Abstract
Herpes simplex virus 1 (HSV-1) ICP27 has been shown to interact with RNA polymerase II (RNAP II) holoenzyme. Here, we show that ICP27 interacts with the C-terminal domain (CTD) of RNAP II and that ICP27 mutants that cannot interact fail to relocalize RNAP II to viral transcription sites, suggesting a role for ICP27 in RNAP II recruitment. Using monoclonal antibodies specific for different phosphorylated forms of the RNAP II CTD, we found that the serine-2 phosphorylated form, which is found predominantly in elongating complexes, was not recruited to viral transcription sites. Further, there was an overall reduction in phosphoserine-2 staining. Western blot analysis revealed that there was a pronounced decrease in the phosphoserine-2 form and in overall RNAP II levels in lysates from cells infected with wild-type HSV-1. There was no appreciable difference in cdk9 levels, suggesting that protein degradation rather than dephosphorylation was occurring. Treatment of infected cells with proteasome inhibitors MG-132 and lactacystin prevented the decrease in the phosphoserine-2 form and in overall RNAP II levels; however, there was a concomitant decrease in the levels of several HSV-1 late proteins and in virus yield. Proteasomal degradation has been shown to resolve stalled RNAP II complexes at sites of DNA damage to allow 3′ processing of transcripts. Thus, we propose that at later times of infection when robust transcription and DNA replication are occurring, elongating complexes may collide and proteasomal degradation may be required for resolution.
ICP27 is a multifunctional regulatory protein that is required for herpes simplex virus 1 (HSV-1) productive infection. This 63-kDa phosphoprotein is expressed with immediate-early kinetics, and it is required for appropriate expression of viral early and late gene products; in addition, it contributes to the shutoff of host protein synthesis (for review, see reference 54). ICP27 has been demonstrated to function posttranscriptionally at the level of RNA processing and export (4, 35, 49-51), and recently evidence for a role in translation initiation has been presented (15, 16). Further, ICP27 also appears to contribute to the transcriptional regulation of HSV-1 early and late genes (23, 61). Thus, ICP27 appears to function in all stages of viral gene expression from transcription through translation. The mechanisms of some of its effects on gene expression have been elucidated. At early times after infection, ICP27 interacts with several splicing factors (4, 51), including members of a family of essential splicing factors termed SR proteins, and affects their phosphorylation. This results in the blockage of prespliceosomal assembly, which in turn contributes to the shutoff of host protein synthesis because cellular pre-mRNAs cannot be properly processed (51). At later times, beginning at about 6 h after infection, ICP27 begins to shuttle between the nucleus and cytoplasm (6, 38, 42, 49, 56). ICP27 binds to viral mRNAs (37, 49, 55) and facilitates their export to the cytoplasm by interacting with the cellular RNA export adaptor protein Aly/REF and the cellular export receptor TAP/NXF (5, 6, 27). ICP27 has also been proposed to have a role in 3′ processing of some viral late RNAs by affecting polyadenylation site selection and recruiting a factor involved in cleavage (34, 35).
ICP27 has also been shown to stimulate expression of some early genes and transcription of some late viral genes (23, 61). Furthermore, ICP27 has been shown to associate with cellular RNA polymerase II (RNAP II) holoenzyme, and this interaction was found to be independent of DNA and RNA (66). A number of cellular proteins that are involved in RNA processing, including 5′ capping, splicing, and 3′ cleavage and polyadenylation, have been found to bind to the C-terminal domain (CTD) of RNAP II, which acts as a platform to bring these factors to sites on the nascent transcript where their action is needed (for review, see references 3 and 21). Therefore, in this study we first asked if ICP27 interacted directly with the RNAP II CTD, in accord with its posttranscriptional activities.
Some cellular RNA processing factors that associate with the RNAP II CTD interact predominantly with a specific phosphorylated form of the CTD, whereas others do not show a marked preference when binding (2, 7, 24, 28). The RNAP II CTD in all eukaryotes is highly conserved and consists of tandem repeats of a heptapeptide, YSPTSPS, which is repeated 52 times in humans. The CTD is reversibly phosphorylated on serine-2 and serine-5 in the heptapeptide repeat (9). Unphosphorylated RNAP II is recruited to the promoter, where a preinitiation complex forms. At initiation of transcription, serine-5 is phosphorylated primarily by cdk7, which is associated with general transcription factor TFIIH (9, 28). Thus, RNAP II found at the promoters of genes is mostly phosphorylated on serine-5 (for review, see reference 26). This form is also referred to as hypophosphorylated or RNAP IIA. During elongation, another kinase, P-TEFb, which consists of the cyclin-dependent kinase cdk9 and one of several cyclin T isoforms, phosphorylates serine-2 (for review, see reference 43). The elongating form of RNAP II is hyperphosphorylated and is called the RNAP IIO form, which has a slower migration (63). In HSV-1-infected cells, an intermediately phosphorylated form has been found beginning around 5 h after infection (48). HSV-1 protein ICP22 and kinase UL13 have been shown to mediate the phosphorylation of RNAP II, resulting in the RNAP IIi form (31, 47). Further, it has been shown that ICP22 interacts with cdk9 in a complex and that the HSV-1 kinase Us3 may also be involved in the intermediate phosphorylation of RNAP II (14). The exact nature of the modifications and the role of the RNAP IIi form during viral transcription have not been elucidated.
It was reported recently that in addition to the modification of RNAP II to the IIi form, HSV-1 infection leads to a loss of serine-2 phosphorylation (17). In this study, a small subfraction remained and was found to colocalize with splicing speckles, leading the authors to postulate that reduced serine-2 phosphorylation and its relocation to nuclear speckles may be demonstrative of the inhibition of cellular gene transcription, which was previously shown to occur on several cellular genes during HSV-1 infection (58). To determine with which phosphorylated forms of the CTD ICP27 associates, we used monoclonal antibodies that are specific for the serine-5 and serine-2 forms. Surprisingly, we found decreased levels of the serine-2 form as well as an overall decrease in RNAP II holoenzyme, which suggested protein degradation rather than reduced phosphorylation. In accord with this postulate, proteasome inhibitors prevented the loss of the phosphoserine-2 form as well as the decrease in overall RNAP II levels, as did treatment with actinomycin D during infection to halt early and late viral transcription. Studies in yeasts have shown that the 26S proteasome associates with the 3′ ends of genes in a transcription-dependent fashion and correlates with the accumulation of transcripts and the buildup of transcription complexes in the same region (19, 57). The results that we present here lead us to postulate that during robust transcription of HSV-1 early and late genes from both strands of the genome, RNAP II complexes may collide and stall and proteasomal degradation may be required to resolve these complexes.
MATERIALS AND METHODS
Cells, viruses, and recombinant plasmids.
HeLa cells and rabbit skin fibroblasts (RSFs) were grown on minimal essential medium containing 10% fetal calf serum. HSV-1 wild-type (WT) strain KOS and ICP27 null mutant 27-LacZ were described previously (53). ICP27 viral mutants dLeu, d1-2, d4-5, m15, m16, n406, and n504 were generously provided by Steve Rice (30, 44, 45). ICP27 mutant plasmids pΔN, pΔNLS, pD2ΔS5, pR1, pH 17, pΔC, pS2, and pS18 have been described previously (5, 6, 20, 51). Plasmids pΔ26-100 and pΔ63-100 were constructed from pSG130B/S (52) containing the WT ICP27 gene by deleting the fragment from AgeI to DraIII and the fragment from DraIII to RsrII, respectively, and inserting oligonucleotides to restore the reading frame and replace flanking amino acids. pEt21-GST-CTD expressing full-length WT murine glutathione transferase (GST)-CTD with 52 repeats was generously provided by David Bentley (33).
Virus infection and immunoprecipitation procedures.
HeLa R19 cells were infected with the viruses indicated in each figure at a multiplicity of infection of 10. Nuclear extracts and whole-cell extracts were prepared as described previously (6, 51). For all immunoprecipitation experiments, extracts were treated with 10 μg/ml DNase I (Sigma) and a 1:100 dilution of RNase cocktail (Ambion) at 30°C for 30 min in the presence of protease inhibitors, 4 mM Pefabloc, and leupeptin at 0.1 μg/μl. Immunoprecipitations, gel electrophoresis, and immunoblotting procedures were performed as described previously (6, 51, 65).
In vitro binding assays.
GST-binding assays were performed at room temperature by combining 20 μl of glutathione-Sepharose-bound GST proteins with 10 μl of 35S-labeled proteins (or as indicated in the figures) from in vitro translation reactions as described previously (6, 51). DNase and RNase were added as described above. Beads were washed five times, and bound proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
In vitro phosphorylation.
Twenty microliters of GST and GST-CTD bound to glutathione-Sepharose beads was incubated with 50 μCi of [γ-32P]ATP and 5 units of cdc-2 kinase (New England Biolabs) under the conditions specified by the manufacturer. Apyrase (Sigma), which is an ATPase, was included in some reactions as a negative control for phosphorylation.
