Abstract
Epithelial cells of the lung are the primary targets for respiratory viruses. Virus-carried single-stranded RNA (ssRNA) can activate Toll-like receptors (TLRs) 7 and 8, whereas dsRNA is bound by TLR3 and a cytoplasmic RNA helicase, retinoic acid-inducible protein I (RIG-I). This recognition leads to the activation of host cell cytokine gene expression. Here we have studied the regulation of influenza A and Sendai virus-induced alpha interferon (IFN-α), IFN-β, interleukin-28 (IL-28), and IL-29 gene expression in human lung A549 epithelial cells. Sendai virus infection readily activated the expression of the IFN-α, IFN-β, IL-28, and IL-29 genes, whereas influenza A virus-induced activation of these genes was mainly dependent on pretreatment of A549 cells with IFN-α or tumor necrosis factor alpha (TNF-α). IFN-α and TNF-α induced the expression of the RIG-I, TLR3, MyD88, TRIF, and IRF7 genes, whereas no detectable TLR7 and TLR8 was seen in A549 cells. TNF-α also strongly enhanced IKKɛ mRNA and protein expression. Ectopic expression of a constitutively active form of RIG-I (ΔRIG-I) or IKKɛ, but not that of TLR3, enhanced the expression of the IFN-β, IL-28, and IL-29 genes. Furthermore, a dominant-negative form of RIG-I inhibited influenza A virus-induced IFN-β promoter activity in TNF-α-pretreated cells. In conclusion, IFN-α and TNF-α enhanced the expression of the components of TLR and RIG-I signaling pathways, but RIG-I was identified as the central regulator of influenza A virus-induced expression of antiviral cytokines in human lung epithelial cells.
Influenza A viruses are negative-strand RNA viruses that are capable of infecting a variety of avian and mammalian species. In humans, influenza A viruses cause widespread epidemics. The primary targets of influenza virus, parainfluenza virus, and other respiratory viral pathogens are the epithelial cells of the upper respiratory tract. Influenza viruses can also infect dendritic cells (DCs) and macrophages, which elicit a strong cytokine production response to the infection. At early phases of infection, influenza A virus-infected macrophages produce alpha/beta interferon (IFN-α/β) and tumor necrosis factor alpha (TNF-α), which are the key cytokines regulating innate immune responses. IFN-α/β and TNF-α directly inhibit viral replication and activate NK cells, DCs, and macrophages. However, influenza A virus nonstructural protein 1 (NS1) (3) has been shown to interfere with host cell IFN production (12, 20). Recently, two novel IFN-α/β-related cytokines, interleukin-28A/B (IL-28A/B; also called IFN-λ2/3) and IL-29 (IFN-λ1), were described (18, 41). Like that of IFN-α/β, IL-28 and IL-29 gene expression is activated during viral infections, and the corresponding proteins have antiviral activity. The IL-28 and IL-29 receptor is a heterodimeric class II cytokine receptor consisting of IL-28Rα and IL-10Rβ. IL-28 and IL-29 activate the Jak-STAT signaling pathway (18, 41). Thus, IL-28 and IL-29 may contribute to the activation of innate immunity by a mechanism similar to but independent from that of IFN-α/β.
In viral infections, innate immune responses are initiated when viruses or their genetic material is recognized by cellular pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) (16, 45). Recently, it was shown that single-stranded RNA (ssRNA) from influenza A virus is recognized by TLR7 and TLR8 (6, 14, 27), leading to the production of IFN-α/β. Viral dsRNA is formed during the replication cycle of many RNA viruses and is recognized by TLR3. TLR3 activation leads to the production of IFN-α/β and other cytokines (2). Although TLRs play important roles in the establishment of the antiviral response, accumulating evidence suggests that other PRRs are involved in the activation of the IFN response in viral infections. Recently, a cytoplasmic RNA helicase, retinoic acid-inducible protein I (RIG-I), was reported to bind viral dsRNA and activate IFN-β gene expression (48).
Signal transduction via TLRs requires a conserved Toll/IL-1 receptor domain, which recruits adapter molecules to the receptor complex (1). Most TLRs utilize a common adapter molecule, MyD88. TLR3, however, signals independently of MyD88 via an adapter called Toll/IL-1 receptor domain-containing adapter inducing IFN-β (TRIF) (1). TRIF is known to associate with IκB kinase ɛ (IKKɛ) and TANK-binding kinase 1 (TBK1), which are the virus-activated kinases that regulate the phosphorylation and activation of IRF3 and subsequent IFN-α/β production (10, 15, 32, 40). In contrast to TLRs, little is known about the signaling molecules involved in RIG-I-induced gene activation.
For the present study, we have analyzed the regulation of IFN-α, IFN-β, IL-28, and IL-29 gene expression in human lung epithelial cells in response to influenza A and Sendai virus infections. Sendai virus infection readily activated the expression of the IFN-α, IFN-β, IL-28, and IL-29 genes, whereas influenza A virus-induced activation of these genes was dependent on pretreatment of A549 cells with IFN-α or TNF-α. IFN-α and TNF-α strongly activated RIG-I and TLR3 gene expression, whereas the TLR7 and TLR8 genes were not expressed in A549 cells. A dominant-negative form of RIG-I inhibited influenza A virus-induced IFN-β promoter activity in TNF-α-pretreated cells. Our results suggest a role for RIG-I in influenza A virus-induced expression of antiviral cytokines in human lung epithelial cells.
MATERIALS AND METHODS
Cell culture and viruses.
A549 human lung carcinoma cells (ATCC CCL185) were maintained in Eagle's minimal essential medium supplemented with 0.6 μg/ml penicillin, 60 μg/ml streptomycin, 2 mM L-glutamine, and 10% heat-inactivated fetal calf serum (Integro, Zaandaam, The Netherlands). Influenza A/Beijing/353/89 (H3N2) virus, wild-type (wt) influenza A/Udorn/72 (H3N2) virus, influenza A/Udorn/72 CPSF mutant virus, which has a mutation in the NS1A gene that prevents the interaction of the NS1A protein with the 30-kDa subunit of the cleavage and polyadenylation specificity factor (CPSF) (31), and Sendai (strain Cantell) virus were grown in 11-day-old embryonated eggs as previously described (34). The infectivity titers of the stock viruses in A549 cells were 2 × 107 PFU/ml for the wt Beijing and Udorn viruses, 1 × 107 PFU/ml for the Udorn CPSF mutant virus, and 4 × 107 PFU/ml for Sendai virus. In all experiments, 2 PFU/cell of influenza A or Sendai virus was used. With these virus doses, all cells were infected (data not shown).
Cytokines.
Human leukocyte IFN-α was provided by the Finnish Red Cross Blood Transfusion Service (Helsinki, Finland) and was used at 100 IU/ml. TNF-α was purchased from R&D Biosystems (Abingdon, United Kingdom) and used at 10 ng/ml.
Transfections.
