WTF (A) or ED (B) or their mock controls was measured by ELISA. The specificity of the DC-SIGN-Fc-MV interaction was determined by measuring binding in the presence of mannan and EGTA. An Fc chimera with an identical Fc domain (ICAM-3-Fc) was used as an Fc control. Data for one representative experiment of three are shown. Errors bars represent standard deviations of triplicates.
DC-SIGN interacts with MV glycoproteins F and H.
The MV envelope contains the viral glycoprotein complex consisting of the hemagglutinin (H) and the fusion (F) protein, which are involved in receptor binding and fusion, respectively. The interactions of DC-SIGN with the viral glycoproteins of HIV-1, hepatitis C virus, and Ebola virus occur through N-linked carbohydrates (25, 26). Both MV glycoproteins H and F contain functional N-glycosylation sites (4, 23), and both glycoproteins are incorporated into MV virions. A DC-SIGN-Fc capture ELISA was used to identify the MV glycoprotein that interacts with DC-SIGN. MV proteins were immunoprecipitated from a MV ED lysate with recombinant DC-SIGN-Fc, and antibodies against F and H were used to identify the captured glycoproteins. Both glycoproteins F and H were detected in the immunoprecipitate using DC-SIGN-Fc (Fig. 2A). Next, F and H were isolated from cell lines expressing either F or H without other viral proteins in order to exclude that DC-SIGN binding to immunoprecipitated F and H was caused by binding to a complex with other viral proteins. The isolated F and H proteins were used to coat fluorescent beads, and binding to DC-SIGN transfectants was measured using the fluorescent bead adhesion assay. DC-SIGN interacted with both isolated F and H, demonstrating that both MV envelope glycoproteins are ligands for DC-SIGN.
FIG. 2.
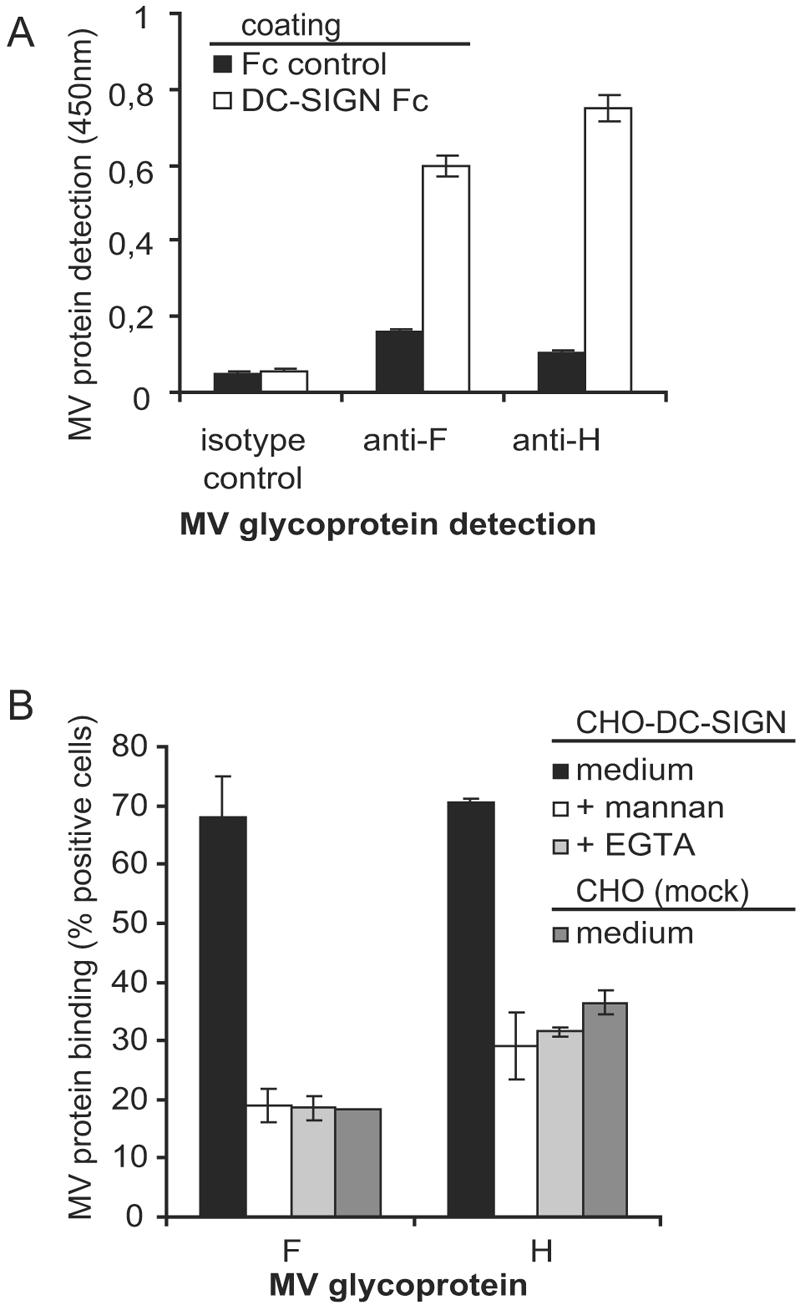
DC-SIGN binds the MV glycoproteins F and H. (A) DC-SIGN-Fc capture ELISA was used to determine the interaction of DC-SIGN-Fc with glycoproteins in MV (ED) lysates. MV proteins were immunoprecipitated with DC-SIGN-Fc and detected with MV-specific antibodies for F (A5047) or H (L77). Error bars represent standard deviations of duplicates. Data for one representative experiment of three are shown. (B) Recombinant F and H interact with DC-SIGN. F and H were expressed in the Meljuso cell line and then used to coat beads. The interactions of these F- and H-coated beads with CHO-DC-SIGN transfectants were measured by a fluorescent bead binding assay. The specificity of the DC-SIGN interaction was determined by measuring binding in the presence of mannan and EGTA. Error bars represent standard deviations of duplicates. Data for one representative experiment of three are shown.
