Abstract
Human epithelial cells infected with the parainfluenza virus simian virus 5 (SV5) show minimal activation of host cell interferon (IFN), cytokine, and cell death pathways. In contrast, a recombinant SV5 P/V gene mutant (rSV5-P/V-CPI−) overexpresses viral gene products and is a potent inducer of IFN, proinflammatory cytokines, and apoptosis in these cells. In this study, we have compared the outcomes of wild-type (WT) SV5 and rSV5-P/V-CPI− infections of primary human dendritic cells (DC), important antigen-presenting cells for initiating adaptive immune responses. We have tested the hypothesis that a P/V mutant which activates host antiviral responses will be a more potent inducer of DC maturation and function than WT rSV5, which suppresses host cell responses. Infection of peripheral blood mononuclear cell-derived immature DC with WT rSV5 resulted in high levels of viral protein and progeny virus but very little increase in cell surface costimulatory molecules or secretion of IFN and proinflammatory cytokines. In contrast, immature DC infected with the rSV5-P/V-CPI− mutant produced only low levels of viral protein and progeny virus, but these infected cells were induced to secrete IFN-α and other cytokines and showed elevated levels of maturation markers. Unexpectedly, DC infected with WT rSV5 showed extensive cytopathic effects and increased levels of active caspase-3, while infection of DC with the P/V mutant was largely noncytopathic. In mixed-culture assays, WT rSV5-infected DC were impaired in the ability to stimulate proliferation of autologous CD4+ T cells, whereas DC infected with the P/V mutant were very effective at activating T-cell proliferation. The addition of a pancaspase inhibitor to DC infected with WT rSV5 reduced cytopathic effects and resulted in higher surface expression levels of maturation markers. Our finding that the SV5 P/V mutant has both a reduced cytopathic effect in human DC compared to WT SV5 and an enhanced ability to induce DC function has implications for the rational design of novel recombinant paramyxovirus vectors based on engineered mutations in the viral P/V gene.
Epithelial cells are often a major cell type that is initially infected by respiratory viruses, and this virus-host cell interaction can result in antiviral responses such as cytokine induction or apoptosis which can influence the spread of progeny viruses to other cells or tissues. Our previous results with epithelial cells have shown a dramatic difference in the outcomes of infections with the parainfluenza virus simian virus 5 (SV5), which suppresses epithelial cell antiviral responses, and an engineered P/V gene mutant that activates antiviral responses (52). Respiratory viruses can also infect professional antigen-presenting cells (APC), a cell type that plays a critical role in coordinating innate and adaptive immune responses (34, 40). This pathogen-cell interaction is particularly important for immune responses to viruses, since whether the virus suppresses antiviral responses in APC or whether the APC suppress the virus infection can be an important determinant of the subsequent adaptive immune response (22, 28). In this study, we have addressed the issues of whether the established phenotypes of wild-type (WT) and mutant SV5 in epithelial cells are also apparent during infections of dendritic cells (DC) and if these viruses have differing effects on the functions of infected DC.
DC are pivotal professional APC that are capable of recognizing microbial products to initiate innate and adaptive immune responses (5, 44). DC can respond to virus infection by activating antiviral innate immune responses such as the production of type I interferon (IFN), which can limit virus growth (7). Most importantly, DC that have sensed a virus infection can also serve as potent activators of other immune cells. A virus infection of immature DC can trigger a series of cell signaling events that result in conversion of DC with an immature phenotype to a mature form that is capable of activating naïve T-cell functions, including proliferation, cytokine secretion, and cytolytic activity. These DC maturation events include increased cell surface expression of costimulatory molecules such as CD40, CD80, and CD86, downregulation of antigen capture receptors, and an increased capacity to secrete immunomodulatory cytokines such as interleukin-12 (IL-12), IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) (5, 44).
Clearance of a viral infection and the subsequent development of long-term adaptive immunity depend on appropriate innate and adaptive responses at the time of infection (6, 7, 22), and DC play a pivotal role in linking these two arms of immunity (5). As such, viruses have evolved mechanisms to limit DC activation. For example, infections with a number of viruses have been shown to prevent DC maturation or function. Examples of this include herpes simplex virus (36, 45), cytomegalovirus (35), vaccinia virus (14), measles virus (18, 28, 48), Lassa fever virus (4), hantavirus (43), poliovirus (51), and Ebola virus (8). Many of these viruses prevent DC maturation by limiting the production of cytokines or preventing the upregulation of costimulatory molecules. In the case of many paramyxoviruses, extensive studies with epithelial and fibroblast-derived cell lines have shown that proteins encoded by the viral P/V/C gene can function in counteracting antiviral responses (reviewed in references 16, 17, 24, and 29), but the specific role that these viral antagonists may play in this regard in professional antigen-presenting cells such as DC is not completely understood.
For SV5, the viral V protein has been shown to target STAT1 for degradation during infection of epithelial cell- and fibroblast-like cells (1, 12), resulting in a block in type I and type II IFN signaling. Recently, the SV5 V protein has also been shown to play a role in limiting the synthesis phase of the IFN response, as translocation of interferon regulatory factor 3 (IRF3) to the nucleus is blocked following SV5 infection (20, 42). The SV5 V protein may also play a role in limiting the induction of some proinflammatory cytokines, since while WT SV5 is a poor inducer of IL-8 and monocyte chemoattractant protein 1 in lung epithelial cells, a recombinant SV5 with mutations in the V protein is a potent activator of these host cell genes (57). Thus, the SV5 V protein plays multiple roles in counteracting host cytokine responses, being involved in blocking of both the IFN induction and signaling pathways (11, 20, 24).
We have previously described the properties of a recombinant SV5 (rSV5) mutant (rSV5-P/V-CPI−) (52) that was engineered to encode six P/V substitutions which disrupt the ability of SV5 to target STAT1 for degradation (9). However, in epithelial cells, the rSV5-P/V-CPI− mutant was shown to have a number of additional unexpected phenotypes that were very different from those of WT rSV5. WT rSV5 blocks IFN signaling (12) and is a poor inducer of IFN as well as other cytokines (11, 20, 57). In addition, infection with WT rSV5 causes a long-term, noncytopathic infection of human cells in vitro (10, 20, 52). In contrast, infection of human epithelial cells with rSV5-P/V-CPI− resulted in premature and elevated levels of viral RNA and protein compared to infection with WT rSV5, and the P/V mutant produced higher yields than the WT (52). In addition, epithelial cells infected with rSV5-P/V-CPI− secreted large amounts of IFN and other antiviral cytokines (53, 57). The most striking contrast between rSV5-P/V-CPI− and WT SV5 is seen in the degree of cytopathic effect, since infections of tissue culture cell lines and cultures of primary epithelial cells with rSV5-P/V-CPI− result in extensive cell death by apoptosis (52, 54).
Given these striking differences between WT and P/V mutant SV5 in epithelial cells, we have addressed the issue of whether the contrasting abilities of WT and P/V mutant SV5 to suppress antiviral responses would also be evident during infections of primary human DC. We have tested the general hypothesis that a P/V mutant paramyxovirus which activates host antiviral responses will be a more potent inducer of DC maturation and function than a WT paramyxovirus which suppresses these host cell responses. Our results have implications for the rational design of safer and more potent recombinant paramyxovirus vectors based on engineered mutations in the viral P/V gene.
MATERIALS AND METHODS
Cells, viruses, and Western blotting.
