Abstract
Similar to its close relative human herpesvirus 8, rhesus monkey rhadinovirus (RRV) persists predominantly in B cells of its natural host. Rhesus monkey B-cell lines immortalized by the Epstein-Barr-related virus from rhesus monkeys (rhEBV) were used as targets for infection by RRV. These cultured B cells were susceptible to infection by RRV and continued to produce low titers of RRV for months of continuous culture. Infection by RRV did not detectably alter the growth rates of these B-cell lines when it was measured at standard or reduced serum concentrations. Depending on the cell line, 5 to 40% of the B cells stained positive for the RRV genome by fluorescence in situ hybridization (FISH). Most RRV-positive cells showed a fine punctate nuclear staining pattern consistent with latent infection, while a small minority of cells (0.2 to 1%) contained large, intensely staining nuclear foci consistent with productive, replicative infection. Greater than 90% of the cells were rhEBV genome positive in a pattern consistent with latent infection, and again only a small minority of cells showed a productive, replicative staining pattern. Dual, two-color FISH staining revealed coinfection of numerous cells with both RRV and rhEBV, but productive replication of RRV and rhEBV was always observed in separate cells, never in the same cell. Thus, productive replication of RRV is unlinked to that of rhEBV; factors that influence activation to productive replication act separately on RRV and rhEBV, even within the same cell. The percentage of B cells expressing green fluorescent protein (GFP) early after infection with a recombinant RRV containing a GFP reporter gene was dose dependent and at a low multiplicity of infection increased progressively over time until 14 to 17 days after infection. These results establish a naturalistic cell culture system for the study of infection and persistence by RRV in rhesus monkey B cells.
Rhesus monkey rhadinovirus (RRV) is a gamma-2 herpesvirus (a rhadinovirus) that is a close relative of human herpesvirus 8 (HHV-8; the Kaposi sarcoma-associated herpesvirus) (1, 6, 20). The vast majority of the open reading frames present in HHV-8 have at least one corresponding homolog in RRV, and there is a close sequence similarity in corresponding genes. Unlike HHV-8, RRV can be grown lytically and to high titers in permissive monolayer cells (6). The CD20+ B lymphocyte is the primary cell type harboring RRV in the peripheral blood of persistently infected rhesus monkeys (2, 13). This situation parallels HHV-8 in humans, where the B lymphocyte has also been found to be the principal cell type that harbors the virus during persistent infection (11, 14, 17). Here we describe a rhesus monkey B-cell culture system for the study of RRV persistence and latency.
Growth of RRV-infected B cells.
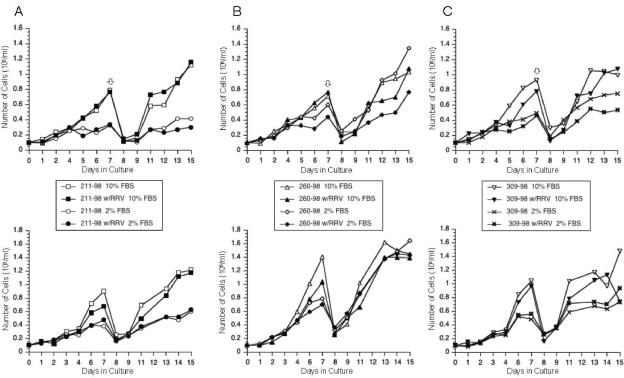
The gamma-2 herpesviruses not only persist in lymphoid cells but often immortalize or otherwise alter the growth properties of these lymphoid target cells (4, 9, 21-23). To examine if RRV infection alters the growth of rhesus monkey B cells, we selected three rhesus monkey B-cell lines, 211-98, 260-98, and 309-98, for RRV infection. These three rhesus monkey B-cell lines have been described previously (18); they were established by immortalization with the Epstein-Barr virus (EBV)-related virus of rhesus monkeys (rhEBV; also called rhesus lymphocryptovirus [LCV]) (15, 18). The three monkeys used as the sources of cells for these immortalizations were rhEBV negative. RRV strain 26-95 was harvested from the supernatant of infected rhesus fibroblasts at the time of complete cell lysis to use for B-cell infections. One million cells from each B-cell line were incubated for 1 h with RRV 26-95 in a volume of 0.5 ml, and control cells were incubated in 0.5 ml of Dulbecco modified Eagle medium (DMEM) in parallel. Cells were diluted with DMEM containing 10% or 2% fetal bovine serum (FBS) to 0.1 × 106 cells per ml, and live-cell counts were determined by trypan blue exclusion for each culture up to day 15 postinfection (Fig. 1, top). On day 7, cultures supplemented