Cellular DC-SIGN is a receptor for measles virus.
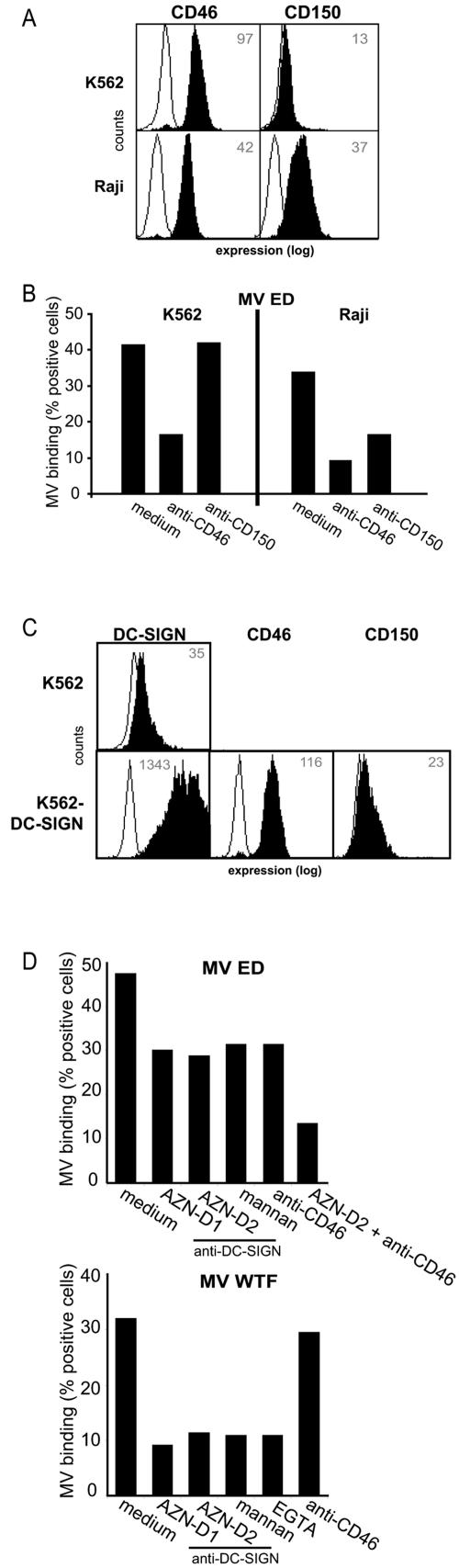
Next, we investigated whether cellular DC-SIGN, in addition to the MV receptors CD46 and CD150, functions as a receptor for MV. CD150 interacts with all MV strains, whereas high-affinity binding to CD46 has only been demonstrated for laboratory-adapted MV strains, including MV ED (2, 10, 24). The erythroleukemic cell line K562 and the B-cell line Raji were used to investigate the differential contributions of CD46, CD150, and DC-SIGN to the MV interaction; K562 cells express CD46 but not CD150, whereas Raji cells express both CD46 and CD150 (Fig. 3A). Binding of MV to mock-transfected K562 and Raji cells was determined using fluorescent beads coated with intact UV-inactivated MV virions. The beads were coated with the inactivated viruses by using an antibody against either MV H or F. We did not observe any differences between beads coated with MV using the different coating antibodies, strongly suggesting that the anti-envelope antibodies do not interfere with receptor binding (data not shown). K562 cells efficiently interacted with the MV ED strain, and the binding was blocked by antibodies against CD46 (Fig. 3B), demonstrating that the binding of MV ED is mediated by CD46. Raji cells express both CD46 and CD150 (Fig. 3A), and the binding of ED to these cells is mediated by both CD46 and CD150, since the observed binding was partially blocked by antibodies against either CD46 or CD150 (Fig. 3B). These results demonstrate that fluorescently labeled beads coated with whole virions are suitable for studying the interactions of MV with different receptors.
FIG. 3.
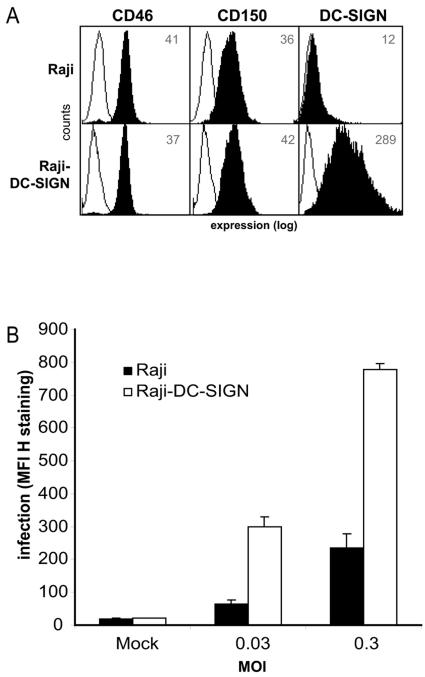
Cellular DC-SIGN is a receptor for MV. (A) K562 cells express the MV receptor CD46, whereas Raji cells express the MV receptors CD46 and CD150. Open histograms represent the isotype controls, and filled histograms represent specific antibody staining. The mean fluorescence intensity of specific staining is depicted. Expression is shown in log scale and ranges from 100 to 104. (B) Specific binding of MV ED-coated beads to the receptor CD46 or CD150 was determined by measuring binding in the presence of blocking antibodies for CD46 (13/42) or CD150 (5C6). Standard deviations for the fluorescent bead adhesion assay were <5%. Data for one representative experiment of three are shown. (C) K562-DC-SIGN transfectants express high levels of DC-SIGN. Open histograms represent the isotype controls, and filled histograms represent specific antibody staining. The mean fluorescence intensity of specific staining is depicted. Expression is shown in log scale and ranges from 100 to 104. (D) MV ED and WTF interactions with K562-DC-SIGN transfectants were determined using the fluorescent bead adhesion assay. Specificity was determined by measuring binding in the presence of blocking antibodies against DC-SIGN (AZN-D1 and AZN-D2), mannan, EGTA, or CD46 (13/42). Standard deviations for the fluorescent bead adhesion assay were <5%. Data for one representative experiment of three are shown.
