The ability of vaccination to empower natural immune defenses against viral infection is well illustrated by the worldwide eradication of smallpox and the partial elimination of polio after global vaccination efforts (74, 98). Unfortunately, these major successes have been infrequent, and diverse viruses continue to threaten our societies. The development of new vaccines, or the improvement of existing ones, is currently hindered by a limited knowledge of the intracellular mechanisms leading to the activation of antiviral adaptive immunity and of the viral elements responsible for its triggering upon infection. The use of the viral RNA analog poly(I:C), the discovery of the highly virus-responsive plasmacytoid dendritic cells (pDCs), and the identification of Toll-like receptors (TLRs) specific for viral elements have led to a greater understanding of the initiation of antiviral immunity. Nevertheless, there is little evidence to suggest that these elements are sufficient—or even necessary—for adaptive antiviral immune responses. A more comprehensive understanding of the mechanisms by which viruses trigger the development of adaptive immunity is therefore still needed.
Recent work led to the identification of a novel pathway for the activation of conventional DCs (cDCs), the primary cells responsible for the generation of adaptive immunity. The triggering of this pathway leads to strong adaptive antiviral immunity in vivo even in the absence of TLR signaling. Here, we review the evidence for the identification and characterization of this pathway. Intracellular molecular candidates that may participate in this pathway are discussed, and a model for the triggering of DC maturation by viruses is presented.
DCs IN ANTIVIRAL IMMUNITY
Efficient antiviral immunity involves both innate and adaptive immune responses. Innate immunity, mediated by proteins (cytokines, acute phase proteins, and complement) and cells (natural killer [NK] cells, phagocytes, and cells that release inflammatory mediators) provides a rapid response that inhibits the progression of the infection. Adaptive immunity, in contrast, leads to viral clearance and generates long-term immunological memory through the generation of specific T and B cells. DCs orchestrate the development of adaptive immunity. Located in all tissues in an immature state, they serve as sentinels in charge of identifying pathogenic invaders. Upon recognition of pathogens, they change their expression pattern of proinflammatory genes, including those coding for cytokines, chemokines, and costimulatory molecules, in a process known as DC maturation. These functional changes are necessary for initiating adaptive immunity (8, 86). Two major DC populations participate in the development of antiviral immune responses: cDCs and pDCs. cDCs are found in peripheral tissues and secondary lymphoid organs (108), mature in the presence of virus, and efficiently process and present antigens in the lymph nodes to initiate the adaptive immune response (16). pDCs, found primarily in blood and secondary lymphoid organs (108), produce high levels of type I interferons (IFNs) upon binding of virus components to TLRs (9, 42). It is speculated that pDCs have an important role in antiviral immunity; however, they have poor antigen presentation ability and their physiological role remains controversial (5, 44, 58).
TLRs IN THE DEVELOPMENT OF ANTIVIRAL IMMUNITY
TLRs are membrane-bound receptors that can be activated by the binding of molecular structures conserved among families of microbes. More than 10 different TLRs have been identified to date. They are highly conserved among mammals and are expressed in a variety of cell types. Numerous reviews describe these molecules and their role in innate immunity (24, 34, 82, 100, 101).
TLRs specifically activated by various viral elements have been identified. TLR3 binds the viral replication intermediary double-stranded RNA (dsRNA) (2). TLR7 binds single-stranded RNA (ssRNA), the genomic material of many viruses, such as influenza virus and Sendai virus (SeV) (20, 33). TLR9 binds CpG motifs found in the genomic DNA of viruses such as herpes simplex virus (HSV) (51, 64) and murine cytomegalovirus (MCMV) (99). Additionally, some TLRs respond to viral proteins, such as the respiratory syncytial virus fusion protein (TLR4) (32, 53) and the measles virus hemagglutinin protein (TLR2) (12).
TLR binding and stimulation by pathogen-associated molecules is followed by a cascade of intracellular events that culminate in the expression of multiple genes. TLR signaling is mediated primarily by the adaptor protein myeloid differentiation factor 88 (MyD88) that triggers the activation of transcription factors, such as NF-κB, that are essential for the expression of proinflammatory genes (77, 100). This pathway also leads to potent production of type I IFNs through the activation of the transcription factor IFN regulatory factor 7 (IRF-7) upon stimulation of TLR7 or TLR9 (48, 77, 91). In contrast, in response to TLR3 or TLR4 stimulation, it is the Toll/interleukin-1 receptor domain-containing adaptor protein inducing IFN-β (TRIF) that mediates the production of type I IFNs primarily through the activation of IRF-3 (36, 113, 114).
Compelling evidence demonstrates that TLR signaling leads to DC maturation (reviewed in references 46, 72, and 87). Based on the crucial role of DCs in the generation of adaptive immune responses, it has been extensively argued that TLR signaling is necessary for adaptive antiviral immunity (13, 37, 46, 87). Nevertheless, most of the existing data showing a role for TLR signaling in the clearance of viral infections may be explained by the innate immune effects of type I IFNs. TLR3 contributes partially to protection against MCMV (99) through innate immune activation. TLR9 leads to type I IFN production in mice infected with HSV (51, 64) and participates in the clearance of MCMV (50, 99). TLR7 signaling results in type I IFN production in response to infection with vesicular stomatitis virus (VSV) (65). Also, there is a generalized effect of TLR3, -4, -5, -7, and -9 signaling in controlling replication of the hepatitis B virus through the induction of type I IFNs (41). A number of reports using mice deficient in the adaptor molecule MyD88 claim an important role for TLRs in the development of adaptive antiviral immunity (69, 115, 120). Since signaling of the Th1-driving cytokines interleukin 18 (IL-18) and IL-1 in these animals is impaired (1), these data do not constitute conclusive evidence for the involvement of TLR signaling in the generation of antiviral adaptive immunity.
