Abstract
The type I interferon (IFN) response plays an important role in the control of many viral infections. However, since there is no rodent animal model for human immunodeficiency virus, the antiviral effect of IFN-α and IFN-β in retroviral infections is not well characterized. In the current study we have used the Friend virus (FV) model to determine the activity of type I interferons against a murine retrovirus. After FV infection of mice, IFN-α and IFN-β could be measured between 12 and 48 h in the serum. The important role of type I IFN in the early immune defense against FV became evident when mice deficient in IFN type I receptor (IFNAR−/−) or IFN-β (IFN-β−/−) were infected. The levels of FV infection in plasma and in spleen were higher in both strains of knockout mice than in C57BL/6 wild-type mice. This difference was induced by an antiviral effect of IFN-α and IFN-β and was most likely mediated by antiviral enzymes as well as by an effect of these IFNs on T-cell responses. Interestingly, the lack of IFNAR and IFN-β enhanced viral loads during acute and chronic FV infection. Exogenous IFN-α could be used therapeutically to reduce FV replication during acute but not chronic infection. These findings indicate that type I IFN plays an important role in the immediate antiviral defense against Friend retrovirus infection.
Cytokine responses play an important role in many viral infections. In particular, the induction of alpha/beta interferon (IFN-α/β) is the most immediate antiviral host response to many viral infections. The current model suggests that IFN-β is induced directly after virus infection, which then leads to the expression of IFN-α genes (8). IFN-α/β bind to a common surface receptor composed of the two subunits IFNAR1 and IFNAR2 (23) on primary target cells and activate numerous intrinsic antiviral factors, such as double-stranded-RNA-dependent protein kinase (PKR), 2′-5′ oligoadenylate synthetase, and Mx proteins, which lead to the inhibition of protein synthesis and block viral replication (10). However, type I interferons not only play an important role in the innate immune response but also influence the generation of the adaptive immune responses: for example, IFN-α/β induce NK cell cytotoxicity, promote CD8 T-cell activity, and enhance Th1-type responses (3). The discovery of the human immunodeficiency virus type 1 (HIV-1) as the causative agent of AIDS has created a fundamental interest in the role of interferons in immunity to retroviral infections. In vitro studies on HIV-1 demonstrated an induction of IFN-α by infection of peripheral blood mononuclear cells (1) and inhibition of the early stages of the HIV-1 replication cycle at the level of provirus formation (32, 33). In addition, IFN-β has been shown to enhance the resistance of peripheral blood leukocytes to HIV-1 infection (38). However, very little information is available on the in vivo role of IFN-α/β in early retrovirus infection. In animal models, such as simian immunodeficiency virus infections of macaques, IFN-α was detected in the serum of monkeys as early as 4 days postinfection (17). In the serum of HIV patients, IFN-α is detectable during the acute phase of infections and at the time of disease progression and opportunistic infectious (39). However, nothing is known about the in vivo antiviral activity of type I IFN and immediate concentrations directly after exposure to HIV. Experiments using mice with specific cytokine gene inactivations have proven to be useful models for obtaining information about the regulation of immune cells or cytokines in response to infection (7, 41). To get more insight into the role of IFN-α/β in immunity to murine retroviruses, we used the Friend virus mouse model. The Friend virus complex (FV) is comprised of a nonpathogenic replication-competent helper virus (Friend murine leukemia virus [F-MuLV]) and a replication-defective but pathogenic virus called the spleen focus-forming virus (SFFV) (16). SFFV encodes a defective envelope protein, gp55, that binds to the erythropoietin receptor of erythroblasts and induces a polyclonal proliferation that subsequently leads to severe splenomegaly (14, 15, 20). In susceptible strains, the disease progresses to lethal erythroleukemia, whereas resistant mice, such as C57BL/6 (B6), control the erythroblast proliferation (25, 27, 40). To determine the biological relevance of the type I IFN system in retroviral immunity, we infected mice of different genetic backgrounds with FV. In addition, we analyzed mice with targeted gene defects in the IFN-α/β receptor or IFN-β after retroviral infection. Mice used for this experiment were on the B6 genetic background because of the availability of knockouts. B6 mice are partially resistant to FV-induced erythroleukemia due to the Fv2 gene, which acts in a nonimmunological manner (14, 30). Mice that carry Fv-2r allele express a full-length Stk, whereas mice with the Fv-2s allele also express a truncated form of the kinase, which interacts with SFFV gp55 and contributes to the development of polyclonal cell activation and splenomegaly (28). Despite their genetic resistance to FV-induced leukemia, wild-type B6 mice fail to completely eliminate FV during acute infection and become persistently infected for life (37). Furthermore, B6 mice deficient in lymphocyte subsets become susceptible and develop erythroleukemia (11, 18, 37), indicating that immune responsiveness is an important factor for the resistance of B6 mice to FV-induced disease. Thus, knockout mice on B6 background are perfect tools for determining the role of immune cells or molecules in Friend retrovirus resistance. The current study shows that type I interferons play an important role in innate immunity to retroviral infection and suggest new approaches for antiretroviral therapy based on type I interferon application.
MATERIALS AND METHODS
Mice.
C57BL/6 and BALB/c mice were purchased from Harlan Laboratories. IFNAR−/− (26) and IFN-β−/− (8) mice, obtained from Paul-Ehrlich-Institut, Langen, Germany, were generated on Sv129 and backcrossed 10 times to B6 mice. Experiments were also done with (B10.A × A.BY)F1 mice (H-2a/b) and (B10 × A.BY)F1 mice (H-2b/b). In all experiments, 3- to 6-month-old mice were used.
Virus infection.
