Abstract
The heat shock cognate protein hsc70 has been implicated as a postattachment cell receptor for rotaviruses. Here we show that hsc70 interacts specifically with rotaviruses through its peptide-binding domain, since a recombinant full-length hsc70 protein and its peptide-binding domain, but not its ATPase domain, bound triple-layered particles in a solid-phase assay, and known ligands of hsc70 competed this binding. The peptide ligands of hsc70 were also shown to block rotavirus infectivity when added to cells before virus infection, suggesting that hsc70 on the surface of MA104 cells also interacts with the virus through its peptide-binding domain and that this interaction is important for virus entry. When purified infectious virus was incubated with soluble hsc70 in the presence of the cochaperone hsp40 and ATP and then pelleted through a sucrose cushion, the recovered virus had lost 60% of its infectivity, even though hsc70 was not detected in the pellet fraction. The hsc70-treated virus showed slightly different reactivities with monoclonal antibodies and was more susceptible to heat and basic pHs than the untreated virus, suggesting that hsc70 induces a subtle conformational change in the virus that results in a reduction of its infectivity. The relevance of the ATPase activity of hsc70 for reducing virus infectivity was demonstrated by the finding that in the presence of a nonhydrolyzable analogue of ATP, virus infectivity was not affected, and a mutant protein lacking ATPase activity failed to reduce virus infection. Altogether, these results suggest that during cell infection, the interaction of the virus with hsc70 on the surface of MA104 cells results in a conformational change of virus particles that facilitates their entry into the cell cytoplasm.
Rotaviruses are the single most important cause of severe dehydrating diarrhea in young children worldwide. These nonenveloped viruses are formed by a triple-layered protein capsid which surrounds the genome, composed of 11 segments of double-stranded RNA (11). The outermost layer, which is responsible for the initial interactions of the virus with the cell surface, consists of the following two proteins: VP7, a glycoprotein that forms the smooth surface of the virion, and VP4, which forms the spikes that extend from the surface of the virus particle. Both proteins play essential roles during the early interactions of the virus with the cell surface, including receptor binding and cell penetration (11, 29). To be infectious, rotaviruses depend on the proteolytic cleavage of VP4 (776 amino acids [aa]) into subunits VP8 (aa 1 to 247) and VP5 (aa 248 to 776); this cleavage does not affect cell binding but is required for entry of the virus into the cell's cytoplasm (17, 25, 29). Rotaviruses have a very specific cell tropism, infecting primarily the mature enterocytes at the tips of the villi of the small intestine, and the susceptibility of these cells seems to be limited to a narrow age window (26). In cell culture, rotavirus binds to a large variety of cell types, although it only efficiently infects some of them, including those of renal and intestinal origin (6, 11, 12). Rotavirus infection seems to be a multistep process in which the virus has to interact with several cell surface molecules to enter the cell (29). We have previously shown that the neuraminidase-sensitive simian rotavirus strain RRV initially attaches to a sialic acid-containing cell molecule through the VP8 subunit of VP4 and then subsequently interacts with integrin α2β1 through VP5 (46). After these initial contacts, RRV interacts with at least three additional proteins located at the cell surface, i.e., integrins αvβ3 and αxβ2 (19, 22) and the heat shock cognate protein hsc70 (20); whether these last three interactions occur sequentially or alternatively has not been determined. The virus interaction with hsc70 is mediated by a domain in VP5 located between amino acids 642 and 659 of the protein, and this interaction takes place at a postattachment step, since a synthetic peptide that mimics this region (peptide KID) and antibodies to hsc70 block the infectivity of rotavirus but not its binding to the cell surface (20, 45).
hsc70 is a constitutive member of the heat shock-induced hsp70 protein family. The proteins in this family are evolutionarily conserved, but they are not functionally interchangeable; the functional diversity of these molecular chaperones may result from variations in their abilities to bind to different target proteins (15). The heat shock proteins have been associated with a number of functions, including protein folding, translocation across membranes, and assembly and disassembly of oligomeric complexes. In particular, hsc70 has been shown to favor the transport of proteins across organelle membranes, binding nascent polypeptides and dissociating clathrin from clathrin coats (3). hsc70 contains two functional domains, namely, an amino-terminal ATPase (A) domain (44 kDa; aa 1 to 383) that contains the nucleotide-binding site and a carboxy-terminal peptide-binding (PB) domain (30 kDa; aa 384 to 650) that contains the substrate-binding pocket determining the specificity for different peptide substrates (16). The ATPase domain is well conserved among the members of the hsp70 family, while the peptide-binding domain is more variable (3, 30).
In this work, we report that soluble, recombinant hsc70 interacts with rotavirus particles through its peptide-binding domain, and we show that this interaction reduces virus infectivity in an ATP-dependent manner, most probably by inducing a conformational change in the virus.
MATERIALS AND METHODS
Cells and viruses.
MA104 cells were cultured in Eagle's minimal essential medium (MEM) supplemented with 10% fetal bovine serum. Rhesus rotavirus (RRV G3P5 [3]) was obtained from H. B. Greenberg, Stanford University, Stanford, CA, and was propagated in MA104 cells as previously described (34). The viral particles were purified by CsCl density gradient centrifugation of a virus lysate preincubated with 10 μg/ml of trypsin (specific activity, 1 unit/250 mg) (Gibco) for 60 min at 37°C and treated as described previously (47).
Proteins and peptides.
A cDNA clone of human hsc70 (in plasmid pETHSC; GenBank accession no. M11717) was originally obtained from R. T. Morimoto, Northwestern University, Evanston, IL. The sequences encoding the complete hsc70 protein as well as its amino (A; aa 1 to 383)- and carboxy (PB; aa 384 to 650)-terminal domains were subcloned into the pET28 expression vector (Novagene) to generate fusion proteins with a six-histidine tail at their carboxy termini. The hsc70 mutant K71M (32) was made using a QuickChange site-directed mutagenesis kit (Promega) and the sense oligonucleotide 5′-193CCACACAGTTTTTGATGCCATGCGTCTGATTGGTCGACG232-3′ (mutated nucleotides are shown in bold). The recombinant proteins produced in bacteria were purified by affinity chromatography on HiTrap chelating columns (Pharmacia). The nucleotide associated with the purified recombinant proteins was most probably ATP, given the high ATP/ADP ratio present in normal cells and the higher affinity of hsc70 for ATP (4). To obtain nucleotide-free hsc70, the purified protein was incubated with 10 mM EDTA for 10 min at room temperature and then was precipitated by the addition of (NH4)2SO4 to 75% saturation. The protein was redissolved in phosphate-buffered saline (PBS), dialyzed to eliminate the excess of (NH4)2SO4, and stored at 4°C, where it is stable for several months (4). The nucleotide-free hsc70 protein was loaded with different nucleotides as described below. Synthetic peptide A (103FYQLALT109), derived from a peptide of the p53 protein (15), peptide C (491KLIGVLSSLFRPC502), derived from the surface glycoprotein of vesicular stomatitis virus (48), and an irrelevant peptide control derived from the RRV VP5 protein (676INNDEVFEAGTDGRY690) were purchased from Invitrogen (Carlsbad, CA) and used at the indicated concentrations.
