Abstract
Expression of the herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 in transfected cells reactivates rep gene expression from integrated adeno-associated virus (AAV) type 2 genomes via a mechanism that requires both its RING finger and USP7 interaction domains. In this study, we found that the rep reactivation defect of USP7-binding-negative ICP0 mutants can be overcome by further deletion of sequences in the C-terminal domain of ICP0, indicating that binding of USP7 to ICP0 is not directly required. Unlike the case in transfected cells, only the RING finger domain of ICP0 was essential for rep gene reactivation during HSV-1 infection. However, mutants unable to bind to USP7 activate HSV-1 gene expression and reactivate rep gene expression with reduced efficiencies. These results further elucidate the role of ICP0 as a helper factor for AAV replication and illustrate that care is required when extrapolating from the properties of ICP0 in transfection assays to events occurring during HSV-1 infection.
We have previously demonstrated that the herpes simplex virus type 1 (HSV-1) ICP0 protein promotes the reactivation of rep gene expression in HA-16 cells latently infected with wild-type adeno-associated virus type 2 (AAV-2) (7). The AAV-2 rep gene encodes regulatory proteins, in particular Rep78 and Rep68, that are essential for AAV replication and gene expression and, consequently, for the onset of the AAV life cycle. The synthesis of these proteins is under the control of the p5 promoter, which is naturally repressed during AAV latency. How repression of the p5 promoter is maintained during latency and how it is relieved in the presence of a helper virus like HSV-1 is for the moment unknown. Two ICP0 regions are important for rep gene activation in transfected cells: the RING finger domain, which confers E3 ubiquitin ligase activity (2), and the domain involved in its interaction with the ubiquitin-specific protease USP7 (6). USP7 contributes to the functions of ICP0, since mutants unable to bind USP7 activate gene expression with reduced efficiency both in transfection reporter assays and during HSV-1 infection (1, 5, 10). Recently, it was demonstrated that USP7 protects ICP0 from autoubiquitination and proteasome-dependent degradation, thereby increasing the efficiency of ICP0 expression during HSV-1 infection (3). In this study, we investigated whether the interaction between ICP0 and USP7 was directly involved in the mechanism by which ICP0 activates rep gene expression.
USP7 is not strictly required for ICP0-induced rep gene expression.
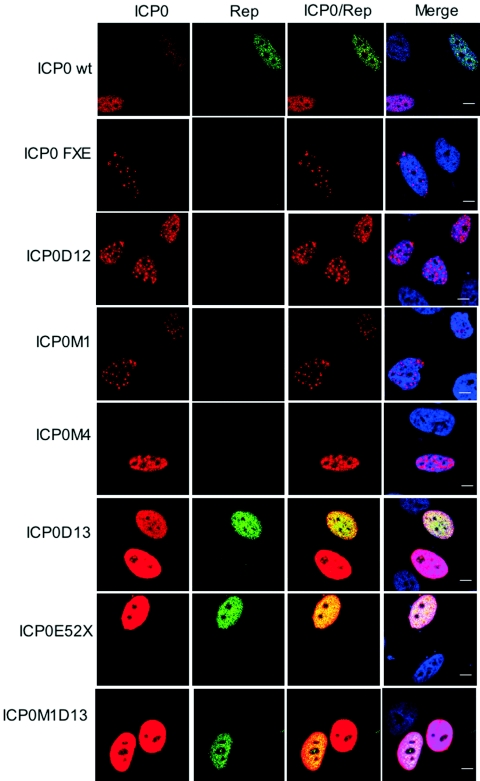
To evaluate the effect of USP7 during ICP0-mediated rep gene reactivation, plasmids expressing a panel of insertion and deletion mutants affecting the USP7 binding domain and regions toward the C terminus of ICP0 (Fig. 1) (5, 10, 11) were transfected into HA-16 cells, and rep gene expression was analyzed by immunofluorescence (7, 13). In comparison with the plasmid expressing wild-type (wt) ICP0, the Rep signal was extremely low or undetectable in cells transfected with ICP0 mutants that have a defect in USP7 binding (M1, M4, and D12) (Fig. 2). In contrast, the ICP0-D13 mutant that has lost sequences required for self multimerization and localization to ND10 but retained the ability to interact with USP7 (10) activated rep gene expression efficiently. Removal of the RING finger from ICP0 (mutant FXE) eliminated rep gene reactivation (Fig. 2), as described in our previous report (7).