Mice were injected intravenously with 0.5 ml phosphate-buffered saline (PBS) containing 3,000 or 10,000 spleen focus-forming units (FFU) of the Friend virus complex. The B-tropic, polycythemia-inducing FV complex used in all experiments was from uncloned virus stocks obtained from 10% spleen cell homogenates as described previously (12). The progression of disease was monitored by spleen weights and virus assays as indicated. Persistently infected mice were mice that had been infected at least 8 weeks prior to experimentation.
Infectious center and viremia assays.
For infectious center assays, single-cell suspensions from infected mouse spleens were cocultivated with Mus dunnis cells at 10-fold dilutions. For viremia assays, freshly frozen plasma samples were titrated on susceptible M. dunni cells pretreated with 4 μg of Polybrene per ml. Cultures were incubated for 5 days, fixed with ethanol, stained with F-MuLV envelope-specific monoclonal antibody 720, and developed with peroxidase-conjugated goat antimouse antibody and aminoethylcarbazol to detect foci.
Interferon inhibition assay.
Mus dunni cells were treated in vitro for 24 h with increasing concentrations of IFN-α/β (1 to 1,000 U/ml). Cells were then infected with 50 FFU of FV, cultivated for 4 days, fixed with ethanol, and stained with F-MuLV-envelope-specific antibody 720.
IFN ELISA.
At various time points postinfection, blood was collected from mice and sera were stored frozen (−72°C) until use. To quantitate the amounts of IFN protein, sera were assayed in duplicates using an enzyme-linked immunosorbent assay (ELISA) specific for mouse IFN-α or IFN-β (PBL Biomedical Laboratories; Piscataway, N.J.). The ELISA was performed in accordance with the manufacturer's protocol and analyzed at an absorbance of 450 nm. The limits of detection of IFN-α and IFN-β were 12.5 pg/ml and 15.6 pg/ml, respectively.
Alpha interferon treatment in vivo.
Mice were treated daily intraperitoneally with 100 μl of 5 × 104 U (treatment prior to infection and postinfection) or 1 × 105 (treatment postinfection) of recombinant human alpha interferon A/D (BglII) (PBL Biomedical Laboratories, Piscataway, N.J.), respectively. Treatments were performed daily from day −1 to day +3 or twice on days +3 and +4 after FV infection. The IFN-α hybrid is active on virtually every mammalian cell, substituting for mouse type I interferon. Control mice were injected with PBS only. Eleven days postinfection, the mice were sacrificed and analyzed for disease progression and viral loads. In a similar experiment, persistently infected mice were treated daily with human alpha interferon A/(BglII D) from day 60 to day 65 postinfection.
Flow cytometry.
Single-cell suspensions of nucleated, live cells were analyzed after red blood cell lyses using a FACSCalibur flow cytometer. Directly labeled fluorescent antibodies specific for CD4 (clone RM4-5), CD8 (clone 53-6.7), CD19 (clone 1P3), NK1.1 (PK136) or CD49b/Pan-NK (clone DX5) were obtained from BD Biosciences (San Diego, CA). FcR block (rat antimouse CD16/26 [FcγIII/II receptor, clone 2.4G2]) (BD Biosciences) was used to prevent nonspecific binding of antibodies to Fc receptors. A total of 1 million cells were analyzed per sample, and 7-amino-actinomycin D (7AAD) (BD Pharmingen) was used to gate out the dead cells.
RESULTS
Antiviral effect of IFN-α and IFN-β in vitro.
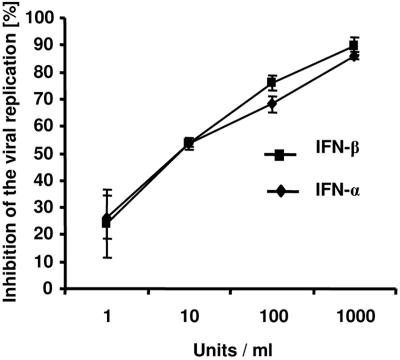
In order to assess the antiviral effect of IFN-α and IFN-β in vitro, we tested both interferons for inhibition of viral replication on Mus dunnis cells. IFN-α and IFN-β inhibited F-MuLV replication in a concentration-dependent manner. One thousand units/ml reduced viral replication by about 90% (Fig. 1). No difference was found in the antiviral activities of IFN-α and IFN-β. Neutralization of IFN-α by antibodies during the IFN-β treatment showed that the antiretroviral effect of IFN-β was independent of a possible IFN-α production by the Mus dunnis cells (data not shown).
FIG. 1.
Antiviral effect of IFN-α and IFN-β in vitro. Mus dunnis cells were treated in vitro for 24 h with increasing concentrations of IFN-α or IFN-β (1 to 1,000 U/ml). Inhibition of viral replication was determined 4 days after infection of the cells with 50 FFU of F-MuLV virus. Each IFN-α/β concentration was titrated in duplicate. Similar results were obtained in two independent experiments.
Kinetics of IFN-α and IFN-β responses after Friend virus infection.