with 10% FBS were arbitrarily diluted 1:6 and those supplemented with 2% FBS were arbitrarily diluted 1:3. No differences in growth properties were detected in the RRV-infected versus the uninfected B-cell lines maintained in the presence of 10% FBS. At the 2% serum concentration, the RRV-infected 260-98 cell line displayed a slightly reduced growth rate compared to that of its uninfected counterpart (Fig. 1B, top), but this effect was marginal for 211-98 and 309-98 (Fig. 1A and C, top). All cell lines were maintained for an additional 8 weeks post RRV infection and then analyzed again for their growth properties. Cultures were diluted with DMEM containing 10% or 2% FBS to 0.1 × 106 cells per ml, and live-cell counts were determined for each culture up to day 15 postinfection (Fig. 1A, B, and C, bottom). No differences in growth properties were detected in RRV-infected versus uninfected B-cell lines independent of the serum concentration.
FIG. 1.
Influence of RRV infection on growth properties of B-cell lines 211-98 (A), 260-98 (B), and 309-98 (C). (Top) Immortalized rhesus B-lymphocyte cell lines were infected with RRV and cultured in the presence of 10% or 2% FBS starting with a cell density of 0.1 × 106 cells per ml of medium. Live-cell counts were determined for each culture up to day 15 postinfection. On day 7 (arrows), cultures were arbitrarily diluted 1:6 for cultures supplemented with 10% FBS and 1:3 for cultures supplemented with 2% FBS. (Bottom) Following 4 weeks of culture postinfection, the same cell lines were diluted again to a density of 0.1 × 106 cells per ml of medium supplemented with 10% or 2% FBS and growth properties were analyzed as outlined above.
Although RRV has been associated with lymphomas in the setting of simian immunodeficiency virus-induced immunodeficiency at one primate center (24), we were not able to detect any alterations in the growth potential of the B cells in our present study, even at reduced serum concentrations. It is, of course, possible that any growth-altering properties of RRV were overshadowed by the potent rhEBV cell growth transformation.
RRV production from persistently infected rhesus B-cell lines.
Following RRV infection, B-cell lines 211-98, 260-98, and 309-98, as well as the uninfected parental cell lines, were continuously maintained in culture for 4 months. At this time point, clarified supernatants from RRV-infected B-cell lines were analyzed for RRV production and supernatants from parental cell cultures were used in parallel as negative controls. As additional controls, RRV titers in supernatant from lytically infected rhesus fibroblast cells and uninfected parental cells were determined in parallel. Fifty percent tissue culture infective dose (TCID50) endpoint titers were determined in 48-well plates in duplicate by serial 10-fold dilution on a rhesus fibroblast line until complete cell lysis by 2 weeks (Table 1). All RRV-infected B-cell lines produced infectious RRV at titers of 103 to 104 per ml, indicating that the rhesus B cells were persistently infected with RRV. These titers were lower by a factor of 1,000 to 10,000 than titers obtained from infected fibroblast cultures (typically, 107 TCID50/ml). Infectious RRV was detected in supernatant taken at earlier and later times as well. No overt cytopathic effects were observed in the RRV-infected B-cell lines. These data demonstrate the persistence of RRV in the B-cell lines and the continuous production of low levels of infectious RRV.
TABLE 1.
RRV titers in culture supernatants of rhEBV-immortalized B-cell linesa
| Cell line | TCID50/ml |
|---|---|
| 211-98 | Negative |
| 211-98 with RRV | 104 |
| 260-98 | Negative |
| 260-98 with RRV | 104 |
| 309-98 | Negative |
| 309-98 with RRV | 103 |
| Rf 388-93 | Negative |
| Rf 388-93 with RRV | 107 |
Supernatants from the indicated cell lines, uninfected and RRV infected, were analyzed for the concentration of infectious RRV. Rf 388-93 is a rhesus monkey fibroblast cell line. TCID50 endpoint titers were determined in duplicate by serial 10-fold dilution on rhesus fibroblast line Rf 388-93 following complete cell lysis after 2 weeks.