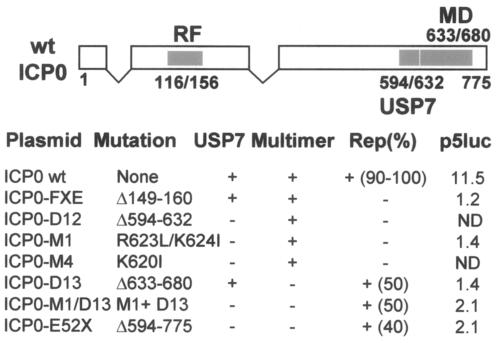
FIG. 1.
Locations and properties of ICP0 mutants used in this study. Numbers refers to amino acid positions. Filled boxes indicate the positions of the RING Finger (RF), the USP7 binding region (USP7) and the self-multimerization (MD) domain. The data below the schematic view of the ICP0 gene indicate the mutations present in each ICP0 expression plasmid and their ability to bind USP7 (USP7), to multimerize (Multimer), to induce rep gene expression in transfected HA-16 cells (Rep), and to activate a cotransfected p5luc plasmid expressing the luciferase gene under the control of the p5 rep gene promoter. The numbers indicate either the percentages of Rep- expressing cells among those expressing ICP0 (as illustrated in Fig. 2) or the p5luc n-fold activation over basal levels by wt ICP0 and the various mutants. ND, not done. The original characterization of these mutant ICP0 proteins can be found in references (5, 10, and 11).
FIG. 2.
Rep gene activation by transfection of different ICP0 mutant constructs. HA-16 cells were transfected with the indicated ICP0-expressing constructs and analyzed 24 h later by immunofluorescence using anti-ICP0 rabbit serum and anti-Rep mouse antibody (76.3) as described previously (7). The ICP0 signal was detected with a secondary tetramethyl rhodamine isothiocyanate-conjugated antirabbit antibody, and the Rep signal was detected with a fluorescein isothiocyanate-conjugated antimouse antibody. The last column shows merged images of both labeling schemes including staining of the nucleus with TO-PRO-3. Bars, 8 μm.
We next analyzed the effect of the ICP0 deletion mutant E52X, which lacks the C-terminal 180 residues that include sequences required for the interaction with USP7, for self-multimerization, and for localization to ND10 (4, 9, 10). Surprisingly, despite being unable to bind to USP7, mutant E52X reactivated rep gene expression efficiently (Fig. 2), indicating that binding of USP7 was not an absolute requirement for rep gene activation by ICP0. This conclusion was confirmed by constructing an ICP0 mutant that combines the M1 and D13 lesions (ICP0-M1D13). This double mutant was able to reactivate rep gene expression as efficiently as D13 (Fig. 2), indicating that the defect conferred by the M1 mutation could be overridden by removal of ICP0 sequences on the C-terminal side of the USP7 binding domain.
Investigation of the properties of rep reactivation-proficient and -deficient ICP0 mutants.
The observation that mutations in the minimal USP7 binding domain, such as M1, M4, and D12, are unable to reactivate rep gene expression in transfected cells, while lesions that affect both the USP7 binding domain and sequences further downstream (mutants E52X and M1D13) regain this activity, prompted us to examine the properties of these mutant proteins in a variety of assays. We found that rep gene reactivation in cells latently infected with AAV-2 did not correlate simply with the transactivation properties of ICP0 in transient assays. Consistent with earlier studies using other reporter genes (5), mutants M1, D12, D13, and E52X were all defective in activating the AAV-2 rep gene promoter (p5) in a cotransfected luciferase reporter plasmid (Fig. 1). Therefore, reactivation of an integrated, repressed rep gene by ICP0 differs in some way from its effects on a cotransfected plasmid containing the p5 promoter.