To investigate whether cellular DC-SIGN binds MV, we used K562 transfectants expressing high levels of DC-SIGN (Fig. 3C). The binding of ED to K562-DC-SIGN cells was similar to that observed for mock-transfected K562 cells; this interaction was sensitive to CD46-specific antibodies, as seen with mock-transfected K562 cells, and, additionally, to DC-SIGN-specific antibodies. Neutralization of MV binding upon combined application of CD46- and DC-SIGN-specific antibodies was additive, and binding was blocked to background levels (Fig. 3D). The involvement of DC-SIGN was further confirmed using the polycarbohydrate mannan and the calcium chelator EGTA. Both C-type lectin inhibitors reduced the binding of ED to K562-DC-SIGN cells to a similar extent as antibodies against DC-SIGN (Fig. 3D). Since high-affinity binding to CD46 is a property of ED, but not WTF, this wild-type MV strain poorly interacted with mock-transfected K562 cells (8.2% ± 3.9%). In contrast, efficient binding of WTF to K562-DC-SIGN cells was observed, which was blocked to background levels by the inclusion of blocking antibodies against DC-SIGN, mannan, and EGTA (Fig. 3D), but not antibodies against CD46. Thus, DC-SIGN functions as a cellular receptor for both vaccine and wild-type MV strains, as it mediates binding of MV to the cell surface, as do CD150 for all strains and CD46 for attenuated strains.
DC-SIGN is not an entry receptor for measles virus.
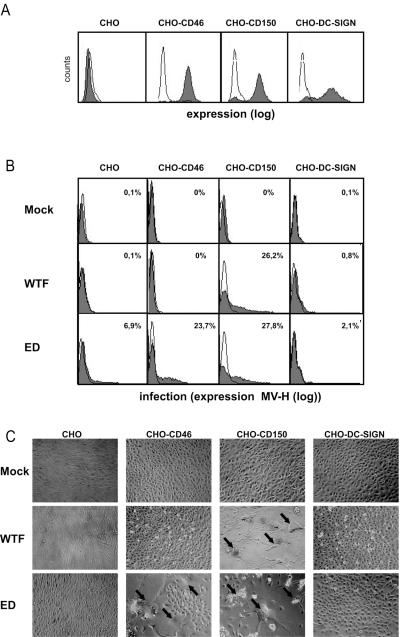
To investigate the role of DC-SIGN in MV uptake, DC-SIGN was stably transfected into CHO cells, which are known to support MV replication after transgenic expression of CD46 or CD150 (Fig. 4A). CHO-DC-SIGN cells and, as controls, CHO-CD46 and CHO-CD150 cells were exposed to live MV ED or WTF, and levels of H protein accumulating on the cell surface after 48 h as well as the formation of syncytia in the cultures were determined as measures of infection. As expected, CHO-CD150 cells supported infection by both WTF and ED, while H protein accumulation and syncytium formation in CHO-CD46 cells were only seen with the ED strain (Fig. 4B and C). When measured 24 and 72 h following infection, both parameters of infection increased (data not shown). In contrast, even high expression levels of DC-SIGN did not confer susceptibility to infection with either ED or WTF on CHO cells at any time analyzed (shown for 48 h postinfection; Fig. 4B and C), as neither the expression of H protein exceeding background levels nor syncytia were detectable. These results demonstrate that DC-SIGN confers binding to but not entry into target cells on its own.
FIG. 4.
DC-SIGN is not an entry receptor for MV. (A) CHO transfectants express CD46, CD150, or DC-SIGN. Open histograms represent the isotype controls, and filled histograms indicate the specific antibody staining. The mean expression levels are depicted. Expression is shown in log scale and ranges from 100 to 104. (B and C) CHO transfectants were infected with MV ED, WTF (MOI, 0.5), or a mock control. MV H expression was measured at 48 h postinfection to determine the level of infection. Open histograms represent the isotype controls. Percentages represent the positive cells compared to the isotype controls (B). Representative phase-contrast photos were taken to show syncytium formation of the different transfectants. Arrows indicate examples of observed syncytia (C).
Measles virus interacts with DC-SIGN on immature dendritic cells.