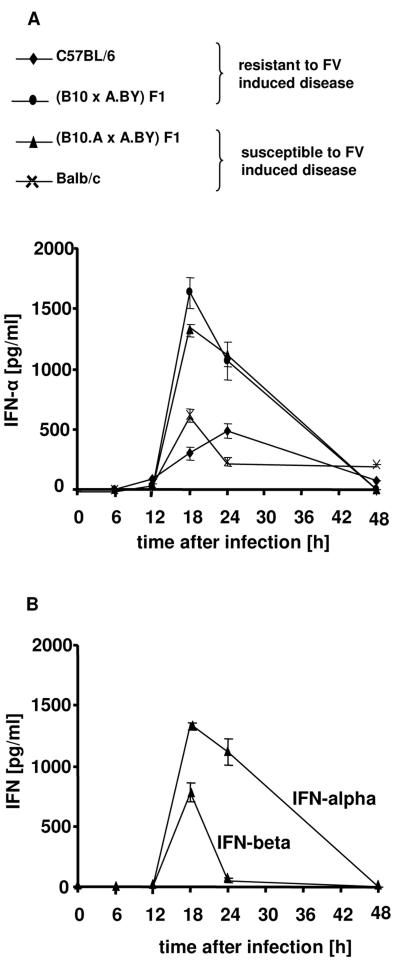
In order to address the role of type I IFN in vivo, IFN-α and -β were measured by ELISA in the serum of FV-infected mice. FV-susceptible (B10.A × A.BY)F1 mice injected with FV demonstrated a rapid production of IFN-α and -β between 12 h and 48 h postinfection (Fig. 2A). The peak levels of IFN-α and IFN-β serum concentration were both at the 18-h time point, but levels of IFN-α were twice as high as IFN-β levels (Fig. 2B). Mouse strains with different susceptibilities to FV-induced disease were compared to evaluate the influence of IFN-α on FV resistance. Peak serum levels of IFN-α were found at 18 h postinfection in susceptible (B10.A × A.BY)F1 and BALB/c mice, and at 18 h to 24 h in the resistant (B10 × A.BY)F1 and C57BL/6 mice (Fig. 2). Each strain produced characteristically different peak levels of IFN-α, but the levels did not correlate with susceptibility of the mice to FV.
FIG. 2.
Kinetics of IFN-α and IFN-β after FV infection. Mice were infected with FV, and blood was collected at the indicated time points postinfection. IFN-α and IFN-β levels were determined in the plasma by ELISA. (A) Kinetics of IFN-α production was determined for two mouse strains susceptible to FV-induced leukemia and two resistant mouse strains. (B) Kinetics of IFN-α production was compared with that of IFN-β production in susceptible (B10.A × A.BY)F1 mice. Four animals per group were analyzed. Standard deviations are indicated by a bar. was documented by spleen weights (A), and viral loads were measured in the spleen (B) and in the blood (C). The mean value for each group is indicated by a bar. Differences between the control group and the two groups of IFN-α-treated mice were analyzed by using Dunnett's multiple-comparison correction test. Statistically significant differences between the groups are indicated.
Effects of IFN-β and IFN receptor deficiency on acute FV infection.
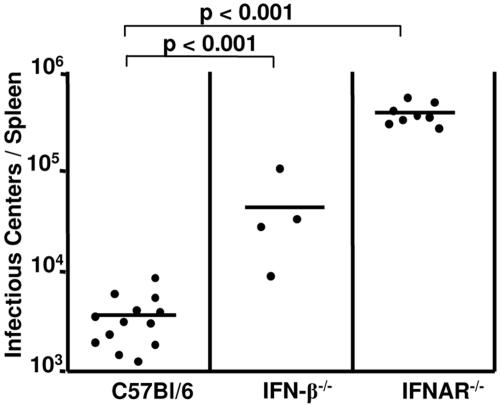
Since no correlation between IFN-α responses and FV susceptibility was found, we investigated whether type I IFNs were dispensable for the control of FV replication in the acute phase of infection. Therefore, we compared the viral loads in plasma and spleen of resistant C57BL/6 mice with those for mice lacking the IFN-β or IFN receptor. At 11 days postinfection, the IFN-β−/− mice had up to 10-fold-higher spleen viral loads than normal C57BL/6 mice (Fig. 3A). For the IFNAR−/− mice, in which both IFN-α and -β cannot signal through their receptor, 15-times-higher spleen virus loads were measured than for wild-type mice.
FIG. 3.
Acute FV infection in mice deficient for IFN-β or IFNAR. C57BL/6 wild-type or knockout mice that lack IFN-β or IFN type I receptor (IFNAR) were infected with FV. Viral loads were measured at 11 days after infection in the spleen (A) and in the blood (B). The results for the knockout mice were compared with those obtained for wild-type mice. The mean value for each group is indicated by a bar. Differences between the group of infected wild-type mice and the groups of knockout mice were analyzed by using Dunnett's multiple-comparison correction test for comparing a control group to several experimental groups. Statistically significant differences between the groups are indicated.
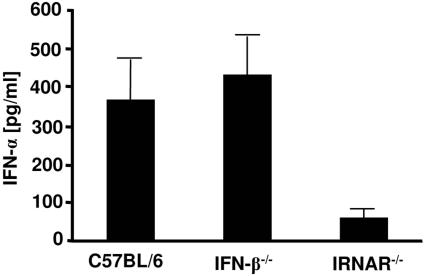
A somewhat different result was found when levels of free virus in the plasma were determined. Although IFN-β knockout mice showed slightly higher viremia than the control group, the differences were not statistically significant (Fig. 3B). However, the levels of viremia in IFNAR−/− mice were significantly higher than those of C57BL/6 wild-type controls. Interestingly, IFN-α production was not impaired in IFN-β−/− mice, since peak levels of IFN-α after infection were not reduced in comparison to those for wild-type mice (Fig. 4). In contrast, IFNAR−/− mice were severely impaired in their ability to produce IFN-α. This might explain the different susceptibilities of the two knockout strains to FV replication. The results indicate the important role of IFN-α in the control of early FV replication in resistant mice.
FIG. 4.