Peripheral blood mononuclear cells (PBMC) were isolated from randomly selected healthy donors by standard density gradient centrifugation with Ficoll-Hypaque (37). CD14+ cells were isolated by positive selection, using CD14 microbeads and a magnetic cell separator according to the manufacturer's specifications (Miltenyi Biotech, Inc., Auburn, Calif.). Typically, the purity of monocytes isolated by this procedure was ≥95%. Enriched CD14+ cells (106 cells/ml in 5 ml) were cultured for 6 days at 37°C in 5% CO2 in culture medium (CM) consisting of RPMI 1640 supplemented with 10% fetal calf serum (Gibco, Grand Island, NY), 2 mM l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, 10 mM HEPES, and 0.1 mM nonessential amino acids (all from BioWhittaker, Walkersville, MD). CM included 10 ng/ml human interleukin-4 (hIL-4) and 10 ng/ml human granulocyte-macrophage colony-stimulating factor. On day 3, half of the medium was replaced with fresh medium supplemented with cytokines to the above final concentrations.
WT rSV5 and rSV5 expressing green fluorescent protein (rSV5-GFP) (19) were recovered as described previously (39) from a cDNA plasmid kindly provided by Robert Lamb and Biao He (Northwestern University). The recovery and growth properties of the P/V mutant rSV5-P/V-CPI− were described previously (52). For DC infections, viruses were purified by centrifugation (25,000 rpm, 6 h, SW28 rotor) through a 20% glycerol cushion, resuspended in Dulbecco's modified Eagle's medium with 0.75% bovine serum albumin, and titrated on CV-1 cells as described previously (54).
For SV5 infections, cells were collected on day 6 and incubated at 106 cells/ml in phosphate-buffered saline containing 10% fetal bovine serum and different multiplicities of infection (MOIs) of SV5 virus for 1 h at 37°C. Cells were pelleted, resuspended in 1 ml CM, and transferred to 24-well tissue culture plates. In some experiments, lipopolysaccharide (LPS; Sigma-Aldrich, St. Louis, MO) was added to a final concentration of 200 ng/ml immediately after cell resuspension in CM. Neutralizing antibodies specific for IFN-β or IFN-α (Chemicon) were used at a final concentration of 104 neutralizing units/ml.
For Western blot analysis, cells that had been previously infected with rSV5-GFP or rSV5-P/V-CPI− were lysed in 1% sodium dodecyl sulfate. Equivalent cell numbers were lysed and analyzed by Western blotting as described previously (39), using rabbit polyclonal antisera to either the SV5 P protein, the cellular STAT1 protein (clone 554; Santa Cruz Biotechnology), or actin (Sigma), followed by horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence.
Cytokine production and cell surface staining.
Levels of secreted IFN were determined by a vesicular stomatitis virus (VSV) challenge bioassay detailed previously (52). The Orsay strain of VSV was a kind gift of D. Lyles, Wake Forest University Medical Center. IFN-α was measured by an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's recommendations (PBL Biomedical Labs, Piscataway, N.J.). All other cytokines, i.e., IL-1β, IL-6, IL-8, IL-10, IL-12 p70, and TNF, were quantified by an inflammatory response cytometric bead array assay (BD Pharmingen, San Diego, CA). For the detection of surface markers, DC were stained with the following conjugated monoclonal antibodies according to the manufacturer's recommendations: anti-CD11c (B-ly6), anti-CD14 (M5E2), anti-CD40 (5C3), anti-CD80 (L307.4), anti-CD86 (FUN-1), anti-HLA-A,B,C (G46-2.6), and anti-HLA-DR (TU39) (all from BD Pharmingen, San Diego, CA). Samples were analyzed on a FACScan instrument using Cell Quest software (Becton Dickinson, San Diego, CA). Paired Student's t test was used to determine significance.
Cytometric analyses of apoptotic cells.
Apoptosis was measured by intracellular detection of active caspase-3. DC samples were fixed and permeabilized with Cytofix and CytoPerm (BD Pharmingen, San Diego, CA) and then stained with a fluorescein isothiocyanate (FITC)-conjugated polyclonal anti-caspase-3 antibody (BD Pharmingen, San Diego, CA) along with CD11c-phycoerythrin-Cy5. The pancaspase inhibitor Z-VAD-FMK (R&D Systems, Minneapolis, MN) was included at a concentration of 100 μM at the time of viral infection. Samples were analyzed by flow cytometry. Paired Student's t test was used to determine significance.
Fluorescence microscopy.
Infected or mock-infected DC were harvested at 24 h postinfection (p.i.), spun onto coverslips, and fixed with paraformaldehyde. Cells were washed, mounted on glass slides with mounting medium containing DAPI (4′,6′-diamidino-2-phenylindole; Molecular Probes, Eugene, OR), and analyzed with a Nikon Eclipse fluorescence microscope using a 20× lens. Images were captured using a QImaging digital camera and processed using QCapture software. Exposure times were manually set to be constant between samples.
T-cell proliferation assay.
Six-day-old DC were mock treated, infected with WT rSV5 or rSV5-P/V-CPI− at an MOI of 5, or treated with LPS (200 ng/ml). After 24 h, DC were washed and counted before being cocultured with autologous T cells that had been labeled with 5 μM CFSE (5,6-carboxyfluorescein diacetate-succinimidyl ester; Molecular Probes, Eugene, OR) at a DC/T-cell ratio of 1:10. Cells were cultured for 3 days in 96-well round-bottomed plates in the presence of 0.01 ng/ml toxic shock syndrome toxin 1 (TSST-1) superantigen (Toxin Technology, Sarasota, FL). A concentration of 0.01 ng/ml TSST-1 was chosen as the minimal concentration giving detectable T-cell proliferation. The proliferation of T-cell receptor (TCR) Vβ2-specific CD4+ T cells was measured by flow cytometry as a decrease in CFSE intensity. Analysis and quantitation of data were performed using FlowJo software (FlowJo, Ashland, OR). The value referred to as “percent divided” is the percentage of the population that entered division.
RESULTS
Differential protein expression following infection of primary human T cells and DC with WT rSV5 and the P/V mutant.
PBMC isolated from whole blood from randomly selected healthy donors were divided by Miltenyi beads into CD14-positive and CD14-negative populations (Fig. 1A). The CD14-negative population consisted largely of T cells, which were used for T-cell proliferation assays in later experiments (see below). For the CD14-positive population, an analysis of surface markers showed that <1% of the cells expressed surface CD11c or the maturation markers CD40, CD80, and CD86 (not shown). These CD14+ cells were then cultured for 6 days with recombinant granulocyte-macrophage colony-stimulating factor and IL-4, conditions which generate myeloid-derived immature DC (46). More than 95% of this immature DC population was CD11c+ (not shown).
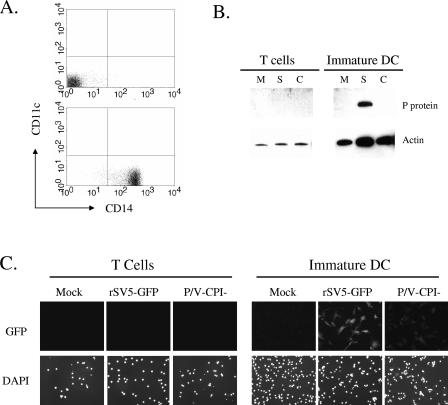
FIG. 1.
SV5 infection of primary human T cells and immature DC. (A) PBMC were separated by selection with CD14 beads, and the unselected (top panel) or selected (bottom panel) populations were analyzed by flow cytometry for surface CD14 and CD11c expression. (B) T cells or immature DC were mock infected (lanes M) or infected at an MOI of 5 with rSV5-GFP (lanes S) or P/V-CPI− (lanes C). At 24 h p.i., cell lysates were prepared and analyzed by Western blotting for SV5 P protein or cellular actin. (C) T cells or immature DC were mock infected or infected at an MOI of 5 with rSV5-GFP or P/V-CPI− and examined at 24 h p.i. by microscopy for nuclei (DAPI panels) or GFP. Panels represent equivalent exposure times.