Immunofluorescence microscopy.
RSFs were grown on coverslips and infected as described in the figure legends. At various times, cells were fixed in 3.7% formaldehyde, and immunofluorescence staining was performed as described previously (6, 51) with anti-ICP27 antibodies H1119 and H1113 (Goodwin Institute); anti-ICP4 antibody H1101 (Goodwin Institute); anti-RNAP II antibodies 8WG16, H5, and H14 (Covance Research Products); ARNA3 (Research Diagnostics); and anti-SC35 hybridoma supernatant (51). The specificity of the RNAP II antibodies is shown in Table 1. Procedures for the serial treatment of cells with different monoclonal antibodies were as described previously (51). Cells were viewed by fluorescence microscopy at a magnification of ×100 with a Zeiss Axiovert S100 microscope. Images were pseudocolored and merged using Adobe Photoshop.
TABLE 1.
Specificity of monoclonal antibodies against RNAP II
| Monoclonal antibody | Epitope | RNAP II form | Reference |
|---|---|---|---|
| 8WG16 | Unphosphorylated and Ser-5-phosphorylated CTD | Unphosphorylated and RNAP IIA | 41 |
| H14 | Ser-5-phosphorylated CTD | RNAP IIA-initiating complex | 41 |
| H5 | Ser-2-phosphorylated CTD | RNAP IIO-elongating complex | 41 |
| ARNA3 | N-terminal epitope in large subunit of RNAP II holoenzyme | All forms, unphosphorylated, RNAP IIA, and RNAP IIO | 28 |
RESULTS
ICP27 interacts with the CTD of RNAP II.
To determine if, like many cellular RNA processing factors, ICP27 binds to the CTD of RNAP II, in vitro binding studies were performed. GST-CTD was found to bind to ICP27 translated in vitro in reticulocyte lysates (Fig. 1A). Further, the translation reactions were treated with DNase and RNase before and during the binding assay, indicating that ICP27 interacted with GST-CTD through protein-protein interactions and that binding was not mediated by nucleic acids. Zhou and Knipe also found that the interaction of ICP27 with RNAP II holoenzyme occurred in the absence of nucleic acids (66). Because bacterially expressed GST-CTD is not phosphorylated and ICP27 was found to bind, this indicates that ICP27 may not have a preference for a specific phosphorylated form of RNAP II, as has been found with the cellular cleavage and polyadenylation specificity factor, which interacts with both unphosphorylated and phosphorylated forms (10). To determine if phosphorylation of the CTD had any effect on the binding by ICP27, GST-CTD was phosphorylated in vitro with cdc-2 kinase (Fig. 1B), which phosphorylates both serine-2 and serine-5 (62). Binding of ICP27 to cdc-2-phosphorylated GST-CTD was found to be equivalent to that seen with unphosphorylated GST-CTD (Fig. 1C). Similarly, ICP27 in virus-infected cells was found to coimmunoprecipitate with both unphosphorylated and phosphorylated forms of RNAP II (Fig. 1D). Specifically, immunoprecipitations were performed with monoclonal antibody 8WG16, which recognizes unphosphorylated RNAP II and the hypophosphorylated IIA form, and with monoclonal antibodies H14 and H5, which recognize the phosphoserine-5 and phosphoserine-2 forms, respectively (41). Western blot analysis of the immunoprecipitated complexes using monoclonal antibody specific for ICP27 demonstrated that ICP27 was coimmunoprecipitated with both unphosphorylated and phosphorylated forms (Fig. 1D) and, further, that both unphosphorylated and phosphorylated forms of RNAP II were coimmunoprecipitated with ICP27 (Fig. 1E). As with the in vitro binding assays, the coimmunoprecipitations were performed in the presence of DNase and RNase, indicating that nucleic acids were not required to bridge these interactions.
FIG. 1.
ICP27 binds to both unphosphorylated and phosphorylated RNAP II CTD in vitro and in vivo. (A) GST binding assays were performed with GST-CTD and in vitro translated ICP27. Two or twenty microliters of the translation reaction was added to the binding assays as indicated. The binding assays were performed in the presence of DNase and RNase to eliminate the possibility of nucleic acid bridging. Luciferase was included as a negative control. (B) GST-CTD was phosphorylated in vitro with cdc2 kinase. GST was included as a negative control. (C) Unphosphorylated or cdc2-phosphorylated GST-CTD was added to binding assays with in vitro translated ICP27 as indicated. The binding reactions were also performed with GST-CTD that was treated with cdc-2 in the presence of the ATPase apyrase as a control. All binding assays were performed in the presence of DNase and RNase. (D) RNAP II was immunoprecipitated with antibody 8WG16 or a combination of H14 and H5 from nuclear extracts (NE) of cells that were infected with HSV-1 KOS for 6 h. ICP27 in the immunoprecipitated complexes was detected by Western blot analysis with antibody H1119, which is specific for ICP27. The intense band beneath the ICP27 band is heavy chain immunoglobulin G from the immunoprecipitation. (E) Immunoprecipitations were performed with ICP27 antibody H1119 on nuclear extracts from cells that were mock-infected (UN) or were infected with 27-LacZ or HSV-1 KOS. Western blot analysis was performed to detect RNAP II and ICP27 with the indicated antibodies. NE, nuclear extracts; Luc, luciferase; IP, immunoprecipitation.
Both the N terminus and C terminus of ICP27 must be intact for efficient interaction with RNAP II CTD.
To map the regions of ICP27 that are involved in its interaction with RNAP II CTD, in vitro binding studies were performed with GST-CTD and a series of ICP27 mutant proteins that harbor deletions or insertions. The positions of the mutations within the ICP27 coding sequence are shown in Fig. 2A. Binding to unphosphorylated GST-CTD was seen to be greatly reduced for ICP27 mutants ΔN, in which N-terminal residues 3 to 28 are deleted (65); ΔC, in which C-terminal residues 450 to 512 are deleted (51); and S2, which has an insertion of four amino acids between residues 465 and 466 (20). C-terminal mutant S18, with an insertion of four amino acids between residues 504 and 505 (20), bound as efficiently as WT ICP27 (Fig. 2B). Thus, as with ICP27's self-interaction (65) and its interaction with several other proteins, including splicing SR proteins (51) and export receptor TAP/NXF1 (5), both the N terminus and C terminus, at least until amino acid 504, must be intact for efficient binding to unphosphorylated GST-CTD (Fig. 2B). It was similarly found that the N terminus and C terminus must be intact for binding of ICP27 to phosphorylated GST-CTD (Fig. 2C); however, mutant S2 bound efficiently to phosphorylated GST-CTD, but binding to unphosphorylated GST-CTD was barely detectable (Fig. 2B). The insertion and deletion mutations undoubtedly cause conformation changes in ICP27, and it is possible that binding to the more extended structure of the phosphorylated CTD (63) is more tolerant of these alterations. It should also be noted that the in vitro binding assays were repeated five times, and the results were similar to the representative results shown in Fig. 2 in all cases.
FIG. 2.
Both the N terminus and the C terminus of ICP27 must be intact for binding to RNAP II CTD in vitro. In vitro binding assays were performed with GST-CTD and WT or mutant forms of ICP27 that were translated in vitro. (A) A schematic representation of the ICP27 coding sequence showing the sites of the mutations. The leucine-rich region (LRR), the nuclear localization signal (NLS), the RGG-box motif (RGG), the arginine-rich region 2 (R2), three predicted KH domains (KH1, KH2, KH3), and a zinc finger-like domain (CCHC) are depicted. (B) In vitro binding assays were performed with unphosphorylated (unphos) GST-CTD and WT or mutant forms of ICP27 as indicated. Luciferase was included as a negative control. Input 35S-labeled proteins are shown in the lower panel. (C) GST-CTD was phosphorylated (phos) in vitro with [γ-32P]ATP by cdc2 kinase, and binding assays were performed with WT and mutant forms of ICP27. Input 35S-labeled proteins are shown in the lower panel. DNase and RNase were added to all binding reactions. Luc, luciferase.
Both the N terminus and C terminus of ICP27 are required to be intact for the interaction with RNAP II in virus-infected cells.