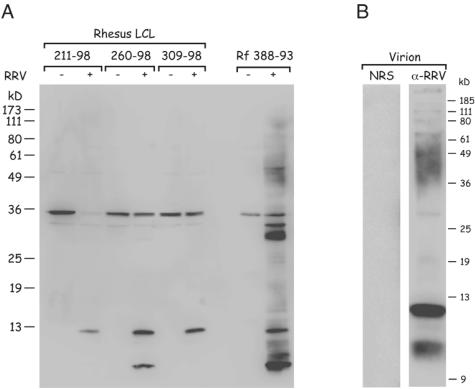
To further analyze the status of RRV persistence, we compared viral protein expression in persistently infected B cells with that in lytically infected fibroblasts. To generate cell lysates of the RRV-infected B cells and the parental cell lines, 10 million cells were lysed in 1 ml of RIPA buffer (phosphate-buffered saline containing 1% NP-40, 0.1% sodium dodecyl sulfate, and 0.5% sodium deoxycholate) and clarified by centrifugation (13,000 rpm for 15 min at 4°C). In addition, lysates were prepared from rhesus fibroblast cells at 81 h post RRV infection and from uninfected control fibroblasts. Protein concentrations were measured by using the bicinchoninic acid protein assay reagent (Pierce, Rockford, IL). For the B-cell lines, 20-μg samples of whole-cell lysates were analyzed by Western blotting and compared to 10 μg of fibroblast-derived lysates. Viral proteins were detected with a polyclonal rabbit serum raised against purified Triton X-100-denatured RRV particles (Fig. 2A). While RRV-infected fibroblast cells displayed a complex pattern of RRV polypeptide expression, only one or two of the bands were detected in the RRV-infected B cells. These polypeptides exhibited mobilities corresponding to approximately 13 and 10 kDa and were specific for RRV since they were absent in the parental B-cell lines and in uninfected fibroblasts. Two bands with the same mobility were detected in column-purified RRV virion preparations with the rabbit anti-RRV serum but not with normal rabbit control serum (Fig. 2B, right and left sides respectively). Mass spectrometry was performed on the excised material of 13 kDa (p13), and a total of nine peptides were identified. Eight peptides matched the gene product of open reading frame 52 (Orf52), and one peptide matched Orf47 of RRV. Orf52 of RRV is a homologue of BLRF2 of EBV, which constitutes one component of the highly immunogenic viral capsid antigen complex (8, 10). All sera from RRV-infected rhesus monkeys that were tested showed good reactivity to p13 by Western blotting (data not shown).
FIG. 2.
Expression of RRV proteins in persistently infected B-cell lines. (A) RRV-infected rhesus B-cell lines and their parental cell lines (211-98, 260-98, and 309-98) were lysed in RIPA buffer, and 20 μg of total cellular protein was loaded per lane. As controls, uninfected rhesus fibroblast cells (Rf 388-93) and fibroblast cells that had been infected for 81 h were lysed in parallel and 10 μg of total cellular protein was loaded per lane. Following electrophoresis, proteins were transferred to membrane filters and RRV proteins were detected by Western blotting with an RRV-specific rabbit serum. (B) Detection of RRV proteins in virus particle preparations. Column-purified RRV particles were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. RRV proteins were detected by Western blotting with an RRV-specific rabbit serum (α-RRV) or normal rabbit serum (NRS) as a control.
RRV genomes in rhesus B cells.