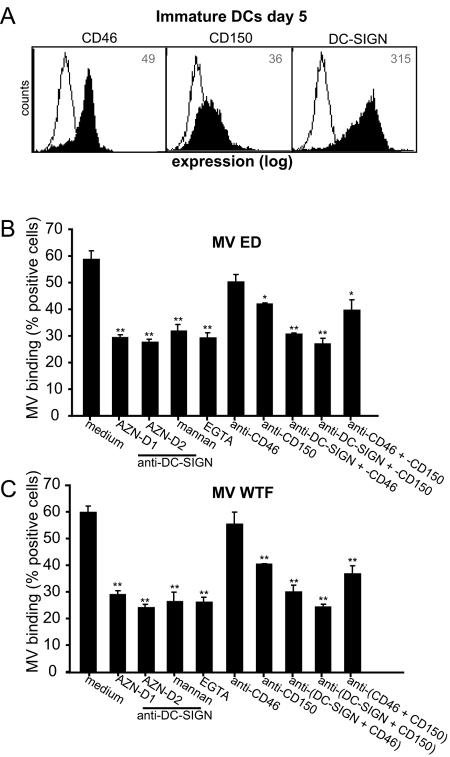
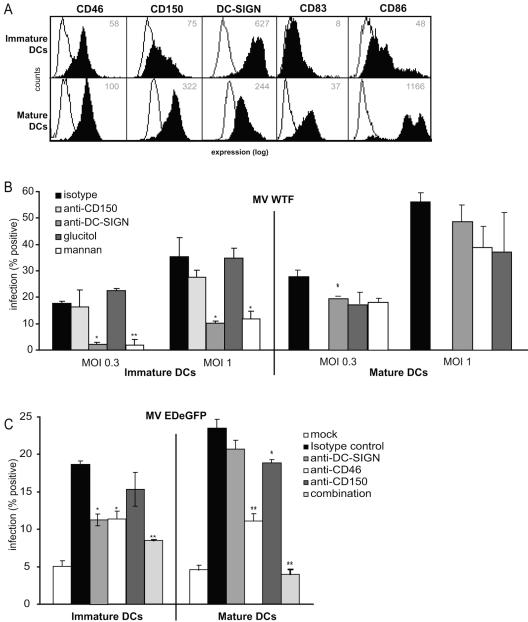
Immature DCs express high levels of both DC-SIGN and CD46 and low levels of CD150 (Fig. 5A) (27, 31). The interactions of different MV strains with immature DCs were investigated using blocking antibodies against the different receptors to determine their contributions. Immature DCs interacted strongly with both the ED and WTF MV strains, and the interactions with both virus strains were blocked to comparable extents by antibodies against DC-SIGN, mannan, and EGTA (Fig. 5B and C), demonstrating that DC-SIGN is the major C-type lectin that interacts with MV. Antibodies against CD150 also partially blocked the interaction. Strikingly, CD46-specific antibodies only marginally affected the binding of MV ED to DCs (Fig. 5B and C). In contrast to what we observed with K562-DC-SIGN cells (Fig. 3D), combinations of antibodies against DC-SIGN and either CD150 or CD46 did not increase the level of inhibition seen with DC-SIGN alone (Fig. 5B and C). A combination of the three antibodies did not further block the interaction with immature dendritic cells (data not shown). Background levels were determined using mock-coated beads (20% ± 0.7%). These data demonstrate that DC-SIGN is essential for the interaction of immature DCs with both MV strains.
FIG. 5.
DC-SIGN is a major attachment receptor for MV on immature DCs. (A) Immature DCs express CD46 and CD150 and high levels of DC-SIGN. Open histograms represent isotype controls, and filled histograms indicate specific antibody staining. The mean fluorescence intensity of specific staining is depicted. Expression is shown in log scale and ranges from 100 to 104. (B and C) Binding of MV ED and WTF to DCs was determined using the fluorescent bead adhesion assay. Specificity was determined by measuring binding in the presence of mannan, EGTA, antibodies to DC-SIGN (AZN-D1 and AZN-D2), CD46 (13/42), or CD150 (5C6) or with a combination of the antibodies. Data for one representative experiment of three are shown. Error bars represent standard deviations of triplicates. *, P < 0.05; **, P < 0.01 (versus medium control).
DC-SIGN facilitates measles virus infection through CD150.
DC-SIGN efficiently interacts with MV but does not mediate entry into target cells. We compared the infectivity of Raji cells to that of Raji cells expressing DC-SIGN to investigate whether DC-SIGN facilitates infection through CD150. Raji cells express CD46 and CD150, and Raji-DC-SIGN cells, in addition, express high levels of DC-SIGN (Fig. 6A). Cells were infected with different concentrations of MV WTF, and infection levels were measured using flow cytometry. Using a low multiplicity of infection (0.03), 25% ± 0.15% of Raji cells were infected, in contrast to 84.3% ±1.9% of Raji-DC-SIGN cells. Infection, as determined by MV H staining, was increased fivefold in Raji-DC-SIGN cells compared to that in Raji cells (Fig. 6B). The increased susceptibility was due to the expression of DC-SIGN, since pretreatment of the cells with mannan reduced the infection to the levels seen with Raji cells (data not shown). Also, at a higher infection ratio (MOI, 0.3), the Raji-DC-SIGN cells were significantly more susceptible to infection (Fig. 6B). These data demonstrate that DC-SIGN facilitates the infection of cells expressing DC-SIGN and CD150 in cis.
FIG. 6.
DC-SIGN enhances the infection of measles virus in cis. (A) Raji-DC-SIGN cells express CD46, CD150, and DC-SIGN. Open histograms represent isotype controls, and filled histograms indicate specific antibody staining. The mean fluorescence intensity of staining is depicted. Expression is shown in log scale and ranges from 100 to 104. (B) Raji cells and Raji-DC-SIGN cells were infected with different dilutions of MV WTF. MV H expression was measured at 48 h postinfection to determine the level of infection. Data for one representative experiment of two are shown. Error bars represent standard deviations of triplicates.