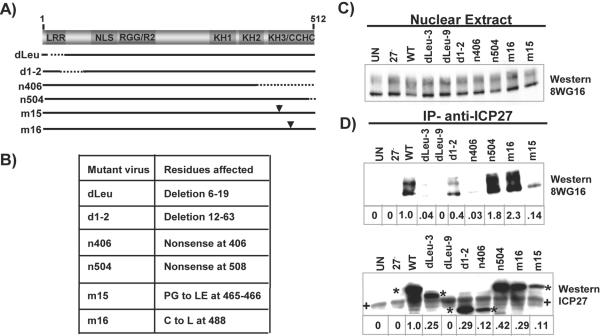
To confirm the in vitro mapping studies in vivo, coimmunoprecipitation studies were performed on nuclear extracts from cells infected with WT HSV-1 KOS and ICP27 viral mutants. The positions of the mutations are shown in Fig. 3A, and the residues affected are listed in Fig. 3B. Immunoprecipitation with monoclonal antibody to ICP27 resulted in the coprecipitation of RNAP II, recognized by antibody 8WG16 (41), in extracts from WT-infected cells and from cells infected with mutant virus d1-2, which has a deletion of residues 12 to 63, and mutant virus n504, which yields a protein that is truncated at residue 504 (30, 46) (Fig. 3D). These data are in agreement with the in vitro mapping data shown in Fig. 2, further narrowing the N-terminal region required to residues 3 to 12 and confirming that residues 505 to 512 are not required. Zhou and Knipe (66) also reported that RNAP II was coprecipitated with mutant n504. There was a barely detectable interaction seen with mutant n406, which lacks the C-terminal 112 amino acids (Fig. 3D and E), and binding was also greatly reduced with mutant dLeu, which has a deletion of N-terminal residues 6 to 19 (30). This includes the epitope for ICP27 monoclonal antibody H1119 (36), and therefore ICP27 monoclonal antibody H1113 was used for coimmunoprecipitation with dLeu extracts (Fig. 3D and E). Interestingly, two different ICP27 substitution mutants, m15 and m16, behaved differently. Mutant m15 has substitutions for residues 465 and 466, and m16 contains a substitution for residue 488 (45), both within the C-terminal region of ICP27. RNAP II was efficiently coimmunoprecipitated with mutant m16 but not with mutant m15. That is, while about one-third as much m15 mutant form of ICP27 was immunoprecipitated compared to the amount of the m16 form, only 14% of the level of RNAP II was precipitated with m15 compared to WT, whereas m16 coprecipitated 2.3 times as much as WT ICP27 (Fig. 3D). Thus, the conformational change in the m15 protein reduced its ability to bind to RNAP II. We conclude that both the N terminus and the C terminus up to residue 504 must be largely intact for efficient interaction with RNAP II.
FIG. 3.
The N terminus and the C terminus of ICP27 must be intact for efficient interaction with RNAP II in virus-infected cells. (A) A schematic diagram of the ICP27 coding sequence showing the positions of the lesions in the ICP27 viral mutants. The domains shown are as described in the legend to Fig. 2. (B) The residues affected in each mutant are indicated. (C) HeLa cells were infected for 6 h with WT HSV-1 KOS and the indicated mutant viruses. A portion of each nuclear extract was analyzed by Western blotting with anti-RNAP II antibody 8WG16 to monitor RNAP II expression. (D) Immunoprecipitations (IP) were performed with antibody H1119 to ICP27, and RNAP II was detected by Western blot analysis with antibody 8WG16. ICP27 mutant dLeu has a deletion in the epitope recognized by H1119 (dLeu-9), and therefore immunoprecipitation was performed in parallel with ICP27 antibody H113, which recognizes a different epitope (dLeu-3). RNAP II was detected with antibody 8WG16, and ICP27 was detected with H1119. Asterisks denote the positions of WT and mutant forms of ICP27. The plus (+) signs indicate heavy chain immunoglobulin G that was present from the immunoprecipitations. The numbers under the lanes indicate the amount of immunoprecipitated protein relative to the WT, which was arbitrarily set to equal 1.0. Protein amounts were quantified using SigmaScan software. UN, untreated.
ICP27 colocalizes with RNAP II, which is relocalized to viral transcription sites, and these sites are poorly formed in infections with ICP27 mutants that do not associate with RNAP II.
Immunofluorescence analysis was performed to determine if ICP27 colocalized with RNAP II in HSV-1-infected cells, as further evidence of a physical interaction. In Fig. 4 and in all subsequent immunofluorescence images, representative cells instead of a field of cells are shown at higher magnification to better illustrate the fluorescence patterns found. In all cases, the cells that are shown represent the pattern observed in the majority (greater than 80%) of the cells in at least 10 fields. At 6 h after infection, ICP27 was seen to colocalize in part with RNAP II stained by antibody 8WG16, which recognizes unphosphorylated and phosphoserine-5-hypophosphorylated RNAP IIA (41) (Fig. 4A). This colocalization was also seen in staining with antibody ARNA3, which recognizes an epitope from amino acids 794 to 822 in the N terminus of the large subunit of RNAP II (29); thus, all forms, phosphorylated and unphosphorylated, are recognized (Fig. 4C). Mutant d4-5, which interacts with RNAP II, also colocalized as did mutant d1-2 (Fig. 4A). In contrast, colocalization was not seen with the N-terminal mutant dLeu and C-terminal mutants m15 and n406 (Fig. 4A and C), in accord with the immunoprecipitation data (Fig. 3).
FIG. 4.
ICP27 mutants that failed to interact with RNAP II also fail to colocalize and recruit RNAP II to viral transcription sites. RSFs were infected for 6 h with WT HSV-1 KOS and the indicated ICP27 mutants. In panel E, WT-infected cells were fixed at 3 and 8 h after infection, and mutant-infected cells were fixed at 8 h after infection. Cells were stained with anti-ICP27, anti-ICP4, and the RNAP II antibodies 8WG16, ARNA3, and H14 as indicated.
It has been shown that RNAP II is redistributed in HSV-1-infected cells to sites of viral transcription and, further, that ICP27 is one of the immediate-early gene products that is required for this to occur (48). Therefore, we looked at the distribution of RNAP II in WT and ICP27 mutant virus-infected cells in replication compartments that contain the HSV-1 major transcriptional activator ICP4. In accord with the results of Rice et al. (48), in WT-infected cells, RNAP II recognized by 8WG16 and ARNA3 was redistributed into ICP4-containing viral transcription-replication compartments by 6 h after infection (Fig. 4B and D). Redistribution was also seen in d4-5- and d1-2-infected cells (Fig. 4B), although in the latter case, replication compartment formation was delayed relative to WT. By 10 h after infection, there was a concordance of RNAP II and ICP4 in d1-2-infected cells (data not shown). In contrast, in cells infected with dLeu, m15, and n406, RNAP II remained diffusely distributed throughout the nucleus, and, further, ICP4-containing transcription-replication compartments were poorly formed (Fig. 4B and D). These data suggest that ICP27 is required for the efficient recruitment of RNAP II to viral transcription sites and, further, that formation of transcription-replication compartments requires RNAP II redistribution.
Next, we looked at the distribution of the phosphoserine-5 form of RNAP II, which is recognized by antibody H14 (41) and which is found predominantly at promoters in initiation complexes (26). H14 staining was not seen to be largely redistributed in WT-infected cells even at 8 h after infection (Fig. 4E). While there was a movement of some of the H14 staining to viral transcription compartments seen at 8 h compared to 3 h, staining was distributed throughout the nucleus. These results are in agreement with those of Fraser and Rice (17), who also found little redistribution with H14 in WT-infected cells. This observation suggests that cellular transcription initiation continues during viral infection even at late times. Interestingly, the H14 staining pattern seen in ICP27 null mutant 27-LacZ-infected cells and in m15-infected cells more closely resembles what is seen in mock-infected cells (Fig. 4E). There was also little evidence of transcription compartment formation in 27-LacZ- and m15-infected cells. These results suggest that viral transcription is greatly reduced even at 8 h after infection with these mutants. In fact, microarray analysis of total RNA extracted 8 h after infection with 27-LacZ and m15 showed that the abundance of viral early and late transcripts was reduced to 10% of WT KOS levels for 27-LacZ and to 15% of KOS levels for m15 (A. Sun, E. K. Wagner, and R. M. Sandri-Goldin, unpublished results). Further, dLeu and n406 also showed greatly decreased levels of late transcripts in microarray analysis, and little redistribution of RNAP II and poor formation of ICP4-transcription compartments were also observed (Fig. 4B, D, and E). We conclude that the interaction of ICP27 with RNAP II is required for efficient redistribution of RNAP II to viral transcription sites and that these sites are poorly formed in infections with ICP27 mutants that do not interact with RNAP II. Further, viral transcription appears to be reduced in ICP27 N-terminal and C-terminal mutants that fail to interact with RNAP II. Thus, ICP27's role in the stimulation of viral transcription may hinge upon its ability to interact with and recruit RNAP II.
ICP27 colocalizes with the phosphoserine-2 form of RNAP II at early times after infection but not at later times.