The IKKɛ expression vector has been described previously (40). RIG-IC and ΔRIG-I plasmids were prepared as described previously (48). Expression constructs were transfected into A549 cells, using FuGENE 6 transfection reagent (Roche Molecular Biochemicals) according to the manufacturer's instructions. For luciferase assays, the cells were transfected with the indicated expression plasmids, a Renilla luciferase expression plasmid, and a firefly luciferase reporter under the control of the IFN-β promoter. The firefly and Renilla luciferase activities were measured using a dual-luciferase reporter assay system (Promega) and a Victor multilabel reader (Wallac).
Real-time PCR.
Real-time PCR quantification of different type I IFNs and IL-29 was done as previously described (5).
RNA isolation and Northern blot analysis.
Total cellular RNA was isolated with an RNeasy kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. Samples containing equal amounts of RNA (10 μg) were size fractionated in 1% formaldehyde-agarose gels, transferred to nylon membranes (Hybond; Amersham, Buckinghamshire, United Kingdom), and hybridized with specific probes. IFN-α (36), IFN-β (35), MyD88 (37), TLR3, TLR7, TLR8 (29), TRIF, IKKɛ, TBK1, IRF7, IL-28, and IL-29 (42) probes have been described previously. The probe for RIG-I was cloned from total cellular RNA obtained from Sendai virus-infected macrophages by reverse transcription-PCR (RT-PCR) using oligonucleotides TGTTTCCAGGGATCCCAGCAATGA and ACTTCACATGGATCCCCCAGTCATGGC. Ethidium bromide staining of rRNA bands was used to ensure equal RNA loading. The probes were labeled with [α-32P]dATP (3,000 Ci/mmol; Amersham), using a random-primed DNA labeling kit (Boehringer, Mannheim, Germany). The membranes were hybridized (Ultrahyb; Ambion, Austin, TX), washed with 1× SSC (0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate (SDS), and exposed to Kodak AR X-Omat films at −70°C using intensifying screens.
Oligonucleotide DNA precipitation.
To analyze the activation and DNA binding of IRF3 and IRF7 in A549 cells, the cells were left unstimulated or stimulated with IFN-α or TNF-α followed by infection with influenza A virus for 6 h. The cells were harvested, nuclear extracts were prepared, and an oligonucleotide precipitation assay was carried out as previously described (42). PRDI-III of the IFN-β gene (GGATCCCACTTTCACTTCTCCCTTTCAGTTTTC) and the PRD-like element of the IFN-α14 gene (GGATCCGGAAAGCCAAAAGAGAAGTAGAAAAAAA) were used to study the DNA binding of IRF3 and IRF7, respectively (25).
TLR3 protein expression.
TLR3 protein expression was studied as previously described (47).
Western blot analysis.
For Western blot analyses, A549 cells were stimulated with IFN-α, TNF-α, or a combination of both. Proteins (10 μg) from cellular extracts were separated by 10% SDS-polyacrylamide gel electrophoresis (10% SDS-PAGE) using the Laemmli buffer system. Separated proteins in gels were transferred to Immobilon-P membranes (Millipore, Bedford, MA) with an Isophor electrotransfer apparatus (Hoefer Scientific Instruments, San Francisco, CA) at 200 mA for 2 h. Binding of primary and secondary antibodies (Abs) was performed in phosphate-buffered saline (pH 7.4) containing 5% nonfat milk for 1 h at room temperature. Goat anti-IKKɛ, rabbit anti-IRF3, and rabbit anti-IRF7 Abs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-RIG-I Abs were prepared by immunizing guinea pigs four times at 4-week intervals with Escherichia coli-expressed glutathione S-transferase-ΔRIG-I (amino acids 1 to 284) (50 mg/immunization). Horseradish peroxidase-conjugated anti-guinea pig (P0141; Dako A/S, Glostrup, Denmark), anti-goat (P0449; Dako), and anti-rabbit (P0448; Dako) immunoglobulins were used as secondary Abs. The protein bands were visualized on Hyper-Max film using the ECL chemiluminescence system (Amersham).
RESULTS
Kinetics of IFN-α, IFN-β, IL-28, and IL-29 gene expression in virus-infected lung epithelial A549 cells.
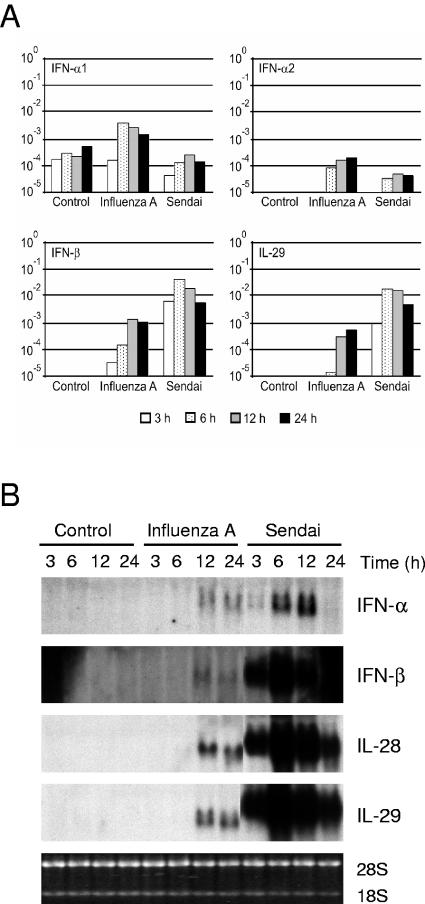
Epithelial cells, DCs, and macrophages in the lungs are the targets for respiratory viruses. We have previously shown that influenza A and Sendai viruses differ in the ability to induce cytokine expression in human macrophages (28, 33, 34). For this report, we studied the expression of the IFN-α, IFN-β, IL-28, and IL-29 genes in A549 lung epithelial cells in response to influenza A and Sendai virus infections. To study the kinetics of type I IFN and IL-29 gene expression, total cellular RNA was isolated at different time points after infection, and the expression of the IFN-α1, IFN-α2, IFN-β, and IL-29 genes was studied by real-time RT-PCR. Influenza A virus was able to induce weak IFN-α1 and IFN-α2 responses and somewhat more expression of IFN-β and IL-29 mRNAs. IFN-β mRNA expression was initially seen 3 h after infection, whereas the other IFN types and IL-29 mRNA started to accumulate 6 h after infection (Fig. 1A). Sendai virus, in contrast, induced IFN-β and IL-29 mRNA expression very rapidly (at 3 h), and 50- to 80-fold higher mRNA expression levels were seen in Sendai virus-infected cells than in influenza A virus-infected cells. Sendai virus infection had little effect on IFN-α mRNA expression (Fig. 1A). To confirm the results obtained by RT-PCR, we performed Northern blot analyses. In influenza A virus-infected A549 cells, weak IFN-α, IFN-β, IL-28, and IL-29 mRNA expression was seen 12 and 24 h after infection (Fig. 1B). In Sendai virus-infected cells, the expression of these genes was rapid, high levels of IFN-α, IFN-β, IL-28, and IL-29 mRNAs were detected 6 h after infection, and the genes persisted in an up-regulated state for up to 24 h (Fig. 1B). As a whole, Sendai virus induced much higher expression levels of the IFN-β, IL-28, and IL-29 genes in A549 cells than did influenza A virus.