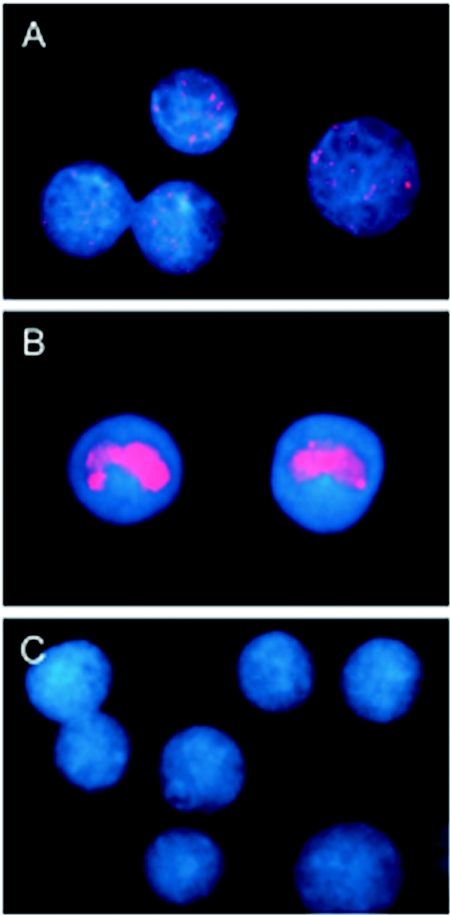
We showed previously that in situ hybridization with pooled rhEBV cosmid probes can reliably identify cells that are lytically or latently infected with rhEBV (12, 18). An analogous approach was used here to detect cells infected with RRV. To first address the question of what percentage of the cells were infected with RRV, fluorescence in situ hybridization (FISH) was performed with cosmid clones spanning the RRV genome. The RRV cosmid library was established by subcloning genomic RRV DNA into modified cosmid vector pSuperCos1 (Stratagene, La Jolla, CA). The derivation of overlapping sets of cosmid clones from RRV 26-95 has been recently described (3). DNA from three overlapping cosmid clones (clones 48, 1, and 43) constituting 90% of the RRV genome was labeled with SpectrumRed according to the manufacturer's recommendations (Vysis, Downers Grove, IL). 4,4′,6′-Diamidino-2-phenylindole (DAPI; Roche, Indianapolis, IN) was used to stain nuclear DNA. DNA labeling and in situ hybridization were carried out as described previously (12, 18). The percentage of cells that stained positively for the RRV genome ranged from 5 to 40%, depending on the B-cell line (Fig. 3 and Table 2). Most positive cells contained fine, punctate nuclear signals (Fig. 3A), a pattern consistent with the presence of viral episomes in latently infected cells (16, 18, 19). A small minority of cells showed intense staining confined to one or several large nuclear foci, a pattern consistent with productive replication (Fig. 3B). No hybridization was observed in the parental cell lines that were not infected with RRV (Fig. 3C). When hybridized with a bacmid DNA probe specific for rhEBV, >90% of the cells from each line were stained in a pattern consistent with latent infection, with a minor fraction of cells demonstrating a productive replicative pattern of staining (not shown).
FIG. 3.
Detection of RRV genomes in persistently infected B-cell lines by FISH. RRV-infected B-cell lines and corresponding parental cell lines were fixed and hybridized in situ with a SpectrumRed-labeled RRV pooled-cosmid probe that covered 90% of the unique coding region. Cell nuclei were counterstained with DAPI (blue). Results are shown for the RRV-infected 211-98 cell line (A and B) and the parental RRV-negative cell line (C). Staining patterns observed were consistent with latent persistence of RRV (A) and, at a lower frequency, productive replication (B). No RRV-specific staining was observed in the RRV-negative 211-98 parental cell line (C).
TABLE 2.
Status of RRV infection in rhEBV-immortalized rhesus B cell linesa
| Cell line | Latent RRV, latent rhEBV | Productive RRV, latent rhEBV | RRV negative, latent rhEBV | RRV negative, productive rhEBV |
|---|---|---|---|---|
| 211-98 with RRV | 40.4 | 0.2 | 59.2 | 0.2 |
| 260-98 with RRV | 4.8 | 0.6 | 94.6 | 0 |
| 309-98 with RRV | 26.6 | 1.2 | 71.6 | 0.6 |
A total of 500 cells of each cell line were evaluated by two-color FISH for RRV and rhEBV latent persistence or productive replication. Only cells that were positive for rhEBV were scored for RRV. Values represent percent cells with the indicated staining pattern. A small percentage of cells (less than 10%) did not show a signal for rhEBV; it is likely that these represent false negatives. None of the cells were rhEBV negative and RRV positive.