We were particularly interested in determining if ICP27 colocalized with the phosphoserine-2 form of RNAP II, which is found predominantly in elongating transcription complexes, and with which factors required for splicing and 3′ end formation have been found to associate (1, 2, 24). Antibody H5 has been reported to specifically recognize serine-2 phosphorylation in the CTD (41). At 4 h after infection with WT KOS, H5 staining was seen to be redistributed compared to mock- and 27-Lac Z-infected cells and to colocalize with ICP27 (Fig. 5A.) This was confirmed by Z-stack analysis. The deconvoluted maximum view of H5 (green) and ICP27 (red) staining is shown to the right of WT panels at 4 h in Fig. 5A. However, by 8 h postinfection, ICP27 was actively shuttling between the nucleus and cytoplasm, and H5 staining was seen to move to structures that resembled splicing speckles (Fig. 5A). Z-stack deconvolution analysis demonstrated that ICP27 and H5 staining were distinct and did not overlap (Fig. 5A, right column). In m15- and n406-infected cells, the speckled staining pattern seen with H5 was not observed, and a pattern more similar to what was seen in mock- and 27-LacZ-infected cells was seen. Further, ICP27, which is confined to the nucleus even at late times in these mutants because they are defective in export (5), did not colocalize with H5 staining (Fig. 5A). The phosphoserine-2 form of RNAP II recognized by H5 was recently reported to be found in splicing speckles rather than in HSV-1 replication compartments marked by ICP8 staining in WT HSV-1-infected cells (17). We sought to determine whether any H5 staining colocalized with ICP4. In WT-infected cells, H5 staining was again observed in structures resembling splicing speckles, and colocalization with ICP4 compartments was not observed (Fig. 5B). In cells infected with ICP27 mutants 27-LacZ, m15, and n406, a diffuse distribution of H5 staining was observed similar to what was seen in mock-infected cells, and, again, there was little formation of ICP4 compartments evident (Fig. 5B). To further determine the nature of the H5 speckled staining pattern in WT-infected cells at late times after infection, we did a colocalization analysis with a monoclonal antibody to splicing factor SC35, which has been shown to be a component of splicing speckles (18). In mock- and n406-infected cells, H5 staining was diffusely distributed throughout the nucleus and was distinct from SC35 speckles (Fig. 5C). In WT KOS-infected cells, H5 staining colocalized with SC35 staining (Fig. 5C). There was also a striking reduction in the overall level of H5 staining compared to what was seen in mock- and n406-infected cells. Antibody H5 has been reported to cross-react with splicing SR proteins, including SC35, when levels of SR proteins are more abundant than levels of the phosphoserine-2 form of RNAP II (13). Because overall H5 staining was greatly reduced, we reasoned that H5 may actually be cross-reacting with SC35 and other SR proteins, which are enriched in splicing speckles. Z-stack analysis supported this supposition because there was a perfect coincidence in the staining patterns (Fig. 5C). This result leads us to conclude that the phosphoserine-2 form of RNAP II becomes depleted at late times after WT HSV-1 infection and that the H5 antibody preferentially reacts with SR splicing proteins whose serine phospho-epitopes can be recognized by H5.
FIG. 5.
H5 staining decreases and colocalizes with splicing speckles at late times in WT HSV-1 infection. RSFs were either mock infected or were infected with WT HSV-1 KOS or the ICP27 mutants 27-LacZ, m15, and n406. Cells were fixed at 4 and 8 h after infection where indicated and at 8 h if not indicated. Cells were stained with anti-phosphoserine-2 antibody H5 and antibodies to ICP27, ICP4, and SC35 as indicated. Z-stack deconvolution analysis was performed where indicated, and the maximum view is shown in each case.
The elongating phosphoserine-2 form along with RNAP II levels in general decrease during WT HSV-1 infection.
To further examine the apparent decrease in the phosphoserine-2 form of RNAP II noted in staining with antibody H5, Western blot analysis was performed because RNAP II has a predicted molecular mass of 220 to 240 kDa, whereas SC35 has an apparent molecular mass of 35 kDa; consequently, cross-reactivity would not be a problem as it was in the immunofluorescence studies. Nuclear extracts were prepared from cells that were mock-infected or were infected with WT HSV-1 KOS or 27-LacZ at different times after infection as indicated in Fig. 6. Probing with antibody ARNA3, which recognizes all forms of RNAP II, demonstrated a decrease in total RNAP II levels in WT KOS-infected cells beginning about 5 h after infection (Fig. 6). A slight decrease was also seen in 27-LacZ-infected cells but not until 7 h after infection, and a more significant decrease was evident at 9 h after infection, as can be seen in the protein quantification (Fig. 6B, top panel). RNAP II levels remained constant in mock-infected cells. The decrease in the phosphoserine-2 form recognized by H5 antibody was evident in WT KOS-infected cells beginning at 3 h after infection, and a dramatic reduction was seen by 9 h (Fig. 6A and B). Again, the decrease in phosphoserine-2 levels was delayed in 27-LacZ infection but became detectable by 5 h after infection (Fig. 6A and B, bottom panel). Levels of the phosphoserine-5 form detected by H14 antibody remained relatively stable in mock- and HSV-1-infected cells although there were differences in migration indicating differences in the level of serine-5 phosphorylation (Fig. 6A and B, middle panel). There were no significant alterations in the levels of cdk7, which phosphorylates serine-5, or cdk9, which phosphorylates serine-2. This finding, coupled with the decreases seen in both phosphorylated and faster-migrating unphosphorylated species detected by ARNA3, led us to postulate that protein degradation rather than dephosphorylation or reduced phosphorylation on serine-2 was occurring. That is, the phosphoserine-2 form appeared to be degraded during the course of infection, and this occurred earlier and to a greater extent in WT infection, where viral transcription is robust, compared to 27-LacZ infection, where viral transcription is greatly reduced compared to WT (49, 59).
FIG. 6.
Phosphoserine-2 levels, as well as RNAP II levels, in general decrease during the course of WT HSV-1 infection. (A) HeLa cells were mock infected (M) or were infected with ICP27 null mutant 27-LacZ or WT HSV-1 KOS, and nuclear extracts were prepared at the times indicated. Western blot analysis was performed with the following anti-RNAP II antibodies: ARNA3, which detects all forms of RNAP II both phosphorylated and unphosphorylated; H14, which is specific for phosphoserine-5; and H5, which is specific for phosphoserine-2. The blots were also probed with antibodies to ICP27, the nuclear transcription factor YY1, and kinases cdk7 and cdk9. (B) Protein quantification is shown for total RNAP II (top panel), phosphoserine-5 (P-Ser5) RNAP II (middle panel), and phosphoserine-2 (P-Ser-2) RNAP II forms (bottom panel).
Dephosphorylation of RNAP II in vitro did not show a net loss of protein, whereas the addition of proteasome inhibitors in vivo prevented loss of phosphoserine-2 RNAP II.
To confirm that protein degradation and not dephosphorylation was the cause of the loss of the serine-2 form, RNAP II was treated with alkaline phosphatase in vitro. Antibody ARNA3 was used to immunoprecipitate RNAP II from HSV-1-infected cells at 5 h after infection, and immunopurified complexes were treated with alkaline phosphatase (Fig. 7A). ICP27 was similarly immunoprecipitated and treated in parallel because we showed previously that dephosphorylation of ICP27 in vitro does not result in loss of protein (64). While 32P radioactivity was reduced for both RNAP II and the ICP27 control when each was treated with alkaline phosphatase, Western blot analysis demonstrated that protein levels did not decrease (Fig. 7A). There was a shift toward faster migrating hypophosphorylated forms for RNAP II (Fig. 7A) but no apparent reduction in the amount of protein. We conclude that loss of the phosphoserine-2 form is not the result of dephosphorylation or hypophosphorylation of RNAP II.
FIG. 7.
Loss of phosphoserine-2 (P-Ser2) and RNAP II can be prevented by the proteasomal inhibitors MG123 and lactacystin. (A) RNAP II was immunoprecipitated from WT HSV-1 KOS-infected cells at 5 h after infection, and dephosphorylation with alkaline phosphatase was performed as described previously (64). ICP27 was immunoprecipitated and treated with alkaline phosphatase in parallel as a control. UN, untreated; Buf, treated with buffer alone; AP, treated with alkaline phosphatase. Upper panels show autoradiographs, and the lower panels show Western blots of the same gels probed with antibody for ICP27 or RNAP II. (B) Cells were mock infected or infected WT KOS. Increasing amounts of the proteasome inhibitor MG132 from 0 to 50 μM were added as indicated at 1 h after infection, and nuclear extracts were prepared at 6 h. Western blots were probed with anti-RNAP II antibody ARNA3, which detects all forms, and H5, which detects phosphoserine-2. Blots were also probed with antibody to nuclear transcription factor YY1 and to ICP27. (C) Lactacystin was added in increasing amounts from 0 to 60 μM to mock- and WT KOS-infected cells at 1 h after infection, and nuclear extracts were prepared 5 h later. Blots were probed with antibodies as indicated in panel B.
In Saccharomyces cerevisiae, the 26S proteasome has been shown to be physically and functionally associated with RNAP II at transcriptional pause sites (19). Because it is the phosphoserine-2 form that was specifically depleted during WT HSV-1 infection (Fig. 6) and because this is the form found in elongating transcription complexes, we reasoned that proteasomal degradation might be occurring during active and robust viral transcription. That is, elongating complexes might collide and pause or stall. To determine if proteasomal degradation was indeed occurring, mock- and WT HSV-1-infected cells were treated with increasing amounts of the proteasome inhibitors MG132 and lactacystin, which were added beginning 1 h after infection. Nuclear extracts were prepared at 8 h after infection, and RNAP II levels were determined by Western blot analysis. Neither total RNAP II levels nor phosphoserine-2 levels were appreciably affected by MG132 or lactacystin in mock-infected cells; however, in WT HSV-1-infected cells, the addition of these drugs prevented the decreases seen in the absence of proteasome inhibitors (Fig. 7B and C). We conclude that loss of phosphoserine-2 RNAP II results from proteasomal degradation.