TYPE I IFN IN VIRUS-INDUCED DC MATURATION
Type I IFNs (IFN-α, -β, -ω, and -λ) can be synthesized by most nucleated cells after activation of the transcription factors IRF-3 or IRF-7, NF-κB, and activator protein 1 (18, 52, 68, 90, 118). Type I IFN production is triggered intracellularly by products of viral replication, such as dsRNA (56, 67) and the viral ribonucleoprotein complex (103). Several intracellular proteins respond to these stimuli by inducing the expression of type I IFNs. These proteins are the dsRNA-dependent protein kinase (PKR) (6, 97, 116), the cellular enzymes TANK-binding kinase 1 (TBK1) (27) and IκB kinase epsilon (IKKɛ) (27, 95), the Rho GTPase Rac-1 (26), the retinoic acid-inducible gene I (RIG-I) (117), the melanoma differentiation-associated gene 5 (mda-5) (3), and the death domain-containing protein FADD (7). TLR signaling mediated by TBK1 and IKKɛ can also induce type I IFN production (91).
Once synthesized, type I IFNs are secreted from the cells and bind their receptor in an autocrine or paracrine manner, initiating a signaling cascade that uses the signal transducers and activators of transcription 1 and 2 (STAT1/STAT2) (70, 96) and culminates in the synthesis of IFN-responsive proteins (57). Genes coding for proteins with direct antiviral activity, such as PKR (which inhibits protein synthesis) (19), MxA (which inhibits transcription) (93), and 2′-5′-oligoadenylate synthetase (which activates RNaseL) (67, 78), are the best-described type I IFN responsive genes. Nevertheless, type I IFNs participate in various other aspects of immunity (reviewed in reference 104). They can directly affect B-cell development and function by influencing the generation of antibodies (15, 55) and can modulate the expression of proinflammatory cytokines, such as IL-6, transforming growth factor β, and IL-15, in other immune cells (14, 75, 119). Type I IFN-induced proteins, such as IRF-1 (83), participate in the development of NK cells (23, 79, 80), in the expression of the inducible nitric oxide synthetase by macrophages (47, 71), and in the development of Th1 immunity by inducing IL-12 p40 expression (59, 102). In addition, the IFN-γ inducible protein 10, also a type I IFN-induced protein, attracts Th1 cells to the site of infection, promoting the inflammatory response (22).
The triggering of DC maturation and type I IFN synthesis upon virus infection are both crucial early events in the course of an antiviral immune response. Therefore, it is reasonable to speculate that type I IFNs may be involved in the induction of DC maturation after virus infection. These cytokines have been proposed as endogenous adjuvants based on their ability to induce the up-regulation of major histocompatibility complex and costimulatory molecules (CD80, CD86) on DCs (29, 55, 63, 89, 105). Nevertheless, these molecules are up-regulated to a much greater extent when the cells are cotreated with dsRNA or lipopolysaccharide, indicating that type I IFNs are not able to fully mature DCs (38, 84). Futhermore, cDCs do not secrete proinflammatory cytokines when treated with type I IFNs alone at various doses (61, 84). Also, the up-regulation of costimulatory molecules and secretion of inflammatory cytokines by cDCs occurs normally after infection with SeV or influenza virus in the presence of type I IFN-neutralizing antibodies or in type I IFN receptor-deficient cDCs (61). In addition, efficient adaptive immunity is developed against these viruses in animals deficient in the type I IFN receptor (62a, 85). This evidence suggests that responsiveness to type I IFNs is neither necessary nor sufficient for the induction of complete DC maturation and subsequent development of adaptive immunity to many viruses.
TLR-INDEPENDENT INDUCTION OF DC MATURATION BY VIRUSES
As discussed in the previous sections, a vast amount of data show that TLR stimulation can lead to DC maturation. In contrast, evidence supporting the requirement of TLRs for the development of antiviral immunity in vivo is limited. In fact, cDCs have been shown to mature normally in the absence of TLR signaling when infected with Newcastle disease virus (NDV) (38), HSV (35), influenza virus, or SeV (62). TLR3 is not necessary for the generation of antiviral immunity against VSV, lymphocytic choriomeningitis virus (LCMV), MCMV, or reovirus (25). Moreover, mice infected with influenza virus or SeV are able to clear the infection (as measured by titers of the virus in the lungs), recover from weight loss, and develop efficient adaptive immunity, including antibodies and cytotoxic T cells, in the absence of TLR signaling (62). The absence of TLR7 and TLR9 expression from conventional human DCs (42, 43, 45) further supports the contention that recognition of viral genomic elements by TLRs is dispensable for the maturation of cDCs. All of these observations support the existence of a TLR-independent pathway for the induction of DC maturation capable of efficiently initiating adaptive antiviral immunity. UV-inactivated virus is unable to mature cDCs (61, 62), suggesting that this pathway is triggered by virus replication intermediaries. Moreover, inactivated SeV or influenza virus fails to trigger Th1 immunity (60, 62), further supporting an essential role for this replication-dependent pathway in the initiation of antiviral immunity.