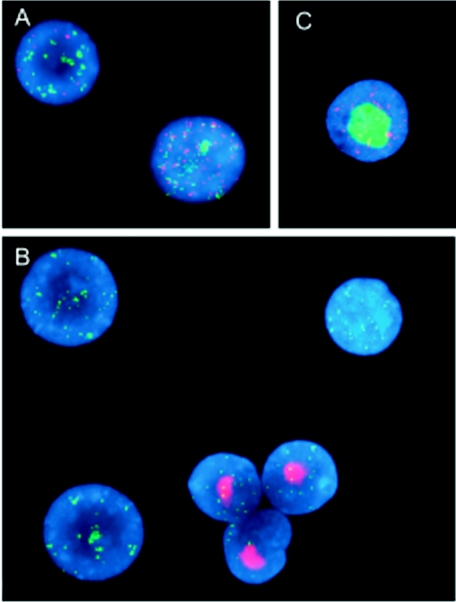
To further study the interaction of RRV and rhEBV in coinfected cells, cells were double stained with SpectrumGreen (Vysis)-labeled rhEBV bacmid DNA and SpectrumRed-labeled RRV DNA. The predominant pattern that was observed was the joint presence of both RRV and rhEBV signals in a punctate nuclear pattern (Fig. 4A), consistent with latent coinfection. Punctate hybridization to RRV in latently infected cells was predominantly distinct from that of rhEBV, with little or no colocalization. Productive replicative infection by RRV or rhEBV, as identified by FISH, was observed at a much lower frequency and never in the same cell (Fig. 4B and C, respectively). To evaluate this relationship more systematically, we scored the rhEBV and RRV staining patterns in 500 cells from each cell line in a second series of slides (Table 2). Again, all cells exhibiting a productive RRV staining pattern showed a latent staining pattern for rhEBV. In this experiment, the few cells that showed a lytic staining pattern for rhEBV were uninfected by RRV. These results indicate that productive replication of RRV in these B-cell lines is separate from and unlinked to productive replication of rhEBV, even within the same cell. Thus, the cascade of gene regulation events leading to productive replication of one virus is not sufficient to trigger productive replication of the other.
FIG. 4.
Productive replication of RRV is independent of that of rhEBV. RRV-infected B-cell lines and corresponding parental cell lines were fixed and hybridized in situ simultaneously with a SpectrumRed-labeled RRV probe and a SpectrumGreen-labeled rhEBV probe. Cell nuclei were counterstained with DAPI (blue). Results are shown for the RRV-infected 211-98 cell line. Panels: A, latent coinfection of RRV and rhEBV; B, productive replication of RRV in the presence of latent rhEBV; C, productive replication of rhEBV in the presence of latent RRV infection. Note that in panel B, three rhEBV-positive cells are present that are apparently not infected by RRV.
It is highly likely that each dot in the predominant punctate staining pattern represents a single RRV DNA molecule. The vast predominance of cells with a small number of these dots for RRV is not consistent with productive, replicative infection but rather is entirely consistent with one form or another of latent or repressed infection. Furthermore, the intense diffuse nuclear staining pattern observed in a small percentage of the cells was associated with intranuclear inclusions, further evidence for productive, replicative infection in these intensely staining cells. FISH is a method that was developed to detect single-copy DNA sequences within complex genomes. It has been used convincingly in previous studies to identify single EBV genomes within cells, for example, in defining the single site of chromosomal integration of EBV DNA in the Namalwa cell line (16). Human B-cell lines that are latently infected with episomal EBV DNA give FISH signals consisting of punctate dots that correspond to individual EBV genomes, whose number can vary from cell line to cell line and even from cell to cell within an individual cell line (16, 19). Kutok et al. (12) have previously shown that lytic replication of rhEBV in epithelial cells is associated with a pattern of intense diffuse nuclear staining that is identical to the pattern observed in the small subset of rhEBV- and RRV-infected B cells that we describe here. Thus, a punctate staining pattern has been shown in previous publications to correspond to individual viral genomes in latent infection, the copy number of latent viral genomes can be variable, and the intense diffuse nuclear staining pattern is associated with productive replicative infection. Our analysis does not, however, specify the nature or level of repressed expression and latent infection with RRV in these cell lines.
Time course and dose dependence of B-cell infection.