DC-SIGN enhances infection of immature DCs with measles virus.
In order to investigate the role of DC-SIGN in MV infection of DCs, we infected immature and mature DCs with measles virus. The mature phenotype was confirmed by flow cytometric analysis showing increased expression of the maturation markers CD83 and CD86. Moreover, the expression of CD150 and CD46 is increased on mature DCs, whereas the expression of DC-SIGN is decreased (Fig. 7A). We infected immature and mature monocyte-derived DCs with different concentrations of WTF and measured the expression of H after 48 h. The fusion inhibitory peptide was added directly after infection in order to prevent viral spread in the cultures so that the percentage of infected cells stained after 2 days represents that of initially infected DCs. Under these conditions, the MV H protein was detected in about 20 to 40% of immature DCs and 25 to 60% of mature DCs (Fig. 6B), confirming previous reports demonstrating that mature DCs are more susceptible to MV infection than immature DCs (27). Strikingly, for immature DCs treated with either the polycarbohydrate mannan or the DC-SIGN blocking antibody AZN-D2, the percentage of MV-infected cells was drastically reduced compared to that for the antibody or carbohydrate control, indicating that DC-SIGN plays an important role in MV uptake in immature DCs. In contrast, blocking DC-SIGN on mature DCs with either mannan or DC-SIGN antibody did not significantly reduce the level of infection. Blocking CD150 by using specific antibodies did not significantly block the infection of immature DCs by WTF. However, mature DCs and, to a lesser extent, immature DCs cultured without the fusion inhibitory protein showed less syncytium formation after pretreatment with an anti-CD150 antibody, confirming previous reports (31; data not shown). Unfortunately, the role of CD150 on mature DCs treated with the fusion inhibitory peptide could not be determined using flow cytometry, since the blocking antibody resulted in high background staining (data not shown). Therefore, we used a recombinant ED virus expressing EGFP (the expression of which is dependent on viral replication) in the presence of either CD46-, CD150-, or DC-SIGN-specific antibodies. While 18% of immature DCs were infected in the absence of antibodies, this percentage was significantly reduced in the presence of either CD46- or DC-SIGN-specific antibodies (Fig. 6C). Similar to the infection of mature DCs with WTF, we did not observe a block by antibodies to DC-SIGN. These data demonstrate that DC-SIGN is essential for binding of MV to immature DCs and that, although it is not functional as an entry receptor per se, when it is highly expressed it enhances the infection of immature DCs in cis.
FIG. 7.
DC-SIGN enhances MV infection of immature DCs. (A) High expression levels of maturation markers CD83 and CD86 on mature DCs indicate a mature phenotype. The expression of the different MV receptors was measured. Open histograms represent isotype controls, and filled histograms indicate specific antibody staining. The mean fluorescence intensity of specific staining is depicted. Expression is shown in log scale and ranges from 100 to 104. (B) Immature and mature DCs were infected with MV WTF (MOI, 0.3 and 1) or a mock control for 48 h. To determine the contribution of DC-SIGN, a specific antibody (AZN-D2) or mannan was used. An antibody (L7) or carbohydrate control (glucitol) was used as a control. With immature DCs, the role of CD150 could be determined by using a specific blocking antibody (5C6). To determine the level of infection, MV H expression was measured by flow cytometry. Percentages represent the numbers of infected cells. (C) Immature and mature DCs were infected with MV EDeGFP (MOI, 0.25) or a mock control for 48 h. Infection was determined by measuring GFP using flow cytometry. The MV receptors DC-SIGN, CD46, and CD150 were specifically blocked by antibodies (AZN-D2, 13/42, and 5C6, respectively) or a combination of the three antibodies to determine their roles in MV infection. Data for one representative experiment of three are shown. Error bars represent standard deviations of triplicates. *, P < 0.05; **, P < 0.01 (versus controls).
DISCUSSION
One of the major features of MV infection is the induction of a transient but severe immune suppression. DCs are thought to be major targets for MV and to be involved in the immunosuppressive effects during and after acute MV infection (35, 39). MV has been demonstrated to interfere with DC development and expansion, CD40 ligand-dependent DC maturation, and interferon production by plasmacytoid DCs (20, 33, 38). Furthermore, MV-infected DCs fail to stimulate and suppress T-cell proliferation (13, 19, 21, 36, 40), and MV enhances FAS-mediated DC apoptosis of DC-T-cell cocultures (37).
DCs express various receptors that interact with pathogens, and recent data suggest that pathogens can target specific receptors to escape immune surveillance. Several pathogens, such as HIV-1, Dengue virus, and Mycobacterium tuberculosis, specifically interact with the DC-specific C-type lectin DC-SIGN to subvert DC function for viral dissemination and immune modulation (43).
Using DC-SIGN-Fc capture ELISA, we demonstrated that recombinant DC-SIGN interacts with both MV strains ED and WTF (Fig. 1). The C-type lectin domain of DC-SIGN has specificities for both mannose-containing carbohydrates and Lewis antigens (15), and interactions with the viral glycoproteins of HIV-1, Ebola virus, and hepatitis C virus are dependent on high-mannose structures presenting N-linked glycosylations (25, 26). Our data demonstrate that DC-SIGN interacts with MV through binding of both the MV F and H glycoproteins (Fig. 2). These MV glycoproteins have been shown to contain N-linked glycosylations (4, 23), suggesting that DC-SIGN interacts with the N-linked glycosylations of these MV glycoproteins.