We next investigated the properties of the mutant forms of ICP0 that are relevant to this study during infection of HeLa cells (from which HA-16 cells were derived [13]). As expected from previous studies (1), mutations in the minimal USP7 binding domain reduced the rate of accumulation of ICP0. Surprisingly, however, this defect was corrected in mutants E52X and M1D13 (Fig. 3A and B). All of the mutants exhibited substantial reductions in the rate of accumulation of UL42, a representative member of the early class of HSV-1 genes. Therefore, defects in ICP0-mediated activation of a reporter gene in transfection assays correlate with reduced rates of accumulation of UL42 during infection. In contrast, only those ICP0 mutant proteins that accumulate with reduced efficiency during HSV-1 infection exhibit a defect in rep gene reactivation in transfected cells.
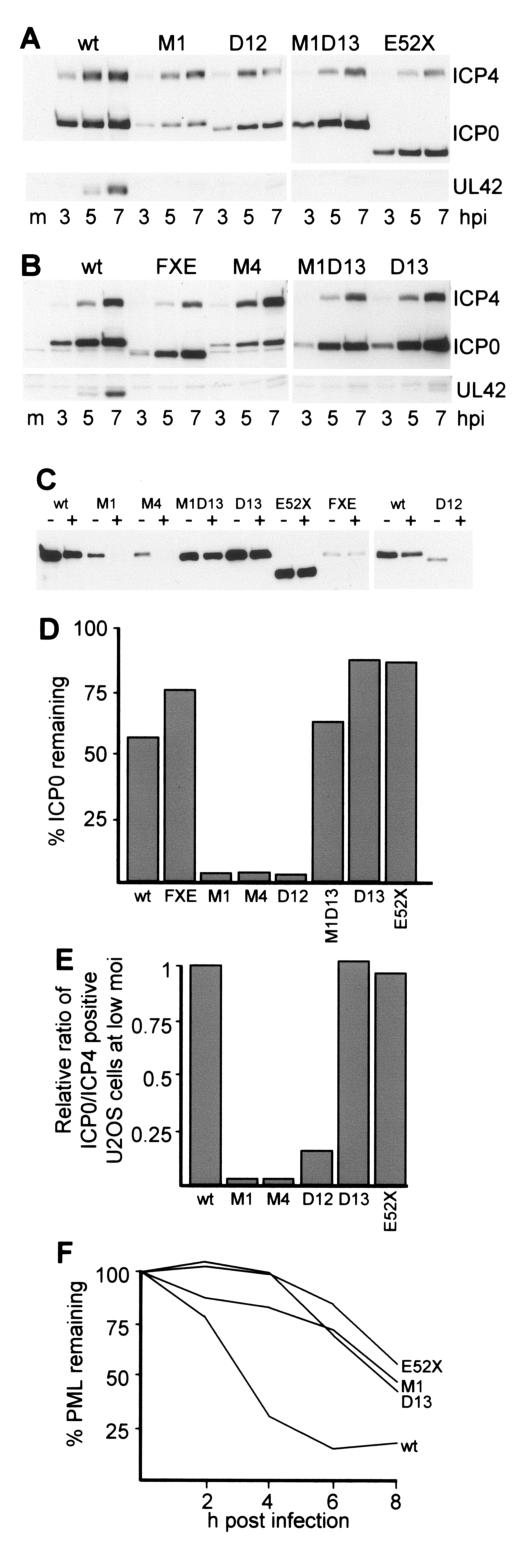
FIG. 3.