IFN-α production after FV infection of mice deficient for IFN-β or IFNAR. Twenty-four hours postinfection, levels of IFN-α were determined by ELISA in plasma samples collected from the indicated knockout and control mice. Four animals per group were analyzed. Standard deviations are indicated by a bar.
Therapeutic effects of human IFN-α A/D on FV-induced disease.
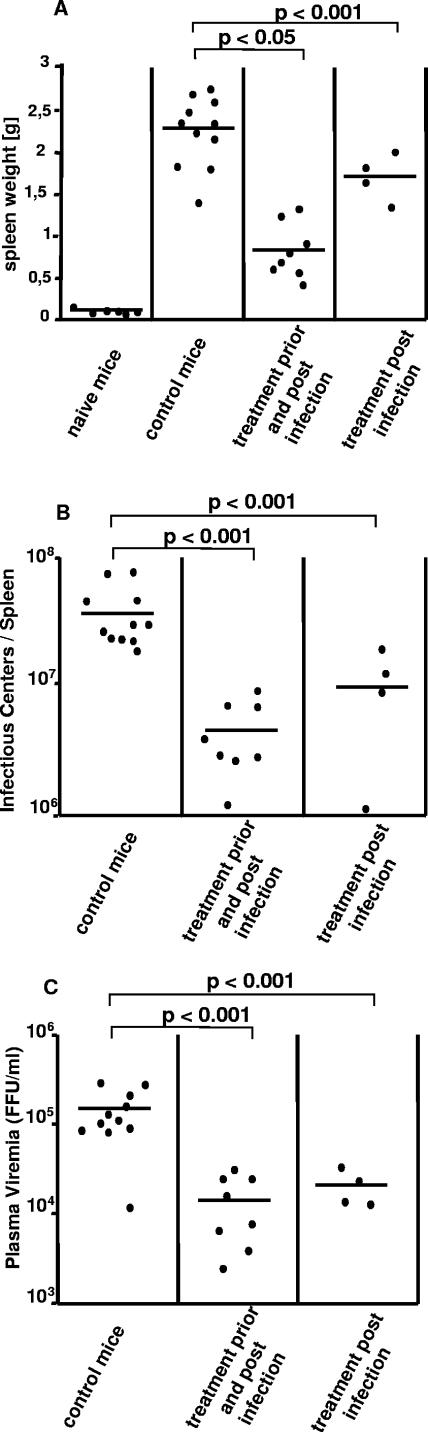
To determine the effect of exogenous IFN-α on acute FV infection, susceptible (B10.A × A.BY)F1 mice were treated daily with recombinant human alpha interferon A/D either from day −1 to days +3 or from day +3 to day +4 after FV infection. At 11 days postinfection, the mean spleen weight of infected, untreated (B10.A × A.BY)F1 mice was 2.24 g. Combined IFN-α treatment prior to infection and postinfection reduced splenomegaly by ∼64% (mean spleen weight = 0.82 g), whereas IFN-α postexposure treatment reduced splenomegaly by ∼27% (mean spleen weight = 1.7 g) (Fig. 5A). As a measure of virus replication, viral loads in spleen and plasma viremia were also assayed. Viral loads in spleen and plasma viremia were significantly lower for both groups of IFN-α-treated mice than for untreated (B10.A × A.BY)F1 mice (Fig. 5B and C). Thus, our findings from the IFN-α treatment experiments support the important effect of IFN-α in FV immunity and imply that an IFN therapy is most effective when performed early during infection.
FIG. 5.
Effect of IFN-α therapy on acute FV infection. Mice were given recombinant IFN-α A/D or (as a control) PBS on days −1 to +3 (treatment pre- and postinfection) or on day +3 and +4 (treatment postinfection) after infection with FV. Eleven days postinfection, disease progression and viral loads were analyzed. Virus-induced disease
Effects of IFN deficiency on leukocytes during acute infection.
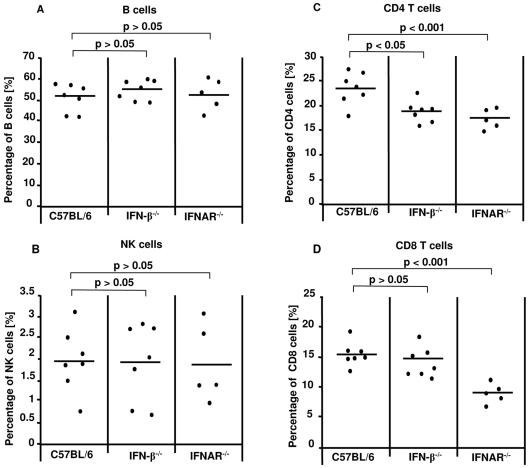
We stained splenic CD8+, CD4+, CD19+, and NK cells in the knockout mice to determine whether the effect of type I interferons on acute FV infection occurs as a result of the effects of IFN caused by antiviral enzymes or is also influenced by secondary effects on leukocytes. At 11 days postinfection, there were no significant differences between the groups of IFN-β−/−, IFNAR−/−, and wild-type mice in the percentage of CD19+ B cells (Fig. 6A), and the titer of virus-specific antibodies was also identical (data not shown). Similar to the case with B cells, no differences were found in the percentage of NK cells (Fig. 6B). We did observe a significant decrease in the percentage of CD4+ T cells in the IFN-β−/− and IFNAR−/− knockout mice compared to the wild-type C57BL/6 controls (Fig. 6C). However, only the lack of IFNAR led also to a significantly lower percentage of CD8+ T cells than that found in C57BL/6 mice (Fig. 6D). These differences were seen only between FV-infected knockout and wild-type mice and were not present in uninfected animals (data not shown). Thus, type I IFN also acts as immune modulators on T cells during acute FV infection.