Although type I IFNs are not necessary or sufficient to induce DC maturation (61), the observation that inactivated virus triggers neither maturation nor the release of type I IFNs from cDCs led us to hypothesize that molecular elements are shared between the induction of type I IFN synthesis and the TLR-independent pathway for DC maturation by viruses (61). Indeed, strains of influenza virus or SeV with different abilities to induce type I IFN expression demonstrate a direct correlation between the strength with which a virus stimulates type I IFN synthesis and the ability of the virus to trigger the maturation of cDCs, independently of the secreted type I IFNs (61). The influenza virus PR8-ΔNS1 strain, which lacks the NS1 protein that confers a type I IFN antagonistic activity to the virus (30), and the SeV strain Cantell, which is used broadly as a potent inducer of type I IFNs, are remarkably better at inducing the expression of costimulatory molecules (CD80 and CD86), proinflammatory cytokines (IL-1, IL-6, IL-12, and tumor necrosis factor alpha), and type I IFNs than their control virus counterparts, wild-type influenza PR8 virus and SeV strain 52 (61).
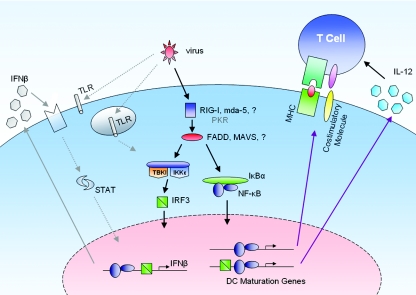
These data support an intracellularly triggered pathway independent of TLR signaling and of secreted type I IFNs, but dependent on virus replication, as a major mechanism for the induction of cDC maturation (Fig. 1). A model for the triggering of cDC maturation, where a virus replication intermediary triggers both type I IFN synthesis and the transcription of genes associated with DC maturation, is shown in Fig. 2.
FIG. 1.
TLR-independent pathway for DC maturation by viruses.
FIG. 2.
Model for cDC maturation upon virus infection. Virus infection triggers induction of both type I IFN and DC maturation through the activation of transcription factors, such as IRF3 and NF-κB. DC maturation is characterized by the up-regulation of major histocompatibility complex and costimulatory molecules and by the secretion of proinflammatory cytokines, such as IL-12. Complete maturation of cDCs in response to virus does not rely on secreted type I IFN or TLR signaling. Nevertheless, the concomitant activation of the type I IFN induction pathway and DC maturation pathway suggests that dsRNA-binding molecules known to be involved in type I IFN induction, such as RIG-I or mda-5, may also lead to DC maturation, while the dsRNA-binding protein PKR has been shown to be unnecessary for complete DC maturation in response to live viruses. Signaling pathways shown in gray are dispensable for complete DC maturation.
VIRAL AND CELLULAR ELEMENTS INVOLVED IN THE TRIGGERING OF TLR-INDEPENDENT DC MATURATION
Several pieces of evidence point toward dsRNA produced during the replication of viral genomes as the trigger for the TLR-independent pathway for DC maturation. First, the inability of inactivated viruses to mature DCs (60-62) indicates that viral replication, and not viral proteins or viral genomes themselves, is essential for the triggering of DC maturation. Second, the synthetic dsRNA analog poly(I:C) can induce DC maturation in the absence of TLR3, the described receptor for viral dsRNA (21, 38). Third, dsRNA binding proteins are capable of antagonizing type I IFN production and DC maturation, strongly suggesting that this viral element is essential for the triggering of immunity (11, 88, 110). Influenza viruses lacking the gene segment coding for the dsRNA-binding protein NS1 induce higher levels of type I IFNs and of DC maturation than influenza viruses coding for this antagonist (30, 61). Moreover, the dsRNA-binding domain of NS1 specifically inhibits type I IFN induction by viral dsRNA (109). Finally, viral defective interfering (DI) particles, incomplete viral genomes that replicate to higher levels than standard virus, while interfering with protein synthesis (40, 54), enhance the DC maturation abilities of virus preparations (J. S. Yount, T. M. Moran, and C. B. López, unpublished data). This suggests that the dsRNA produced during the replication of the virus genome and not the dsRNA produced during mRNA synthesis participates in the triggering of DC maturation and type I IFN production.
A number of dsRNA-activated cellular proteins that mediate type I IFN synthesis have been identified. These proteins, including PKR, TBK1, IKKɛ, RIG-I, mda-5, and FADD (7, 27, 52, 103, 117, 118), activate NF-κB and IRF-3, both of which are involved in type I IFN synthesis and in the transcription of IL-12p35, an essential component of bioactive IL-12 (31, 76). Thus, because the TLR-independent pathway for the triggering of DC maturation by live viruses shares common elements with the pathway resulting in type I IFN production, these cellular proteins must be viewed as good candidates for mediating virus-induced DC maturation (Fig. 2).
PKR was the first cellular enzyme identified to respond to viral infection. It induces the activation of transcription factors essential for both type I IFNs and proinflammatory cytokine synthesis (52, 118). Although PKR has been shown to participate in the induction of type I IFNs after the transfection of dsRNA into mouse DCs (21), it is not required for type I IFN induction or DC maturation after infection with live influenza virus, SeV, or NDV (10, 38, 61). This contradiction illustrates the fundamental differences between an actual virus infection and the use of virus analogs, such as poly(I:C), that lack crucial characteristics such as the ability to replicate and concomitantly amplify the stimuli.
The helicase RIG-I binds dsRNA and is involved in the type I IFN response to virus infection (117). This molecule activates the transcription factors IRF-3 and NF-κB through the recently identified adaptor protein mitochondrial antiviral signaling protein (also called VISA, IPS-1, or Cardif) (49, 73, 94, 112). RIG-I has been implicated in the induction of IL-12 and IL-6 in response to hepatitis C virus infection in transfected cell lines (28). Although a direct effect of RIG-I in the maturation of DCs has not been yet demonstrated, this molecule is a promising candidate for triggering the induction of DC maturation by live viruses. In agreement with this idea, the enhanced production of dsRNA by virus in the presence of DI particles results in a heightened activation of the RIG-I signaling pathway. In fact, RIG-I is necessary for efficient type I IFN induction by DI particle-rich viruses that are also potent inducers of DC maturation (J. S. Yount, T. M. Moran, and C. B. López, unpublished data). Further studies are necessary to evaluate the role of TBK1, IKKɛ, FADD, and any other potential candidates for the triggering of DC maturation and initiation of adaptive immunity upon virus infection.