Luciferase-refolding assay.
Luciferase (0.5 mg/ml; Sigma) was denatured by incubation in buffer B (6 M guanidinium chloride, 5 mM dithiothreitol [DTT], 50 mM KCH3COO, 25 mM HEPES, pH 7.2) for 60 min at room temperature. The denatured samples were then diluted 40-fold with buffer C (50 mM KCH3COO, 5 mM DTT, 1 mg/ml bovine serum albumin [BSA], 25 mM HEPES, pH 7.2). For the renaturation step, the samples were further diluted 25-fold with buffer D [120 mM KCH3COO, 1.2 mM Mg(CH3COO)2, 2 mM DTT, 1 mM ATP, 1 mg/ml BSA, 28 mM HEPES, pH 7.5], with or without the chaperones hsc70 (0.5 μM) and hsp40 (Sigma) (0.025 μM). The mixture was incubated for 90 min at 37°C, and then 1 μl of the reaction mixture was withdrawn and mixed with 50 μl of luciferase substrate (Roche), and the light produced was detected in a scintillation counter (Beckman). An equal amount of nondenatured luciferase in buffer C was measured, and its activity was taken as 100% (5, 41).
Infectivity assays.
Confluent monolayers of MA104 cells grown in 96-well plates were washed twice with MEM, and 50-μl aliquots of different concentrations of peptide diluted in MEM were added to the cells for 30 min at 37°C. After this incubation period, the peptide solution was removed, and RRV (2,000 focus-forming units/well) was then added and adsorbed to the cell surfaces for 1 h at 37°C. After the adsorption period, the virus inoculum was removed, the cells were washed twice with MEM, and the infection was left to proceed for 14 h at 37°C. The infected cells were fixed and immunostained as previously described (1). About 200 focus-forming units were counted in each well with the aid of a Visiolab 1000 station (21).
hsc70 binding assay.
Ninety-six-well enzyme-linked immunosorbent assay (ELISA) plates (Costar) were coated with a polyclonal goat anti-rotavirus hyperimmune serum (diluted 1:10,000) that was used as a capture antibody. To avoid nonspecific binding, the plates were then incubated with a solution of 1% BSA in PBS for 2 h at 37°C. Then, 200 ng of purified rotavirus triple-layered particles (TLPs) was added per well and incubated for 1 h at 37°C. The plates were washed twice and then incubated with the indicated concentration of recombinant proteins for 1 h at 37°C. The recombinant hsc70 protein bound to the viral particles was detected with a rabbit anti-hsc70 hyperimmune serum (diluted 1:2,000) (20), followed by a mouse anti-rabbit antibody conjugated to alkaline phosphatase (diluted 1:1,500) and the substrate Sigma 104 (1 mg/ml) diluted in diethanolamine buffer (100 mM diethanolamine [pH 9.4], 1 mM MgCl2, 5 mM sodium azide). The absorbance at 405 nm was recorded in an EL311 microplate autoreader (Bio-Tek Instruments). The recombinant proteins eluted from the HiTrap column were employed in this assay without further treatment. As controls, the absorbance values obtained for wells having all components of the assay but virus or having virus but lacking hsc70 were used.
Permeabilization assay.
Confluent monolayers of MA104 cells in 96-well plates were infected with trypsin-activated RRV at a multiplicity of infection (MOI) of 8 in the presence of 2 mg/ml of peptide C and 150 μg/ml of α-sarcin (Sigma) for 1 h at 37°C. After this time, the virus inoculum was removed; fresh serum-free MEM, supplemented with 25 μCi of EXPRE35S35S protein-labeling mix (>1,000 Ci/mmol) per ml, was added; and the cells were further incubated for 1 h at 37°C. After the labeling period, the cells were washed with PBS, treated with 5% trichloroacetic acid for 5 min at room temperature, and washed three times with ethanol. The cell monolayer was allowed to dry under a lamp before the addition of 50 μl of 0.1% sodium dodecyl sulfate (SDS) in 0.1 N NaOH. Total radioactivity in the samples was determined by liquid scintillation counting after solubilization of the samples in Ecolite (ICN) (7).
hsc70-TLP interaction in solution.
Nucleotide-free hsc70 (1.6 μg) was incubated with 1 mM of ATP, ADP, or ATPγS for 15 min at room temperature and then with 1.2 μg of virus in 50 μl of PBS or under refolding conditions with 0.025 μM of hsp40 (Sigma) for 90 min at 37°C. After incubation, the samples were pelleted through a sucrose cushion (40% sucrose in PBS containing 0.01% BSA) by centrifugation for 40 min at 4°C in an Airfuge (Beckman) at 25 psi (133,000 × g). The resultant pellet was dissolved in PBS, and the infectivity of the viral particles recovered in the pellet, as well as their protein composition, was determined by immunoblot and infectivity assays.
Effect of pH and temperature on virus infectivity.
TLPs incubated with hsc70 and centrifuged through a sucrose cushion as described above were incubated in a PBS solution adjusted to the indicated pH with either HCl or NaOH (pH 2, 2.5, 3, 3.5, 4 to 8, 8.5, 9, or 9.5) for 1 h at 37°C, and the virus samples were then diluted 10-folded in MEM without serum (13). To evaluate the effect of temperature on virus infectivity, the hsc70-treated TLPs were incubated for 20 min at 37, 40, 45, or 50°C in PBS. After the pH or temperature treatment, the viruses were adsorbed to confluent monolayers of MA104 cells on 96-well plates for 1 h at 37°C. After the adsorption period, the virus inoculum was removed, the cells were washed twice with MEM, the infection was left to proceed for 14 h at 37°C, and the infected cells were detected by immunocytochemistry as described above. As a control, we used TLPs that were treated as described above except that the incubation buffer lacked hsc70.
Immunoblots.
The proteins recovered in the pellets after centrifugation of the TLP-hsc70 mixture through a sucrose cushion (see above) were separated by SDS-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes (Millipore, Bedford, MA). Membranes were blocked with 5% nonfat dried milk in PBS and incubated at 4°C with a mixture of rabbit anti-rotavirus and anti-hsc70 sera diluted in PBS-0.01% milk, followed by incubation with a horseradish peroxidase-conjugated mouse anti-rabbit antibody. The peroxidase activity was developed by Western Lightning Chemiluminescence Reagent Plus (Perkin-Elmer Life Sciences) following the manufacturer's instructions.
Antibody ELISA.
Ninety-six-well ELISA plates (Costar) were coated with a goat anti-rotavirus serum and blocked with BSA as described above. Then, 200 ng of rotavirus TLPs, preincubated or not with hsc70 and centrifuged through a sucrose cushion, was added to each well and incubated for 1 h at 37°C. The plates were washed twice, and the bound virus was detected using the following panel of antibodies: rabbit polyclonal antibodies to rotavirus (anti-TLPs [1:4,000]) and the carboxy-terminal region of VP5 (474 [1:4,000]) and monoclonal antibodies (MAbs) to VP8 (MAb 7A12 [1:800]), VP5 (MAbs HS2 [1:100] and 2G4 [1:800]), and VP7 (MAbs 159 [1:400], 4F8 [1:800], and 60 [1:100]). The bound primary antibodies were detected by the addition of the corresponding alkaline phosphatase-conjugated antibody and substrate, and the absorbance at 405 nm was recorded in an EL311 microplate autoreader (Bio-Tek Instruments). The differences in reactivity obtained with the antibodies were evaluated using a two-tailed, paired t test.