To determine the ability of WT and mutant SV5 to infect primary T cells, cells were mock infected or infected at an MOI of 5 with an rSV5 carrying the GFP gene as an extra gene; this virus has been shown to have WT growth and gene expression properties (19). Alternatively, DC were infected with rSV5-P/V-CPI−, which also encodes GFP between the HN and L genes (52). As shown in Fig. 1B, Western blot analysis of lysates from infected T cells showed no evidence of SV5 protein expression. In support of this result, microscopy analysis of infected T cells showed no GFP fluorescence above background levels (Fig. 1C). These data indicate that T cells are not efficiently infected in vitro by either WT or P/V mutant SV5.
In contrast to the lack of infection of T cells by SV5, immature DC were efficiently infected by rSV5-GFP. This is evident in the results for a representative donor shown in Fig. 1B, where the SV5 P protein and other viral proteins (see below) were detected in lysates of infected DC at 24 h p.i. Unexpectedly, the level of viral protein expression following infection of DC with the P/V-CPI− mutant was very low compared to that seen with rSV5-GFP (Fig. 1B, DC panel) and was typically only seen with long exposure times. The low P/V mutant protein expression level was not due to infection of only a small number of DC in the cell population, since all DC were found to express GFP at 24 h p.i. (Fig. 1C). The GFP fluorescence intensity was much lower than that seen for DC infected with rSV5-GFP (Fig. 1C), consistent with the above differences in protein levels detected by Western blotting.
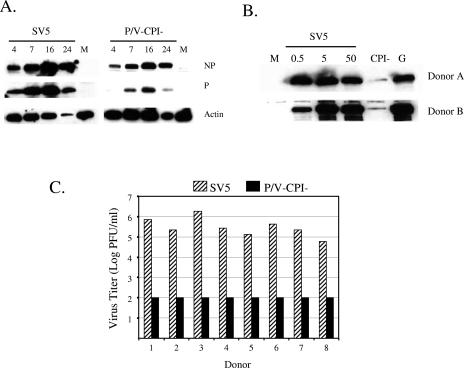
To determine the kinetics of SV5 protein expression in infected DC, cells were mock infected or infected at an MOI of 5 with WT rSV5 or the P/V mutant, and lysates that were harvested at different times p.i. were analyzed for accumulation of proteins by Western blotting. As shown in Fig. 2A, the accumulation of NP and P proteins in cells infected with WT rSV5-GFP was much higher than that seen with the P/V mutant. In the example shown in Fig. 2A, there was a loss of viral and cellular protein at 24 h p.i., but this was not reproducibly seen. As shown in Fig. 2B for cells from two representative donors, DC expressed high levels of P protein after infection at a range of MOIs with WT rSV5 and rSV5-GFP, but infection with the P/V-CPI− mutant resulted in much lower levels of protein expression. For all donors tested, P/V mutant protein accumulation was always less than that seen with WT rSV5 infection, although the differences were not always as dramatic as those shown for the individual donor DC in Fig. 2B.
FIG. 2.
DC infected by the SV5 P/V mutant show low levels of viral protein expression and low virus yields. (A) Time course of protein expression. DC isolated from a representative donor were mock infected (lanes M) or infected at an MOI of 5 with rSV5-GFP or the P/V-CPI− mutant. Protein extracts were collected at the indicated times (h) p.i. and analyzed by Western blotting for SV5 NP and P or cellular actin. (B) Dose-dependent protein expression. Immature DC isolated from two representative donors were mock infected (lane M), infected at the indicated MOI with rSV5, or infected at an MOI of 5 with rSV5-P/V-CPI− (lane CPI−) or WT rSV5-GFP (lane G). Protein extracts were analyzed by Western blotting with a polyclonal antiserum specific for the SV5 P protein. (C) Virus yields. DC isolated from eight individual donors were infected at an MOI of 5 with WT rSV5 or with the P/V-CPI− mutant, and virus yields at 24 h p.i. were determined by a plaque assay.
Yields of progeny WT rSV5 at 24 h p.i. ranged from ∼105 to ∼106 PFU/ml per 106 infected DC, which is 100- to 1,000-fold lower than that seen with the prototypic A549 lung epithelial cell line (52). In contrast, virus yields from P/V mutant-infected DC were ≤102 PFU/ml per 106 cells, which is ∼106-fold lower than that typically seen with epithelial cell lines infected with this P/V mutant (52) and 103- to 104-fold less than that seen with WT rSV5-infected DC.
Taken together, these data demonstrate that rSV5 does not productively infect primary human T cells in vitro but that infection of immature DC results in high-level protein and virion production. While immature DC can also be productively infected with the P/V mutant, our above results with DC differ significantly from previous results with epithelial cells since the P/V mutant is highly restricted to the production of only low levels of viral protein and virus.
STAT1 and type I IFN levels following infection of DC by WT rSV5 and the P/V mutant.
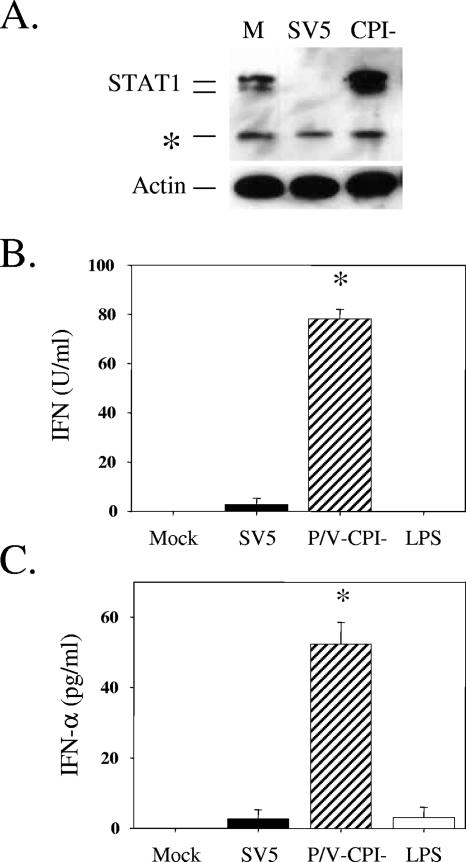
WT SV5 counteracts IFN signaling by targeting STAT1 for degradation (12). In contrast, our previous results have shown that rSV5-P/V-CPI−, which contains six amino acid substitutions in the shared N-terminal region of the P and V proteins, has lost the ability to target STAT1 for degradation in epithelial cells (52). As shown in Fig. 3A, DC from all donors showed STAT1 degradation following SV5 infection. DC from some individual donors were less sensitive to SV5-induced STAT1 degradation and required very high MOIs to induce complete degradation. Since the basis for variability in the sensitivity to STAT1 degradation of some primary cells is not presently known, the data presented here were derived using DC from donors that showed SV5-induced STAT1 degradation following infection at MOIs of 5 to 10. In the case of the P/V-CPI− mutant, STAT1 levels were not reduced following infection and were often increased over those in mock-infected samples, as shown previously for infected A549 epithelial cells (due to IFN signaling). The inability of the P/V mutant to target STAT1 degradation in DC is consistent with the V protein mutations (9, 52), although the low levels of viral protein expression in mutant-infected DC could also contribute to this phenotype.
FIG. 3.