TLR-DEPENDENT TYPE I IFN IN THE DEVELOPMENT OF ANTIVIRAL IMMUNITY
Although DC maturation is independent of secreted type I IFNs for some viruses, other viruses, such as NDV, need to be complemented by this cytokine in order to induce complete DC maturation (38). Because NDV stimulates mouse DC maturation very weakly compared with other viruses, such as SeV, it can be speculated that the strength of the signaling determines the requirement for type I IFN adjuvancy. pDCs that respond to virus infection by producing large amounts of type I IFN could provide this adjuvant effect through an intracellular pathway similar to that described for conventional DCs (39) and via TLR7- and TLR9-mediated signaling. Cells infected by viruses carrying a type I IFN antagonist, such as influenza virus, may also rely on exogenous type I IFNs for complete maturation.
Despite the effects of type I IFNs on different aspects of immunity, their in vivo role in the development of adaptive antiviral immune response is unclear. Type I IFN receptor-deficient mice clear SeV, influenza virus, mouse mammary tumor virus, and rotavirus and generate efficient adaptive immunity against these viruses (4, 62a, 66, 85). In contrast, mice deficient in the type I IFN receptor have an increased susceptibility to infection with LCMV, Semliki Forest virus, Theiler's virus, Venezuelan equine encephalitis (VEE) virus, and vesicular stomatitis virus (106, 107). Evidence obtained from studies using different strains of LCMV and VEE virus suggests that the high virulence of some of the strains corresponds with low sensitivity to the antiviral effects of type I IFNs (81, 111). This resistance to type I IFNs permits the virus to grow to high titers and to persist in the animals, exhausting the effector cells and thus rendering the immune response inadequate for the clearance of the virus (81). Further studies are necessary to determine the actual role of TLR-dependent type I IFNs in antiviral immunity. It can be speculated that the generation of a type I IFN-rich environment by TLR-mediated pathways might be crucial for overcoming the ability of viruses to antagonize the innate type I IFN system and the TLR-independent pathway for DC maturation, thus allowing the development of adaptive immune responses.
PERSPECTIVES
The discovery of a novel pathway for the triggering of DC maturation and subsequent initiation of antiviral immunity represents the beginning of exciting research into the mechanisms utilized by the host to initiate an immune response upon virus infection. This pathway is independent of TLR signaling and secreted type I IFN but depends on virus replication, shares common elements with the type I IFN induction pathway, and seems to be essential for the activation of conventional DCs in the event of a virus infection. Although good cellular candidate molecules and prospective viral elements involved in this pathway are emerging, detailed studies are needed to determine their definitive role in this process. The identification of specific cellular molecules responsible for these events could be of enormous value in the development of antiviral therapies and in the design of effective vaccines.
Acknowledgments
We acknowledge all those contributions from many laboratories that were inadvertently not cited in this review.
The work from our laboratory presented in this paper was supported by grants 1R01AI41111 and U19AI062623-01 (to T.M.M.) from the National Institute of Allergy and Infectious Diseases and by funds granted by the Charles H. Revson Foundation (to C.B.L.). The responsibility for the statements made and views expressed, however, is solely that of the authors.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-β promoter. Proc. Natl. Acad. Sci. USA 101:17264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angel, J., M. A. Franco, H. B. Greenberg, and D. Bass. 1999. Lack of a role for type I and type II interferons in the resolution of rotavirus-induced diarrhea and infection in mice. J. Interferon Cytokine Res. 19:655-659. [DOI] [PubMed] [Google Scholar]
- 5.Angelov, G. S., M. Tomkowiak, A. Marcais, Y. Leverrier, and J. Marvel. 2005. Flt3 ligand-generated murine plasmacytoid and conventional dendritic cells differ in their capacity to prime naive CD8 T cells and to generate memory cells in vivo. J. Immunol. 175:189-195. [DOI] [PubMed] [Google Scholar]
- 6.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. [DOI] [PubMed] [Google Scholar]
- 7.Balachandran, S., E. Thomas, and G. N. Barber. 2004. A FADD-dependent innate immune mechanism in mammalian cells. Nature 432:401-405. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 9.Barchet, W., M. Cella, and M. Colonna. 2005. Plasmacytoid dendritic cells—virus experts of innate immunity. Semin. Immunol. 17:253-261. [DOI] [PubMed] [Google Scholar]