To investigate MV binding to cellular receptors, the previously described bead adhesion assay was adapted, using MV virions to coat fluorescent beads (17). MV-coated beads bind specifically to the known MV receptors CD46 and CD150 on different cell lines (Fig. 3), demonstrating that the fluorescent bead adhesion assay can be used to investigate the interactions of MV with cellular receptors specifically. Using DC-SIGN transfectants, we identified the DC-specific C-type lectin DC-SIGN as a novel receptor for MV (Fig. 3). The high-affinity binding of CD46 with vaccine strains (2, 10, 24) was confirmed, since the erythroleukemic cell line K562, expressing CD46 but not CD150, interacted with the vaccine strain ED but not with the wild-type strain WTF. Strikingly, WTF did interact with K562 transfectants expressing DC-SIGN, and the interaction was completely dependent on DC-SIGN, demonstrating that DC-SIGN is a receptor for WTF (Fig. 3). The expression of DC-SIGN by K562 transfectants did not increase binding to ED compared to that of mock-transfected K562 cells. This might be due to a saturation of bead binding by K562 cells. However, only the combination of antibodies against DC-SIGN and CD46 could efficiently inhibit binding. This demonstrates that DC-SIGN contributes to the interaction of ED with these cells.
Immature DCs express both MV receptors CD46 and CD150 and high levels of DC-SIGN (Fig. 5 and 7) (31). Both WTF and ED interacted strongly with immature DCs, and the interaction was blocked by antibodies against DC-SIGN. This was due to the inhibition of DC-SIGN, since the antibodies against DC-SIGN are specific and do not inhibit binding of MV to CD46 or CD150 (data not shown). Our results demonstrate that DC-SIGN is an important attachment receptor for MV on immature DCs, in addition to CD150 (Fig. 5). Although CD46 has been shown to support MV infection of human DCs (27, 34), we did not observe any significant block of MV binding to DCs with antibodies against CD46, suggesting that CD46 is not involved as an attachment receptor for MV, in contrast to CD150 and DC-SIGN. The incomplete block using the combination of antibodies suggests that another receptor might be present; however, we cannot exclude that the presence of two or more receptors prevents a complete block using the respective antibodies. Although the MV-coated bead binding assay is useful for investigating the participation of different receptors on cells, a comparison between affinities of different receptors cannot be made. Binding affinity studies comparing the binding of MV to CD46, CD150, and DC-SIGN should be made to ultimately draw this conclusion.
Next, we investigated the contribution of DC-SIGN to the infection of DCs, using infectious MV. DCs are efficiently infected by MV (Fig. 7) (19). Since DCs express the MV entry receptor CD150 and the MV vaccine strain receptor CD46, CHO transfectants had to be used to address the issue of whether DC-SIGN supports the uptake of MV by itself. We demonstrated that DC-SIGN does not function as an entry receptor such as CD46 and CD150, since CHO cells expressing high levels of DC-SIGN were not infected with MV (Fig. 4). However, different viruses, such as Ebola virus and dengue virus, have been shown to target DC-SIGN to facilitate DC infection (1, 30). Raji cells expressing CD46 and CD150 can be infected by MV. Using Raji transfectants expressing high levels of DC-SIGN, we demonstrated that DC-SIGN efficiently enhances infection by MV WTF in cis (Fig. 6). Moreover, blocking studies demonstrated that DC-SIGN is crucial for immature DC infection by MV WTF and is important, in addition to CD46, for infection by MV ED (Fig. 7). These data support an important function for DC-SIGN as an attachment receptor for MV that enhances the infection of immature DCs in cis through CD46 or CD150. Our data suggest that MV uses a two-step mechanism to infect immature DCs: MV targets DC-SIGN to interact with the MV receptors CD150 and CD46 that mediate direct infection of DCs. The expression levels of CD46, CD150, and DC-SIGN are variable throughout the different activation stages of a DC. Monocyte-derived immature DCs express intermediate levels of both CD150 and CD46 and high levels of DC-SIGN, whereas immature DCs in vivo express low levels of CD150, intermediate levels of CD46, and high levels of DC-SIGN (22). This supports the importance of DC-SIGN as an attachment receptor for MV. MV infection of immature DCs in the periphery most likely depends on the initial interaction with DC-SIGN, which mediates infection in cis through the MV receptors CD150 and CD46. Mature DCs express high levels of both CD150 and CD46 and low levels of DC-SIGN (Fig. 7) (22). Indeed, we observed that the antibodies against DC-SIGN were not effective at blocking infection of mature DCs with both ED and WTF (Fig. 7). This may be a combined effect of the decreased expression of DC-SIGN and the increased expression of CD46 and CD150. This was supported by our data showing that CD46 and CD150 antibodies block the infection of mature DCs by ED and that CD150 antibodies block syncytium formation by WTF in mature DCs.
Viruses such as HIV-1 exploit DC-SIGN for their dissemination (17); DC-SIGN captures virus in the periphery, protects it from the innate immune system, and transports it to the lymphoid tissues, where lymphoid target cells are efficiently infected. MV enters the body at the respiratory tract, where it either first infects the epithelial cells or directly infects immature DCs. Our data suggest that DC-SIGN might be important in the first interaction of MV with these immature DCs to facilitate infection. This is further supported by the expression of DC-SIGN by DCs localized in the respiratory tract (41; data not shown). Further studies will be needed to specifically investigate the function of DC-SIGN in MV spread by these DCs.