RESULTS
hsc70 binds to rotavirus through its peptide-binding domain.
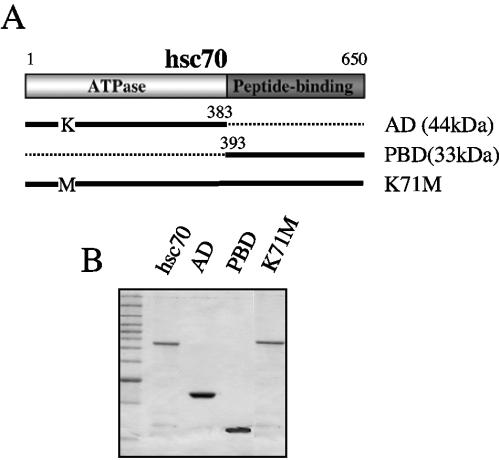
We have previously shown that rotavirus TLPs, but not double-layered particles, bind to purified hsc70 in ELISA (20). hsc70 has two functional domains, the amino-terminal A domain that comprises amino acid residues 1 to 383 and the carboxy-terminal PB domain represented by amino acids 384 to 650 (Fig. 1A). To determine which of these two domains of hsc70 is involved in binding the virus, the complete hsc70 protein and its A and PB domains were expressed in Escherichia coli as fusions to histidine tails, and the resultant proteins were purified by nitrilotriacetic acid-affinity chromatography and analyzed by gel electrophoresis (Fig. 1B). ELISA plates coated with RRV TLPs were then incubated with increasing concentrations of the recombinant proteins, and the protein bound to TLPs was detected using a rabbit polyclonal serum to hsc70. The complete hsc70 protein and its PB domain were found to bind TLPs in a concentration-dependent manner, while the A domain did not bind TLPs at any of the concentrations tested (Fig. 2A). As previously shown for hsc70 (20), preincubation of the recombinant PB domain of hsc70 with the virus reduced virus infectivity about 40%; in contrast, the A domain of hsc70 had no effect (results not shown).
FIG. 1.
Synthesis of peptide-binding and ATPase domains of hsc70 in bacteria. (A) Schematic representation of the two domains of hsc70. The amino-terminal ATPase domain (AD) is represented by a light gray box, while the carboxy-terminal peptide-binding domain (PBD) is shown as a dark gray box. The thick lines represent the two domains expressed independently in E. coli. The amino acid change in the hsc70 K71M mutant is also shown. (B) SDS-polyacrylamide gel electrophoresis of the purified recombinant hsc70, AD, PBD, and K71M proteins, stained with Coomassie blue.
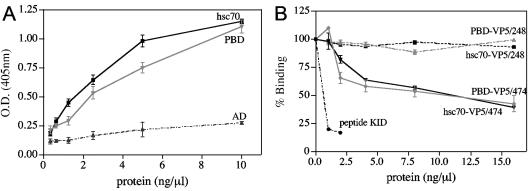
FIG. 2.
The binding domain of hsc70 interacts with rotavirus TLPs. (A) Ninety-six-well ELISA plates coated with purified RRV TLPs (200 ng/well) were incubated with the indicated concentrations of affinity-purified recombinant human hsc70 or its ATPase (AD) or peptide-binding (PBD) domain for 60 min at 37°C, and bound recombinant protein was detected by incubation with a rabbit hyperimmune serum to hsc70 as described in Materials and Methods. The concentration of recombinant protein present in each well was plotted against the optical density (O.D.) reading at 405 nm obtained in the ELISA plate. (B) The indicated concentrations of affinity-purified recombinant VP5-248 or VP5-474 protein were preincubated with 500 ng of hsc70 or PBD protein for 30 min at 37°C. After incubation, the mixtures were added to ELISA plates coated with purified RRV TLPs (200 ng/well), and bound hsc70 or PBD was detected as described above. Data are expressed as percentages of hsc70 or PBD protein bound to TLPs when the protein was incubated with PBS as a control. The arithmetic means and standard deviations of three independent experiments performed in duplicate are shown.
We have previously established that rotavirus particles interact with hsc70 through a region near the carboxy-terminal region of VP5 (45). To determine whether the interaction of TLPs with the PB domain of hsc70 was through the same region of VP5, the amino-terminal portion of VP5 (VP5-248; residues 274 to 474) or the carboxy-terminal part of this protein (VP5-474; amino acids 474 to 776) was expressed in bacteria and purified by affinity chromatography (45). These recombinant polypeptides were preincubated with either the complete hsc70 protein or its PB domain, and these mixtures were then added to ELISA plates coated with TLPs. We found that protein VP5-474, but not VP5-248, was able to compete the binding of both hsc70 and its PB domain to TLPs (Fig. 2B). Also, the VP5-474 and VP5-248 proteins were used to coat ELISA plates and were tested for the ability to bind complete hsc70 or its two independent domains. hsc70 and its PB domain bound to the carboxy-terminal part of VP5 in a concentration-dependent manner, but not to the amino-terminal region of VP5 (results not shown). Altogether, these results suggest that viral particles bind to the PB domain of hsc70.
Known ligands of hsc70 compete the interaction of TLPs with hsc70.
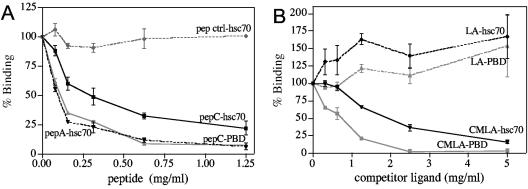
In vitro studies have shown that hsc70 binds to carboxymethylated lactalbumin (CMLA), the denatured and reduced form of lactalbumin (LA), while it does not recognize the native protein (33). hsc70 also binds short synthetic peptides with hydrophobic internal residues, particularly those enriched in Leu but also those enriched in Ile, Val, Phe, and Tyr, and flanking regions of basic residues (36, 37, 40), such as peptide C, derived from the surface glycoprotein of vesicular stomatitis virus (48), and peptide A, derived from a peptide of the p53 protein (15). To establish if hsc70 binds TLPs through its peptide-binding site, the ability of CMLA and peptide C to compete this interaction was tested. In these assays, hsc70 or the PB domain was preincubated with different amounts of peptide C or proteins LA and CMLA and then added to ELISA plates that had been previously coated with TLPs. Increasing concentrations of peptide C and CMLA decreased the amounts of recombinant proteins bound to TLPs, while LA did not affect this interaction (Fig. 3A and B). These results suggest that hsc70 interacts with the viral particles through its peptide-binding site, which is used by this chaperone to bind other ligands.
FIG. 3.