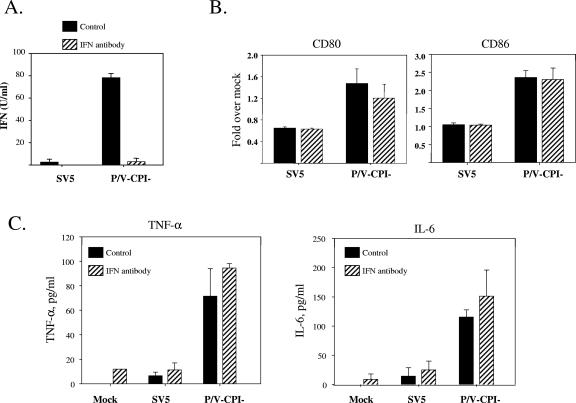
STAT1 and IFN levels following infection of DC by WT rSV5 and the P/V mutant. (A) STAT1 levels. Immature DC from a representative donor were mock infected (lane M) or infected at an MOI of 5 with rSV5-GFP or the P/V-CPI− mutant virus. Protein extracts were analyzed by Western blotting with a polyclonal antiserum specific for STAT1 or cellular actin. *, cross-reactive host protein. (B and C) Type I IFN levels. DC isolated from individual donors were mock infected, infected with rSV5 or the P/V-CPI− mutant at an MOI of 5, or treated with LPS (200 ng/ml). At 24 h p.i., supernatants were analyzed by a VSV challenge assay for levels of type I IFN (B) or by ELISA for levels of IFN-α (C). Data are the mean values from three donors, with standard deviations indicated by bars. *, significantly different from WT SV5-infected samples.
Previous results with human epithelial and fibroblast cell lines have shown that WT SV5 is a poor inducer of type I IFNs (11, 20, 42), while the P/V mutant activates IFN secretion (52). To determine if these same properties also apply to human DC, type I IFN secretion was assayed after infection of immature DC with WT and P/V mutant SV5. Using a bioassay for type I IFN (Fig. 3B), DC infected with the P/V-CPI− mutant secreted ∼80 U/ml IFN per 106 cells, while WT SV5-infected DC produced levels very similar to those seen with mock-infected or control LPS-treated cells. In an ELISA (Fig. 3C), the P/V-CPI− mutant induced ∼50 pg/ml IFN-α during a 24-h infection, while infection with WT rSV5 induced levels that were not above those of control samples. The lack of IFN-α induction in LPS-treated DC is consistent with previous results showing that myeloid DC express Toll-like receptor 4 (TLR4) but produce very little IFN-α when challenged with LPS (25). The increased production of IFN-α following rSV5-P/V-CPI− infection was consistent with the inability of the P/V mutant to induce STAT1 degradation (31).
DC maturation and function are induced by infection with the rSV5 P/V mutant but not with WT rSV5.
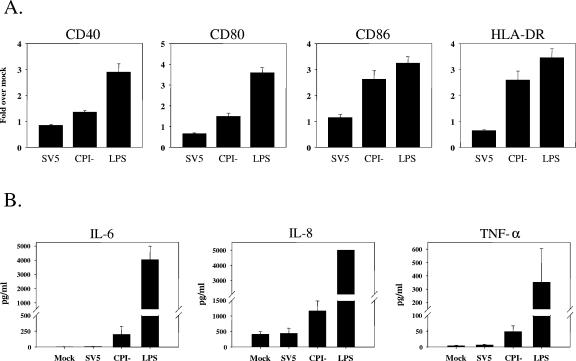
Virus infection of immature DC can result in host cell responses that lead to DC maturation, including the secretion of immunomodulatory cytokines such as IL-8 and TNF-α and the upregulation of cell surface molecules such as CD40, HLA, CD80, and CD86 (5, 44). To determine if rSV5 infection induces DC maturation, immature DC were mock infected, infected at an MOI of 5 with WT rSV5 or the P/V mutant, or treated with LPS as a positive control. Levels of CD40, CD86, CD80, and class II molecules were then determined by flow cytometry at 24 h p.i. As shown in Fig. 4A, LPS treatment efficiently upregulated cell surface expression of these four maturation markers to levels ∼2.5- to ∼3.5-fold higher than those in mock-treated DC. In contrast, DC infected with WT rSV5 did not show increased expression of maturation markers, and in some cases surface expression was lower than that seen with mock-infected cells (e.g., CD80 and HLA). Infection with the P/V mutant had a differential effect on these four cell surface maturation markers. The expression of some maturation markers (e.g., CD86 and HLA) was increased to levels similar to those after LPS treatment, while other markers (e.g., CD40 and CD80) were induced significantly over mock-infected DC but were still low compared to those in the LPS control. As shown in Fig. 4B, DC were also induced to secrete IL-6, IL-8, and TNF-α following infection with the P/V mutant, although the levels of secreted cytokines were much lower than those seen with LPS-treated DC. As with the cell surface maturation markers (Fig. 4A), WT rSV5 infection did not significantly induce DC to secrete these cytokines.
FIG. 4.
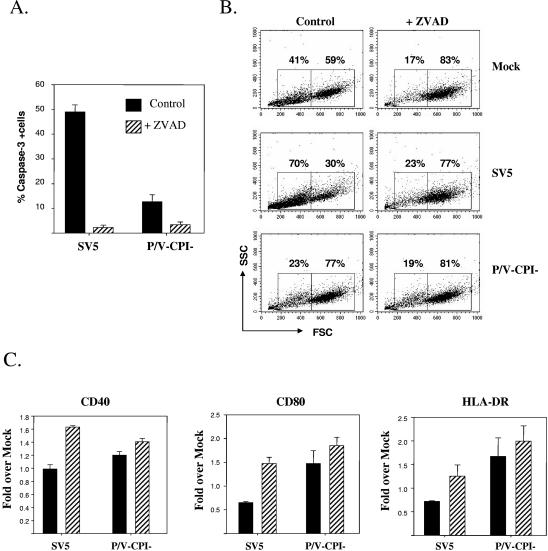
Increases in maturation markers following infection of immature DC with the P/V-CPI− mutant but not with WT rSV5. DC from individual donors were mock infected, infected at an MOI of 5 with WT rSV5 or the P/V-CPI− mutant, or treated with LPS. After 24 h, cells were stained with antibodies to the indicated maturation marker proteins and analyzed by flow cytometry (A). Mean fluorescence intensities (with standard errors) are expressed relative to those of mock samples and are from four independent experiments with DC from four donors. The upregulation of costimulatory molecules following CPI− mutant infection compared to that after SV5 infection was statistically significant, with the following P values: for CD40, P = 0.00034; for CD80, P = 0.0069; for CD86, P = 0.0086; for HLA-DR, P = 0.01. Alternatively, cell-free supernatants were assayed by a cytometric bead array for the indicated cytokines (B). Values are means ± standard errors of three independent experiments with DC from four donors.
The above findings that infection of immature DC with the P/V mutant induced IFN and did not result in STAT1 degradation raised the possibility that virus-induced DC maturation was dependent on IFN signaling through the STAT1 pathway. To test this hypothesis, we determined the effect of anti-IFN neutralizing antibodies on SV5-induced DC maturation. The addition of antibodies specific for both IFN-α and IFN-β resulted in neutralization of extracellular IFN (Fig. 5A) but did not significantly alter the levels of the maturation markers CD80 and CD86 (Fig. 5B) or CD40 and HLA (not shown) on the surfaces of DC infected with either WT or P/V mutant SV5. Likewise, under conditions of neutralized extracellular IFN, the levels of cytokines induced by infection with the WT or P/V mutant virus did not change significantly (Fig. 5C). Since UV light treatment of the P/V mutant reduced the ability of virus infection to mature DC (not shown), our data suggest that the mechanism by which the P/V mutant activates DC maturation is largely independent of IFN secretion but dependent on virus replication.
FIG. 5.