The interaction of MV with DC-SIGN could directly lead to an immunosuppressive phenotype. Viruses seem to target DC-SIGN primarily for infection of DCs and/or viral transmission, whereas DC-SIGN interactions with nonviral pathogens modulate DC-induced immune responses, leading to immune suppression by regulatory T cells or shifting of the Th1/Th2 balance away from a favorable (for the host) outcome (9). The interaction of the cell wall component of M. tuberculosis with DC-SIGN leads to a suppression of DC maturation and the induction of IL-10, probably through cross talk between DC-SIGN and Toll-like receptors (16). Interestingly, MV infection of DCs is characterized by a block in maturation and induces IL-10 (38, 39). Moreover, MV WTF interacts with Toll-like receptor 2 (3), suggesting that the DC-SIGN interaction with MV might modulate DC function, similar to the case seen for M. tuberculosis. In addition to its role in enhancing MV infection of DCs in cis (and eventually T-cell infection in trans), this triggering of DC-SIGN by MV might regulate DC functions and thus be important in modulating T-cell responses on a quantitative and qualitative basis.
Acknowledgments
This work was supported by a grant from the Dutch Scientific Research program (NWO; no. 917-46-367).
We thank Paul Duprex for providing us with the EDeGFP virus and Rik de Swart for critical comments and providing us with the Meljuso transfectants.
REFERENCES
- 1.Alvarez, C. P., F. Lasala, J. Carrillo, O. Muniz, A. L. Corbi, and R. Delgado. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartz, R., R. Firsching, B. Rima, V. ter Meulen, and J. Schneider-Schaulies. 1998. Differential receptor usage by measles virus strains. J. Gen. Virol. 79:1015-1025. [DOI] [PubMed] [Google Scholar]
- 3.Bieback, K., E. Lien, I. M. Klagge, E. Avota, J. Schneider-Schaulies, W. P. Duprex, H. Wagner, C. J. Kirschning, V. ter Meulen, and S. Schneider-Schaulies. 2002. Hemagglutinin protein of wild-type measles virus activates Toll-like receptor 2 signaling. J. Virol. 76:8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolt, G., I. R. Pedersen, and M. Blixenkrone-Moller. 1999. Processing of N-linked oligosaccharides on the measles virus glycoproteins: importance for antigenicity and for production of infectious virus particles. Virus Res. 61:43-51. [DOI] [PubMed] [Google Scholar]
- 5.Borrow, P., and M. B. Oldstone. 1995. Measles virus-mononuclear cell interactions. Curr. Top. Microbiol. Immunol. 191:85-100. [DOI] [PubMed] [Google Scholar]
- 6.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383-387. (Erratum, 257:1848.) [DOI] [PubMed] [Google Scholar]
- 7.Clements, C. J., and F. T. Cutts. 1995. The epidemiology of measles: thirty years of vaccination. Curr. Top. Microbiol. Immunol. 191:13-33. [DOI] [PubMed] [Google Scholar]
- 8.de Swart, R. L., H. W. Vos, F. G. UytdeHaag, A. D. Osterhaus, and R. S. van Binnendijk. 1998. Measles virus fusion protein- and hemagglutinin-transfected cell lines are a sensitive tool for the detection of specific antibodies by a FACS-measured immunofluorescence assay. J. Virol. Methods 71:35-44. [DOI] [PubMed] [Google Scholar]
- 9.de Witte, L., Y. Kooyk, and T. B. Geijtenbeek. 2005. Pathogen interactions with DC-SIGN modulate immune responses: a Toll tale? Curr. Immunol. Rev. 1:157-164. [Google Scholar]
- 10.Dorig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 11.Duprex, W. P., S. McQuaid, L. Hangartner, M. A. Billeter, and B. K. Rima. 1999. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J. Virol. 73:9568-9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlenhoefer, C., W. J. Wurzer, S. Loffler, S. Schneider-Schaulies, V. ter Meulen, and J. Schneider-Schaulies. 2001. CD150 (SLAM) is a receptor for measles virus but is not involved in viral contact-mediated proliferation inhibition. J. Virol. 75:4499-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M. C. Rissoan, Y. J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geijtenbeek, T. B., G. C. van Duijnhoven, S. J. van Vliet, E. Krieger, G. Vriend, C. G. Figdor, and Y. van Kooyk. 2002. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J. Biol. Chem. 277:11314-11320. [DOI] [PubMed] [Google Scholar]
- 15.Geijtenbeek, T. B., S. J. van Vliet, A. Engering, B. A. 't Hart, and Y. van Kooyk. 2004. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu. Rev. Immunol. 22:33-54. [DOI] [PubMed] [Google Scholar]
- 16.Geijtenbeek, T. B., S. J. van Vliet, E. A. Koppel, M. Sanchez-Hernandez, C. M. Vandenbroucke-Grauls, B. Appelmelk, and Y. van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geijtenbeek, T. B. H., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. F. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 18.Geijtenbeek, T. B. H., R. Torensma, S. J. van Vliet, G. C. F. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 19.Grosjean, I., C. Caux, C. Bella, I. Berger, F. Wild, J. Banchereau, and D. Kaiserlian. 1997. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J. Exp. Med. 186:801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahm, B., M. J. Trifilo, E. I. Zuniga, and M. B. Oldstone. 2005. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity 22:247-257. [DOI] [PubMed] [Google Scholar]