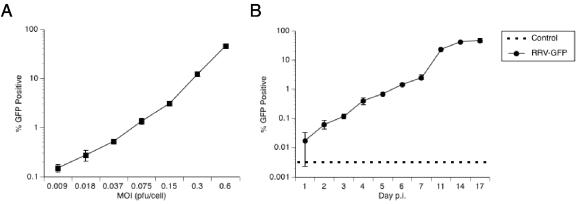
LCL211-98 cells were infected at increasing multiplicities of infection (MOIs) with a replication-competent, recombinant RRV expressing green fluorescent protein (GFP), RRV-GFP. RRV-GFP was generated and its titer was determined in rhesus monkey fibroblasts as described by Bilello et al. (3). At day 4 postinfection, triplicate cultures of LCL211-98 cells, either uninfected or infected with increasing numbers of PFU of RRV-GFP per cell, were analyzed by fluorescence-activated cell sorting (FACS) to determine the percentage of cells that were GFP positive at each MOI. As displayed in Fig. 5A, the percentage of GFP-positive cells was MOI dependent, ranging from approximately 0.2% to 47% GFP positive at day 4 postinfection, with MOIs of 0.009 and 0.6 PFU/cell, respectively. To determine if RRV was actively replicating and spreading within the cultures, LCL211-98 cultures were infected at a low MOI with 0.037 PFU of RRV-GFP/cell. Each day postinfection, cultures were examined for GFP positivity by FACS analysis until day 17. The percentage of GFP-positive cells increased steadily over time after infection with RRV-GFP to a maximum of approximately 47% at day 17 postinfection, when the experiment was concluded. Decreases in cell number (flow cytometry) and viability (trypan blue exclusion) were noted after 14 days postinfection (data not shown). Thus, RRV-GFP was able to replicate and spread slowly through the B-cell culture. The maximal number of GFP-positive cells (47% in Fig. 5) was similar to the number of RRV-positive cells obtained by FISH in separate experiments (41% in Table 2).
FIG. 5.
Dose and time dependence. (A) The percentage of GFP-positive LCL211-98 cells after infection with RRV-GFP is MOI dependent. LCL211-98 cells were infected with increasing MOIs of RRV-GFP. The number of PFU of RRV-GFP was determined on rhesus monkey fibroblasts. At day 4 postinfection, cultures were examined for GFP expression by FACS analysis. The results show the percentage of viable GFP-positive cells along with the standard deviation at the indicated number of PFU per cell. Where no error bar is shown, the error falls within the size of the symbol. (B) The percentage of GFP-positive LCL211-98 cells increases over time postinfection (p.i.). Triplicate cultures of LCL211-98 cells were mock infected or infected with RRV-GFP at 0.037 PFU/cell. The number of PFU of RRV-GFP was determined on rhesus monkey fibroblasts. Each day postinfection, 1 ml of the suspension culture was removed and replaced with 1 ml of medium. The aliquoted cells were pelleted by centrifugation and examined by FACS analysis to determine the percentage of GFP-positive cells.
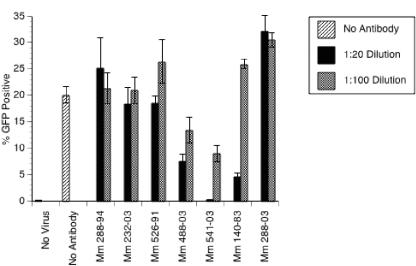
Bilello et al. (3) have recently described a neutralization assay in which sera from RRV-positive monkeys, but not RRV-negative monkeys, were able to block infection of rhesus fibroblasts by RRV-GFP. To examine the capacity of sera from rhesus monkeys naturally infected with RRV to neutralize infection of B cells by RRV-GFP, RRV-GFP (0.3 PFU/cell) was incubated with either medium alone or heat-inactivated sera from RRV-negative and RRV-positive rhesus monkeys at a dilution of 1:20 or 1:100. Following this 3-h incubation, LCL211-98 cells were inoculated with the virus-serum mixture. At day 4 postinfection, the percentage of GFP-positive cells was determined by FACS analysis. Sera from RRV-negative monkeys 288-94 and 232-03 did not neutralize RRV-GFP infection of LCL211-98 cells (Fig. 6). However, sera from rhesus monkeys 541-03, 140-83, and 488-03, which were naturally infected with RRV, neutralized RRV-GFP infection by 99%, 77%, and 63%, respectively, at a 1:20 dilution (Fig. 6). Sera from naturally infected monkeys 288-03 and 526-91 did not show neutralizing activity in this B-cell assay. The sera from these two monkeys also had the weakest neutralizing activity for RRV infection of rhesus fibroblasts (3). In fact, there was a strong correlation of the rank order of neutralizing activity on the two cell types. Of the five RRV-positive sera compared, 541-03 had the highest neutralizing activity on both cell types.