Ligands of hsc70 compete with the interaction of rotavirus with hsc70. The indicated concentrations of peptide C (A) or CMLA and LA (B) were preincubated with 500 ng of recombinant hsc70 or PBD protein for 30 min at 37°C. After incubation, the mixtures were added to ELISA plates coated with purified RRV TLPs (200 ng/well), and the recombinant protein bound to TLPs was detected by incubation with a rabbit hyperimmune serum to hsc70 as described in Materials and Methods. Data are expressed as percentages of hsc70 or PBD protein bound to TLPs when the protein was incubated with PBS as a control. The arithmetic means and standard deviations of three independent experiments performed in duplicate are shown.
Ligands of hsc70 block rotavirus infectivity.
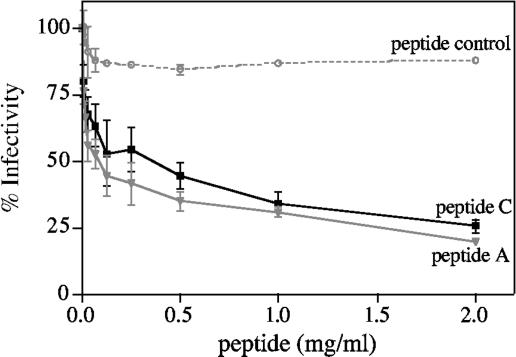
hsc70 has been detected on the surface of MA104 cells, and it has been shown that this protein is involved in rotavirus cell infection at a postattachment step (20). To evaluate if the interaction of the virus with hsc70 on the cell surface was similarly affected by known ligands of the chaperone, the effect of peptides A and C on the infectivity of the virus was tested. Preincubation of MA104 cells with these peptides prior to the addition of the virus resulted in an inhibition of infectivity of about 70%, while an irrelevant peptide did not block rotavirus infection (Fig. 4). To discard a nonspecific inhibitory effect of the peptides on the cell that would prevent rotavirus replication, the peptides were added after the virus had been allowed to enter the cells for 1 h at 37°C; under these conditions, none of the peptides had an effect on the infectivity of the virus (results not shown). Altogether, these results suggest that during cell infection, the virus particle interacts with the peptide-binding domain of hsc70, which is important for the entry process of the virus into the cell.
FIG. 4.
Effects of hsc70 ligands on infectivity of RRV. Confluent monolayers of MA104 cells grown in 96-well plates were incubated with the indicated concentrations of peptide A, peptide C, or a control peptide for 30 min at 37°C, and then trypsin-activated RRV (2,000 focus-forming units per well) was added and allowed to adsorb for 1 h at 37°C. The excess virus was removed, and the infection was allowed to proceed for 14 h at 37°C. Finally, the cells were fixed and immunostained, and the infectious foci were counted as described in Materials and Methods. Data are expressed as percentages of the virus infectivity obtained when the cells were preincubated with PBS as a control. The arithmetic means and standard deviations for three independent experiments performed in duplicate are shown.
The interaction between hsc70 and rotavirus does not prevent the coentry of α-sarcin.
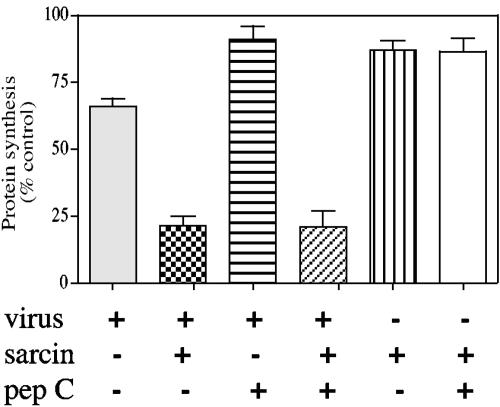
It has been shown for several viruses, including rotaviruses, that the entry of viral particles into the cell induces early permeabilization of the cell membrane (7, 14, 28). The early cell permeabilization induced by rotavirus allows the coentry of the toxin α-sarcin, a potent inhibitor of the cell translation machinery, whose activity in the cytosol results in a severe arrest of protein synthesis (10, 38). To determine if peptide C blocks the infectivity of rotavirus by interfering with its entry into the cell, an α-sarcin coentry assay was carried out. When α-sarcin was added to the cells either alone or in the presence of peptide C, but in the absence of virus, no effect on protein synthesis was observed, as measured by the incorporation of 35S-labeled amino acids into the protein (Fig. 5). However, when the toxin was added in the presence of virus, a drastic reduction in protein synthesis of 75 to 80%, compared to uninfected cells, was observed. The same level of inhibition of protein synthesis was observed when the virus was added in the presence of peptide C (Fig. 5), indicating that this peptide did not affect the coentry of α-sarcin, although under these conditions (an MOI of 8 and 2 mg/ml peptide C), peptide C blocked virus infectivity by 70% (Fig. 4). Similarly, incubation of the cell with antibodies to hsc70 did not block the coentry of α-sarcin, despite inhibiting virus infectivity by 50% (results not shown). These data suggest that peptide C interferes with the interaction of the virus with hsc70, blocking virus infection at a step that takes place after early permeabilization of the cell membrane.
FIG. 5.
The coentry of α-sarcin is not blocked by peptide C. MA104 cells in 96-well plates were infected with trypsin-activated RRV at an MOI of 8 in the presence or absence of 2 mg/ml of peptide C and in the presence or absence of 150 μg/ml of α-sarcin, as indicated. Cells were then labeled with an 35S protein-labeling mix for 1 h at 37°C, and the radioactivity in the trichloroacetic acid-precipitable material was determined as described in Material and Methods. Data are expressed as percentages of the amount of 35S-labeled amino acids incorporated into mock-infected cells in the absence of α-sarcin and peptide C. The arithmetic means and standard deviations for three independent experiments performed in duplicate are shown.
Transient interaction of hsc70 with the viral particle permanently modifies its infectivity.
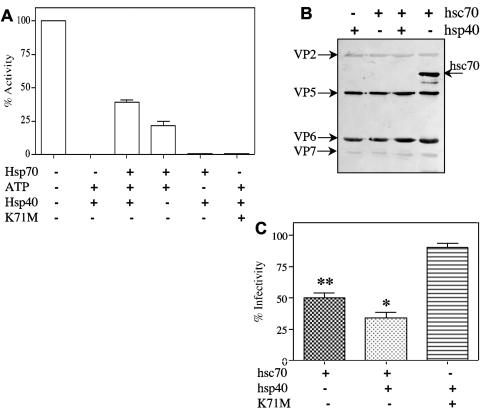
The chaperone activity of hsc70 depends on the hydrolysis of ATP and is enhanced by the presence of the cochaperone hsp40. To test if the purified recombinant hsc70 protein used in these assays was active, an in vitro, hsc70-dependent luciferase-refolding assay was performed. For this assay, native firefly luciferase was denatured with guanidinium hydrochloride and then incubated with hsc70 in the presence of hsp40 and ATP; the activity of the refolded luciferase was then measured and compared with those of the native and denatured proteins. Forty percent of the denatured luciferase activity was recovered after refolding of the protein in the presence of hsc70, hsp40, and ATP (Fig. 6A), a level of activity similar to that recovered in previously reported hsc70 refolding assays (5, 41).
FIG. 6.