FIG. 6.
B-, NK-, and T-cell responses in mice deficient in IFN-β or IFNAR. C57BL/6 wild-type and knockout mice that lack IFN-β or IFNAR were infected with FV. Eleven days postinfection, live, nucleated spleen cells were stained for CD19 (A), NK1.1/DX5 (B), CD4 (C), and CD8 (D). Dead cells were excluded by DAAD staining. Percentages from total splenocytes are shown. The mean value for each group is indicated by a bar. Differences between the group of infected wild-type mice and the groups of knockout mice were analyzed by using Dunnett's multiple-comparison correction test. Statistically significant differences between the groups are indicated.
Effects of IFN deficiency on chronic FV infection.
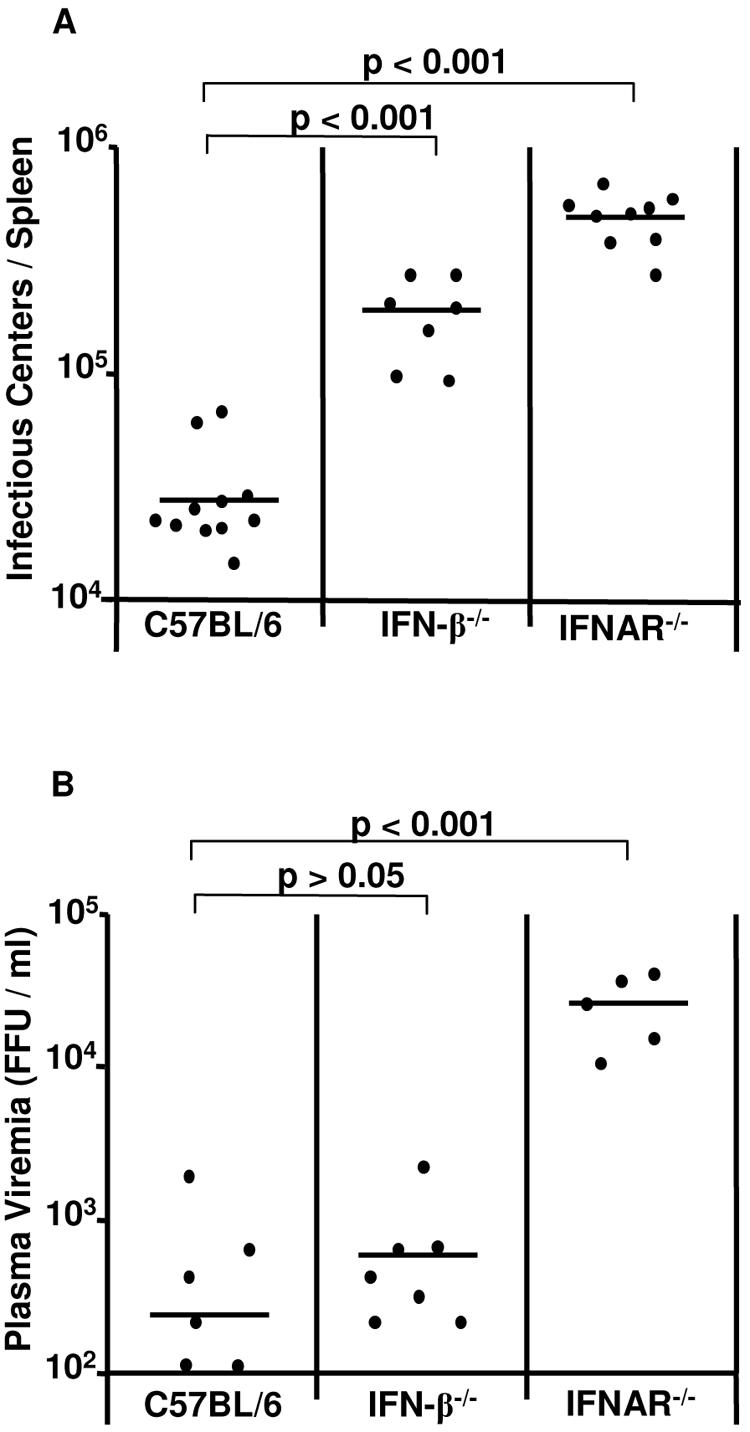
Since the previous experiments showed that IFN-α/β play an important role during acute FV infection, the question came up of whether IFN deficiency can also influence the chronic phase of FV infection. C57BL/6 mice, which are resistant to FV-induced leukemia, develop chronic infections after FV inoculations. An infectious center assay was performed to compare viral loads of C57BL/6, IFN-β−/−, and IFNAR−/− mice at 8 weeks postinfection. The lack of IFNAR and IFN-β resulted in significantly higher spleen virus loads than in C57BL/6 mice (Fig. 7). The levels of spleen infection in the IFN-β−/− mice were approximately 10 times, and in the IFNAR−/− mice 20 times, higher than in wild-type mice. These results indicate that the effects of type I interferons on acute viremia can subsequently influence the levels of chronic virus infections.
FIG. 7.
Persistent FV infection in mice deficient in IFN-β or IFNAR. C57BL/6 wild-type or knockout mice that lack IFN-β or IFNAR were infected with FV. Viral loads were measured at 8 weeks after infection in the spleen. The results for knockout mice were compared with those for wild-type mice. The mean value for each group is indicated by a bar. Differences between the group of infected wild-type mice and the groups of knockout mice were analyzed by using Dunnett's multiple-comparison correction test. Statistically significant differences between the groups are given.