FIG. 1.
Kinetics of IFN-α, IFN-β, IL-28, and IL-29 gene expression in virus-infected lung epithelial cells. A549 cells were infected with influenza A H3N2 (A/Beijing/353/89) or Sendai (strain Cantell) virus (multiplicity of infection, 2). At the times indicated, the cells were collected and total cellular RNA was isolated. (A) Quantitative RT-PCR analysis was carried out for virus-induced expression of the IFN-α1 and IFN-α2 subtypes, IFN-β, and IL-29 (IFN-λ1). All quantification data are presented as ratios to the glyceraldehyde-3-phosphate dehydrogenase level. (B) RNA samples (10 μg/lane) were subjected to Northern blot analysis with IFN-α2, IFN-β, IL-28, and IL-29 probes. Ethidium bromide staining of rRNA bands was used to control for equal RNA loading.
IFN-α and TNF-α pretreatment enhances influenza A virus-induced IFN-β, IL-28, and IL-29 gene expression in A549 cells.
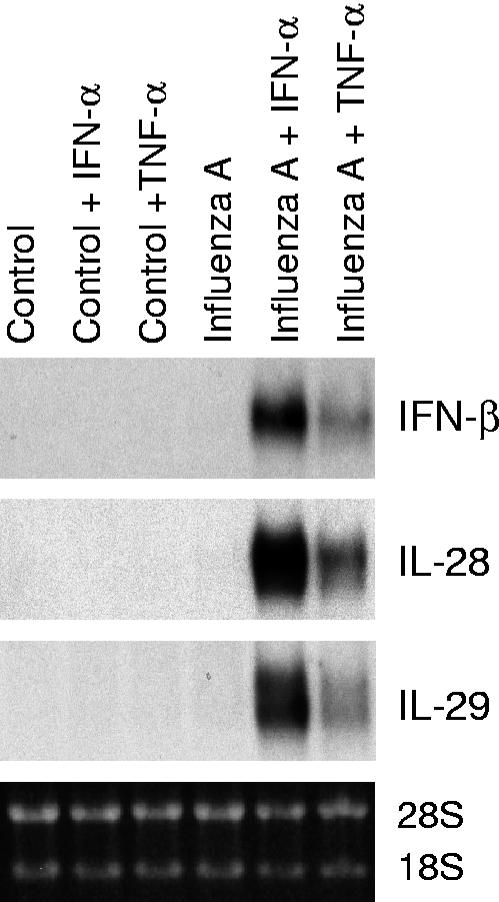
It is well established that IFN-α genes are regulated by a positive feedback mechanism by IFN-α and IFN-β during virus infection (23, 46). We have previously shown that IFN-α enhances influenza A virus-induced expression of antiviral cytokines in human primary macrophages (42). Like IFN-α/β, TNF-α is produced at early times of infection and has an important role in enhancing innate immune responses. To study whether IFN-α and TNF-α would positively regulate virus-induced IFN-β, IL-28, and IL-29 gene expression in lung epithelial cells, A549 cells were pretreated with IFN-α and TNF-α for 24 h, followed by infection with influenza A virus for 6 h. IFN-α and TNF-α clearly enhanced influenza A virus-induced expression of the IFN-β, IL-28, and IL-29 genes in A549 cells (Fig. 2).
FIG. 2.
IFN-α and TNF-α enhance influenza A virus-induced IFN-β, IL-28, and IL-29 gene expression. A549 cells were left untreated or pretreated with IFN-α (100 IU/ml) or TNF-α (10 ng/ml) for 24 h, followed by infection with influenza A virus (A/Beijing/353/89; multiplicity of infection, 2) for 6 h. The cells were collected, total cellular RNA was prepared, and IFN-β, IL-28, and IL-29 mRNA expression was studied by Northern blotting.
Influenza A NS1A CPSF mutant virus is a more potent activator of IL-28 and IL-29 gene expression than wild-type virus.
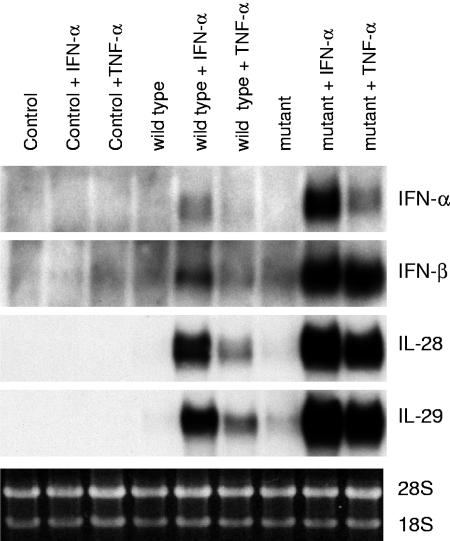
It has been shown that the influenza A virus NS1A protein interferes with the host cell IFN response by inhibiting the posttranscriptional processing of cellular mRNAs (20). To address the role of NS1A in influenza A virus-induced IFN-α, IFN-β, IL-28, and IL-29 responses, wt and CPSF-binding-defective NS1A mutant Udorn viruses were used (31). A549 cells were infected with wt or CPSF mutant Udorn virus for 6 h, total cellular RNA was isolated, and IFN-α, IFN-β, IL-28, and IL-29 mRNA expression was analyzed by Northern blotting (Fig. 3). Strongly enhanced IFN-α, IFN-β, IL-28, and IL-29 mRNA expression was seen in IFN-α- and TNF-α-pretreated cells that were infected with wild-type or CPSF mutant virus. In addition, the CPSF mutant virus was a more potent activator of IFN-α, IFN-β, IL-28, and IL-29 mRNA expression than the wild-type virus (Fig. 3).
FIG. 3.
Expression of IFN-α, IFN-β, IL-28, and IL-29 mRNAs in wild-type and CPSF-binding-defective NS1A mutant Udorn virus-infected cells. A549 cells were left untreated or pretreated with IFN-α (100 IU/ml) or TNF-α (10 ng/ml) for 24 h, followed by infection with wild-type or CPSF mutant influenza A/Udorn/72 virus for 6 h. IFN-α, IFN-β, IL-28, and IL-29 gene expression was studied by Northern blotting.
IFN-α and TNF-α activate RIG-I and TLR3 mRNA and protein expression in A549 cells.