Role of type I IFN secretion in DC maturation following infection with WT and P/V mutant SV5. DC from individual donors were mock infected or infected at an MOI of 5 with WT rSV5 or the P/V-CPI− mutant and incubated with (hatched bars) or without (black bars) a mixture of neutralizing antibodies against IFN-α and IFN-β. After 24 h, samples were harvested, and supernatants were analyzed by a VSV challenge assay for levels of type I IFN (A). Alternatively, cells were stained with antibodies to the indicated maturation marker proteins and analyzed by flow cytometry (B). Mean fluorescence intensities (with standard errors) are expressed relative to those of mock samples and are from four independent experiments with cells from four donors. Cell-free supernatants were also assayed by a cytometric bead array for TNF-α and IL-6 (C). Values are means ± standard errors of three independent experiments with DC from three donors.
Cytopathic effects are induced following infection of primary DC with WT rSV5 but not with the P/V mutant.
During our analysis of DC infections, we noted that immature DC infected with WT rSV5 showed high levels of cytopathic effects that were not seen during infection with the P/V mutant. This is evident in Fig. 6A, where DC infected with WT rSV5 showed a decrease in forward and side scatter in flow cytometric assays, which reflects a cell size and granularity that are consistent with an increased cytopathic effect. In contrast, forward/side scatter in DC infected with the P/V-CPI− mutant was similar to that seen with mock-infected or LPS-treated samples.
FIG. 6.
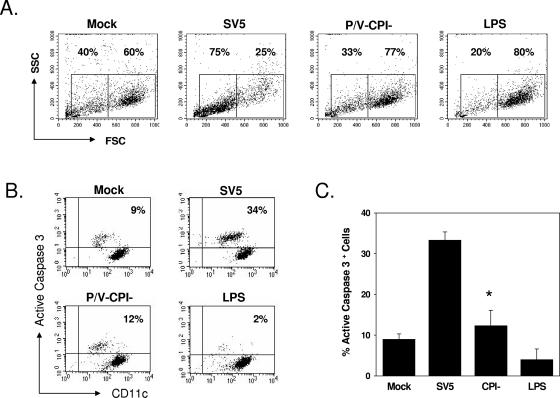
Increased cytopathic effect and active caspase-3 following infection of DC with WT rSV5 but not with the P/V-CPI− mutant. DC from individual donors were mock infected, infected at an MOI of 5 with WT rSV5 or the P/V-CPI− mutant, or treated with LPS (200 ng/ml). At 24 h p.i., cells were analyzed by flow cytometry for intracellular levels of active caspase-3. Panel A shows results from a representative experiment plotting forward and side scatter. Panel B shows results from a representative experiment plotting levels of staining with FITC-anti-caspase-3 antibody (y axis) versus staining with phycoerythrin-Cy5-anti-CD11c antibody (x axis). Panel C shows the mean percentages of cells with elevated caspase-3 staining from four individual donors. *, P value of 0.026 between SV5- and CPI− mutant-infected samples.
To determine if apoptotic pathways were activated by WT rSV5, mock-infected and virus-infected cells were analyzed for the presence of active caspase-3. As shown for the representative example in Fig. 6B, mock-infected or LPS-treated cells showed only ∼4 to 9% of cells with elevated active caspase-3. In contrast, ∼34% of the WT rSV5-infected DC showed elevated staining for active caspase-3. Infection with the rSV5-P/V-CPI− mutant resulted in only ∼12% of the DC showing high levels of active caspase-3 staining. Mean values from the analysis of DC from four individual donors showed that ∼33% of the WT rSV5-infected DC population had elevated active caspase-3 staining compared to ∼10 to 15% for both mock-infected and rSV5-P/V-CPI−-infected cells (Fig. 6C). Thus, infection of DC with WT rSV5, but not with the P/V mutant, leads to the activation of cytopathic effects and apoptotic pathways.
The addition of a pancaspase inhibitor reduced apoptotic markers and cytopathic effects found in DC infected with WT rSV5. This is evident in Fig. 7, where incubation of DC with 100 μM Z-VAD-FMK led to reduced levels of active caspase-3 (Fig. 7A) and resulted in a forward/side scatter profile detected by flow cytometry that was similar to that of mock-infected control cells (Fig. 7B). To determine if the inhibition of cytopathic effects would lead to increased maturation of SV5-infected DC, immature cells were mock infected or infected at an MOI of 5 with WT rSV5 or the P/V mutant and then incubated with or without 100 μM Z-VAD-FMK. Cell surface maturation markers were assayed at 24 h p.i. As shown in Fig. 7C, the addition of Z-VAD-FMK had little effect on the levels of the surface maturation markers CD40, CD80, and HLA-DR in the case of DC infected with the P/V mutant. In contrast, the addition of the pancaspase inhibitor to DC infected with WT rSV5 resulted in slightly higher levels of all three markers than those in control-treated WT rSV5-infected cells. These data indicate that the activation of DC apoptotic pathways following infection with WT rSV5 contributes in part to the low levels of virus-induced maturation markers. However, since the levels of maturation markers on Z-VAD-FMK-treated virus-infected cells were still well below those seen for control LPS-treated DC (Fig. 4), these data indicate that additional mechanisms contribute to limited DC maturation following SV5 infection.
FIG. 7.
Z-VAD-FMK treatment reduces active caspase-3 and cytopathic effects in SV5-infected DC. (A) Active caspase-3 levels. DC were infected at an MOI of 5 with WT rSV5 or the P/V-CPI− mutant and incubated for 24 h with or without 100 μM Z-VAD-FMK (pancaspase inhibitor) before analysis of caspase-3 levels by flow cytometry. Data are the means ± standard errors from three individual experiments with three donors. The values for SV5-infected DC with and without Z-VAD-FMK treatment were significantly different (P = 0.0001) by a paired t test. (B and C) Cytopathic effect and maturation markers. Mock-infected or infected DC were treated as described for panel A and analyzed by flow cytometry for changes in forward and side scatter (B). In addition, cells were analyzed for cell surface maturation marker levels by flow cytometry (C). Mean fluorescence intensities (with standard errors) are expressed relative to those of mock samples and are from four independent experiments with four donors.
DC infected with the SV5 P/V mutant are effective activators of T-cell proliferation.
The ability of SV5-infected DC to stimulate T-cell functions was tested in an in vitro coculture assay. Immature DC from individual donors were mock infected, infected with WT rSV5 or the P/V mutant, or treated with LPS as a positive control. After 24 h, DC were then cocultured for 3 days with CFSE-labeled autologous T cells in the presence of 0.01 ng/ml TSST-1, a superantigen that preferentially activates CD4+ T cells bearing the TCR Vβ2 chain. The proliferation of CD4+ Vβ2+ T cells was assayed by measuring the CFSE intensity. In this assay, T cells that are stimulated to proliferate by mature DC show a loss of CFSE fluorescence with each cell division.
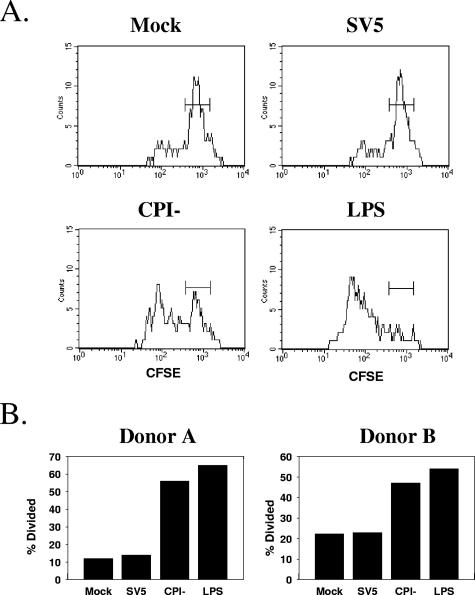
Figure 8A shows a representative example of CFSE staining from one of four individual experiments. T cells cocultured with WT rSV5-infected DC showed high levels of CFSE, with the staining profile being very similar to that seen with T cells cocultured with mock-infected DC. In contrast, a large percentage of T cells that were cocultured with P/V mutant-infected DC showed a decrease in CFSE intensity. The decrease in CFSE staining in samples with P/V mutant-infected DC was greater than that in mock-infected samples but less than that in LPS-treated samples. In four individual experiments, the percentage of T cells cocultured with P/V mutant-infected DC that were induced to divide (percent divided) was always higher than that seen for T cells cocultured with WT rSV5-infected DC (two examples are shown in Fig. 8B). However, the absolute percent divided values varied somewhat between individual donor cells (compare the values for donors A and B in Fig. 8B). T cells were not directly infected by WT rSV5 (Fig. 1), indicating that the lack of T-cell division in the samples cocultured with WT rSV5 was not due to direct inhibitory effects of SV5 infection on T cells themselves.