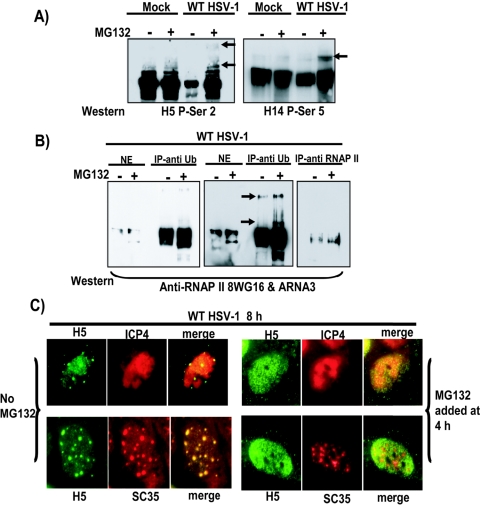
Ubiquitination of RNAP II can be detected in HSV-1-infected cells in the presence of MG132, which also prevents decreased H5 staining and its relocalization to splicing speckles.
To determine if ubiquitinated forms of RNAP II could be detected, Western blot analysis was performed on samples from mock- and HSV-1 WT KOS-infected cells that were or were not treated with MG132. The blots were probed with H5, specific for serine-2 phosphorylation, and H14, specific for serine-5 phosphorylation. The blots were overexposed to allow detection of the labile ubiquitinated forms (Fig. 8A). Slower-migrating species indicative of ubiquitinated forms were detected in WT HSV-1 samples that were treated with MG132 but not in the absence of the inhibitor. In fact, reduced levels of the phosphoserine-2 form were again seen in the absence of MG132 in KOS-infected cells (Fig. 8A). To directly demonstrate ubiquitination, WT HSV-1-infected cells were treated with MG132 as described above. Infected cell lysates were immunoprecipitated with anti-ubiquitin antibody (12), and Western blot analysis was performed with anti-RNAP II antibodies 8WG16 and ARNA3 to detect all forms of RNAP II (Fig. 8B). Slower-migrating forms of RNAP II that coprecipitated with anti-ubiquitin antibody were readily detected in the longer exposure seen in the middle panel of Fig. 8B (marked by arrows). Ubiquitinated forms were more apparent in the MG132-treated sample, as would be expected with a proteasome inhibitor. To verify that RNAP II was coprecipitated with anti-ubiquitin antibody, in a parallel experiment proteins bound to the antibody-protein A Sepharose beads were eluted, and the eluate was immunoprecipitated with anti-RNAP II antibodies 8WG16 and ARNA3 (right panel). Western blot analysis confirmed that RNAP II was precipitated with anti-ubiquitin antibody. Further, addition of MG132 to KOS-infected cells at 4 h after infection prevented the decrease in H5 staining seen at 8 h after infection when no MG132 was added (Fig. 8C). Additionally, H5 staining was not relocalized to splicing speckles in the presence of MG132 but, instead, remained diffusely nuclear. These findings lend further support to our conclusion that H5 staining of splicing speckles resulted from cross-reactivity of the H5 antibody with SR proteins, rather than from a redistribution of the phosphoserine-2 form to these sites.
FIG. 8.
MG132 prevents the decrease in H5 staining and its relocalization to splicing speckles. (A) Mock- and HSV-1 WT KOS-infected cells were left untreated (−) or were treated with 50 μM MG132 added 1 h after infection, and nuclear extracts were prepared at 6 h. Samples of the nuclear extracts were fractionated by SDS-PAGE, and Western blot analysis was performed with H5 antibody, specific for phosphoserine-2, and H14 antibody, specific for phosphoserine-5 (P-Ser2). The blots were overexposed to detect the faint, slower-migrating species that are marked by arrows. (B) WT KOS-infected cells were either left untreated (−) or were treated with MG132 added 1 h after infection as indicated. At 6 h after infection, samples of nuclear extracts (NE) were either fractionated directly by SDS-PAGE or were immunoprecipitated (IP) with anti-ubiquitin antibody (left and middle panels) (12). The blot was probed with anti-RNAP II antibodies 8WG16 and ARNA3 to detect all species of RNAP II. The panel in the middle is a darker exposure of the panel on the left. Arrows indicate the position of slower-migrating ubiquitinated forms of RNAP II. In a parallel experiment, after immunoprecipitation with anti-ubiquitin antibody, precipitated proteins bound to the antibody-protein A Sepharose beads were eluted in 1% SDS in phosphate-buffered saline at 60°C for 15 min as previously described (12). The eluate was diluted with 20 volumes of phosphate-buffered saline containing 100 mM NaCl and1% Triton X-100. Samples were then immunoprecipitated with anti-RNAP II antibodies 8WG16 and ARNA3 and were fractioned by SDS-PAGE. Western blot analysis was performed with 8WG16 and ARNA3. (C) WT HSV-1 KOS-infected cells were either left untreated (left panels) or were treated with 50 μM MG132 at 4 h after infection (right panels). Cells were fixed at 8 h and stained with phosphoserine-2 antibody H5, antibody to ICP4, and antibody to SR splicing factor SC35.
Inhibition of transcription prevents loss of RNAP II phosphoserine-2.
Proteasomal degradation of RNAP II has been found to occur on stalled elongation complexes (19, 57), and therefore we reasoned that inhibiting transcription elongation by adding actinomycin D might prevent degradation of RNAP II just as proteasomal inhibitors prevented degradation. Mock- and WT HSV-1 KOS-infected cells were either treated with actinomycin D at 4 h after infection or left untreated, and samples were analyzed at 8 h. In HSV-1-infected cells treated with the actinomycin D, H5 staining remained diffuse throughout the nucleus and resembled the pattern seen in mock-infected cells treated with actinomycin D (Fig. 9A). Further, relocalization to SC35 speckles was not observed (Fig. 9A). The addition of actinomycin D did disrupt the formation of ICP4 transcription compartments (Fig. 9B), but H5 staining did not decrease and remained diffuse throughout the nucleus; further, RNAP II stained by 8WG16 remained diffuse and was not redistributed to ICP4 sites, as was seen in the absence of actinomycin D (Fig. 9B). This indicates that viral transcription was halted by actinomycin D.
FIG. 9.
Inhibition of viral transcription prevents the loss of phosphoserine-2, as well as its relocalization to splicing speckles. (A and B) Mock- and WT HSV-1 KOS-infected cells were either untreated or were treated with actinomycin D (10 μg/ml) at 4 h after infection, and cells were fixed at 8 h. Cells were stained with antibody H5 and with antibodies to SC35 (A) and ICP4 (B) as indicated. (C) Mock (M)- and WT HSV-1 KOS-infected cells were either untreated or were treated with actinomycin D (10 μg/ml) at 4 h after infection; total cell extracts were prepared at 8 h, and samples were fractioned by SDS-PAGE. Western blots were probed with antibodies to RNAP II (ARNA3 and H5); HSV-1 IE proteins ICP4, ICP0 and ICP27; and viral late protein gC. Antibody to YY1 was also used as a loading control. (D) Cells were mock (M)-infected or were infected with WT HSV-1 KOS or ICP27 mutants n406 and m15. Nuclear extracts were prepared at the times indicated; samples were fractionated by SDS-PAGE, and Western blot analysis was performed with antibodies to RNAP II large subunit (ARNA3), phosphoserine-5 (H14), and phosphoserine-2 (H5). YY1 was also probed as a loading control. P-Ser, phosphoserine.
To look at protein levels directly, Western blot analysis was performed. Actinomycin D added at 4 h to WT KOS-infected cells partly rescued the degradation of RNAP II levels as well as phosphoserine-2 levels (Fig. 9C). In contrast, there was little effect on RNAP II levels in mock-infected cells, suggesting that it is active viral transcription that creates stalled transcription complexes. That viral transcription was effectively inhibited by this treatment was verified by probing for HSV-1 proteins. Expression of the immediate-early proteins ICP4, ICP0, and ICP27 was only slightly reduced when actinomycin D was added at 4 h; however, expression of the late protein gC was undetectable (Fig. 9C). Additional evidence that phosphoserine-2 degradation occurs as a result of active HSV-1 transcription is shown in Fig. 9D. Western blot analysis of a time course of infection was performed on mock-, mutant n406-, mutant m15-, and WT HSV-1-infected cells. In n406- and m15-infected cells, late transcript levels were found to be reduced to less than 10% of WT levels by microarray analysis (A. Sun et al. unpublished results), and levels of late gene products were shown previously to be severely reduced in these mutants (44, 45). Similar to what was seen for the ICP27 null mutant 27-LacZ (Fig. 6), decreased levels of RNAP II detected by ARNA3 and of phosphoserine-2 detected by H5 were seen but at later times after infection compared to WT, and the decreases were not as profound (Fig. 9D). We interpret these results to mean that robust viral transcription must be occurring for the degradation of phosphoserine-2. Decreased viral transcription due to treatment with actinomycin D or in cells infected with ICP27 viral mutants that fail to interact with RNAP II results in decreased RNAP II degradation.