- 10.Barchet, W., A. Krug, M. Cella, C. Newby, J. A. Fischer, A. Dzionek, A. Pekosz, and M. Colonna. 2005. Dendritic cells respond to influenza virus through TLR7- and PKR-independent pathways. Eur. J. Immunol. 35:236-242. [DOI] [PubMed] [Google Scholar]
- 11.Basler, C. F., and A. Garcia-Sastre. 2002. Viruses and the type I interferon antiviral system: induction and evasion. Int. Rev. Immunol. 21:305-337. [DOI] [PubMed] [Google Scholar]
- 12.Bieback, K., E. Lien, I. M. Klagge, E. Avota, J. Schneider-Schaulies, W. P. Duprex, H. Wagner, C. J. Kirschning, V. Ter Meulen, and S. Schneider-Schaulies. 2002. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J. Virol. 76:8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowie, A. G., and I. R. Haga. 2005. The role of Toll-like receptors in the host response to viruses. Mol. Immunol. 42:859-867. [DOI] [PubMed] [Google Scholar]
- 14.Brassard, D. L., M. J. Grace, and R. W. Bordens. 2002. Interferon-α as an immunotherapeutic protein. J. Leukoc. Biol. 71:565-581. [PubMed] [Google Scholar]
- 15.Braun, D., I. Caramalho, and J. Demengeot. 2002. IFN-α/β enhances BCR-dependent B cell responses. Int. Immunol. 14:411-419. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. 2004. Progress toward global eradication of poliomyelitis, January 2003-April 2004. Morb. Mortal. Wkly. Rep. 53:532-535. [PubMed] [Google Scholar]
- 17.Carbone, F. R., and W. R. Heath. 2003. The role of dendritic cell subsets in immunity to viruses. Curr. Opin. Immunol. 15:416-420. [DOI] [PubMed] [Google Scholar]
- 18.Chu, W. M., D. Ostertag, Z. W. Li, L. Chang, Y. Chen, Y. Hu, B. Williams, J. Perrault, and M. Karin. 1999. JNK2 and IKKβ are required for activating the innate response to viral infection. Immunity 11:721-731. [DOI] [PubMed] [Google Scholar]
- 19.de Haro, C., R. Mendez, and J. Santoyo. 1996. The eIF-2α kinases and the control of protein synthesis. FASEB J. 10:1378-1387. [DOI] [PubMed] [Google Scholar]
- 20.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 21.Diebold, S. S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L. E. Haswell, A. Al-Shamkhani, R. Flavell, P. Borrow, and C. Reis e Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424:324-328. [DOI] [PubMed] [Google Scholar]
- 22.Dufour, J. H., M. Dziejman, M. T. Liu, J. H. Leung, T. E. Lane, and A. D. Luster. 2002. IFN-γ-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 168:3195-3204. [DOI] [PubMed] [Google Scholar]
- 23.Duncan, G. S., H. W. Mittrucker, D. Kagi, T. Matsuyama, and T. W. Mak. 1996. The transcription factor interferon regulatory factor-1 is essential for natural killer cell function in vivo. J. Exp. Med. 184:2043-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunne, A., and L. A. O'Neill. 2005. Adaptor usage and Toll-like receptor signaling specificity. FEBS Lett. 579:3330-3335. [DOI] [PubMed] [Google Scholar]
- 25.Edelmann, K. H., S. Richardson-Burns, L. Alexopoulou, K. L. Tyler, R. A. Flavell, and M. B. Oldstone. 2004. Does Toll-like receptor 3 play a biological role in virus infections? Virology 322:231-238. [DOI] [PubMed] [Google Scholar]
- 26.Ehrhardt, C., C. Kardinal, W. J. Wurzer, T. Wolff, C. von Eichel-Streiber, S. Pleschka, O. Planz, and S. Ludwig. 2004. Rac1 and PAK1 are upstream of IKK-ɛ and TBK-1 in the viral activation of interferon regulatory factor-3. FEBS Lett. 567:230-238. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald, K. A., S. M. McWhirter, K. L. Faia, D. C. Rowe, E. Latz, D. T. Golenbock, A. J. Coyle, S. M. Liao, and T. Maniatis. 2003. IKKɛ and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4:491-496. [DOI] [PubMed] [Google Scholar]
- 28.Foy, E., K. Li, R. Sumpter, Jr., Y. M. Loo, C. L. Johnson, C. Wang, P. M. Fish, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale, Jr. 2005. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc. Natl. Acad. Sci. USA 102:2986-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallucci, S., M. Lolkema, and P. Matzinger. 1999. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5:1249-1255. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Sastre, A., A. Egorov, D. Matassov, S. Brandt, D. E. Levy, J. E. Durbin, P. Palese, and T. Muster. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324-330. [DOI] [PubMed] [Google Scholar]
- 31.Goriely, S., C. Molle, M. Nguyen, V. Albarani, N. Ouled Haddou, R. Lin, D. De Wit, V. Flamand, F. Willems, and M. Goldman. 11 October 2005, posting date. Interferon regulatory factor 3 is involved in Toll like receptor (TLR)4- and TLR3-induced IL-12p35 gene activation. Blood [Online.] http://www.bloodjournal.org/cgi/reprint/2005-06-2416v1. [DOI] [PubMed]
- 32.Reference deleted.