FIG. 8.
DC infected with the P/V mutant, but not with WT rSV5, are effective activators of T-cell proliferation. DC were mock treated, infected at an MOI of 5 with WT SV5 or the P/V-CPI− mutant, or treated with LPS (200 ng/ml) for 24 h before being cocultured with CFSE-labeled autologous T cells in the presence of 0.01 ng/ml TSST-1 superantigen. After 3 days, cells were stained with TCR Vβ2-APC antibodies (y axis), and the proliferation of CD4+ cells was measured by changes in CFSE intensity (x axis). (A) Representative CFSE staining results for an individual donor's DC. (B) Percentages of T cells undergoing division (% divided) for samples from two individual donors.
Taken together, these data indicate that WT rSV5 is a poor inducer of DC maturation pathways that lead to the upregulation of surface costimulatory molecules and cytokine secretion and that these infected DC are poor activators of T-cell proliferation. In contrast, the SV5 P/V mutant induces increased expression of some DC maturation markers, and these infected DC are more effective activators of T-cell proliferation.
DISCUSSION
The paramyxovirus SV5 is unusual among this family of viruses since it is a poor inducer of host antiviral responses in human epithelial and fibroblast-derived cells. This is evident from studies of WT rSV5 infections, which result in high-level virus production with minimal cytopathic effects and limited secretion of proinflammatory cytokines (56) or type I IFN (20, 42, 52). In contrast, the rSV5-P/V-CPI− mutant is highly cytopathic to epithelial and fibroblast-derived cells, and host antiviral cytokines and IFN are induced to high levels (52, 56). Our previous results have shown that these phenotypes are not limited to tissue culture cell lines but also largely apply to infections of normal primary human epithelial cells isolated directly ex vivo with only minimal passage (54). Here we have tested the hypothesis that the rSV5-P/V-CPI− mutant is a more potent inducer of DC maturation and function than WT rSV5. Table 1 provides a summary of the results presented here for primary DC compared to our previous results with epithelial cells infected with WT rSV5 and the P/V-CPI− mutant.
TABLE 1.
Summary of SV5-infected DC and epithelial cells
| Parameter | Value for dendritic cells
|
Value for epithelial cells
|
||
|---|---|---|---|---|
| WT rSV5 | P/V-CPI− | WT rSV5 | P/V-CPI− | |
| Gene expression | High | Low | High | Higher |
| Virus yield (PFU/ml)a | ∼105-106 | <102 | ∼107 | ∼108 |
| IFN-α/β secretionb | <10 | ∼80 | NDe | 5,000-10,000 |
| STAT1 statusc | Decreased | Increased | Decreased | Increased |
| Cytopathic effectd | 30-50% | ∼12% | NDe | ∼20% |
Typical PFU/ml titers from 106 cells. Epithelial cell yields were at 16 h p.i. (52).
Units of type I IFN per ml determined by a VSV-based bioassay.
STAT1 levels detected by Western blotting; P/V-CPI−-infected cells usually showed slightly increased levels.
Percentage of cells with elevated active caspase-3 at 24 h p.i. Unpublished data are given for P/V-CPI− infection in A549 cells.
ND, none detectable.
PBMC-derived DC from eight individual donors showed efficient in vitro infection by WT rSV5, with abundant viral protein synthesis and progeny virus production (Table 1). The P/V mutant also efficiently infected DC in vitro; however, viral protein expression and virus production were low compared to those seen with WT rSV5. This was an unexpected result, since our previous data with epithelial cells showed that the rSV5-P/V-CPI− mutant expresses higher-than-WT levels of viral mRNA and protein (52, 53) and that virus yields from single-step growth cycles are ∼1 log higher than that of WT SV5 (Table 1). These phenotypes were also seen with primary human epithelial cells isolated directly ex vivo (54). The low level of P/V virus gene expression in DC was not restored to WT expression levels by the addition of neutralizing anti-IFN antibodies (not shown), indicating that the restriction on P/V mutant replication was due to intracellular antiviral pathways that were independent of IFN signaling. Many antiviral gene products are activated by pathways that are IRF dependent but independent of IFN signaling (13). Our results suggest that either different intracellular antiviral pathways are selectively activated by the P/V mutant in DC and epithelial cells or that the same pathway is activated in these two cell types but is much more sensitive to activation in DC.
Type I IFN was induced by infection of DC with the P/V mutant but not with WT rSV5 (Table 1). Thus, the ability of WT rSV5 to limit the induction of IFN and the loss of this function in the case of the P/V mutant are common phenotypes shared between epithelial cells and DC. Activation of the IFN promoter can involve pathways that include the cytoplasmic RNA helicases RIG-I and MDA-5 (2, 27, 55) or some Toll-like receptors that are activated during virus infection (21, 47). Some types of professional APC such as DC have high constitutive levels of IRF-7 (26), allowing these cells to respond rapidly to infection by producing high levels of IFN-α without the need for RIG-I- or MDA-5-mediated IFN-β synthesis to prime cells (3, 31). In fibroblast-derived cells, the SV5 V protein has been shown to prevent IFN-β promoter activation following exposure to double-stranded RNA (42) and through MDA-5 (2). Based on the recent finding that respiratory syncytial virus and measles virus prevent TLR-mediated IFN synthesis during infections of plasmacytoid cells (47), work is in progress to determine if WT rSV5 limits IFN induction during the productive infection of DC by targeting either IRF-7- or TLR-activated pathways.
In some experimental systems, secreted type I IFN can induce DC maturation (23, 30). This raised the possibility that differences in IFN induction, STAT1 degradation, and DC maturation by the WT and P/V mutant viruses may be linked mechanistically. However, the addition of neutralizing anti-IFN antibodies during infection with the P/V mutant did not reduce the levels of DC maturation markers or cytokines (Fig. 5), suggesting that P/V mutant-induced maturation was not solely dependent on autocrine IFN signaling. Our results are similar to those found with murine DC infected with influenza virus and Sendai virus (32), where it was proposed that the maturation of infected DC was due to the IFN-inducing properties of a virus but that maturation was not due to secreted IFN per se.
Mouse DC infected with WT rSV5 respond very differently from human DC infected with WT rSV5. Our previous studies have shown that WT rSV5 infection of mouse DC induces efficient upregulation of the maturation markers CD40 and CD86 and the secretion of proinflammatory cytokines such as IL-6 and IL-12 (41). This contrasts with the results shown here for WT rSV5 infection of human DC, which induces very little upregulation of maturation markers or cytokine secretion. These species-specific differences in the ability of WT rSV5 to induce DC maturation are consistent with previous results for WT SV5 infections of mouse and human epithelial and fibroblast cells (11) and may be related, at least in part, to the differing abilities of WT rSV5 to counteract antiviral pathways in human and mouse cells (56). In the case of human DC infected with the P/V mutant, antiviral pathways are not effectively blocked, and the DC show some maturation phenotypes. Thus, the outcome of infection of mouse DC with WT rSV5 shares common properties with that of infection of human DC with the rSV5-P/V-CPI− mutant, i.e., low-level viral protein synthesis, induction of IFN synthesis, and upregulation of a subset of costimulatory markers (CD40 and CD86). Interestingly, in both systems (mouse DC infected with WT SV5 and human DC infected with the P/V mutant), increased DC surface expression of the CD80 maturation marker is minimal. Since CD80 expression by mature DC can be an important factor for stimulating T cells (15, 33), it may be possible to engineer additional SV5 mutants with an enhanced ability to upregulate CD80 expression and further stimulate T-cell functions.