MG132 rescues phosphoserine-2 degradation but results in reductions in viral late proteins and in virus yields.
We postulated that the elongating transcription complex containing the phosphoserine-2 form became arrested or stalled during active HSV-1 transcription and DNA replication. This could result from colliding elongation complexes, because HSV-1 transcripts are transcribed from both strands in some regions of the genome, from simultaneous transcription and DNA replication on the same template, from DNA damage due to HSV-1 recombination events, or from a combination of these possible events. If ubiquitination and proteasomal degradation of RNAP II were occurring to resolve stalled complexes and allow appropriate 3′ end processing, inhibition of this process by proteasome inhibitors could result in diminished levels of late viral products. To determine whether that was the case, WT HSV-1-infected cells were either treated with MG132 at 1 h after infection or left untreated, and total RNA (Fig. 10A) and proteins were extracted at 4 and 8 h (Fig. 10B). Viral RNA was analyzed by microarray analysis as described previously (60). Although there were some differences seen in the accumulation of early (green) and late (blue) transcripts in infected cells treated with MG132 compared to untreated cells (Fig. 10A), these increases or decreases were in the range of less than twofold for each viral transcript. This indicates that MG132 did not inhibit transcript accumulation. In contrast to the modest increases or reductions seen in the levels of viral early and late transcripts, MG132 treatment had a greater effect on late protein levels. Levels of gB and gD were significantly reduced, and gC was undetectable in MG132-treated cells (Fig. 10B). Further, virus yields were reduced 25- to 60-fold when MG132 or lactacystin was added (Fig. 10C). These data suggest that proteasomal degradation of RNAP II is beneficial for HSV-1 gene expression and replication.
FIG. 10.
Proteasomal inhibitors rescued phosphoserine-2 (P-Ser 2), but reduced levels of viral late proteins and virus yields result. (A) WT HSV-1 KOS-infected cells were either left untreated or treated with MG132 as indicated beginning 1 h after infection. Total RNA was isolated at 8 h after infection, and microarray analysis was performed against an array of HSV-1 transcript-specific probes as described previously (60). Immediate-early genes are shown in red, early genes are shown in green, and late genes are shown in blue. The experiment was performed twice, and the hybridizations were performed in triplicate in each experiment. Error bars represent the standard deviations. (B) WT HSV-1 KOS-infected cells were treated with increasing amounts of MG132 as indicated, beginning at 1 h after infection. Total cell lysates were prepared at 4 and 8 h after infection, and samples were fractionated by SDS-PAGE. Western blot analysis was performed with H5 antibody and antibodies to HSV-1 late proteins gB, gD, and gC and to immediate-early protein ICP4. (C) WT KOS-infected cells were either untreated or were treated with MG132 (20 μM) or lactacystin (20 μM), which was added at 1 h after infection. Cells were harvested at 24 h, and virus titers were determined.
DISCUSSION
HSV-1 ICP27 is a multifunctional regulatory protein with demonstrated posttranscriptional activities, including host cell splicing inhibition, stimulation of cleavage and polyadenylation of some viral poly(A) sites, viral RNA export, and translation initiation (6, 15, 16, 27, 34, 35, 51). In addition, ICP27 has been shown to stimulate the transcription of some late transcripts (23), and the accumulation of early and late gene products is severely reduced in ICP27 viral mutant infections (23, 30, 44-46). The mechanism by which ICP27 stimulates transcription has not been elucidated. We present evidence here that the interaction of ICP27 with RNAP II (66), and specifically with the CTD of RNAP II (Fig. 1), is important for the recruitment of RNAP II to sites of HSV-1 transcription marked by ICP4 staining. It was shown previously that RNAP II is redistributed to viral replication compartments during HSV-1 infection and that there was redundancy among HSV-1 immediate-early gene products in effecting this relocalization (48). We have mapped the regions of ICP27 that bind to the CTD in vitro and that are required for coimmunoprecipitation and colocalization with RNAP II in virus-infected cells. Both the N terminus and the C terminus are required to be largely intact for the association of ICP27 with RNAP II (Fig. 2 to 4). Furthermore, RNAP II is not redistributed to viral replication compartments in infections with ICP27 N-terminal and C-terminal mutants, and these compartments are poorly formed (Fig. 4). Levels of viral transcripts are reduced to 10 to 15% of WT KOS levels in infections with ICP27 mutants that cannot interact with RNAP II, and this may be because mutant ICP27 cannot recruit RNAP II to viral transcription sites. In the absence of the recruitment of abundant amounts of RNAP II, viral transcription-replication compartments do not fully form but resemble prereplication compartments (8, 11, 32). Thus, ICP27's role in transcription may be linked to its role in posttranscriptional regulation. That is, like many cellular RNA processing factors, ICP27 binds to the CTD of RNAP II, which acts as a platform for RNA processing and export factors (3). ICP27 has also been shown to interact with ICP8, which is required for HSV-1 DNA replication and which also associates with RNAP II (39, 66), and evidence has also been presented that ICP27 binds to ICP4 (40). Thus, ICP27 bound to the RNAP II CTD may direct RNAP II to sites containing ICP8 as well as ICP4. The failure of ICP27 mutants to bind to the CTD might therefore result in a reduced recruitment of RNAP II to viral replication sites, such that viral transcription is diminished and replication compartment formation is impaired.
Interestingly, recruitment of RNAP II was observed with antibodies 8WG16 and ARNA3, which recognize both unphosphorylated and different phosphorylated forms of RNAP II, but not with H14, which recognizes phosphoserine-5, or with H5, which recognizes phosphoserine-2. H14 staining was seen to remain diffusely localized throughout the nucleus even at late times after infection (Fig. 4), and only a fraction of the staining was seen to overlap with ICP4-containing compartments. Serine-5 phosphorylation occurs during transcription initiation at the promoters of genes (7, 21, 33), and the finding that serine-5 phosphorylation can be found throughout the nucleus during HSV-1 infection implies that cellular transcription is an ongoing process. In contrast to the pattern seen with H14, H5 staining was reduced and relocalized to splicing speckles marked by SC35 staining. H5 is specific for phosphoserine-2, which is found in elongating RNAP II (41). A similar result has been reported by Fraser and Rice (17). These authors interpreted this result to mean that the partial depletion of serine-2 phosphorylation along with relocalization of the remaining H5 staining material to splicing speckles occurred as a result of the inhibition of cellular transcription. It has been reported previously that HSV-1 infection causes repression of transcription for some cellular genes (58). These authors also speculated that serine-2 phosphorylation was not required for HSV-1 transcription because of the disappearance of the H5 staining and its relocalization to splicing speckles and that HSV-1-induced phosphorylation of the CTD through the action of ICP22 and UL13 could substitute for serine-2 phosphorylation. Although the sites of CTD phosphorylation mediated by ICP22 and UL13 have not been elucidated, Durand et al. (14) have shown recently that ICP22 associates with cdk9 in a complex that can phosphorylate the CTD in vitro. Because cdk9 phosphorylates serine-2 on the CTD, it seems unlikely that the HSV-1-induced changes would not involve serine-2 phosphorylation. Furthermore, 3′ end formation and transcription have been shown to be coupled through the binding of factors required for cleavage and polyadenylation to the serine-2-phosphorylated CTD (2). Therefore, we sought to determine if the loss of H5 staining was due to degradation of phosphoserine-2 RNAP II.
It has been reported that antibody H5 cross-reacts with SR splicing proteins, including SC35 when these factors are more abundant than phosphoserine-2 of RNAP II (13). Z-stack analysis demonstrated that H5 and SC35 staining were strikingly coincident (Fig. 5), leading us to speculate that we were observing H5 cross-reacting with SC35 and other SR proteins. Western blot analysis confirmed that the phosphoserine-2 form of RNAP II was significantly reduced in WT HSV-1-infected cells and, furthermore, that there was also a reduction in RNAP II levels overall (Fig. 6). This suggested that protein degradation rather than dephosphorylation was occurring. Treatment with proteasome inhibitors MG132 and lactacystin prevented the loss of RNAP II and the serine-2 phosphorylated form, and relocalization to splicing speckles also was not observed (Fig. 7 and 8). Because the degradation occurred faster and more dramatically in WT HSV-1-infected cells compared to infections with ICP27 null mutant 27-LacZ, whose viral transcriptional program is greatly reduced, we considered the possibility that active viral transcription was responsible. It has been shown in yeasts that stalled transcription complexes at sites of DNA damage or due to accumulating transcription complexes undergo proteasomal degradation of RNAP II to resolve stalled complexes and to rescue 3′ end formation (2, 19). Therefore, at times of active viral transcription, elongating RNAP II might stall because of accumulating complexes during robust transcription and possibly simultaneous DNA replication. Rescue of these RNAs through proteasomal degradation might be a necessary aspect of viral replication. If that is the case, then, first, inhibiting transcriptional elongation by actinomycin D should also rescue phosphoserine-2, and we observed this result (Fig. 9). Second, blocking proteasomal degradation with proteasome inhibitors should interfere with viral replication, at least to some extent because transcripts in stalled complexes would not undergo correct processing and 3′ end formation. While we did not look directly at 3′ ends of viral transcripts, we did find that while early and late transcripts were altered in abundance by less than twofold, viral late proteins were reduced more significantly, and virus yields were reduced 25- to 60-fold (Fig. 10). These findings indicate that proteasome inhibitors do not inhibit transcription per se but prevent the degradation of stalled RNAP II complexes, which could result in improper 3′ ends. These inappropriately processed transcripts may make poor substrates for export and/or translation, such that viral protein levels are reduced, in turn leading to reductions in virus yields.