- 33.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 34.Hertzog, P. J., L. A. O'Neill, and J. A. Hamilton. 2003. The interferon in TLR signaling: more than just antiviral. Trends Immunol. 24:534-539. [DOI] [PubMed] [Google Scholar]
- 35.Hochrein, H., B. Schlatter, M. O'Keeffe, C. Wagner, F. Schmitz, M. Schiemann, S. Bauer, M. Suter, and H. Wagner. 2004. Herpes simplex virus type-1 induces IFN-α production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 101:11416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoebe, K., X. Du, P. Georgel, E. Janssen, K. Tabeta, S. O. Kim, J. Goode, P. Lin, N. Mann, S. Mudd, K. Crozat, S. Sovath, J. Han, and B. Beutler. 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424:743-748. [DOI] [PubMed] [Google Scholar]
- 37.Hoebe, K., E. Janssen, and B. Beutler. 2004. The interface between innate and adaptive immunity. Nat. Immunol. 5:971-974. [DOI] [PubMed] [Google Scholar]
- 38.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-α/β signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hornung, V., J. Schlender, M. Guenthner-Biller, S. Rothenfusser, S. Endres, K. K. Conzelmann, and G. Hartmann. 2004. Replication-dependent potent IFN-α induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J. Immunol. 173:5935-5943. [DOI] [PubMed] [Google Scholar]
- 40.Huang, A. S., and D. Baltimore. 1970. Defective viral particles and viral disease processes. Nature 226:325-327. [DOI] [PubMed] [Google Scholar]
- 41.Isogawa, M., M. D. Robek, Y. Furuichi, and F. V. Chisari. 2005. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J. Virol. 79:7269-7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito, T., Y. H. Wang, and Y. J. Liu. 2005. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9. Springer Semin. Immunopathol. 26:221-229. [DOI] [PubMed] [Google Scholar]
- 43.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388-3393. [DOI] [PubMed] [Google Scholar]
- 44.Jefford, M., M. Schnurr, T. Toy, K. A. Masterman, A. Shin, T. Beecroft, T. Y. Tai, K. Shortman, M. Shackleton, I. D. Davis, P. Parente, T. Luft, W. Chen, J. Cebon, and E. Maraskovsky. 2003. Functional comparison of DCs generated in vivo with Flt3 ligand or in vitro from blood monocytes: differential regulation of function by specific classes of physiologic stimuli. Blood 102:1753-1763. [DOI] [PubMed] [Google Scholar]
- 45.Kadowaki, N., S. Ho, S. Antonenko, R. W. Malefyt, R. A. Kastelein, F. Bazan, and Y. J. Liu. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaisho, T., and S. Akira. 2001. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 22:78-83. [DOI] [PubMed] [Google Scholar]
- 47.Kamijo, R., H. Harada, T. Matsuyama, M. Bosland, J. Gerecitano, D. Shapiro, J. Le, S. I. Koh, T. Kimura, S. J. Green, et al. 1994. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science 263:1612-1615. [DOI] [PubMed] [Google Scholar]
- 48.Kawai, T., S. Sato, K. J. Ishii, C. Coban, H. Hemmi, M. Yamamoto, K. Terai, M. Matsuda, J. Inoue, S. Uematsu, O. Takeuchi, and S. Akira. 2004. Interferon-α induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5:1061-1068. [DOI] [PubMed] [Google Scholar]
- 49.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6:981-988. [DOI] [PubMed] [Google Scholar]
- 50.Krug, A., A. R. French, W. Barchet, J. A. Fischer, A. Dzionek, J. T. Pingel, M. M. Orihuela, S. Akira, W. M. Yokoyama, and M. Colonna. 2004. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21:107-119. [DOI] [PubMed] [Google Scholar]
- 51.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 103:1433-1437. [DOI] [PubMed] [Google Scholar]
- 52.Kumar, A., J. Haque, J. Lacoste, J. Hiscott, and B. R. Williams. 1994. Double-stranded RNA-dependent protein kinase activates transcription factor NF-κB by phosphorylating IκB. Proc. Natl. Acad. Sci. USA 91:6288-6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reference deleted.
- 54.Lazzarini, R. A., J. D. Keene, and M. Schubert. 1981. The origins of defective interfering particles of the negative-strand RNA viruses. Cell 26:145-154. [DOI] [PubMed] [Google Scholar]
- 55.Le Bon, A., G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli, and D. F. Tough. 2001. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14:461-470. [DOI] [PubMed] [Google Scholar]
- 56.Lepe-Zuniga, J. L., J. Rotbein, and J. U. Gutterman. 1989. Production of interferon-α induced by dsRNA in human peripheral blood mononuclear cell cultures: role of priming by dsRNA-induced interferons-γ and -β. J. Interferon Res. 9:445-456. [DOI] [PubMed] [Google Scholar]
- 57.Levy, D. E., D. S. Kessler, R. Pine, and J. E. Darnell, Jr. 1989. Cytoplasmic activation of ISGF3, the positive regulator of interferon-α-stimulated transcription, reconstituted in vitro. Genes Dev. 3:1362-1371. [DOI] [PubMed] [Google Scholar]
- 58.Liu, Y. J. 2004. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. [DOI] [PubMed]
- 59.Lohoff, M., D. Ferrick, H. W. Mittrucker, G. S. Duncan, S. Bischof, M. Rollinghoff, and T. W. Mak. 1997. Interferon regulatory factor-1 is required for a T helper 1 immune response in vivo. Immunity 6:681-689. [DOI] [PubMed] [Google Scholar]
- 60.López, C. B., A. Fernandez-Sesma, J. L. Schulman, and T. M. Moran. 2001. Myeloid dendritic cells stimulate both Th1 and Th2 immune responses depending on the nature of the antigen. J. Interferon Cytokine Res. 21:763-773. [DOI] [PubMed] [Google Scholar]
- 61.López, C. B., A. Garcia-Sastre, B. R. Williams, and T. M. Moran. 2003. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J. Infect. Dis. 187:1126-1136. [DOI] [PubMed] [Google Scholar]
- 62.López, C. B., B. Moltedo, L. Alexopoulou, L. Bonifaz, R. A. Flavell, and T. M. Moran. 2004. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J. Immunol. 173:6882-6889. [DOI] [PubMed] [Google Scholar]