Some remarkable findings from the studies presented here are the contrasting cytopathic effects of WT rSV5 and the P/V-CPI− mutant (Table 1), since these phenotypes are largely reversed when these two viruses infect human DC or epithelial cells. WT rSV5 establishes a generally noncytopathic infection of human epithelial cells and is a poor inducer of host cell death pathways (10, 20, 52). In A549 lung epithelial cells infected with WT rSV5, only ∼2% of the cell population shows signs of apoptosis at late times p.i. (52). In contrast, our rSV5-P/V-CPI− mutant is highly cytopathic for epithelial cells, and most of the cell population shows signs of apoptosis by 36 h p.i. Coinfection studies with WT rSV5 and the P/V-CPI− mutant in A549 cells have shown that WT rSV5 can block the cell rounding, loss of cell volume, and DNA fragmentation induced by rSV5-P/V-CPI−, which are three late events in the apoptotic pathway (53). However, WT rSV5 is not able to block the loss of mitochondrial membrane potential, an early event in the cell death process induced by the P/V mutant (53). Our present finding that WT rSV5-infected DC show signs of activated apoptotic death pathways suggests that either (i) different apoptotic pathways are primed for activation in DC versus epithelial cells, and the WT virus is ineffective at blocking the DC death pathways; or (ii) the threshold for activation of a common pathway is lower in DC than in epithelial cells. Importantly, our mechanistic studies with a pancaspase inhibitor show that activation of caspase-dependent pathways can only partially account for the lack of DC maturation following WT SV5 infection and suggest that additional factors contribute to WT SV5 preventing DC maturation.
Our results for DC infection with the SV5 P/V mutant have implications for the use of paramyxoviruses as viral vectors for eliciting adaptive immune responses. It has been proposed that recombinant negative-strand RNA viruses harboring mutations in viral IFN antagonist genes could represent novel viral vectors that have a reduced capacity to cause disease but retain the ability to elicit adaptive immune responses (38, 49). For example, Palese and coworkers have shown with a mouse model system that an influenza virus harboring deletions in the NS1 protein is less pathogenic but still elicits strong adaptive antibody and T-cell responses (49). In the case of bovine respiratory syncytial virus, IFN-inducing mutants which lack expression of the NS proteins have been shown to be highly attenuated in vivo and to induce protective immunity to challenge (50). In addition to our data shown here that the SV5 P/V mutant is largely noncytopathic in primary DC, we have previously shown that this mutant is also restricted for low-MOI spread through an epithelial cell population (54). Thus, these findings are consistent with a general hypothesis that the safety of paramyxovirus vectors can be improved by engineering mutations into the P/V gene (38, 49, 50). The SV5 P/V mutant was also more effective than WT rSV5 at inducing DC to express maturation markers and to gain functions necessary for the activation of T cells. As a caveat to extending our cell culture data to in vivo results, it is unclear whether SV5 directly infects DC in whole animals. Taken together, the available data raise the possibility that paramyxoviruses with engineered P/V mutations could form the basis for new viral vaccine vectors that are both safe and effective activators of adaptive immune responses.
Acknowledgments
We thank members of the Parks and Alexander-Miller labs for helpful comments on the manuscript. We are grateful to Doug Lyles for the kind gift of the Orsay strain of VSV.
This work was supported by NIH grants AI-42023 (G.D.P.), HL-071985 (M.A.-M.), and AI-060642 (S.B.M.).
REFERENCES
- 1.Andrejeva, J., D. F. Young, S. Goodbourn, and R. E. Randall. 2002. Degradation of STAT1 and STAT2 by the V proteins of SV5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons. J. Virol. 76:2159-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase Mda-5 and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. USA 101:17264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asselin-Paturel, C., and G. Trinchieri. 2005. Production of type I interferons: plasmacytoid dendritic cells and beyond. J. Exp. Med. 202:461-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baize, S., J. Kaplon, C. Faure, D. Pannetier, M. C. Georges-Courbot, and V. Deubel. 2004. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J. Immunol. 172:2861-2869. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 6.Biron, C. A. 1999. Initial and innate responses to viral infections—pattern setting in immunity or disease. Curr. Opin. Microbiol. 2:374-381. [DOI] [PubMed] [Google Scholar]
- 7.Biron, C. A., and G. C. Sen. 2001. Interferons and other cytokines, p. 321-349. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 8.Bosio, C. M., M. J. Aman, C. Grogan, R. Hogan, G. Ruthel, D. Negley, M. Mohamadzadeh, S. Bavari, and A. Schmaljohn. 2003. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J. Infect. Dis. 188:1630-1638. [DOI] [PubMed] [Google Scholar]
- 9.Chatziandreou, N., D. Young., J. Andrejeva, S. Goodbourn, and R. E. Randall. 2002. Differences in interferon sensitivity and biological properties of two related isolates of simian virus 5; a model for virus persistence. Virology 293:234-242. [DOI] [PubMed] [Google Scholar]
- 10.Choppin, P. W. 1964. Multiplication of a myxovirus (SV5) with minimal cytopathic effects and without interference. Virology 23:224-233. [DOI] [PubMed] [Google Scholar]
- 11.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. Sendai virus and SV5 block activation of IFN-responsive genes: importance of virus pathogenesis. J. Virol. 73:3125-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. The V protein of SV5 inhibits interferon signaling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 73:9928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elco, C. P., J. M. Guenther, B. R. Williams, and G. C. Sen. 2005. Analysis of genes induced by Sendai virus infection of mutant cell lines reveals essential roles of IRF3, NFκB, and interferon but not Toll-like receptor 3. J. Virol. 79:3920-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelmayer, J., M. Larsson, M. Subklewe, A. Chahorudi, W. I. Cox, R. M. Steinman, and N. Bhardwaj. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762-6768. [PubMed] [Google Scholar]
- 15.Fields, P. E., R. J. Finch, G. S. Gray, R. Zollner, J. L. Thomas, K. Sturmhoefel, K. Lee, S. Wolf, T. F. Gajewski, and F. W. Fitch. 1998. B7.1 is a quantitatively stronger costimulus than B7.2 in the activation of naïve CD8+ TCR-transgenic T cells. J. Immunol. 161:5268-5275. [PubMed] [Google Scholar]
- 16.Garcia-Sastre, A. 2001. Inhibition of interferon-mediated antiviral responses by influenza A viruses and other negative strand RNA viruses. Virology 279:375-384. [DOI] [PubMed] [Google Scholar]
- 17.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signaling, immune modulation, antiviral responses and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 18.Grosjean, I., C. Caux, C. Bella, I. Berger, F. Wild, J. Banchereau, and D. Kaiserlian. 1997. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J. Exp. Med. 186:801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He, B., R. G. Paterson, C. D. Ward, and R. A. Lamb. 1997. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology 237:249-260. [DOI] [PubMed] [Google Scholar]