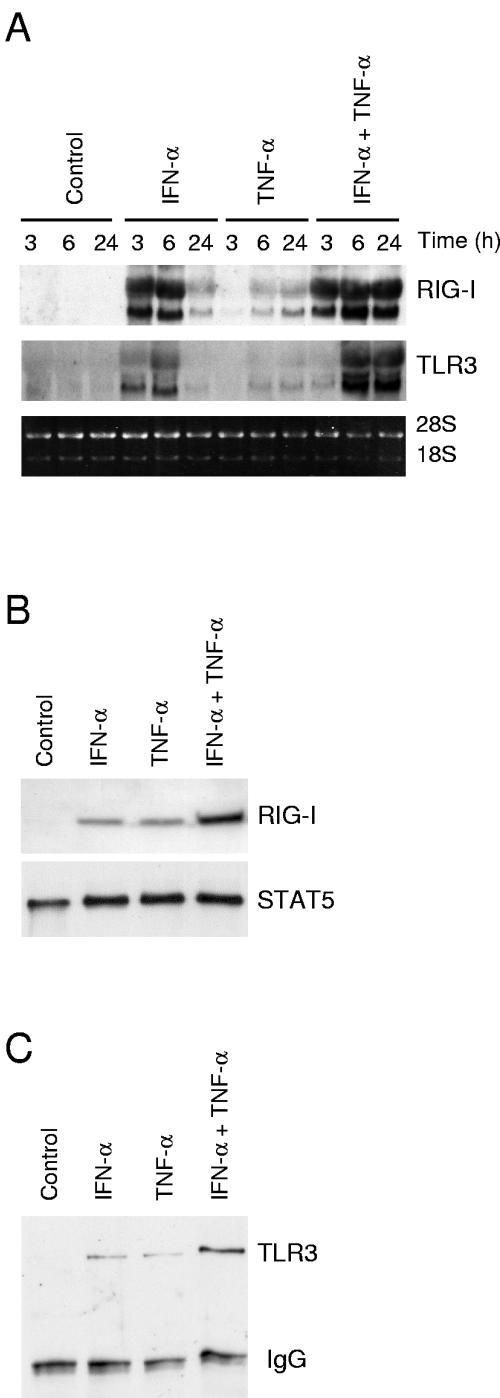
RIG-I, TLR3, TLR7, and TLR8 are PRRs that detect viral RNAs. TLR7 and TLR8 are involved in ssRNA recognition, whereas RIG-I and TLR3 mediate dsRNA-induced IFN gene expression (2, 6, 14, 27, 48). Since IFN-α and TNF-α pretreatment enhanced virus-induced IFN-α/β, IL-28, and IL-29 gene expression, we studied whether these cytokines would stimulate RIG-I or TLR expression in A549 cells. In line with previous studies (29, 42, 47, 48), IFN-α activated RIG-I and TLR3 gene expression in A549 cells (Fig. 4A). Surprisingly, TNF-α also clearly enhanced RIG-I and TLR3 mRNA synthesis, although with delayed kinetics compared to that of IFN-α (Fig. 4A). Furthermore, IFN-α and TNF-α had an additive effect on RIG-I and TLR3 mRNA expression. Similarly, both IFN-α and TNF-α induced RIG-I and TLR3 protein expression in A549 cells (Fig. 4B and C). In line with our previous observations (47), no detectable expression of TLR7 or TLR8 mRNA was seen in untreated or cytokine-stimulated A549 cells (data not shown).
FIG. 4.
IFN-α and TNF-α activate RIG-I and TLR3 mRNA and protein expression in A549 lung epithelial cells. (A) A549 cells left untreated or stimulated with IFN-α (100 IU/ml), TNF-α (10 ng/ml), or their combination for the times indicated. Total cellular RNA was prepared, and RNA samples (10 μg/lane) were subjected to Northern blot analysis with RIG-I and TLR3 probes. Ethidium bromide staining of rRNA bands was used to control for equal RNA loading. (B) A549 cells left untreated or stimulated with IFN-α (100 IU/ml), TNF-α (10 ng/ml), or their combination for 24 h. RIG-I protein expression was studied by Western blotting. To control for equal loading, the membranes were stained with anti-STAT5 Ab. (C) The cells were left untreated or treated with IFN-α (100 IU/ml), TNF-α (10 ng/ml), or their combination for 24 h. After treatment, the cells were collected and prepared for immunoprecipitation with anti-TLR3 Ab. After immunoprecipitation, the samples were run in 10% SDS-PAGE gels under reducing conditions. To control for equal loading, the membranes were stained with Ponceau S and then immunoblotted with anti-TLR3 Ab.
IFN-α and TNF-α enhance the expression of genes involved in the TLR/RIG-I signaling pathway.
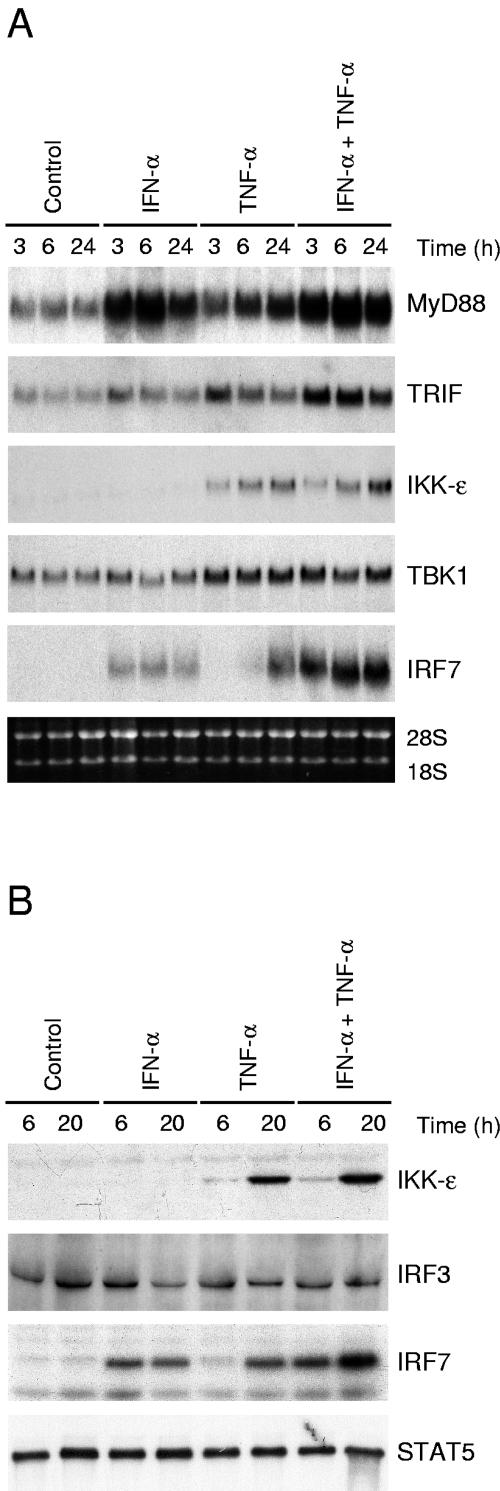
The recognition of viruses by PRRs activates antiviral genes via recruitment of adapter proteins and protein kinases to the receptor complex. Most TLRs utilize a common adapter, MyD88, whereas TLR3 also signals via TRIF (1). TRIF associates with kinases IKKɛ and TBK1, which phosphorylate the transcription factors IRF3 and IRF7 (10, 40). To further study putative positive feedback mechanisms in IFN-α- and TNF-α-pretreated cells, we carried out Northern blot analyses of cytokine-stimulated cells (Fig. 5A). IFN-α and TNF-α enhanced MyD88 mRNA synthesis 3 h and 6 h after cytokine stimulation, respectively. IFN-α- and TNF-α-induced TRIF expression was seen already 3 h after the cytokine treatment. IFN-α had no effect on IKKɛ and TBK1 mRNA expression, whereas TNF-α strongly activated both IKKɛ mRNA and protein expression in A549 cells (Fig. 5A and B). TNF-α also up-regulated TBK1 mRNA expression. IRF3 was expressed at a basal level in A549 cells, and its expression was not affected by cytokine stimulation (Fig. 5B). In IFN-α- and TNF-α-stimulated cells, IRF7 mRNA and protein expression was clearly activated (Fig. 5A and B). In conclusion, we observed that IFN-α and TNF-α enhanced the expression of the signaling components known to be involved in the activation of IFN-α/β gene expression.