- 62a.López, C. B., J. S. Yount, T. Hermesh, and T. M. Moran. Sendai virus infection induces efficient adaptive immunity independently of type I interferons. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 63.Luft, T., K. C. Pang, E. Thomas, P. Hertzog, D. N. Hart, J. Trapani, and J. Cebon. 1998. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 161:1947-1953. [PubMed] [Google Scholar]
- 64.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maillard, I., P. Launois, I. Xenarios, J. A. Louis, H. Acha-Orbea, and H. Diggelmann. 1998. Immune response to mouse mammary tumor virus in mice lacking the alpha/beta interferon or the gamma interferon receptor. J. Virol. 72:2638-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malmgaard, L. 2004. Induction and regulation of IFNs during viral infections. J. Interferon Cytokine Res. 24:439-454. [DOI] [PubMed] [Google Scholar]
- 68.Mamane, Y., C. Heylbroeck, P. Genin, M. Algarte, M. J. Servant, C. LePage, C. DeLuca, H. Kwon, R. Lin, and J. Hiscott. 1999. Interferon regulatory factors: the next generation. Gene 237:1-14. [DOI] [PubMed] [Google Scholar]
- 69.Mansur, D. S., E. G. Kroon, M. L. Nogueira, R. M. Arantes, S. C. Rodrigues, S. Akira, R. T. Gazzinelli, and M. A. Campos. 2005. Lethal encephalitis in myeloid differentiation factor 88-deficient mice infected with herpes simplex virus 1. Am. J. Pathol. 166:1419-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marie, I., J. E. Durbin, and D. E. Levy. 1998. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 17:6660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin, E., C. Nathan, and Q. W. Xie. 1994. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J. Exp. Med. 180:977-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mazzoni, A., and D. M. Segal. 2004. Controlling the Toll road to dendritic cell polarization. J. Leukoc. Biol. 75:721-730. [DOI] [PubMed] [Google Scholar]
- 73.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167-1172. [DOI] [PubMed] [Google Scholar]
- 74.Minor, P. D. 2004. Polio eradication, cessation of vaccination and re-emergence of disease. Nat. Rev. Microbiol. 2:473-482. [DOI] [PubMed] [Google Scholar]
- 75.Mitani, Y., A. Takaoka, S. H. Kim, Y. Kato, T. Yokochi, N. Tanaka, and T. Taniguchi. 2001. Cross talk of the interferon-α/β signalling complex with gp130 for effective interleukin-6 signalling. Genes Cells 6:631-640. [DOI] [PubMed] [Google Scholar]
- 76.Morelli, A. E., A. T. Larregina, R. W. Ganster, A. F. Zahorchak, J. M. Plowey, T. Takayama, A. J. Logar, P. D. Robbins, L. D. Falo, and A. W. Thomson. 2000. Recombinant adenovirus induces maturation of dendritic cells via an NF-κB-dependent pathway. J. Virol. 74:9617-9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moynagh, P. N. 2005. TLR signalling and activation of IRFs: revisiting old friends from the NF-κB pathway. Trends Immunol. [DOI] [PubMed]
- 78.Nilsen, T. W., P. A. Maroney, and C. Baglioni. 1982. Synthesis of (2′-5′)oligoadenylate and activation of an endoribonuclease in interferon-treated HeLa cells infected with reovirus. J. Virol. 42:1039-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ogasawara, K., S. Hida, N. Azimi, Y. Tagaya, T. Sato, T. Yokochi-Fukuda, T. A. Waldmann, T. Taniguchi, and S. Taki. 1998. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature 391:700-703. [DOI] [PubMed] [Google Scholar]
- 80.Ohteki, T., H. Yoshida, T. Matsuyama, G. S. Duncan, T. W. Mak, and P. S. Ohashi. 1998. The transcription factor interferon regulatory factor 1 (IRF-1) is important during the maturation of natural killer 1.1+ T cell receptor-α/β+ (NK1+ T) cells, natural killer cells, and intestinal intraepithelial T cells. J. Exp. Med. 187:967-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ou, R., S. Zhou, L. Huang, and D. Moskophidis. 2001. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 75:8407-8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pasare, C., and R. Medzhitov. 2004. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 6:1382-1387. [DOI] [PubMed] [Google Scholar]
- 83.Pine, R., T. Decker, D. S. Kessler, D. E. Levy, and J. E. Darnell, Jr. 1990. Purification and cloning of interferon-stimulated gene factor 2 (ISGF2): ISGF2 (IRF-1) can bind to the promoters of both beta interferon- and interferon-stimulated genes but is not a primary transcriptional activator of either. Mol. Cell. Biol. 10:2448-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pollara, G., M. Jones, M. E. Handley, M. Rajpopat, A. Kwan, R. S. Coffin, G. Foster, B. Chain, and D. R. Katz. 2004. Herpes simplex virus type-1-induced activation of myeloid dendritic cells: the roles of virus cell interaction and paracrine type I IFN secretion. J. Immunol. 173:4108-4119. [DOI] [PubMed] [Google Scholar]
- 85.Price, G. E., A. Gaszewska-Mastarlarz, and D. Moskophidis. 2000. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J. Virol. 74:3996-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reis e Sousa, C. 2004. Activation of dendritic cells: translating innate into adaptive immunity. Curr. Opin. Immunol. 16:21-25. [DOI] [PubMed] [Google Scholar]
- 87.Reis e Sousa, C. 2004. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin. Immunol. 16:27-34. [DOI] [PubMed] [Google Scholar]
- 88.Rodriguez, J. J., and C. M. Horvath. 2004. Host evasion by emerging paramyxoviruses: Hendra virus and Nipah virus V proteins inhibit interferon signaling. Viral Immunol. 17:210-219. [DOI] [PubMed] [Google Scholar]
- 89.Santini, S. M., C. Lapenta, M. Logozzi, S. Parlato, M. Spada, T. Di Pucchio, and F. Belardelli. 2000. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 191:1777-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 91.Sato, S., M. Sugiyama, M. Yamamoto, Y. Watanabe, T. Kawai, K. Takeda, and S. Akira. 2003. Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-κB and IFN-regulatory factor-3, in the Toll-like receptor signaling. J. Immunol. 171:4304-4310. [DOI] [PubMed] [Google Scholar]
- 92.Reference deleted.