- 20.He, B., R. G. Paterson, N. Stock, J. E. Durbin, R. K. Durbin, S. Goodbourn, R. E. Randall, and R. A. Lamb. 2002. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology 303:15-32. [DOI] [PubMed] [Google Scholar]
- 21.Hengel, H., U. H. Koszinowski, and K. K. Conzelmann. 2005. Viruses know it all: new insights into IFN networks. Trends Immunol. 26:396-401. [DOI] [PubMed] [Google Scholar]
- 22.Hilleman, M. R. 2004. Strategies and mechanisms for host and pathogen survival in acute and persistent viral infections. Proc. Natl. Acad. Sci. USA 101(Suppl. 2):14560-14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horvath, C. M. 2004. Silencing STATs: lessons from paramyxovirus interferon evasion. Cytokine Growth Factor Rev. 15:117-127. [DOI] [PubMed] [Google Scholar]
- 25.Ito, T., R. Amakawa, T. Kaisho, H. Hemmi, K. Tajima, K. Uehira, Y. Ozaki, H. Tomizawa, S. Akira, and S. Fukuhara. 2002. Interferon-α and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J. Exp. Med. 195:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izaguirre, A., B. J. Barnes, S. Amrute, W. S. Yeow, N. Megjugorac, J. Dai, D. Feng, E. Chung, P. M. Pitha, and P. Fitzgerald-Bocarsly. 2003. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 74:1125-1138. [DOI] [PubMed] [Google Scholar]
- 27.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19-28. [DOI] [PubMed] [Google Scholar]
- 28.Klagge, I. M., and S. Schneider-Schaulies. 1999. Virus infections with dendritic cells. J. Gen. Virol. 80:823-833. [DOI] [PubMed] [Google Scholar]
- 29.Lamb, R. A., and D. Kolakofsky. 2001. Paramyxoviridae: the viruses and their replication, p. 1305-1340. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 30.Lebon, A., and D. F. Tough. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432-436. [DOI] [PubMed] [Google Scholar]
- 31.Levy, D. E., I. Marie, E. Smith, and A. Prakash. 2002. Enhancement and diversification of IFN induction by IRF-7 mediated positive feedback. J. Interferon Cytokine Res. 22:87-93. [DOI] [PubMed] [Google Scholar]
- 32.Lopez, C. B., A. Garcia-Sastre, B. R. Williams, and T. M. Moran. 2003. Type I interferon induction pathways, but not released interferon, participate in the maturation of dendritic cells induced by negative strand RNA viruses. J. Infect. Dis. 187:1126-1136. [DOI] [PubMed] [Google Scholar]
- 33.Matulonis, U., C. Dosiou, G. Freeman, C. Lamont, P. Mauch, L. M. Nadler, and J. D. Griffin. 1996. B7-1 is superior to B7-2 costimulation in the induction and maintenance of T-cell-mediated antileukemia immunity. Further evidence that B7-1 and B7-2 are functionally distinct. J. Immunol. 156:1126-1131. [PubMed] [Google Scholar]
- 34.Mckenna, K., A. S. Beingnon, and N. Bhardwaj. 2005. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J. Virol. 79:17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moutaftsi, M., A. M. Mehl, L. K. Borysiewicz, and Z. Tabi. 2002. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood 99:2913-2921. [DOI] [PubMed] [Google Scholar]
- 36.Muller, D. B., M. J. Raftery, A. Kather, T. Giese, and G. Schonrich. 2004. Frontline: induction of apoptosis and modulation of c-FLIPL and p53 in immature dendritic cells infected with herpes simplex virus. Eur. J. Immunol. 34:941-951. [DOI] [PubMed] [Google Scholar]
- 37.Nat, R., E. Radu, T. Regalia, and L. M. Popescu. 2002. Apoptosis in the immune system. 1. Fas-induced apoptosis in monocyte-derived human dendritic cells. J. Cell Mol. Med. 6:223-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palese, P., and A. Garcia-Sastre. 2002. Influenza vaccines: present and future. J. Clin. Investig. 110:9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parks, G. D., K. R. Ward, and J. C. Rassa. 2001. Increased readthrough transcription across the simian virus 5 M-F gene junction leads to growth defects and a global inhibition of viral mRNA synthesis. J. Virol. 75:2213-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasare, C., and R. Medzhitov. 2004. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 6:1382-1387. [DOI] [PubMed] [Google Scholar]
- 41.Pejawar, S. S., G. D. Parks, and M. A. Alexander-Miller. 2005. Abortive versus productive viral infection of dendritic cells with a paramyxovirus results in differential upregulation of select costimulatory molecules. J. Virol. 79:7544-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poole, E., B. He, R. A. Lamb, R. E. Randall, and S. Goodbourn. 2002. The V proteins of simian virus 5 and other paramyxoviruses inhibit induction of interferon-β. Virology 303:33-46. [DOI] [PubMed] [Google Scholar]
- 43.Raftery, M. J., A. A. Kraus, R. Ulrich, D. H. Kruger, and G. Schonrich. 2002. Hantavirus infection of dendritic cells. J. Virol. 76:10724-10733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reis e Sousa, C. 2001. Dendritic cells as sensors of infection. Immunity 14:495-498. [DOI] [PubMed] [Google Scholar]
- 45.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245-3253. [DOI] [PubMed] [Google Scholar]
- 46.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlender, J., V. Hornung, S. Finke, M. Gunthner-Biller, S. Marozin, K. Brzozka, S. Moghim, S. Endres, G. Hartmann, and K.-K. Conzelmann. 2005. Inhibition of Toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 79:5507-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider-Schaulies, S., I. M. Klagge, and V. ter Meulen. 2003. Dendritic cells and measles virus infection. Curr. Top. Microbiol. Immunol. 276:77-101. [DOI] [PubMed] [Google Scholar]
- 49.Talon, J., M. Salvatore, R. E. O'Neill, Y. Nakaya, H. Zheng, T. Muster, A. García-Sastre, and P. Palese. 2000. Influenza A and B viruses expressing altered NS1 proteins: a vaccine approach. Proc. Natl. Acad. Sci. USA 97:4309-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valarcher, J. F., J. Furze, S. Wyld, R. Cook, K. K. Conzelmann, and G. Taylor. 2003. Role of alpha/beta interferons in the attenuation and immunogenicity of recombinant bovine respiratory syncytial viruses lacking NS proteins. J. Virol. 77:8426-8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wahid, R., M. J. Cannon, and M. Chow. 2005. Dendritic cells and macrophages are productively infected by poliovirus. J. Virol. 79:401-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wansley, E. K., and G. D. Parks. 2002. Naturally occurring substitutions in the P/V gene convert the noncytopathic paramyxovirus simian virus 5 into a virus that induces alpha/beta interferon synthesis and cell death. J. Virol. 76:10109-10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wansley, E. K., J. M. Grayson, and G. D. Parks. 2003. Apoptosis induction and interferon signaling but not IFN-beta promoter induction by an SV5 P/V mutant are rescued by coinfection with wild-type SV5. Virology 316:41-54. [DOI] [PubMed] [Google Scholar]
- 54.Wansley, E. K., P. J. Dillon, M. D. Gainey, J. Tam, S. D. Cramer, and G. D. Parks. 2005. Growth sensitivity of a recombinant simian virus 5 P/V mutant to type I interferon differs between tumor cell lines and normal primary cells. Virology 335:131-144. [DOI] [PubMed] [Google Scholar]
- 55.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 56.Young, D. F., N. Chatziandreou, B. He, S. Goodbourn, R. A. Lamb, and R. E. Randall. 2001. Single amino acid substitution in the V protein of simian virus 5 differentiates its ability to block interferon signaling in human and murine cells. J. Virol. 75:3363-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Young, V. A., and G. D. Parks. 2003. Simian virus 5 is a poor inducer of chemokine secretion from human lung epithelial cells: identification of viral mutants that activate IL-8 secretion by distinct mechanisms. J. Virol. 77:7124-7130. [DOI] [PMC free article] [PubMed] [Google Scholar]