FIG. 6.
Neutralization of RRV-GFP infection. RRV-GFP (MOI = 0.3 PFU/cell) was incubated with either medium alone (No Antibody) or sera from RRV-negative rhesus monkeys (Mm 288-94 and 232-03) or rhesus monkeys naturally infected with RRV (Mm 526-91, 488-03, 541-03, 140-83, and 288-03) at a dilution of 1:20 or 1:100. The number of PFU of RRV-GFP was determined on rhesus monkey fibroblasts. After incubating of the virus-serum mixture for 3 h at 37°C with gentle rocking, LCL211-98 cells were inoculated with either medium alone (No Virus) or the virus-serum mixture. At day 4 postinfection, cultures were examined for GFP expression by FACS analysis. The results shown are the percentage of GFP-positive cells along with the standard deviation for each serum dilution tested.
In summary, we have described here a rhesus monkey B-cell culture system that will allow the study of RRV's ability to infect and persist in rhesus monkey B cells. Persistence of RRV and Kaposi sarcoma-associated herpesvirus in human B-cell lines has recently been described (5, 7). One line of investigation that will be made possible by the availability of this system is the extent to which different RRV glycoproteins and different cellular receptors may be used for infection of B cells versus fully permissive fibroblasts. It will now also be possible to investigate the ordered regulation of gene expression in the context of rhesus monkey B cells.
Acknowledgments
This work was supported by Public Health Service grants 1P01DE14388, 1RO1AI063928, RR00168, and 5T32AI0724522.
We thank Charles Lee for assistance with the FISH studies.
REFERENCES
- 1.Alexander, L., L. Denekamp, A. Knapp, M. R. Auerbach, B. Damania, and R. C. Desrosiers. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 74:3388-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergquam, E. P., N. Avery, S. M. Shiigi, M. K. Axthelm, and S. W. Wong. 1999. Rhesus rhadinovirus establishes a latent infection in B lymphocytes in vivo. J. Virol. 73:7874-7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilello, J. P., J. S. Morgan, B. Damania, S. M. Lang, and R. C. Desrosiers. 2006. A genetic system for rhesus monkey rhadinovirus: use of recombinant virus to quantitate antibody-mediated neutralization. J. Virol. 80:1549-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 5.Chen, L., and M. Lagunoff. 2005. Establishment and maintenance of Kaposi's sarcoma-associated herpesvirus latency in B cells. J. Virol. 79:14383-14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desrosiers, R. C., V. G. Sasseville, S. C. Czajak, X. Zhang, K. G. Mansfield, A. Kaur, R. P. Johnson, A. A. Lackner, and J. U. Jung. 1997. A herpesvirus of rhesus monkeys related to the human Kaposi's sarcoma-associated herpesvirus. J. Virol. 71:9764-9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeWire, S. M., M. A. McVoy, and B. Damania. 2002. Kinetics of expression of rhesus monkey rhadinovirus (RRV) and identification and characterization of a polycistronic transcript encoding the RRV Orf50/Rta, RRV R8, and R8.1 genes. J. Virol. 76:9819-9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farber, I., W. Hinderer, M. Rothe, D. Lang, H. H. Sonneborn, and P. Wutzler. 2001. Serological diagnosis of Epstein-Barr virus infection by novel ELISAs based on recombinant capsid antigens p23 and p18. J. Med. Virol. 63:271-276. [DOI] [PubMed] [Google Scholar]