FIG. 5.
IFN-α and TNF-α induce the expression of genes involved in the TLR/RIG-I signaling pathway. (A) A549 cells left unstimulated or stimulated with IFN-α (100 IU/ml) and/or TNF-α (10 ng/ml) for the times indicated. Total cellular RNA was prepared, and RNA samples (10 μg/lane) were subjected to Northern blot analysis with MyD88, TRIF, IKKɛ, TBK1, and IRF7 probes. (B) A549 cells were stimulated with IFN-α, TNF-α, or their combination for the times indicated. IKKɛ, IRF3, and IRF7 protein expression was studied by Western blotting. To control for equal loading, the membranes were stained anti-STAT5 Ab.
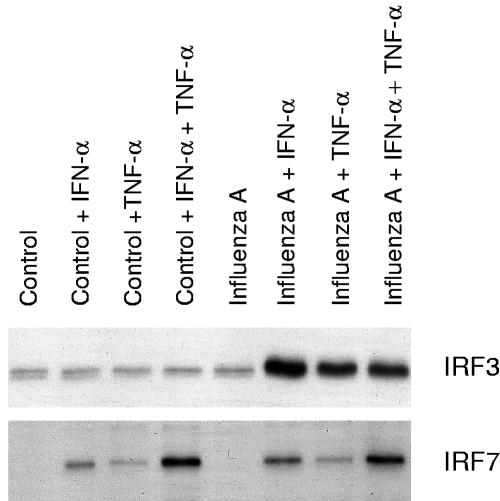
IFN-α or TNF-α pretreatment is essential for enhanced influenza A virus-induced activation of IRF3.
IFN-α and TNF-α enhanced IRF7 expression, whereas IRF3 expression was not affected by these cytokines. The effects of IFN-α and TNF-α on influenza A virus-induced activation of IRF3 and IRF7 were further studied with the oligonucleotide immunoprecipitation method. Low basal DNA binding of IRF3 to the IFN-β promoter PRDI-PRDIII region was seen in untreated A549 cells. Influenza A virus infection itself did not clearly enhance IRF3 binding to the IFN-β promoter PRDI-PRDIII region, whereas a strong enhancement in IRF3 binding was seen in influenza A virus-infected cells that had been pretreated with IFN-α or TNF-α (Fig. 6). IRF7 binding appeared to be associated with the overall expression of IRF7 (compare Fig. 6 to Fig. 5), and no further enhancement of its binding to the IFN-α14 PRD-like element was seen in influenza A virus-infected cell extracts (Fig. 6).
FIG. 6.
IFN-α and TNF-α enhance influenza A virus-induced IRF3 and IRF7 DNA binding. A549 cells were left untreated or pretreated with IFN-α, TNF-α, or their combination for 24 h, after which the cells were infected with influenza A virus (A/Beijing/353/89). After 6 h of infection, nuclear extracts were prepared and precipitated with oligonucleotides containing the promoter elements of the IFN-α14 or IFN-β gene. IRF3 and IRF7 binding was visualized by Western blotting.
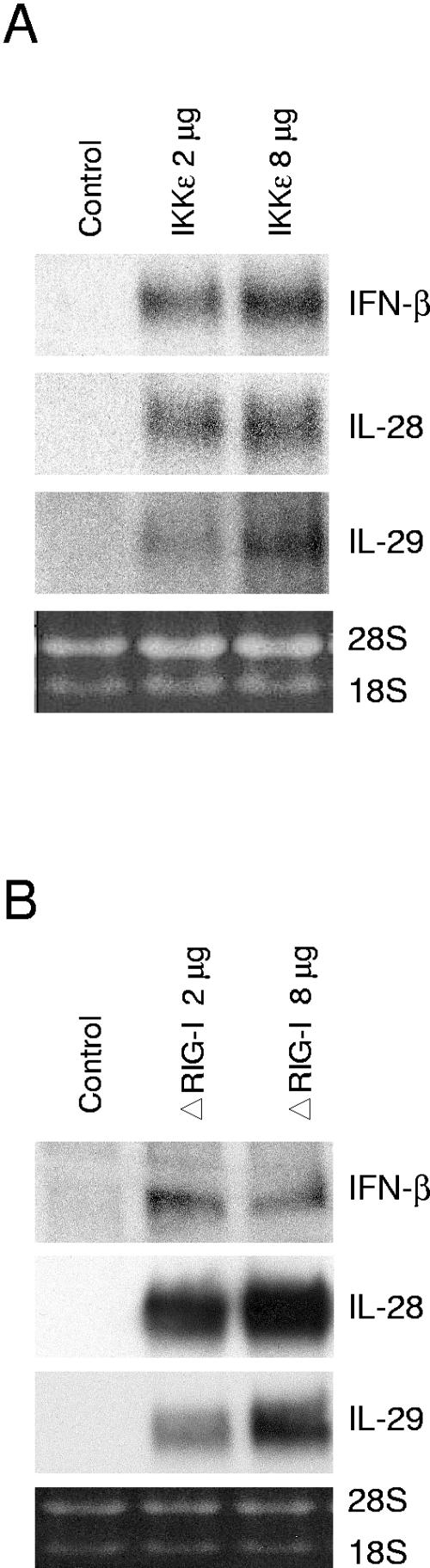
IKKɛ and a constitutively active form of RIG-I induce IFN-β, IL-28, and IL-29 gene expression.
It has previously been shown that ectopic expression of IKKɛ (40) and a constitutively active form of RIG-I, ΔRIG-I, that contains the two caspase recruitment domains induces IFN-β expression (48). To study whether overexpression of IKKɛ or ΔRIG-I would result in the activation of IL-28 and IL-29 gene expression, we transfected A549 cells with IKKɛ and ΔRIG-I expression plasmids. As shown in Fig. 7, overexpression of IKKɛ or ΔRIG-I activated the endogenous IFN-β, IL-28, and IL-29 genes in A549 cells.
FIG. 7.