The interaction of hsc70 with rotaviral particles decreases their infectivity. (A) In vitro hsc70-dependent luciferase-refolding assay, carried out as described in Materials and Methods. The presence in the reaction mixture of 0.5 μM hsc70, 0.5 μM K71M protein, and 0.025 μM hsp40 is indicated. The activity of refolded luciferase was determined and compared with those of the native and denatured proteins. Data are expressed as percentages of the activity detected with native luciferase. The arithmetic means and standard deviations of two independent experiments performed in duplicate are shown. (B) Purified and trypsin-activated RRV (1.2 μg) was incubated with 1.6 μg of either recombinant hsc70 or K71M mutant (not shown in the figure) protein under refolding conditions (see Materials and Methods) for 1 h at 37°C, in the absence or presence of 0.025 μM hsp40, and then pelleted through a 40% sucrose cushion. The viruses recovered from the pellets were analyzed by immunoblotting with a mixture of rabbit hyperimmune sera to purified rotavirus TLPs and hsc70. The last lane in the immunoblot shows the input amounts of RRV and hsc70 used in the assay, analyzed before the centrifugation step. (C) The infectivities of the viruses recovered from the pellets (obtained as described for panel B) were determined by an immunoperoxidase assay as described in Materials and Methods. The difference between the infectivities of untreated virus and virus treated with hsc70 alone was statistically significant (** [P < 0.0001]), as was the difference in infectivities of the virus treated with hsc70 in the presence or absence of hsp40 (* [P < 0.05]). Data are expressed as percentages of the virus infectivity obtained when the virus was incubated without hsc70 as a control. The arithmetic means and standard deviations for three independent experiments performed in duplicate are shown.
We have previously shown that incubation of hsc70 with virus particles decreases the infectivity of the virus about 50% (20). To determine if this hsc70-induced reduction in virus infectivity requires the chaperone activity of the protein, an assay was set up in solution. In this assay, purified TLPs were incubated with hsc70 in either the absence or presence of hsp40 for 90 min at 37°C, and the viral particles were then pelleted through a 40% sucrose cushion to remove the soluble proteins. The virus particles recovered in the pellet were analyzed by Western blotting using antibodies to the viral structural proteins and hsc70, and the infectivity of the virus particles present in the pellet was also determined. The immunoblots showed that the stoichiometry of the structural proteins present in the viral particles incubated with hsc70 in the presence or absence of hsp40 was similar to that of control TLPs incubated in the absence of the chaperone (Fig. 6B), i.e., there were no detectable losses of any particular viral protein, including the surface proteins VP5 and VP7. VP8 was not detected in the immunoblots, but the fact that MAb 7A12, directed to VP8, recognized TLPs treated or not treated with hsc70 suggests that this VP4 subunit was not lost from the virion (see below). On the other hand, hsc70 was not detected in the pellet under any of the experimental conditions tested, indicating that the interaction of this protein with TLPs was either transient or weak, with the protein remaining in the soluble fraction at the top of the sucrose cushion upon centrifugation of the samples. When the infectivity of the viral particles recovered in the pellet was determined, a reduction of about 50% in the infectivity of the virus preincubated with hsc70 was observed compared to that of TLPs preincubated in the absence of the chaperone (Fig. 6C). The infectivity of the virus was found to be further reduced (about 70%) when the virus was preincubated in the presence of both hsc70 and its cochaperone, hsp40 (Fig. 6C). The fact that the virus particles preincubated with hsc70 and centrifuged through a sucrose cushion showed a decreased infectivity even in the absence of detectable hsc70 suggests that under the conditions employed, the interaction of hsc70 with virus particles might cause a conformational change in the virus that results in reduced infectivity.
The reduction of rotavirus infectivity induced by hsc70 is dependent on the hydrolysis of ATP.
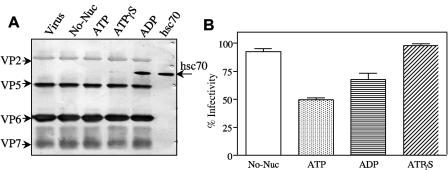
The chaperone activity of hsc70 requires the hydrolysis of ATP. To evaluate whether this activity is required for the reduction of virus infectivity, purified hsc70 was depleted of the associated nucleotide by incubation with EDTA, and the nucleotide-free protein was incubated with TLPs in the presence of either ATP, ADP, or ATPγS, a nonhydrolyzable analogue of ATP. After the incubation period, the viruses were centrifuged through a sucrose cushion, and the infectivities of the TLPs in the pellets were determined. In the presence of ATP, hsc70 induced a 50% reduction in the infectivity of the virus, while a reduction of about 30% was observed in the presence of ADP (Fig. 7B). The infectivity of TLPs incubated with hsc70-ATPγS was not affected, being equivalent to that observed for control TLPs incubated in the absence of the chaperone (Fig. 7B). Interestingly, when the virus particles recovered in the pellet were analyzed by immunoblotting, hsc70 was found in this fraction when the TLPs were incubated with hsc70-ADP, suggesting that under these conditions the chaperone remained associated with the virus (Fig. 7A). This was not the case when TLPs were incubated with hsc70 in the presence of either ATP or ATPγS. These results suggest that the transient interaction of hsc70 with the virus that leads to inactivation of its infectivity requires the hydrolysis of ATP, while hsc70-ADP is able to block the infectivity of the virus through a more stable interaction with TLPs, probably causing steric interference during virus infection.
FIG. 7.
Effect of different nucleotides on interaction of hsc70 with viral particles. Purified and trypsin-activated RRV (1.2 μg) was incubated with 1.6 μg of hsc70 for 1 h at 37°C in the presence of 1 mM of either ATP, ADP, or ATPγS and then pelleted through a 40% sucrose cushion. No-Nuc, no nucleotide was present in the reaction mixture. (A) Immunoblot of viruses recovered from pellets with a mixture of rabbit hyperimmune sera to purified rotavirus TLPs and hsc70. The first and last lanes of the immunoblot show the input amounts of virus and hsc70, respectively, used in these assays. (B) The infectivities of the viruses recovered from the pellets were determined by an immunoperoxidase assay as described in Materials and Methods. Data are expressed as percentages of the virus infectivity obtained when the virus was incubated without hsc70 as a control. The arithmetic means and standard deviations of four independent experiments performed in duplicate are shown.
To further confirm the relevance of the ATPase activity of hsc70 to the inactivation of the virus, we constructed and expressed in bacteria a mutant hsc70 protein (mutant K71M) with a single amino acid change in the A domain (Fig. 1A), which was previously shown to abolish the ATPase activity of the chaperone (32). As expected, the recombinant K71M protein was not able to restore the activity of denatured luciferase in the luciferase-refolding assay (Fig. 6A). However, it bound TLPs in a concentration-dependent manner in ELISA, and this binding was competed by incubation with CMLA, indicating that the mutant recombinant protein was still able to interact with TLPs through its peptide-binding domain (results not shown). When TLPs were incubated with the K71M protein in the presence of ATP and hsp40 and then centrifuged through a sucrose cushion as described before, the infectivity of the virus recovered from the pellet was not decreased compared to that of control TLPs (Fig. 6C). These results confirm that the ATPase activity of hsc70 is required to inactivate the infectivity of the virus.
hsc70 induces a conformational change in rotavirus particles.