Properties of ICP0 mutants with lesions in the USP7 binding and C-terminal domains. A and B. Rate of ICP0 accumulation. HeLa cells were infected with the indicated HSV-1 viruses at a MOI of 5 PFU/ml (based on titrations in U2OS cells), and then whole-cell extracts were harvested at 3, 5, and 7 h postinfection (hpi). The samples were analyzed by Western blotting for ICP4, ICP0, and UL42 as described previously (1). C. Accumulation and stability of ICP0. HeLa cells were infected in duplicate with viruses expressing the wt and indicated mutant forms of ICP0 at a MOI of 2. In this experiment, wt ICP0 was expressed by dl1403R, the wt rescuant of the ICP0-null mutant virus dl1403, from which all the other ICP0 mutant viruses were derived. At 3 h pi, one well of each duplicate was harvested and the other was treated with cycloheximide at 100 μg/ml. These wells were harvested 1 h later. ICP0 levels were analyzed by Western blotting. D. Nonsaturated exposures of the Western blot results in C were scanned, and the relative levels of ICP0 were determined by densitometry. E. FACS analysis of ICP0- and ICP4-positive cells after infection of U2OS cells at a MOI of 0.1 PFU/cell. Cells in 35-mm dishes were harvested 5 h after infection, and duplicate samples were processed for detection of ICP4- and ICP0-positive cells as described previously (3). In all infections about 50% of the cells were ICP4 positive. The ratio of ICP0- to ICP4-positive cells in each infection was calculated and was expressed as a fraction of that obtained with wt HSV-1. F. Relative rates of PML degradation induced by wt and mutant ICP0 proteins. HeLa cells were infected with the indicated viruses at a MOI of 10 PFU/cell, and then parallel samples were harvested at 2, 4, 6, and 8 h after infection. Whole-cell extracts were analyzed for PML by Western blotting. The intensity of the major PML band was determined by densitometry, and the amount remaining was expressed as a proportion of that in the mock-infected control.
The increased levels of expression of the ICP0 mutant proteins D13, M1D13, and E52X compared to those of M1, M4, and D12 (Fig. 3A and B) can be attributed to the former group of proteins being more stable than the latter group at early times of infection and at their respective levels of synthesis in HeLa cells (Fig. 3C and D). The defect in accumulation of mutant M1 ICP0 is also strikingly illustrated by a substantial defect in the number of ICP0-positive cells compared to those expressing ICP4 as detected by fluorescence-activated cell sorting analysis after low-multiplicity-of-infection (MOI) infection of U2OS cells (3). Analysis of a selection of the relevant mutants using this assay clearly demonstrated that M1, M4, and to a lesser extent D12 exhibit a substantial underrepresentation of ICP0-positive cells after low-MOI infection. This defect does not occur in deletion mutant D13 and E52X infections (Fig. 3E).
There is no obvious explanation of why removal of C-terminal sequences of ICP0 results in a more stable protein, even in the absence of USP7 binding. The D13, E52X, and M1D13 mutant proteins lack sequences required for multimerization of ICP0 and are more diffusely spread through the nucleoplasm (Fig. 2). Therefore, it is possible that autoubiquitination of ICP0 is increased either by its self-interaction or when the protein is initially resident in ND10. Although there is a correlation between the stabilities of these ICP0 mutant proteins at early times of infection and their ability to reactivate rep gene expression in transfected HA-16 cells, we cannot conclude that ICP0 stability is a crucial factor, because all of the ICP0 proteins analyzed here accumulate to similar levels in transfection experiments analogous to those of Fig. 2 (data not shown). We can conclude, however, that ICP0 mutant proteins that fail to reactivate rep gene expression in transfected HA-16 cells also accumulate less efficiently during HSV-1 infection.