Activation of endogenous IFN-β, IL-28, and IL-29 gene expression by ectopic expression of IKKɛ and a constitutively active form of RIG-I. A549 cells were mock transfected or transfected with an IKKɛ expression vector (A) or ΔRIG-I expression vector (B). After 24 h of transfection, the cells were collected, and total cellular RNA was prepared. Endogenous IFN-β, IL-28, and IL-29 mRNA expression was studied by Northern blotting.
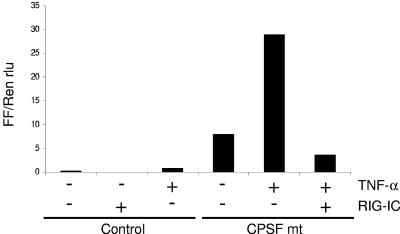
RIG-I is involved in influenza A virus-induced expression of IFN-β.
Gene targeting studies have shown that TLR3 is not required for IFN-α/β production in virus-infected cells (6, 7, 9, 27). Similarly, in our experiments, transfection of TLR3 into A549 cells did not enhance the expression of antiviral cytokines in response to influenza A virus infection (data not shown). To study the role of RIG-I in influenza A virus-induced expression of antiviral cytokines, we used a dominant-negative form of RIG-I, RIG-IC. To enhance the expression of endogenous RIG-I, the cells were treated with TNF-α, followed by transfection with the RIG-IC expression plasmid and infection with the CPSF mutant influenza A virus (Fig. 8). IFN-β mRNA synthesis was measured using a dual-luciferase assay. RIG-IC expression clearly reduced virus-induced IFN-β reporter activity in TNF-α-pretreated A549 cells. These results suggest that RIG-I is involved in influenza A virus-induced expression of IFN-β.
FIG. 8.
A dominant-negative form of RIG-I (RIG-IC) inhibits IFN-β reporter activity in TNF-α-pretreated and influenza A virus-infected cells. A549 cells were left untreated or pretreated with TNF-α (10 ng/ml) for 24 h and then transfected with IFN-β reporter and Renilla luciferase constructs, with or without a RIG-IC expression plasmid. After 24 h, cells were infected with influenza A/Udorn CPSF mutant virus for 18 h. Firefly and Renilla luciferase activities were measured, and firefly/Renilla luciferase ratios (FF/Ren rlu) were calculated.
DISCUSSION
We have previously shown that A549 lung epithelial cells are highly susceptible to influenza A virus infection and that low levels of IFN-α/β are secreted from virus-infected cells (35). Recently, two IFN-α/β-related cytokines, IL-28A/B and IL-29, were described (18, 41). We have previously shown that IFN-α enhances TLR- and virus-induced expression of the IL-28 and IL-29 genes in human primary macrophages (42). In the present study, we analyzed the regulation of IFN-α, IFN-β, IL-28, and IL-29 gene expression in human lung epithelial cells in response to influenza A and Sendai virus infection. Sendai virus infection in A549 cells resulted in rapid and efficient expression of the IFN-α, IFN-β, IL-28, and IL-29 genes. In contrast, influenza A virus-stimulated A549 cells expressed these genes at high levels only when the cells were pretreated with IFN-α or TNF-α. This up-regulation correlated with enhanced expression of RIG-I and TLR3. Similarly, the expression of MyD88, TRIF, IKKɛ, TBK1, and IRF7, signaling components involved in the activation of IFN gene expression, was up-regulated in response to TNF-α stimulation. It is well established that most IFN-α genes are regulated by a positive feedback mechanism by IFN-α/β during virus infection (23, 46). Our finding that TNF-α can also prime cells for high IFN-α/β production is a novel one and suggests an important role for TNF-α in enhancing innate immune responses during viral infections.
TLRs play a crucial role in the mammalian host defense against microbes. TLRs recognize conserved structural components of microbes and activate intracellular signaling pathways leading to the production of cytokines (1). TLRs that have been associated with virus-induced cytokine production include TLR4, which recognizes the fusion protein of respiratory syncytial virus (22), and TLR2, which induces chemokine production in response to measles virus infection (3). TLR2 and TLR9 are involved in the activation of the innate immune response against herpes simplex virus (19, 21, 26). TLR3 is a receptor for dsRNA, whereas TLR7 and TLR8, which are preferentially expressed in macrophages or DCs, are activated by ssRNA originating from influenza A virus or other RNA viruses (2, 6, 14, 27). Our results show that in human lung epithelial cells, TLR7 and TLR8 are not the crucial components in virus-induced IFN-β, IL-28, and IL-29 gene expression, since A549 cells appear to be devoid of TLR7 and TLR8 expression (47; data not shown) and yet virus-induced cytokine gene expression occurred at a high level. In addition to TLRs, it was recently shown that the RNA helicase RIG-I is a cytoplasmic receptor for dsRNA (48). In our experimental setting, IFN-α and TNF-α induced high expression levels of RIG-I and TLR3 in A549 cells, which correlated with an enhanced responsiveness of the cells to influenza A virus infection. Therefore, RIG-I or TLR3 could be involved in the activation of IFN-α/β, IL-28, and IL-29 genes in response to influenza A virus infection. However, gene targeting studies have shown that TLR3 is not required for IFN-α/β production in virus-infected cells (6, 7, 9, 27). In accordance with these studies, transfection of TLR3 into A549 cells did not enhance the expression of antiviral cytokines in response to influenza A virus infection (data not shown). In our transfection experiments, a constitutively active form of RIG-I (ΔRIG-I) induced IFN-β, IL-28, and IL-29 gene expression. In addition, a dominant-negative form of RIG-I (RIG-IC) effectively inhibited influenza A virus-induced IFN-β promoter activity in TNF-α-pretreated A549 cells. These results show that TNF-α-induced RIG-I, not TLR3, mediates influenza A virus-induced IFN-β gene expression in human lung epithelial cells. Previously, it has been shown that RIG-I is involved in Newcastle disease virus and hepatitis C virus recognition (4, 11, 44). Our results suggest that RIG-I is involved in influenza A virus recognition.
The binding of microbial components to TLRs activates target genes via adapter proteins and protein kinases that are associated with or recruited to the receptor complex (1). Most TLRs utilize a common adapter, MyD88, whereas TLR3 and TLR4 also signal independently of MyD88 via TRIF. TRIF associates with IKKɛ and TBK1, which phosphorylate the transcription factors IRF3 and IRF7. Gene targeting studies have shown that IKKɛ and TBK1 are essential for TLR- and virus-induced activation of IFN-α/β genes (10, 15, 32, 40). Here we show that both IFN-α and TNF-α up-regulate MyD88 and TRIF expression. In contrast to IFN-α, TNF-α also up-regulated TBK1 and especially IKKɛ expression in epithelial cells. Furthermore, in transfection experiments, enhanced IKKɛ expression resulted in the activation of IFN-β, IL-28, and IL-29 gene expression. Previously, it has been shown that IFN-β promoter activity is induced by ectopic expression of IKKɛ (10, 40). Our results show that IFN-β as well as IL-28 and IL-29 gene expression can be activated via an IKKɛ-dependent pathway.