- 9.Fickenscher, H., and B. Fleckenstein. 2001. Herpesvirus saimiri. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:545-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinderer, W., D. Lang, M. Rothe, R. Vornhagen, H. H. Sonneborn, and H. Wolf. 1999. Serodiagnosis of Epstein-Barr virus infection by using recombinant viral capsid antigen fragments and autologous gene fusion. J. Clin. Microbiol. 37:3239-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, Y. Q., J. J. Li, B. J. Poiesz, M. H. Kaplan, and A. E. Friedman-Kien. 1997. Detection of the herpesvirus-like DNA sequences in matched specimens of semen and blood from patients with AIDS-related Kaposi's sarcoma by polymerase chain reaction in situ hybridization. Am. J. Pathol. 150:147-153. [PMC free article] [PubMed] [Google Scholar]
- 12.Kutok, J. L., S. Klumpp, M. Simon, J. J. MacKey, V. Nguyen, J. M. Middeldorp, J. C. Aster, and F. Wang. 2004. Molecular evidence for rhesus lymphocryptovirus infection of epithelial cells in immunosuppressed rhesus macaques. J. Virol. 78:3455-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mansfield, K. G., S. V. Westmoreland, C. D. DeBakker, S. Czajak, A. A. Lackner, and R. C. Desrosiers. 1999. Experimental infection of rhesus and pig-tailed macaques with macaque rhadinoviruses. J. Virol. 73:10320-10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mesri, E. A., E. Cesarman, L. Arvanitakis, S. Rafii, M. A. Moore, D. N. Posnett, D. M. Knowles, and A. S. Asch. 1996. Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J. Exp. Med. 183:2385-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rangan, S. R., L. N. Martin, B. E. Bozelka, N. Wang, and B. J. Gormus. 1986. Epstein-Barr virus-related herpesvirus from a rhesus monkey (Macaca mulatta) with malignant lymphoma. Int. J. Cancer 38:425-432. [DOI] [PubMed] [Google Scholar]
- 16.Reisinger, J., S. Rumpler, T. Lion, and P. F. Ambros. 2005. Visualization of episomal and integrated Epstein-Barr virus DNA by fiber fluorescence in situ hybridization. Int. J. Cancer 118:1603-1608. [DOI] [PubMed] [Google Scholar]
- 17.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 18.Rivailler, P., A. Carville, A. Kaur, P. Rao, C. Quink, J. L. Kutok, S. Westmoreland, S. Klumpp, M. Simon, J. C. Aster, and F. Wang. 2004. Experimental rhesus lymphocryptovirus infection in immunosuppressed macaques: an animal model for Epstein-Barr virus pathogenesis in the immunosuppressed host. Blood 104:1482-1489. [DOI] [PubMed] [Google Scholar]
- 19.Rose, C., M. Green, S. Webber, L. Kingsley, R. Day, S. Watkins, J. Reyes, and D. Rowe. 2002. Detection of Epstein-Barr virus genomes in peripheral blood B cells from solid-organ transplant recipients by fluorescence in situ hybridization. J. Clin. Microbiol. 40:2533-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Searles, R. P., E. P. Bergquam, M. K. Axthelm, and S. W. Wong. 1999. Sequence and genomic analysis of a Rhesus macaque rhadinovirus with similarity to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 73:3040-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunil-Chandra, N. P., J. Arno, J. Fazakerley, and A. A. Nash. 1994. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am. J. Pathol. 145:818-826. [PMC free article] [PubMed] [Google Scholar]
- 22.Tarakanova, V. L., F. Suarez, S. A. Tibbetts, M. A. Jacoby, K. E. Weck, J. L. Hess, S. H. Speck, and H. W. Virgin. 2005. Murine gammaherpesvirus 68 infection is associated with lymphoproliferative disease and lymphoma in BALB β2-microglobulin-deficient mice. J. Virol. 79:14668-14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong, E. L., and B. Damania. 2005. Linking KSHV to human cancer. Curr. Oncol. Rep. 7:349-356. [DOI] [PubMed] [Google Scholar]
- 24.Wong, S. W., E. P. Bergquam, R. M. Swanson, F. W. Lee, S. M. Shiigi, N. A. Avery, J. W. Fanton, and M. K. Axthelm. 1999. Induction of B cell hyperplasia in simian immunodeficiency virus-infected rhesus macaques with the simian homologue of Kaposi's sarcoma-associated herpesvirus. J. Exp. Med. 190:827-840. [DOI] [PMC free article] [PubMed] [Google Scholar]