Thus, our model for the results that we have described is as follows. During viral infection, ICP27 interacts with the CTD of RNAP II, which acts as a platform for RNA processing factors. ICP27 also interacts with ICP8 and ICP4, which are part of HSV-1 replication compartments, and ICP27 recruits mostly unphosphorylated RNAP II to these sites. ICP27 mutants that cannot interact with RNAP II show reductions in viral transcription, and this may result from the inability of these mutants to recruit sufficient amounts of RNAP II to viral transcription-replication sites, which in turn do not assemble into full-fledged compartments. In WT HSV-1-infected cells, transcription is robust following immediate-early gene expression and the commencement of DNA replication. Furthermore, transcription occurs on both strands of the viral genome in some regions, and there is not a tight clustering of genes of different kinetic classes. Instead, immediate-early, early, and late genes can be found juxtaposed to each other, with relatively short intergenic regions between genes. It is also possible that DNA replication and transcription could be occurring on the same template simultaneously. Finally, DNA breaks may occur as a result of recombination events. Thus, it is likely that elongating transcription complexes could pause or become stalled. It has been demonstrated in yeast and in mammalian cells that resolution of these complexes requires proteasomal degradation (2, 19, 22, 25). Therefore, we propose that proteasomal degradation of stalled RNAP II complexes occurs as part of the HSV-1 robust transcriptional program and, furthermore, that viral gene expression and replication are enhanced by this process.
Acknowledgments
We thank Steve Rice for the generous gift of ICP27 viral mutants and Jeff Corden for valuable suggestions.
These studies were supported by U.S. Public Health Service grant AI21515 from the National Institute of Allergy and Infectious Diseases. J. Q. Dai-Ju was supported by a Virology Training Grant from the National Institute of Allergy and Infectious Diseases. L. A. Johnson was supported for part of these studies by a Structure and Macromolecular Synthesis Training Grant from the National Institute of General Medical Sciences.
REFERENCES
- 1.Adamson, T. E., D. C. Shutt, and D. H. Price. 2005. Functional coupling of cleavage and polyadenylation with transcription of mRNA. J. Biol. Chem. 280:32262-32271. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, S. H., M. Kim, and S. Buratowski. 2004. Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3′ end processing. Mol. Cell 13:67-76. [DOI] [PubMed] [Google Scholar]
- 3.Bentley, D. L. 2005. Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors. Curr. Opin. Cell Biol. 17:251-256. [DOI] [PubMed] [Google Scholar]
- 4.Bryant, H. E., S. Wadd, A. I. Lamond, S. J. Silverstein, and J. B. Clements. 2001. Herpes simplex virus IE63 (ICP27) protein interacts with spliceosome-associated protein 145 and inhibits splicing prior to the first catalytic step. J. Virol. 75:4376-4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, I. B., L. Li, L. Silva, and R. M. Sandri-Goldin. 2005. ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export. J. Virol. 79:3949-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, I. B., K. S. Sciabica, and R. M. Sandri-Goldin. 2002. ICP27 interacts with the export factor Aly/REF to direct herpes simplex virus 1 intronless RNAs to the TAP export pathway. J. Virol. 76:12877-12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho, E.-J., T. Takagi, C. R. Moore, and S. Buratowski. 1997. mRNA capping enzyme is recruited to the transcription complex by phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 11:3319-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtin, K. D., and D. M. Knipe. 1993. Altered properties of the herpes simplex virus ICP8 DNA-binding protein in cells infected with ICP27 mutant viruses. Virology 196:1-14. [DOI] [PubMed] [Google Scholar]
- 9.Dahmus, M. E. 1996. Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J. Biol. Chem. 271:19009-19012. [DOI] [PubMed] [Google Scholar]
- 10.Dantonel, J.-C., D. Kanneganti, G. K. Murthy, J. L. Manley, and L. Tora. 1997. Transcription factor TFIID recruits factor CPSF for formation of 3′ end of mRNA. Nature 389:399-402. [DOI] [PubMed] [Google Scholar]
- 11.de Bruyn Kops, A., S. L. Uprichard, M. Chen, and D. M. Knipe. 1998. Comparison of the intranuclear distributions of herpes simplex virus proteins involved in various viral functions. Virology 252:162-178. [DOI] [PubMed] [Google Scholar]
- 12.de Virgilio, M., H. Weninger, and N. E. Ivessa. 1998. Ubiquitination is required for the reto-translocation of a short-lived luminal endoplasmic reticulum glycoprotein to the cytosol for degradation by the proteasome. J. Biol. Chem. 273:9734-9743. [DOI] [PubMed] [Google Scholar]
- 13.Doyle, O., J. L. Corden, C. Murphy, and J. G. Gall. 2002. The distribution of RNA polymerase II largest subunit (RPB1) in the Xenopus germinal vesicle. J. Struct. Biol. 140:154-166. [DOI] [PubMed] [Google Scholar]
- 14.Durand, L. O., S. J. Advani, A. P. W. Poon, and B. Roizman. 2005. The carboxyl-terminal domain of RNA polymerase II is phosphorylated by a complex containing cdk9 and infected-cell protein 22 of herpes simplex virus 1. J. Virol. 79:6757-6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellison, K. S., R. A. Maranchuk, K. L. Mottet, and J. R. Smiley. 2005. Control of VP16 translation by the herpes simplex virus type 1 immediate-early protein ICP27. J. Virol. 79:4120-4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontaine-Rodriquez, E. C., T. J. Taylor, M. Olesky, and D. M. Knipe. 2004. Proteomics of herpes simplex virus infected cell protein 27: association with translation initiation factors. Virology 330:487-492. [DOI] [PubMed] [Google Scholar]
- 17.Fraser, K. A., and S. A. Rice. 2005. Herpes simplex virus type 1 infection leads to loss of serine-2 phosphorylation on the carboxyl-terminal domain of RNA polymerase II. J. Virol. 79:11323-11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu, X. D., and T. Maniatis. 1990. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature 343:437-441. [DOI] [PubMed] [Google Scholar]
- 19.Gillette, T. G., F. Gonzalez, A. Delahodde, S. A. Johnston, and T. Kodadek. 2004. Physical and functional association of RNA polymerase II and the proteasome. Proc. Natl. Acad. Sci. USA 101:5904-5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardwicke, M. A., P. J. Vaughan, R. E. Sekulovich, R. O'Conner, and R. M. Sandri-Goldin. 1989. The regions important for the activator and repressor functions of the HSV-1 alpha protein ICP27 map to the C-terminal half of the molecule. J. Virol. 63:4590-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirose, Y., and J. L. Manley. 2000. RNA polymerase II and the integration of nuclear events. Genes Dev. 14:1415-1429. [PubMed] [Google Scholar]
- 22.Inukai, N., Y. Yamaguchi, I. Kuraoka, T. Yamada, S. Kamijo, J. Kato, K. Tanaka, and H. Handa. 2004. A novel hydrogen peroxide-induced phosphorylation and ubiquitination pathway leading to RNA polymerase II proteolysis. J. Biol. Chem. 279:8190-8195. [DOI] [PubMed] [Google Scholar]
- 23.Jean, S., K. M. LeVan, B. Song, M. Levine, and D. M. Knipe. 2001. Herpes simplex virus 1 ICP27 is required for transcription of two viral late (γ2) genes in infected cells. Virology 283:273-284. [DOI] [PubMed] [Google Scholar]
- 24.Kim, E., L. Du, D. B. Bregman, and S. L. Warren. 1997. Splicing factors associate with hyperphosphorylated RNA polymerase II in the absence of pre-mRNA. J. Cell Biol. 136:19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleiman, F. E., F. Wu-Baer, D. Fonseca, S. Kaneko, and R. Baer. 2005. BRCA1/BARD1 inhibition of mRNA 3′ processing involves targeted degradation of RNA polymerase II. Genes Dev. 19:1227-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kober, M. S., and J. Greenblatt. 2002. Regulation of transcription elongation by phosphorylation. Biochim. Biophys. Acta 1577:261-275. [DOI] [PubMed] [Google Scholar]
- 27.Koffa, M. D., J. B. Clements, E. Izaurralde, S. Wadd, S. A. Wilson, I. W. Mattaj, and S. Kuersten. 2001. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 20:5769-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komarnitsky, P., E.-J. Cho, and S. Buratowski. 2000. Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev. 14:2452-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramer, A., R. Haars, R. Kabisch, F. A. Bautz, and E. K. Bautz. 1980. Monoclonal antibody directed against RNA polymerase II of Drosophila melanogaster. Mol. Gen. Genet. 180:193-199. [DOI] [PubMed] [Google Scholar]