The molecular mechanisms of IFN-α/β gene regulation have been extensively studied. Gene disruption studies have shown the critical roles of IRF3 and IRF7 in the activation of IFN-α/β genes (38). IRF3 is mainly responsible for the initial activation of IFN-β gene expression, whereas enhanced expression of IRF7 leads to the production of the majority of IFN-α subtypes (24, 46). Our results show that like that of IFN-α genes, efficient expression of the IL-28 and IL-29 genes during influenza A virus infection is also dependent on IFN-α or TNF-α priming. IRF3 activation and enhanced binding to the IFN-β promoter PRDI-III element were seen only in influenza A virus-infected cells that had been primed with IFN-α or TNF-α. In contrast to that of IRF3, the DNA binding of IRF7 appeared to be dependent on IFN-α- and TNF-α-induced IRF7 expression, suggesting that the IRF7 protein itself has some intrinsic DNA binding activity. Similar to that of IRF3, the transcriptional activity of IRF7 does, however, require activation by virus infection (17, 39, 43).
Many viruses have evolved mechanisms to interfere with the activation of host antiviral responses. Sendai virus C proteins target STAT proteins for degradation, leading to defective IFN signaling (13), whereas the influenza A virus NS1A protein has been shown to interfere with the production of IFN-α/β (12, 20). Initially, we observed that influenza A virus-infected A549 cells express the IFN-α, IFN-β, IL-28, and IL-29 genes at low levels (Fig. 1). However, after IFN-α or TNF-α priming, A549 cells expressed these genes very well. To address the role of NS1A in influenza A virus-induced cytokine gene expression, we used an NS1A mutant in which the NS1A protein is defective in its ability to bind to CPSF and interfere with host cell mRNA processing (31). In these experiments, we observed that in spite of a functionally defective NS1A protein, influenza A virus-induced IFN-α, IFN-β, IL-28, and IL-29 gene expression was greatly dependent on IFN-α or TNF-α priming (Fig. 4). Our results strongly suggest that IFN-α and/or TNF-α priming, followed by enhanced expression of PRRs and their signaling molecules, is a crucial factor in influenza A virus-induced expression of antiviral cytokine genes. This does not, however, rule out the role of the wild-type NS1A protein as an antagonist of IFN-α/β synthesis (8) or as a destabilizer of IFN mRNAs (30), since in our experiments we also observed higher cytokine mRNA levels in influenza A CPSF mutant virus-infected cells.
In conclusion, our results show that IFN-α and/or TNF-α priming of human lung epithelial cells is an essential factor for influenza A virus-induced production of antiviral cytokines. IL-28 and IL-29 were found to be produced by epithelial cells at high levels, which may have an important role in activating the innate immune response and in restricting the spread of influenza A virus infection. Our results also suggest that RIG-I, but not TLR3, is involved in influenza A virus recognition in epithelial cells.
Acknowledgments
We thank Robert Krug for kindly providing us with the wt and CPSF mutant Udorn influenza viruses. We thank Mari Aaltonen and Hanna Valtonen for expert technical assistance.
This study was supported by grants from the Medical Research Council of the Academy of Finland, the Sigrid Juselius Foundation, and the Finnish Cancer Foundation.
REFERENCES
- 1.Akira, S. 2003. Toll-like receptor signaling. J. Biol. Chem. 278:38105-38108. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.Bieback, K., E. Lien, I. M. Klagge, E. Avota, J. Schneider-Schaulies, W. P. Duprex, H. Wagner, C. J. Kirschning, V. Ter Meulen, and S. Schneider-Schaulies. 2002. Hemagglutinin protein of wild-type measles virus activates Toll-like receptor 2 signaling. J. Virol. 76:8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breiman, A., N. Grandvaux, R. Lin, C. Ottone, S. Akira, M. Yoneyama, T. Fujita, J. Hiscott, and E. F. Meurs. 2005. Inhibition of RIG-I-dependent signaling to the interferon pathway during hepatitis C virus expression and restoration of signaling by IKKepsilon. J. Virol. 79:3969-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coccia, E. M., M. Severa, E. Giacomini, D. Monneron, M. E. Remoli, I. Julkunen, M. Cella, R. Lande, and G. Uze. 2004. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur. J. Immunol. 34:796-805. [DOI] [PubMed] [Google Scholar]
- 6.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and E. S. C. Reis. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 7.Diebold, S. S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L. E. Haswell, A. Al-Shamkhani, R. Flavell, P. Borrow, and C. Reis e Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424:324-328. [DOI] [PubMed] [Google Scholar]
- 8.Donelan, N. R., C. F. Basler, and A. Garcia-Sastre. 2003. A recombinant influenza A virus expressing an RNA-binding-defective NS1 protein induces high levels of beta interferon and is attenuated in mice. J. Virol. 77:13257-13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelmann, K. H., S. Richardson-Burns, L. Alexopoulou, K. L. Tyler, R. A. Flavell, and M. B. Oldstone. 2004. Does Toll-like receptor 3 play a biological role in virus infections? Virology 322:231-238. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald, K. A., S. M. McWhirter, K. L. Faia, D. C. Rowe, E. Latz, D. T. Golenbock, A. J. Coyle, S. M. Liao, and T. Maniatis. 2003. IKKɛ and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4:491-496. [DOI] [PubMed] [Google Scholar]
- 11.Foy, E., K. Li, R. Sumpter, Jr., Y. M. Loo, C. L. Johnson, C. Wang, P. M. Fish, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale, Jr. 2005. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc. Natl. Acad. Sci. USA 102:2986-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Sastre, A. 2004. Identification and characterization of viral antagonists of type I interferon in negative-strand RNA viruses. Curr. Top. Microbiol. Immunol. 283:249-280. [DOI] [PubMed] [Google Scholar]
- 13.Garcin, D., J. B. Marq, L. Strahle, P. le Mercier, and D. Kolakofsky. 2002. All four Sendai virus C proteins bind Stat1, but only the larger forms also induce its mono-ubiquitination and degradation. Virology 295:256-265. [DOI] [PubMed] [Google Scholar]
- 14.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 15.Hemmi, H., O. Takeuchi, S. Sato, M. Yamamoto, T. Kaisho, H. Sanjo, T. Kawai, K. Hoshino, K. Takeda, and S. Akira. 2004. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J. Exp. Med. 199:1641-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiscott, J. 2004. Another detour on the Toll road to the interferon antiviral response. Nat. Struct. Mol. Biol. 11:1028-1030. [DOI] [PubMed] [Google Scholar]