DISCUSSION
The innate immune response plays a significant role as the first response to an invading pathogen insofar as either the pathogen is eliminated or its expansion is held in check until the adaptive immune response can attack. During viral infections, a very important part of the innate immune response is constituted by type I interferons. Alpha/beta interferons are produced within a few hours postinfection and prevent the spread of the virus by autocrine or paracrine pathways. For example, peak levels of IFN-α were found at 24 h in B6 mice which had been infected by encephalomyocarditis virus (9). In the Friend virus model, the different mouse strains all showed an IFN-α/β response with peak levels either at 18 h or 24 h postinfection, with the level of IFN-β measured being markedly lower than that of IFN-α (Fig. 2A and B). Interestingly, the peak IFN-α concentrations did not correlate with the susceptibility of the four different mouse strains to Friend virus-induced leukemia.
During the last few years, it has been revealed how important the role of interferons is in controlling viral replication, and this was best demonstrated in mice lacking a functional interferon type I system. These studies include infections of knockout mice with vaccina virus, Semliki Forest virus, lymphocytic choriomeningitis virus, vesicular stomatitis virus (26), and Murray Valley encephalitis virus (21). Similar to the results with other models, we found that the lack of type I IFN receptors led to significantly higher viral loads in the spleen and plasma during the acute phase of infection with Friend retrovirus (Fig. 3A and B). However, the previous studies failed to differentiate between the cytokines IFN-α and IFN-β. There are 14 nonallelic IFN-α genes, but only one single IFN-β gene exists. Whereas the various murine IFN-α subtypes themselves indicate homologies of around 80%, IFN-α has only a 30% homology to IFN-β, and it is still not known exactly how the functions of IFN-α and IFN-β actually differ (29). The infection of IFN-β−/− mice with FV enabled us to get an impression of the in vivo role of IFN-β. Knockout mice showed enhanced spleen viral loads, and plasma viremia was slightly higher (Fig. 3A and B). However, compared to the FV-infected IFNAR−/− mice, the IFN-β−/− mice were less susceptible (Fig. 3A, and B). Thus, both IFN-α and IFN-β played a significant role in the innate immune defense against retroviral infections. This was also supported by analyzing the in vitro activity of IFN-α and IFN-β against FV (Fig. 1). Our results readily concur with findings from other virus models. Vaccina virus-infected IFN-β−/− mice also showed a greater susceptibility than wild-type mice (4), but the level of susceptibility was nevertheless lower than that of IFNAR−/− mice (36). The high susceptibility of the IFN-β−/− mice was surprising, since we detected the same peak concentration of IFN-α in IFN-β−/− mice as in the C57BL/6 wild-type mice (Fig. 4). In earlier in vitro studies, it was shown that Sendai virus-infected mouse embryonic fibroblasts from IFN-β-deficient mice were not able to produce IFN-α (8). Based on this result, it follows a model, in that IFN-β has a unique role in the induction of type I IFN. In vivo, however, where various different cell types produce IFN-α and IFN-β, this feedback regulation does not appear to apply. Our results showing that IFN-β−/− mice produce normal amounts of IFN-α upon infection are supported by other studies in which IFN-β-deficient mice inoculated with UV-irradiated herpes simplex virus also produced IFN-α (2). A possible explanation for the induction of IFN-α independently of IFN-β could be the described autocrine loop of IFN-α production initiated by the IFN-α4 subtype, which is expressed as one of the first IFN during an innate immune response (22). Consequently, IFN-β seems to have a decisive influence in controlling the replication of FV that is independent of IFN-α induction. Experiments with coxsackievirus B3, which induces myocarditis, also indicated that IFN-β−/− mice showed an increased mortality rate as well as a reduced regulation of IFN-stimulated genes (2′-5′ oligoadenylate synthetase, serine/threonine protein kinase, the GTPase Mx) (5). These results imply that IFN-β activates a specific antiviral response which might be distinct from the antiviral pathway of IFN-α (31).
That not only IFN-β has a decisive influence on the kinetics of FV replication was suggested by the greater susceptibility of IFNAR−/− mice than IFN-β−/− mice during infection. The successful therapy with IFN-α proved that IFN-α also played an important antiviral role (Fig. 5). Previous studies reported long-term therapies (12 to 25 days) for FV infection or murine AIDS in which IFN-α had an effect on virus replication (13, 34, 35). We were able to show that a short-term postexposure therapy with IFN-α during acute infection induced an antiviral status sufficient to control virus replication and disease progression. However, when animals were treated that were persistently infected with FV, the viral load could not be reduced (data not shown). Thus, the administration of IFN-α during the acute phase of infection appears to regulate the virus-host balance, whereas administrations during the chronic retrovirus infection had no effect. IFN-α can affect the viral load in various ways; on the one hand it can inhibit viral replication at a very early stage in the immune response by the production of antiviral enzymes (10); on the other hand it influences the adaptive immune response that develops (10). In vitro experiments demonstrated an inhibitory effect of IFN-α and IFN-β on FV replication that was most likely mediated by antiviral enzymes (Fig. 1). In addition, both the IFN-β−/− mice and the IFNAR−/− mice had lowered percentages of total T cells in their spleens during acute FV infection, suggesting an influence of type I interferons on antiviral immune responses (Fig. 6). Dendritic cells could be regarded as the key figure between the innate and the adaptive immune response. For example, IFN induces the maturation of dendritic cells (19), which is important for the stimulation of T cells. Previous in vitro studies show that dendritic cells from the bone marrow of IFNAR−/− mice present less CD40, CD80, CD86, and major histocompatibility complex class II on their surfaces and the dendritic cells are less effective in presenting peptides to CD4+ and CD8+ T cells (24). These reports provide a good explanation for our findings that IFN-β−/− and IFNAR−/− mice have fewer T cells during retroviral infection than wild-type animals. In contrast, it has been reported that type I IFN negatively regulates CD8+ T-cell responses after DNA vaccination (6). However, we did not observe significant differences in the numbers of FV-specific class I tetramer-positive CD8+ T cells between IFNAR and wild-type mice after FV infection (data not shown). This indicates that type I interferons play a positive rather than a negative role in retroviral immunity. These studies indicate that although both IFN-α and IFN-β each possess the potential for antiviral activity, it is only when they are combined together that they are able to fulfill their entire potential for antiretroviral activity. This mechanism seems to be an antiviral effect through IFN-induced enzymes as well as an effect on the adaptive immune response. Type I interferons therefore play an important role in the antiviral immune response against retroviral infections and might be of use in therapeutic approaches.