- 21.Klagge, I. M., V. ter Meulen, and S. Schneider-Schaulies. 2000. Measles virus-induced promotion of dendritic cell maturation by soluble mediators does not overcome the immunosuppressive activity of viral glycoproteins on the cell surface. Eur. J. Immunol. 30:2741-2750. [DOI] [PubMed] [Google Scholar]
- 22.Kruse, M., E. Meinl, G. Henning, C. Kuhnt, S. Berchtold, T. Berger, G. Schuler, and A. Steinkasserer. 2001. Signaling lymphocytic activation molecule is expressed on mature CD83+ dendritic cells and is up-regulated by IL-1 beta. J. Immunol. 167:1989-1995. [DOI] [PubMed] [Google Scholar]
- 23.Lamb, R. A. 1993. Paramyxovirus fusion: a hypothesis for changes. Virology 197:1-11. [DOI] [PubMed] [Google Scholar]
- 24.Lecouturier, V., J. Fayolle, M. Caballero, J. Carabana, M. L. Celma, R. Fernandez-Munoz, T. F. Wild, and R. Buckland. 1996. Identification of two amino acids in the hemagglutinin glycoprotein of measles virus (MV) that govern hemadsorption, HeLa cell fusion, and CD46 downregulation: phenotypic markers that differentiate vaccine and wild-type MV strains. J. Virol. 70:4200-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, G., G. Simmons, S. Pohlmann, F. Baribaud, H. Ni, G. J. Leslie, B. S. Haggarty, P. Bates, D. Weissman, J. A. Hoxie, and R. W. Doms. 2003. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 77:1337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lozach, P. Y., H. Lortat-Jacob, A. De Lacroix De Lavalette, I. Staropoli, S. Foung, A. Amara, C. Houles, F. Fieschi, O. Schwartz, J. L. Virelizier, F. Arenzana-Seisdedos, and R. Altmeyer. 2003. DC-SIGN and L-SIGN are high-affinity binding receptors for hepatitis C virus glycoprotein E2. J. Biol. Chem. 278:20358-20366. [DOI] [PubMed] [Google Scholar]
- 27.Murabayashi, N., M. Kurita-Taniguchi, M. Ayata, M. Matsumoto, H. Ogura, and T. Seya. 2002. Susceptibility of human dendritic cells (DCs) to measles virus (MV) depends on their activation stages in conjunction with the level of CDw150: role of Toll stimulators in DC maturation and MV amplification. Microbes Infect. 4:785-794. [DOI] [PubMed] [Google Scholar]
- 28.Murray, C. J., and A. D. Lopez. 1997. Mortality by cause for eight regions of the world. Global Burden of Disease Study. Lancet 349:1269-1276. [DOI] [PubMed] [Google Scholar]
- 29.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarro-Sanchez, E., R. Altmeyer, A. Amara, O. Schwartz, F. Fieshi, J. L. Virelizier, F. Arenzana-Seisdedos, and P. Despres. 2003. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 4:723-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohgimoto, S., K. Ohgimoto, S. Niewiesk, I. M. Klagge, J. Pfeuffer, I. C. Johnston, J. Schneider-Schaulies, A. Weidmann, V. ter Meulen, and S. Schneider-Schaulies. 2001. The haemagglutinin protein is an important determinant of measles virus tropism for dendritic cells in vitro. J. Gen. Virol. 82:1835-1844. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlender, J., V. Hornung, S. Finke, M. Gunthner-Biller, S. Marozin, K. Brzozka, S. Moghim, S. Endres, G. Hartmann, and K. K. Conzelmann. 2005. Inhibition of Toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 79:5507-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider, U., V. von Messling, P. Devaux, and R. Cattaneo. 2002. Efficiency of measles virus entry and dissemination through different receptors. J. Virol. 76:7460-7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider-Schaulies, S., I. M. Klagge, and V. ter Meulen. 2003. Dendritic cells and measles virus infection. Curr. Top. Microbiol. Immunol. 276:77-101. [DOI] [PubMed] [Google Scholar]
- 36.Schnorr, J. J., S. Xanthakos, P. Keikavoussi, E. Kampgen, V. ter Meulen, and S. Schneider-Schaulies. 1997. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc. Natl. Acad. Sci. USA 94:5326-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Servet-Delprat, C., P. O. Vidalain, O. Azocar, F. Le Deist, A. Fischer, and C. Rabourdin-Combe. 2000. Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus. J. Virol. 74:4387-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Servet-Delprat, C., P. O. Vidalain, H. Bausinger, S. Manie, F. Le Deist, O. Azocar, D. Hanau, A. Fischer, and C. Rabourdin-Combe. 2000. Measles virus induces abnormal differentiation of CD40 ligand-activated human dendritic cells. J. Immunol. 164:1753-1760. [DOI] [PubMed] [Google Scholar]
- 39.Servet-Delprat, C., P. O. Vidalain, H. Valentin, and C. Rabourdin-Combe. 2003. Measles virus and dendritic cell functions: how specific response cohabits with immunosuppression. Curr. Top. Microbiol. Immunol. 276:103-123. [DOI] [PubMed] [Google Scholar]
- 40.Steineur, M. P., I. Grosjean, C. Bella, and D. Kaiserlian. 1998. Langerhans cells are susceptible to measles virus infection and actively suppress T cell proliferation. Eur. J. Dermatol. 8:413-420. [PubMed] [Google Scholar]
- 41.Tailleux, L., O. Schwartz, J. L. Herrmann, E. Pivert, M. Jackson, A. Amara, L. Legres, D. Dreher, L. P. Nicod, J. C. Gluckman, P. H. Lagrange, B. Gicquel, and O. Neyrolles. 2003. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893-897. [DOI] [PubMed] [Google Scholar]
- 43.van Kooyk, Y., and T. B. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3:697-709. [DOI] [PubMed] [Google Scholar]
- 44.Wu, L., T. D. Martin, M. Carrington, and V. N. KewalRamani. 2004. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology 318:17-23. [DOI] [PubMed] [Google Scholar]