- 17.Iwamura, T., M. Yoneyama, K. Yamaguchi, W. Suhara, W. Mori, K. Shiota, Y. Okabe, H. Namiki, and T. Fujita. 2001. Induction of IRF-3/-7 kinase and NF-kappaB in response to double-stranded RNA and virus infection: common and unique pathways. Genes Cells 6:375-388. [DOI] [PubMed] [Google Scholar]
- 18.Kotenko, S. V., G. Gallagher, V. V. Baurin, A. Lewis-Antes, M. Shen, N. K. Shah, J. A. Langer, F. Sheikh, H. Dickensheets, and R. P. Donnelly. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4:69-77. [DOI] [PubMed] [Google Scholar]
- 19.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 103:1433-1437. [DOI] [PubMed] [Google Scholar]
- 20.Krug, R. M., W. Yuan, D. L. Noah, and A. G. Latham. 2003. Intracellular warfare between human influenza viruses and human cells: the roles of the viral NS1 protein. Virology 309:181-189. [DOI] [PubMed] [Google Scholar]
- 21.Kurt-Jones, E. A., M. Chan, S. Zhou, J. Wang, G. Reed, R. Bronson, M. M. Arnold, D. M. Knipe, and R. W. Finberg. 2004. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. USA 101:1315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 23.Levy, D. E., I. Marie, and A. Prakash. 2003. Ringing the interferon alarm: differential regulation of gene expression at the interface between innate and adaptive immunity. Curr. Opin. Immunol. 15:52-58. [DOI] [PubMed] [Google Scholar]
- 24.Levy, D. E., I. Marie, E. Smith, and A. Prakash. 2002. Enhancement and diversification of IFN induction by IRF-7-mediated positive feedback. J. Interferon Cytokine Res. 22:87-93. [DOI] [PubMed] [Google Scholar]
- 25.Lin, R., P. Genin, Y. Mamane, and J. Hiscott. 2000. Selective DNA binding and association with the CREB binding protein coactivator contribute to differential activation of α/β interferon genes by interferon regulatory factors 3 and 7. Mol. Cell. Biol. 20:6342-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matikainen, S., J. Pirhonen, M. Miettinen, A. Lehtonen, C. Govenius-Vintola, T. Sareneva, and I. Julkunen. 2000. Influenza A and Sendai viruses induce differential chemokine gene expression and transcription factor activation in human macrophages. Virology 276:138-147. [DOI] [PubMed] [Google Scholar]
- 29.Miettinen, M., T. Sareneva, I. Julkunen, and S. Matikainen. 2001. IFNs activate Toll-like receptor gene expression in viral infections. Genes Immun. 2:349-355. [DOI] [PubMed] [Google Scholar]
- 30.Nemeroff, M. E., S. M. Barabino, Y. Li, W. Keller, and R. M. Krug. 1998. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′end formation of cellular pre-mRNAs. Mol. Cell 1:991-1000. [DOI] [PubMed] [Google Scholar]
- 31.Noah, D. L., K. Y. Twu, and R. M. Krug. 2003. Cellular antiviral responses against influenza A virus are countered at the posttranscriptional level by the viral NS1A protein via its binding to a cellular protein required for the 3′ end processing of cellular pre-mRNAs. Virology 307:386-395. [DOI] [PubMed] [Google Scholar]
- 32.Perry, A. K., E. K. Chow, J. B. Goodnough, W. C. Yeh, and G. Cheng. 2004. Differential requirement for TANK-binding kinase-1 in type I interferon responses to Toll-like receptor activation and viral infection. J. Exp. Med. 199:1651-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pirhonen, J., S. Matikainen, and I. Julkunen. 2002. Regulation of virus-induced IL-12 and IL-23 expression in human macrophages. J. Immunol. 169:5673-5678. [DOI] [PubMed] [Google Scholar]
- 34.Pirhonen, J., T. Sareneva, M. Kurimoto, I. Julkunen, and S. Matikainen. 1999. Virus infection activates IL-1β and IL-18 production in human macrophages by a caspase-1-dependent pathway. J. Immunol. 162:7322-7329. [PubMed] [Google Scholar]
- 35.Ronni, T., S. Matikainen, T. Sareneva, K. Melen, J. Pirhonen, P. Keskinen, and I. Julkunen. 1997. Regulation of IFN-α/β, MxA, 2′,5′-oligoadenylate synthetase, and HLA gene expression in influenza A-infected human lung epithelial cells. J. Immunol. 158:2363-2374. [PubMed] [Google Scholar]
- 36.Ronni, T., T. Sareneva, J. Pirhonen, and I. Julkunen. 1995. Activation of IFN-α, IFN-γ, MxA, and IFN regulatory factor 1 genes in influenza A virus-infected human peripheral blood mononuclear cells. J. Immunol. 154:2764-2774. [PubMed] [Google Scholar]
- 37.Sareneva, T., I. Julkunen, and S. Matikainen. 2000. IFN-α and IL-12 induce IL-18 receptor gene expression in human NK and T cells. J. Immunol. 165:1933-1938. [DOI] [PubMed] [Google Scholar]
- 38.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 39.Servant, M. J., B. ten Oever, C. LePage, L. Conti, S. Gessani, I. Julkunen, R. Lin, and J. Hiscott. 2001. Identification of distinct signaling pathways leading to the phosphorylation of interferon regulatory factor 3. J. Biol. Chem. 276:355-363. [DOI] [PubMed] [Google Scholar]
- 40.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148-1151. [DOI] [PubMed] [Google Scholar]
- 41.Sheppard, P., W. Kindsvogel, W. Xu, K. Henderson, S. Schlutsmeyer, T. E. Whitmore, R. Kuestner, U. Garrigues, C. Birks, J. Roraback, C. Ostrander, D. Dong, J. Shin, S. Presnell, B. Fox, B. Haldeman, E. Cooper, D. Taft, T. Gilbert, F. J. Grant, M. Tackett, W. Krivan, G. McKnight, C. Clegg, D. Foster, and K. M. Klucher. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4:63-68. [DOI] [PubMed] [Google Scholar]
- 42.Siren, J., J. Pirhonen, I. Julkunen, and S. Matikainen. 2005. IFN-α regulates TLR-dependent gene expression of IFN-α, IFN-β, IL-28, and IL-29. J. Immunol. 174:1932-1937. [DOI] [PubMed] [Google Scholar]
- 43.Smith, E. J., I. Marie, A. Prakash, A. Garcia-Sastre, and D. E. Levy. 2001. IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA-dependent protein kinase R or Ikappa B kinase but is blocked by vaccinia virus E3L protein. J. Biol. Chem. 276:8951-8957. [DOI] [PubMed] [Google Scholar]
- 44.Sumpter, R., Jr., Y. M. Loo, E. Foy, K. Li, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale, Jr. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79:2689-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 46.Taniguchi, T., and A. Takaoka. 2002. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr. Opin. Immunol. 14:111-116. [DOI] [PubMed] [Google Scholar]
- 47.Tissari, J., J. Siren, S. Meri, I. Julkunen, and S. Matikainen. 2005. IFN-α enhances TLR3-mediated antiviral cytokine expression in human endothelial and epithelial cells by up-regulating TLR3 expression. J. Immunol. 174:4289-4294. [DOI] [PubMed] [Google Scholar]
- 48.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]