- 30.Lengyel, J., C. Guy, V. Leong, S. Borge, and S. A. Rice. 2002. Mapping of functional regions in the amino-terminal portion of the herpes simplex virus ICP27 regulatory protein: importance of the leucine-rich nuclear export signal and RGG box RNA-binding domain. J. Virol. 76:11866-11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long, M. C., V. Leong, P. A. Schaffer, C. A. Spencer, and S. A. Rice. 1999. ICP22 and the UL13 protein kinase are both required for herpes simplex virus-induced modification of the large subunit of RNA polymerase II. J. Virol. 73:5593-5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukonis, C. J., and S. K. Weller. 1997. Formation of herpes simplex virus type 1 replication compartments by transfection: requirements and localization to nuclear domain 10. J. Virol. 71:2390-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCracken, S., N. Fong, K. Yankulov, S. Ballantyne, G. Pan, J. Greenblatt, S. D. Patterson, M. Wickens, and D. L. Bentley. 1997. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature 385:357-361. [DOI] [PubMed] [Google Scholar]
- 34.McGregor, F., A. Phelan, J. Dunlop, and J. B. Clements. 1996. Regulation of herpes simplex virus poly(A) site usage and the action of immediate-early protein IE63 in the early-late switch. J. Virol. 70:1931-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLauchlan, J., A. Phelan, C. Loney, R. M. Sandri-Goldin, and J. B. Clements. 1992. Herpes simplex virus IE63 acts at the posttranscriptional level to stimulate viral mRNA 3′ processing. J. Virol. 66:6939-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mears, W. E., V. Lam, and S. A. Rice. 1995. Identification of nuclear and nucleolar localization signals in the herpes simplex virus regulatory protein ICP27. J. Virol. 69:935-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mears, W. E., and S. A. Rice. 1996. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J. Virol. 70:7445-7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mears, W. E., and S. A. Rice. 1998. The herpes simplex virus immediate-early protein ICP27 shuttles between the nucleus and cytoplasm. Virology 242:128-137. [DOI] [PubMed] [Google Scholar]
- 39.Olesky, M., E. E. McNamee, C. Zhou, T. J. Taylor, and D. M. Knipe. 2005. Evidence for a direct interaction between HSV-1 ICP27 and ICP8 proteins. Virology 331:94-105. [DOI] [PubMed] [Google Scholar]
- 40.Panagiotidis, C. A., E. K. Lium, and S. Silverstein. 1997. Physical and functional interactions between herpes simplex virus immediate-early proteins ICP4 and ICP27. J. Virol. 71:1547-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patturajan, M., R. J. Schulte, B. M. Sefton, R. Berezney, M. Vincent, O. Bensaude, S. L. Warren, and J. L. Corden. 1998. Growth-related changes in phosphorylation of yeast RNA polymerase II. J. Biol. Chem. 273:4689-4694. [DOI] [PubMed] [Google Scholar]
- 42.Phelan, A., and J. B. Clements. 1997. Herpes simplex virus type 1 immediate early protein IE63 shuttles between nuclear compartments and the cytoplasm. J. Gen. Virol. 78:3327-3331. [DOI] [PubMed] [Google Scholar]
- 43.Price, D. H. 2000. P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol. Cell. Biol. 20:2629-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rice, S. A., and D. M. Knipe. 1990. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J. Virol. 64:1704-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rice, S. A., and V. Lam. 1994. Amino acid substitution mutations in the herpes simplex virus ICP27 protein define an essential gene regulation function. J. Virol. 68:823-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rice, S. A., V. Lam, and D. M. Knipe. 1993. The acidic amino-terminal region of herpes simplex virus type 1 alpha protein ICP27 is required for an essential lytic function. J. Virol. 67:1778-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rice, S. A., M. C. Long, V. Lam, P. A. Schaffer, and C. A. Spencer. 1995. Herpes simplex virus immediate-early protein ICP22 is required for viral modification of host RNA polymerase II and establishment of the normal viral transcription program. J. Virol. 69:5550-5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rice, S. A., M. C. Long, V. Lam, and C. A. Spencer. 1994. RNA polymerase II is aberrantly phosphorylated and localized to viral replication compartments following herpes simplex virus infection. J. Virol. 68:988-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandri-Goldin, R. M. 1998. ICP27 mediates herpes simplex virus RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 12:868-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandri-Goldin, R. M. 1998. Interactions between an HSV regulatory protein and cellular mRNA processing pathways. Methods 16:95-104. [DOI] [PubMed] [Google Scholar]
- 51.Sciabica, K. S., Q. J. Dai, and R. M. Sandri-Goldin. 2003. ICP27 interacts with SRPK1 to mediate HSV-1 inhibition of pre-mRNA splicing by altering SR protein phosphorylation. EMBO J. 22:1608-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sekulovich, R. E., K. Leary, and R. M. Sandri-Goldin. 1988. The herpes simplex virus type 1 alpha protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J. Virol. 62:4510-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith, I. L., M. A. Hardwicke, and R. M. Sandri-Goldin. 1992. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology 186:74-86. [DOI] [PubMed] [Google Scholar]
- 54.Smith, R. W. P., P. Malik, and J. B. Clements. 2005. The herpes simplex virus ICP27 protein: a multifunctional post-transcriptional regulator of gene expression. Biochem. Soc. Trans. 33:499-501. [DOI] [PubMed] [Google Scholar]
- 55.Sokolowski, M., J. E. Scott, R. P. Heaney, A. H. Patel, and J. B. Clements. 2003. Identification of herpes simplex virus RNAs that interact specifically with regulatory protein ICP27 in vivo. J. Biol. Chem. 278:33540-33549. [DOI] [PubMed] [Google Scholar]
- 56.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 71:9188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Somesh, B., J. Reid, W. Liu, T. M. M. Sogaard, H. Erdjument-Bromage, P. Tempst, and J. Q. Svejstrup. 2005. Multiple mechanisms confining RNA polymerase II ubiquitylation to polymerases undergoing transcriptional arrest. Cell 121:913-923. [DOI] [PubMed] [Google Scholar]
- 58.Spencer, C. A., M. E. Dahmus, and S. A. Rice. 1997. Repression of host RNA polymerase II transcription by herpes simplex virus type 1. J. Virol. 71:2031-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stingley, S. W., J. J. Ramirez, S. A. Aguilar, K. Simmen, R. M. Sandri-Goldin, P. Ghazal, and E. K. Wagner. 2000. Global analysis of herpes simplex virus type 1 transcription using an oligonucleotide-based DNA microarray. J. Virol. 74:9916-9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun, A., G. V. Devi-Rao, M. K. Rice, L. W. Gary, D. C. Bloom, R. M. Sandri-Goldin, P. Ghazal, and E. K. Wagner. 2004. Immediate-early expression of the herpes simplex virus type 1 ICP27 transcript is not critical for efficient replication in vitro or in vivo. J. Virol. 78:10470-10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uprichard, S. L., and D. M. Knipe. 1996. Herpes simplex ICP27 mutant viruses exhibit reduced expression of specific DNA replication genes. J. Virol. 70:1969-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, J., and J. L. Corden. 1991. Identification of the phosphorylation sites in the repetitive carboxyl-terminal domain of the mouse RNA polymerase II largest subunits. J. Biol. Chem. 266:2290-2296. [PubMed] [Google Scholar]
- 63.Zhang, J., and J. L. Corden. 1991. Phosphorylation causes a conformational change in the carboxyl-terminal domain of the mouse RNA polymerase II largest subunit. J. Biol. Chem. 266:2297-2302. [PubMed] [Google Scholar]
- 64.Zhi, Y., and R. M. Sandri-Goldin. 1999. Analysis of the phosphorylation sites of the herpes simplex virus type 1 regulatory protein ICP27. J. Virol. 73:3246-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhi, Y., K. S. Sciabica, and R. M. Sandri-Goldin. 1999. Self interaction of the herpes simplex virus type 1 regulatory protein ICP27. Virology 257:341-351. [DOI] [PubMed] [Google Scholar]
- 66.Zhou, C., and D. M. Knipe. 2001. Association of herpes simplex virus 1 ICP8 and ICP27 with cellular RNA polymerase II holoenzyme. J. Virol. 76:5893-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]