Acknowledgments
This work was supported by a grant to N.G. and U.D. from the Deutsche Forschungsgemeinschaft (GK 1045/1).
REFERENCES
- 1.Ankel, H., R. M. Capobianchi, C. Castiletti, and F. Dianzanni. 1994. Interferon induction by HIV glycoprotein 120: role of the V3 loop. J. Interferon Res. 14:S209-P8-4. [DOI] [PubMed] [Google Scholar]
- 2.Barchet, W., M. Cella, B. Odermatt, C. Asselin-Paturel, M. Colonna, and U. Kalinke. 2002. Virus-induced interferon α production by a dendritic cell subset in the absence of feedback signalling in vivo. J. Exp. Med. 195:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biron, C. A. 1998. Role of early cytokines, including alpha and beta interferons (IFN-α/β), in innate and adaptive immune responses to viral infections. Immunology 10:383-390. [DOI] [PubMed] [Google Scholar]
- 4.Deonarain, R., A. Alcamí, M. Alexiou, M. J. Dallmann, D. R. Gewert, and A. C. G. Porter. 2000. Impaired antiviral response and alpha/beta interferon induction in mice lacking beta interferon. J. Virol. 74:3404-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deonarain, R., D. Cerullo, K. Fuse, P. P. Liu, and E. N. Fish. 2004. Protective role for interferon-beta in coxsackievirus B3 infection. Circulation 110:3540-3543. [DOI] [PubMed] [Google Scholar]
- 6.Dikopoulos, N., A. Bertoletti, A. Kröger, H. Hauser, R. Schirmbeck, and J. Reimann. 2005. Type I IFN negatively regulates CD8+ T cell responses through IL-10-producung CD4+ T regulatory 1 cells. J. Immunol. 174:99-109. [DOI] [PubMed] [Google Scholar]
- 7.Dittmer, U., K. E. Peterson, R. Messer, I. M. Stromnes, B. Race, and K. J. Hasenkrug. 2001. Role of interleukin 4 (IL-4), Il-12, and gamma interferon in primary and vaccine-primed immune responses to Friend retrovirus infection. J. Virol. 75:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erlandsson, L., R. Blumenthal, M. L. Eloranta, H. Engel, G. Alm, S. Weiss, and T. Leanderson. 1998. Interferon-beta is required for interferon-alpha production in mouse fibroblasts. Curr. Biol. 12:223-226. [DOI] [PubMed] [Google Scholar]
- 9.Gaines, K. L., S. G. Kayes, and G. L. Wilson. 1987. Factors affecting the infection of the D variant of encephalomyocarditis virus in the B cells of C57BL/6J mice. Diabetologica 30:419-425. [DOI] [PubMed] [Google Scholar]
- 10.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral responses and viral countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 11.Hasenkrug, K. J. 1999. Lymphocyte deficiencies increase susceptibility to Friend virus-induced erythroleukemia in Fv-2 genetically resistant mice. J. Virol. 73:6468-6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasenkrug, K. J., D. M. Brooks, M. N. Robertson, R. V. Srinivas, and B. Chesebro. 1998. Immunoprotective determinants in Friend murine leukemia virus envelope protein. Virology 248:66-73. [DOI] [PubMed] [Google Scholar]
- 13.Heng, J. K. M., P. Price, C. M. Lai, and M. W. Beilharz. 1996. Alpha/beta interferons increase host resistance to murine AIDS. J. Virol. 70:4517-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoatlin, M. E., and D. Kabat. 1995. Host-range control of a retroviral disease: Friend erythroleukemia. Trends Microbiol. 3:51-57. [DOI] [PubMed] [Google Scholar]
- 15.Hoatlin, M. E., S. L. Kozak, F. Lilly, A. Chakraborti, C. A. Kozak, and D. Kabat. 1990. Activation of erythropoietin receptors by Friend viral gp55 and by erythropoietin and downmodulation by the murine Fv-2r resistance gene. Proc. Natl. Acad. Sci. USA 87:9985-9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabat, D. 1989. Molecular biology of Friend viral erythroleukemia. Curr. Top. Microbiol. Immunol. 148:1-42. [DOI] [PubMed] [Google Scholar]
- 17.Khatissian, E., M. G. Tovey, M. C. Cumont, V. Monceaux, P. Lebon, L. Montagnier, B. Hurtrel, and L. Chakrabarti. 1996. The relationship between the interferon alpha response and viral burden in primary SIV infection. AIDS Res. Hum. Retrovir. 12:1273-1278. [DOI] [PubMed] [Google Scholar]
- 18.Kitagawa, M., O. Matsubara, and T. Kasuga. 1986. Dynamics of lymphocytic subpopulations in friend leukemia virus-induced leukemia. Cancer Res. 46:3034-3039. [PubMed] [Google Scholar]
- 19.Le Bon, A., and D. F. Tough. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432-436. [DOI] [PubMed] [Google Scholar]
- 20.Li, J. P., A. D. D'Andrea, H. F. Lodish, and D. Baltimore. 1990. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature 343:762-764. [DOI] [PubMed] [Google Scholar]