- 93.Schwemmle, M., K. C. Weining, M. F. Richter, B. Schumacher, and P. Staeheli. 1995. Vesicular stomatitis virus transcription inhibited by purified MxA protein. Virology 206:545-554. [DOI] [PubMed] [Google Scholar]
- 94.Seth, R. B., L. Sun, C. K. Ea, and Z. J. Chen. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell 122:669-682. [DOI] [PubMed] [Google Scholar]
- 95.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148-1151. [DOI] [PubMed] [Google Scholar]
- 96.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 97.Stojdl, D. F., N. Abraham, S. Knowles, R. Marius, A. Brasey, B. D. Lichty, E. G. Brown, N. Sonenberg, and J. C. Bell. 2000. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J. Virol. 74:9580-9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Strassburg, M. A. 1982. The global eradication of smallpox. Am. J. Infect Control. 10:53-59. [DOI] [PubMed] [Google Scholar]
- 99.Tabeta, K., P. Georgel, E. Janssen, X. Du, K. Hoebe, K. Crozat, S. Mudd, L. Shamel, S. Sovath, J. Goode, L. Alexopoulou, R. A. Flavell, and B. Beutler. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 101:3516-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takeda, K., and S. Akira. 2004. TLR signaling pathways. Semin. Immunol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 101.Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1-14. [DOI] [PubMed] [Google Scholar]
- 102.Taki, S., T. Sato, K. Ogasawara, T. Fukuda, M. Sato, S. Hida, G. Suzuki, M. Mitsuyama, E. H. Shin, S. Kojima, T. Taniguchi, and Y. Asano. 1997. Multistage regulation of Th1-type immune responses by the transcription factor IRF-1. Immunity 6:673-679. [DOI] [PubMed] [Google Scholar]
- 103.tenOever, B. R., S. Sharma, W. Zou, Q. Sun, N. Grandvaux, I. Julkunen, H. Hemmi, M. Yamamoto, S. Akira, W. C. Yeh, R. Lin, and J. Hiscott. 2004. Activation of TBK1 and IKKvarepsilon kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity. J. Virol. 78:10636-10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Theofilopoulos, A. N., R. Baccala, B. Beutler, and D. H. Kono. 2005. Type I interferons (α/β) in immunity and autoimmunity. Annu. Rev. Immunol. 23:307-336. [DOI] [PubMed]
- 105.Tough, D. F. 2004. Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk. Lymphoma 45:257-264. [DOI] [PubMed] [Google Scholar]
- 106.van den Broek, M. F., U. Muller, S. Huang, M. Aguet, and R. M. Zinkernagel. 1995. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 69:4792-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van den Broek, M. F., U. Muller, S. Huang, R. M. Zinkernagel, and M. Aguet. 1995. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol. Rev. 148:5-18. [DOI] [PubMed] [Google Scholar]
- 108.Villadangos, J. A., and W. R. Heath. 2005. Life cycle, migration and antigen presenting functions of spleen and lymph node dendritic cells: limitations of the Langerhans cells paradigm. Semin. Immunol. 17:262-272. [DOI] [PubMed] [Google Scholar]
- 109.Wang, X., M. Li, H. Zheng, T. Muster, P. Palese, A. A. Beg, and A. Garcia-Sastre. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 74:11566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weber, F., G. Kochs, and O. Haller. 2004. Inverse interference: how viruses fight the interferon system. Viral Immunol. 17:498-515. [DOI] [PubMed] [Google Scholar]
- 111.White, L. J., J. G. Wang, N. L. Davis, and R. E. Johnston. 2001. Role of alpha/beta interferon in Venezuelan equine encephalitis virus pathogenesis: effect of an attenuating mutation in the 5′ untranslated region. J. Virol. 75:3706-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu, L. G., Y. Y. Wang, K. J. Han, L. Y. Li, Z. Zhai, and H. B. Shu. 2005. VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol. Cell 19:727-740. [DOI] [PubMed] [Google Scholar]
- 113.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640-643. [DOI] [PubMed] [Google Scholar]
- 114.Yamamoto, M., S. Sato, K. Mori, K. Hoshino, O. Takeuchi, K. Takeda, and S. Akira. 2002. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J. Immunol. 169:6668-6672. [DOI] [PubMed] [Google Scholar]
- 115.Yang, R., F. M. Murillo, H. Cui, R. Blosser, S. Uematsu, K. Takeda, S. Akira, R. P. Viscidi, and R. B. Roden. 2004. Papillomavirus-like particles stimulate murine bone marrow-derived dendritic cells to produce alpha interferon and Th1 immune responses via MyD88. J. Virol. 78:11152-11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 118.Zamanian-Daryoush, M., T. H. Mogensen, J. A. DiDonato, and B. R. Williams. 2000. NF-κB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-κB-inducing kinase and IκB kinase. Mol. Cell. Biol. 20:1278-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang, X., S. Sun, I. Hwang, D. F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8:591-599. [DOI] [PubMed] [Google Scholar]
- 120.Zhou, S., E. A. Kurt-Jones, L. Mandell, A. Cerny, M. Chan, D. T. Golenbock, and R. W. Finberg. 2005. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur. J. Immunol. 35:822-830. [DOI] [PubMed] [Google Scholar]