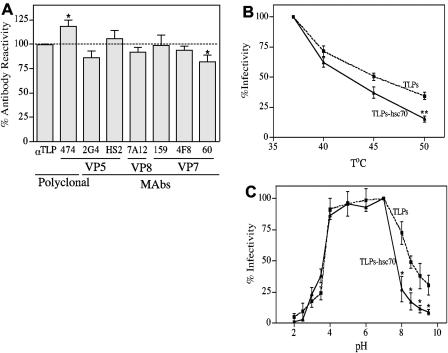
To determine if the interaction of hsc70 with TLPs induces a conformational change in the virus, the reactivities of hsc70-treated TLPs with antibodies directed to the virus surface proteins were characterized by ELISA. In these assays, the plates were coated with a goat anti-rotavirus antibody, and then TLPs that had been preincubated or not with hsc70 and pelleted through a sucrose cushion were added. The reactivities of three MAbs that recognize different epitopes of VP7 (159, 4F8, and 60), two MAbs against different epitopes in VP5 (HS2 and 2G4), one MAb directed to VP8 (7A12), and a polyclonal antibody (474) that recognizes the carboxy-terminal region of VP5 (aa 474 to 776) were determined. A polyclonal anti-rotavirus antibody was used to normalize the amount of input virus used in the assay. We found that antibodies 474 to VP5 and 60 to VP7 showed higher and lower reactivities, respectively, with TLPs incubated with hsc70 than viral particles that were not incubated with the chaperone (Fig. 8A). Although the difference in reactivities obtained with these antibodies was not very pronounced, it was very reproducible and statistically significant (P < 0.05).
FIG. 8.
Antibody reactivities and effects of pH and temperature on the infectivity of virus treated with hsc70. (A) Virus recovered from the pellet after centrifugation through a sucrose cushion was added to a microtiter plate coated with goat hyperimmune serum to rotavirus and incubated for 1 h at 37°C. The indicated antibodies were then added, followed by the appropriate alkaline phosphatase-conjugated secondary antibodies. The phosphatase activity was detected as described in Materials and Methods. A polyclonal anti-rotavirus antibody (αTLP) was used to normalize the amount of input virus used in the assay. Data are expressed as percentages of the reactivity of each antibody obtained when the virus was incubated without hsc70 as a control. The arithmetic means and standard deviations of six independent experiments performed in duplicate are shown. Statistically significant differences (by a paired t test) in the reactivities of antibodies with hsc70-treated and untreated viruses are indicated (* [P < 0.05]). (B) TLPs that were treated or not treated with hsc70 were incubated for 20 min at the indicated temperatures and then allowed to adsorb to cells for 1 h at 37°C. The infection was allowed to proceed for 14 h at 37°C, and the infectious foci were detected as described in Materials and Methods. Data are expressed as percentages of the virus infectivity obtained when the virus was preincubated at 37°C as a control. The arithmetic means and standard deviations for three independent experiments performed in duplicate are shown (**, P < 0.01). (C) TLPs that were treated or not treated with hsc70 were incubated for 1 h at 37°C at the indicated pHs, as described in Materials and Methods. After a 10-fold dilution in MEM without serum, the viruses were allowed to adsorb to cells for 1 h at 37°C. The infection was allowed to proceed for 14 h at 37°C, and the infectious foci were detected as described in Materials and Methods. Data are expressed as percentages of the virus infectivity obtained when the virus was preincubated at pH 7 as a control. The arithmetic means and standard deviations for four independent experiments performed in duplicate are shown (*, P < 0.05).
To evaluate by different criteria if the hsc70-treated virus could be distinguished structurally from untreated TLPs, we tested the effects of pH and temperature on their infectivities. As shown in Fig. 8B, the infectivity of hsc70-treated virus was more sensitive to heat than that of the untreated control virus, being significantly different after incubation for 20 min at 50°C (P < 0.01). Likewise, after incubation of TLPs with hsc70, the virus particles became more sensitive to basic pHs than were control TLPs, showing a decreased stability of infectivity at pHs in the range of 8 to 9.5 (P < 0.05) (Fig. 8C). Altogether, these results suggest that the interaction of hsc70 with TLPs induces a slight conformational change in the outer-layer proteins of the virus that is sufficient, however, to reduce the virus infectivity.
DISCUSSION
hsc70, a constitutive member of the heat shock-induced hsp70 protein family, has been reported to be present on the surface of several types of cells (20, 31), including MA104 cells, where it has been proposed to serve as a postattachment receptor for rotavirus infection (20). The virus interacts with hsc70 through a domain in VP5 located between amino acid residues 642 and 658, near the carboxyl terminus of VP4 (29, 45). In this work, we have shown that hsc70 interacts specifically with rotavirus through its peptide-binding domain, since (i) a recombinant full-length hsc70 protein and its peptide-binding domain, but not its ATPase domain, bound TLPs in a solid-phase assay; (ii) this binding was competed by preincubation of the protein with the carboxy-terminal region of VP5 (which contains the VP5 domain known to interact with hsc70), but not with its amino-terminal portion; and (iii) known ligands of hsc70 competed the binding of this protein to TLPs in the solid-phase assay. The peptide ligands of hsc70 were also shown to block rotavirus infectivity when added to cells before virus infection, suggesting that hsc70 on the surface of MA104 cells also interacts with the virus through its peptide-binding domain.
The interaction of hsc70 with the virus during cell entry has been shown to occur at a postattachment level during virus infection, since either preincubation of cells with antibodies to hsc70 or preincubation of the virus with the chaperone blocks virus infection but not the binding of the virus to the cell surface (20). Furthermore, the early permeabilization of the cell membrane induced during the entry of rotavirus into MA104 cells has been shown to allow the coentry of α-sarcin into the cells, thus inhibiting the synthesis of proteins (7, 28). Interestingly, the coentry of the toxin was not prevented by preincubation of the cells with peptide C or with antibodies to hsc70 (results not shown). These results indicate that either the interaction of hsc70 with the virus occurs at a step during virus entry subsequent to permeabilization of the plasma cell membrane or the remaining 30% infectivity of the virus that still occurs in the presence of peptide C is responsible for the toxic effect detected. The first explanation seems more likely, since the effect of α-sarcin has been shown to correlate with the multiplicity of infection used (7; results not shown), and the 70% reduction in infectivity caused by peptide C should also have resulted in a less pronounced inhibition of protein synthesis by α-sarcin compared to the inhibition observed in the absence of peptide C.