- 21.Lobigs, M., A. Müllbacher, A. Wang, M. Pavy, and E. Lee. 2003. Role of type I and type II interferon responses in recovery from infection with an encephalitic flavivirus. J. Gen. Virol. 84:567-572. [DOI] [PubMed] [Google Scholar]
- 22.Marié I., J. E. Durbin, and D. E. Levy. 1998. Differential viral induction of distinct interferon α genes by positive feedback through interferon regulatory factor-7. EMBO 17:6660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mogenson, K. E., M. Lewerenz, J. Reboul, G. Lutfalla, and G. Uzé. 1999. The type I interferon receptor: structure, function, and evolution of a family business. J. Interferon Cytokine Res. 19:1069-1098. [DOI] [PubMed] [Google Scholar]
- 24.Montoya, M., G. Schiavoni, F. Mattei, I. Gresser, F. Belardelli, P. Borrow, and D. F. Tough. 2002. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 99:3263-3271. [DOI] [PubMed] [Google Scholar]
- 25.Moreau-Gachelin, F., A. Tavitia, and P. Tambourin. 1988. Spi-1 is a putative oncogene in virally induced murine erythroleukemias. Nature 331:277-280. [DOI] [PubMed] [Google Scholar]
- 26.Müller, U., U. Steinhoff, L. F. L. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1920. [DOI] [PubMed] [Google Scholar]
- 27.Munroe, D. G., J. W. Peacock, and S. Benchimol. 1990. Inactivation of the cellular p53 gene is a common feature of Friend virus-induced erythroleukemia: relationship of inactivation to dominant transforming alleles. Mol. Cell. Biol. 10:3307-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishigaki, K., D. Thompson, C. Hanson, T. Yugawa, and S. Ruscetti. 2001. The envelope glycoprotein of Friend spleen focus-forming virus covalent interacts with and constitutively activates a truncated form of the receptor tyrosine kinase Stk. J. Virol. 75:7893-7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oritani, K., P. W. Kincade, C. Zhang, Y. Tomiyama, and Y. Matsuzawa. 2001. Type I interferons and limitin: a comparison of structures, receptors, and functions. Cytokine Growth Factors Rev. 12:337-348. [DOI] [PubMed] [Google Scholar]
- 30.Persons, D. A., R. F. Paulson, M. R. Loyd, M. T. Herley, S. M. Bodner, A. Bernstein, P. H. Correll, and P. A. Ney. 1999. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat. Genet. 23:159-165. [DOI] [PubMed] [Google Scholar]
- 31.Platanias, L. C., S. Uddin, P. Domanski, and O. R. Colamonici. 1996. Differences in interferon α and β signalling. J. Biol. Chem. 271:23630-23633. [DOI] [PubMed] [Google Scholar]
- 32.Shirazi, Y., and P. M. Pitha. 1992. Alpha interferon inhibits early stages of the human immundeficiency virus type 1 replication cycle. J. Virol. 66:1321-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shirazi, Y., and P. M. Pitha. 1993. Interferon-α-mediated inhibition of human immunodeficiency virus type 1 provirus synthesis in T-cells. Virology 193:303-312. [DOI] [PubMed] [Google Scholar]
- 34.Sidwell, R. W., J. D. Morrey, K. M. Okleberry, R. A. Burger, and R. P. Warren. 1993. Immunomodulator effects on the Friend virus infection in genetically defined mice. Ann. N. Y. Acad. Sci. 23:432-446. [DOI] [PubMed] [Google Scholar]
- 35.Sidwell, R. W., R. P. Warren, K. M. Okleberry, R. A. Burger, and J. D. Morrey. 1995. Effect of combination of interferon-α and staduvine on Friend virus infection in (B10.A × A.By)F1 mice. J. Infect. Dis. 171:S93-S98. [DOI] [PubMed] [Google Scholar]
- 36.Van den Broek, M. F., U. Müller, S. Huang, M. Aguet, and R. M. Zinkernagel. 1995. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 69:4792-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van der Gaag, H. C., and A. A. Axelrad. 1990. Friend virus replication in normal and immunosuppressed C57BL/6 mice. Virology 177:837-839. [DOI] [PubMed] [Google Scholar]
- 38.Vieillard, E. L., L. Gazzolo, V. Rousseau, I. Cremer, and E. De Maeyer. 1994. Enhanced resistance against HIV-1 infection in cell populations transformed to constitutively express interferon-beta. J. Interferon Res. 14:S209-P8-3. [Google Scholar]
- 39.Von Sydow M., A. Sönneborg, H. Gaines, and Ö. Strannegård. 1991. Interferon-alpha and tumor necrosis factor-alpha in serum of patients in various stages of HIV-1 infection. AIDS Res. Hum. Retrovir. 7:375-380. [DOI] [PubMed] [Google Scholar]
- 40.Wendling, F., and P. E. Tambourin. 1978. Oncogenicity of Friend-virus-infected cells: determination of origin of spleen colonies by the H-2 antigen as genetic markers. Int. J. Cancer 26:101-106. [DOI] [PubMed] [Google Scholar]
- 41.Zelinskyy, G., B. Balkow, S. Schimmer, K. Schepers, M. M. Simon, and U. Dittmer. 2004. Independent roles of perforin, granzymes and fas control of Friend retrovirus infection. Virology 20:365-367. [DOI] [PubMed] [Google Scholar]