Finally, we investigated the effects of selected mutations on ICP0-induced PML degradation. Consistent with results of an earlier study using HFFF-2 cells (1), the M1 mutation decreased the rate at which PML was degraded in HeLa cells (Fig. 3F). Previous work had established that mutants M1, M4, and D12 disrupt ND10 more slowly than the wt (5). These effects are likely to be due to reduced rates of accumulation of the mutant ICP0 proteins (Fig. 3A), resulting in inefficient PML degradation. Despite their normal rates of accumulation, mutants D13 and E52X also degraded PML with reduced kinetics (Fig. 3F), probably due to their inefficient localization at ND10 (9, 10). Therefore, the gene expression and reporter gene activation defects caused by the D13 and E52X mutations correlate with reduced effects on PML and hence ND10, but despite these defects, these mutant ICP0 proteins reactivate rep gene expression efficiently in transfected cells.
Comparison of reactivation of rep gene expression by ICP0 in transfected and infected cells.
The results presented above suggest that there is a clear difference between the abilities of selected ICP0 mutants to activate rep gene expression in transfected cells and to activate HSV-1 early gene expression efficiently during infection. Therefore, we investigated reactivation of rep gene expression during HSV-1 infection of HA-16 cells, rather than after transfection of ICP0 alone. At 8 h postinfection and with the notable exception of mutant FXE, all the HSV-1 mutants reactivated rep gene expression, in contrast to the results using ICP0 alone in transfected cells. However, all the mutants carrying lesions in the USP7 binding and C-terminal domains induced Rep protein synthesis at a lower level than wt HSV-1, despite the wt, D13, M1D13, and E52X ICP0 proteins accumulating to similar levels (Fig. 4A). Consistent with the experiment of Fig. 3A, expression of UL29, a major HSV-1 helper factor for AAV replication (8, 12, 14), was reduced at this time point in the mutant virus infections (Fig. 4A). The differences between wt HSV-1 and the mutant viruses (except FXE) were essentially eliminated after 24 h of infection (Fig. 4B). Therefore, at the multiplicity used here and in these HeLa-derived cells, the HSV-1 mutant viruses with lesions in the USP7 binding domain and adjacent regions exhibit only a delay in the accumulation of HSV-1 replication proteins and reactivation of AAV rep expression.
FIG. 4.
Effect of wt and mutated HSV strains on the induction of Rep protein synthesis in HA-16 cells. A and B. HA-16 cells were infected in duplicate at a MOI of 5 with HSV-1 viruses as indicated. At 8 h (panel A) and 24 h (panel B) after infection, the cells were washed and lysed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer and analyzed by Western blotting as described previously (7). ICP0, UL29 (detected using a rabbit polyclonal antibody), and tubulin (monoclonal antibody T5168; Sigma) were detected after successive reprobing of the same membrane, whereas ICP4 was detected on a different membrane.
The apparent discrepancy between rep gene reactivation by ICP0 mutants D13, M1D13, and E52X in transfected cells and during HSV-1 infection can be explained as follows. In transfected HA-16 cells expressing ICP0, the Rep proteins are expressed, but this is not sufficient to initiate AAV DNA replication. However, in infected cells the presence of other HSV-1 proteins enables productive replication of AAV genomes and a corresponding increase in rep gene copy number. Therefore, the expression of the Rep proteins will be amplified by a factor that will be proportional to the expression levels of the relevant helper HSV-1 DNA replication proteins. Because the M1D13, D13, and E52X mutations all delay HSV-1 early gene expression in the infection protocol used, AAV replication will in turn be delayed. Therefore, the intrinsic ability of these mutant proteins to activate Rep78 expression as efficiently as wt ICP0 when expressed in transfected cells will be masked during HSV-1 infection by the cognate virus mutants being less able to stimulate AAV replication.
The experiments presented here illustrate an experimental issue that may complicate many investigations, namely, that the behavior of wt and mutant ICP0 proteins in transfection assays may not simply correlate with the situation in virus-infected cells. In transfected cells, we have shown that interaction with USP7 is not directly required for ICP0 to reactivate rep gene transcription from quiescent, integrated AAV genomes and that this activity does not simply correlate with the ability of ICP0 to transactivate gene expression from cotransfected reporter plasmids. Reactivation of rep gene expression in HSV-1-infected cells also requires ICP0, but once reactivation has occurred, the level of Rep protein expression is influenced by the expression of other HSV-1 proteins.