Preincubation of the virus with hsc70 reduces its infectivity. This effect was shown to depend on the ATPase activity of the chaperone, since in the presence of a nonhydrolyzable analogue of ATP, hsc70 failed to inhibit viral infectivity. The observed reduction in infectivity was slightly more pronounced in the presence of the cochaperone hsp40, which is known to enhance the chaperone function of hsc70, although it is not essential for its activity (8, 27, 42). Interestingly, hsc70 also inhibited the infectivity of the virus in the presence of ADP, although to a lesser extent. These apparently conflicting data could be the results of differential interactions of hsc70 with the virus in the presence of either ATP or ADP, since it is known that the binding of hsc70 to its substrate is regulated by the type of nucleotide bound to the chaperone (39). Two substantially different conformations for peptide-free hsc70 exist in the presence of either ATP or ADP (43). When ATP is present, there is a significant interaction between the carboxyl-terminal PB domain and the amino-terminal A domain of hsc70, such that the effect of peptide binding is transmitted to the ATPase domain, resulting in an increased rate of ATP hydrolysis and ADP and Pi release, and reciprocally, the ATPase domain constrains the peptide binding domain to a low-peptide-affinity conformation (39). The hydrolysis of ATP stabilizes the hsc70-protein complex until ADP/ATP exchange allows protein release, closing the catalytic cycle (4). In the presence of ADP, the PB domain is not as constrained by the A domain and could capture the peptide to form a high-affinity complex (23, 39); ADP can be removed from the hsc70-ADP substrate only by ADP/ATP exchange.
Our results also indicate differential interactions of hsc70 with rotavirus TLPs in the presence of either ATP or ADP, as judged by the stability of the hsc70-virus complex under different conditions. In the presence of ADP, the chaperone protein was recovered from the pellet after centrifugation of the virus-hsc70 mixture through a sucrose cushion, suggesting that hsc70 was associated with the virus particles during the centrifugation step. On the other hand, the chaperone was not found in association with the virus upon centrifugation of a virus-hsc70 mixture incubated in the presence of ATP, suggesting that the interaction of hsc70-ATP with TLPs is of a lower affinity than that of hsc70-ADP. The reduction in virus infectivity induced by incubation of hsc70 in the presence of either nucleotide might result from two different mechanisms of inhibition. In the presence of ADP, the reduction in viral infectivity might be due to hsc70 blocking, by steric hindrance, the interaction of the virus with its receptors on the cell membrane. On the other hand, in the presence of ATP, hsc70 might induce a conformational change in the viral particle that irreversibly inactivates the virus. The differential reactivities of antibodies with TLPs incubated or not with hsc70, as well as the differential susceptibilities of the hsc70-treated and untreated viruses to heat and pH, support this hypothesis. The relevance of a functional ATPase activity of hsc70 to the reduction in virus infectivity was further shown by the fact that a mutant hsc70 protein lacking ATPase activity failed to decrease virus infectivity. In light of these results, it is tempting to hypothesize that during cell infection, the interaction of the virus with hsc70 on the surface of MA104 cells results in a conformational change of the virus particle that facilitates, or is required for, virus entry. However, when this interaction takes place with soluble hsc70 in the absence of cells, it triggers a change that inactivates the virion. A similar observation has been reported for other viruses, such as human immunodeficiency virus (24, 44), avian leucosis and sarcoma viruses (9, 18), and poliovirus (2, 35). In the case of poliovirus, upon interaction of the virion with the poliovirus receptor in solution, the virus suffers a conformational rearrangement in which VP4 is released from the virion, changing its density and rendering the virus particle noninfectious (2, 35). Altogether, these results suggest that hsc70 might have an active role in rotavirus cell infection, not only serving as an anchor for viruses on the cell membrane during their transit to the cell's cytoplasm but also helping the virus to enter the cell by possibly modifying the conformation of its surface proteins.
Acknowledgments
This work was partially supported by grants 55003662 and 55000613 from the Howard Hughes Medical Institute and by grant G37621N from CONACYT Mexico. J.P.V. was supported by a scholarship (no. 128410) granted by CONACYT.
REFERENCES
- 1.Arias, C. F., M. Lizano, and S. Lopez. 1987. Synthesis in Escherichia coli and immunological characterization of a polypeptide containing the cleavage sites associated with trypsin enhancement of rotavirus SA11 infectivity. J. Gen. Virol. 68:633-642. [DOI] [PubMed] [Google Scholar]
- 2.Belnap, D. M., D. J. Filman, B. L. Trus, N. Cheng, F. P. Booy, J. F. Conway, S. Curry, C. N. Hiremath, S. K. Tsang, A. C. Steven, and J. M. Hogle. 2000. Molecular tectonic model of virus structural transitions: the putative cell entry states of poliovirus. J. Virol. 74:1342-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukau, B., and A. L. Horwich. 1998. The hsp70 and hsp60 chaperone machine. Cell 92:351-366. [DOI] [PubMed] [Google Scholar]
- 4.Buxbaum, E., and P. Woodman. 1996. Binding of ATP and ATP analogues to the uncoating ATPase hsc70 (70 kDa heat shock cognate protein). J. Biochem. 318:923-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, T. C., C. D. Hsiao, S. J. Wu, and C. Wang. 2001. The effect of mutating arginine-469 on the substrate binding and refolding activities of 70-kDa heat shock cognate protein. Arch. Biochem. Biophys. 386:30-36. [DOI] [PubMed] [Google Scholar]
- 6.Ciarlet, M., S. E. Crawford, E. Cheng, S. E. Blutt, D. A. Rice, J. M. Bergelson, and M. K. Estes. 2002. VLA-2 (α2β1) integrin promotes rotavirus entry into cells but is not necessary for rotavirus attachment. J. Virol. 76:1109-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuadras, M., C. Arias, and S. López. 1997. Rotaviruses induce an early membrane permeabilization of MA104 cells and do not require a low intracellular Ca2+ concentration to initiate their replication cycle. J. Virol. 71:9065-9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cyr, D. M., T. Langer, and M. G. Douglas. 1994. DnaJ-like proteins: molecular chaperones and specific regulators of hsp70. Trends Biochem. Sci. 19:176-181. [DOI] [PubMed] [Google Scholar]
- 9.Damico, R., and P. Bates. 2000. Soluble receptor-induced retroviral infection of receptor-deficient cells. J. Virol. 74:6469-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endo, Y., and I. G. Wool. 1982. The site of action of alpha-sarcin on eukaryotic ribosome. J. Biol. Chem. 257:9054-9060. [PubMed] [Google Scholar]
- 11.Estes, M. K. 2001. Rotaviruses and their replication, p. 1747-1785. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 12.Estes, M. K., and J. Cohen. 1989. Rotavirus gene structure and function. Microbiol. Rev. 53:410-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estes, M. K., D. Y. Graham, E. M. Smith, and C. P. Gerba. 1979. Rotavirus stability and inactivation. J. Gen. Virol. 43:403-409. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Puentes, C., and L. Carrasco. 1980. Viral infection permeabilizes mammalian cells to protein toxins. Cell 20:769-775. [DOI] [PubMed] [Google Scholar]