Acknowledgments
We thank Juergen Kleinschmidt for providing the 303.9 and 76.3 antibodies and Sandra Weller and Mark Challberg for the anti-UL29 antibody.
This work was supported by the U.K. Medical Research Council, INSERM, the Association Française contre les Myopathies (AFM), Vaincre les Maladies Lysosomales (VML), Association Nantaise de Thérapie Génique (ANTG), and the Fondation pour la Thérapie Génique en Pays de la Loire.
REFERENCES
- 1.Boutell, C., M. Canning, A. Orr, and R. D. Everett. 2005. Reciprocal activities between herpes simplex virus type 1 regulatory protein ICP0, a ubiquitin E3 ligase, and ubiquitin-specific protease USP7. J. Virol. 79:12342-12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canning, M., C. Boutell, J. Parkinson, and R. D. Everett. 2004. A RING finger ubiquitin ligase is protected from autocatalyzed ubiquitination and degradation by binding to ubiquitin-specific protease USP7. J. Biol. Chem. 279:38160-38168. [DOI] [PubMed] [Google Scholar]
- 4.Everett, R. D., and G. G. Maul. 1994. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 13:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everett, R. D., M. Meredith, and A. Orr. 1999. The ability of herpes simplex virus type 1 immediate-early protein Vmw110 to bind to a ubiquitin-specific protease contributes to its roles in the activation of gene expression and stimulation of virus replication. J. Virol. 73:417-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everett, R. D., M. Meredith, A. Orr, A. Cross, M. Kathoria, and J. Parkinson. 1997. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 16:1519-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geoffroy, M. C., A. L. Epstein, E. Toublanc, P. Moullier, and A. Salvetti. 2004. Herpes simplex virus type 1 ICP0 protein mediates activation of adeno-associated virus type 2 rep gene expression from a latent integrated form. J. Virol. 78:10977-10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heilbronn, R., M. Engstler, S. Weger, A. Krahn, C. Schetter, and M. Boshart. 2003. ssDNA-dependent colocalization of adeno-associated virus Rep and herpes simplex virus ICP8 in nuclear replication domains. Nucleic Acids Res. 31:6206-6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maul, G. G., and R. D. Everett. 1994. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J Gen. Virol. 75:1223-1233. [DOI] [PubMed] [Google Scholar]
- 10.Meredith, M., A. Orr, M. Elliott, and R. Everett. 1995. Separation of sequence requirements for HSV-1 Vmw110 multimerisation and interaction with a 135-kDa cellular protein. Virology 209:174-187. [DOI] [PubMed] [Google Scholar]
- 11.Meredith, M., A. Orr, and R. Everett. 1994. Herpes simplex virus type 1 immediate-early protein Vmw110 binds strongly and specifically to a 135-kDa cellular protein. Virology 200:457-469. [DOI] [PubMed] [Google Scholar]
- 12.Stracker, T. H., G. D. Cassell, P. Ward, Y. M. Loo, B. van Breukelen, S. D. Carrington-Lawrence, R. K. Hamatake, P. C. van der Vliet, S. K. Weller, T. Melendy, and M. D. Weitzman. 2004. The Rep protein of adeno-associated virus type 2 interacts with single-stranded DNA-binding proteins that enhance viral replication. J. Virol. 78:441-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walz, C., and J. R. Schlehofer. 1992. Modification of some biological properties of HeLa cells containing adeno-associated virus DNA integrated into chromosome 17. J. Virol. 66:2990-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weindler, F. W., and R. Heilbronn. 1991. A subset of herpes simplex virus replication genes provides helper functions for productive adeno-associated virus replication. J. Virol. 65:2476-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]