- 15.Fourie, A., J. Sambrook, and M.-J. Gething. 1994. Common and divergent peptide binding specificities of hsp70 molecular chaperones. J. Biol. Chem. 269:30470-30478. [PubMed] [Google Scholar]
- 16.Frydman, J. 2001. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 70:603-647. [DOI] [PubMed] [Google Scholar]
- 17.Fukuhara, N., O. Yoshie, S. Kitaoka, and T. Konno. 1988. Role of VP3 in human rotavirus internalization after target cell attachment via VP7. J. Virol. 62:2209-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert, J. M., L. D. Hernandez, J. W. Balliet, P. Bates, and J. M. White. 1995. Receptor-induced conformational changes in the subgroup A avian leukosis and sarcoma virus envelope glycoprotein. J. Virol. 69:7410-7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham, K., P. Halasz, Y. Tan, M. Hewish, Y. Takada, E. Mackow, M. Robinson, and B. Coulson. 2003. Integrin-using rotaviruses bind α2β1 integrin α2 I domain via VP4 DGE sequence and recognize αXβ2 and αVβ3 by using VP7 during cell entry. J. Virol. 77:9969-9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerrero, C. A., D. Bouyssounade, S. Zarate, P. Isa, T. Lopez, R. Espinosa, P. Romero, E. Mendez, S. Lopez, and C. F. Arias. 2002. Heat shock cognate protein 70 is involved in rotavirus cell entry. J. Virol. 76:4096-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerrero, C. A., S. Zarate, G. Corkidi, S. Lopez, and C. F. Arias. 2000. Biochemical characterization of rotavirus receptors in MA104 cells. J. Virol. 74:9362-9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerrero, C. A., S. Zarate, P. Isa, E. Mendez, S. Lopez, and C. Arias. 2000. Integrin alpha(v)beta(3) mediates rotavirus cell entry. Proc. Natl. Acad. Sci. USA 97:14644-14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ha, J. H., and D. McKay. 1995. Kinetics of nucleotide-induced changes in the tryptophan fluorescence of the molecular chaperone hsc70 and its subfragments suggest the ATP-induce conformational change follow initial ATP binding. Biochemistry 34:11635-11644. [DOI] [PubMed] [Google Scholar]
- 24.Jones, P. L., T. Korte, and R. Blumenthal. 1998. Conformational changes in cell surface HIV-1 envelope glycoproteins are triggered by cooperation between cell surface CD4 and co-receptors. J. Biol. Chem. 273:404-409. [DOI] [PubMed] [Google Scholar]
- 25.Kaljot, K. T., R. D. Shaw, D. H. Rubin, and H. B. Greenberg. 1988. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J. Virol. 62:1136-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapikian, A. Z., Y. Hoshino, and R. M. Chanock. 2001. Rotavirus, p. 1787-1833. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 27.Kelley, W. L. 1998. The J-domain family and the recruitment of chaperone power. Trends Biochem. Sci. 23:222-227. [DOI] [PubMed] [Google Scholar]
- 28.Liprandi, F., Z. Moros, M. Gerder, J. E. Ludert, F. H. Pujol, M. C. Ruiz, F. Michelangeli, A. Charpilienne, and J. Cohen. 1997. Productive penetration of rotavirus in cultured cells induces coentry of the translation inhibitor alpha-sarcin. Virology 237:430-438. [DOI] [PubMed] [Google Scholar]
- 29.Lopez, S., and C. F. Arias. 2004. Rotavirus entry into cells: a Versaillesque dance. Trends Microbiol. 12:251-300. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Buesa, P., C. Pfund, and E. Craig. 1998. The biochemical properties of the ATPase activity of a 70-kDa heat shock protein (hsp70) are governed by the C-terminal domains. Proc. Natl. Acad. Sci. USA 95:15253-15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Multhoff, G., and L. E. Hightower. 1996. Cell surface expression of heat shock proteins and the immune response. Cell Stress Chaperones 1:167-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Brien, M., K. Flaherty, and D. McKay. 1996. Lysine 71 of the chaperone protein hsc70 is essential for ATP hydrolysis. J. Biol. Chem. 271:15874-15878. [DOI] [PubMed] [Google Scholar]
- 33.Palleros, D. R., W. J. Welch, and A. L. Fink. 1991. Interaction of hsp70 with unfolded proteins: effects of temperature and nucleotides on the kinetics of binding. Proc. Natl. Acad. Sci. USA 88:5719-5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pando, V., P. Isa, C. F. Arias, and S. Lopez. 2002. Influence of calcium on the early steps of rotavirus infection. Virology 295:190-200. [DOI] [PubMed] [Google Scholar]
- 35.Racaniello, V. R. 1996. The poliovirus receptor: a hook, or an unzipper? Structure 4:769-773. [DOI] [PubMed] [Google Scholar]
- 36.Rudiger, S., A. Buchberger, and B. Bukau. 1997. Interaction of hsp70 chaperones with substrates. Nat. Struct. Biol. 4:342-349. [DOI] [PubMed] [Google Scholar]
- 37.Rudiger, S., L. Germeroth, J. Schneider-Mergener, and B. Bukau. 1997. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 16:1501-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sperti, S., M. Zamboni, M. Brigotti, F. Rambelli, and L. Montanaro. 1989. Alpha-sarcin impairs the N-glycosidase activity of ricin on ribosomes. Biochem. Biophys. Res. Commun. 160:857-861. [DOI] [PubMed] [Google Scholar]
- 39.Takeda, S., and D. McKay. 1996. Kinetics of peptide binding to the bovine 70 kDa heat shock cognate protein, a molecular chaperone. Biochemistry 35:4636-4644. [DOI] [PubMed] [Google Scholar]
- 40.Takenaka, I., S. Leung, S. J. McAndrew, J. P. Brown, and L. E. Hightower. 1995. Hsc70-binding peptides selected from a phage display peptide library that resemble organellar targeting sequences. J. Biol. Chem. 270:19839-19844. [DOI] [PubMed] [Google Scholar]
- 41.Terada, K., M. Kanazawa, B. Bukau, and M. Mori. 1997. The human DnaJ homologue dj2 facilitates mitochondrial protein import and luciferase refolding. J. Cell Biol. 139:1089-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh, P., D. Bursac, Y. C. Law, D. Cyr, and T. Lithgow. 2004. The J-protein family: modulating protein assembly, disassembly and translocation. EMBO Rep. 5:567-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilbanks, S. W., and D. Mckay. 1993. How potassium affects the activity of the molecular chaperone hsc70. J. Biol. Chem. 270:2251-2257. [DOI] [PubMed] [Google Scholar]
- 44.Wyatt, R., J. Moore, M. Accola, E. Desjardin, J. Robinson, and J. Sodroski. 1995. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J. Virol. 69:5723-5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zarate, S., M. Cuadras, R. Espinosa, P. Romero, K. Juárez, M. Camacho-Nuñez, C. Arias, and S. López. 2003. Interaction of rotavirus with hsc70 during cell entry is mediated by VP5. J. Virol. 77:7254-7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zarate, S., R. Espinosa, P. Romero, C. A. Guerrero, C. F. Arias, and S. Lopez. 2000. Integrin alpha2beta1 mediates the cell attachment of the rotavirus neuraminidase-resistant variant nar3. Virology 278:500-504. [DOI] [PubMed] [Google Scholar]
- 47.Zarate, S., R. Espinosa, P. Romero, E. Mendez, C. F. Arias, and S. Lopez. 2000. The VP5 domain of VP4 can mediate attachment of rotaviruses to cells. J. Virol. 74:593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, J., and G. Walker. 1996. Identification of elements of the peptide binding site of DnaK by peptide cross-linking. J. Biol. Chem. 271:19668-19674. [PubMed] [Google Scholar]