Abstract
Although Novirhabdovirus viruses, like the Infectious hematopietic necrosis virus (IHNV), have been extensively studied, limited knowledge exists on the route of IHNV entry during natural infection. A recombinant IHNV (rIHNV) expressing the Renilla luciferase gene was generated and used to infect trout. A noninvasive bioluminescence assay was developed so that virus replication in live fish could be followed hours after infection. We provide here evidence that the fin bases are the portal of entry into the fish. Confirmation was brought by the use of a nonpathogenic rIHNV, which was shown to persist in fins for 3 weeks postinfection.
The Infectious hematopoietic necrosis virus (IHNV) belongs to the Novirhabdovirus genus in the Rhabdoviridae family and is the etiological agent of a serious disease in salmonids, mainly in yearling trout. In addition to the five genes encoding the N, P, M, G, and L structural proteins, the negative-stranded RNA genome (∼12 kb) of Novirhabdovirus contains an additional small gene encoding a nonstructural NV protein (1, 17) which has been described as playing a role in IHNV pathogenicity in trout (28). In the IHNV-infected trout, the hematopoietic tissues are the most frequently affected tissues, although most organs and tissues are affected in later stages of the disease (7, 8). Leukocytes and endothelial cells from the intestine and gills have been suggested to be the primary sites of infection (15). However, this study and some others (7, 18, 26, 29) were conducted days postinfection using detection methods in selected organs that require large amounts of virus to be sensitive. Furthermore, key sites of virus replication may have gone undetected if appropriate samples were not taken. Recent advances in bioluminescence imaging (BLI) have enabled the in vivo imaging of luciferase (LUC) in living animals by using a cooled charge-coupled device (CCD) camera (2, 9; for a review, see references 10 and 22). This global detection method offers the advantage of revealing unsuspected sites of pathogen multiplication that could be missed if traditional methods for assaying the infection are used (11, 12). Thus, here we report the development of this noninvasive assay to reinvestigate the spread of a novirhabdovirus infection in live rainbow trout at early times postinfection.
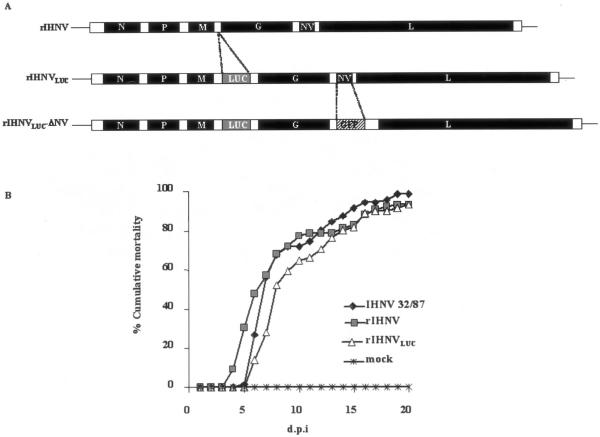
An IHNV-based reverse genetics system (5) has been used to generate a recombinant IHNV that expresses the Renilla reniformis luciferase reporter gene (20), rIHNVLUC (Fig. 1A, middle). A full-length pIHNV cDNA clone (4) was modified such that an additional expression cassette, containing the M gene-derived start and stop transcription sequences, was inserted into the unique EagI restriction enzyme site located between the M and G genes. The Renilla luciferase open reading frame was inserted into the expression cassette, leading to a pIHNVLUC construct. Recovery of rIHNVLUC was achieved as previously described (3, 4). Expression of the LUC gene was first monitored in rIHNVLUC-infected EPC cell lysates by using a luminometer. In a time course experiment, we found that luciferase expression started as early as 2 hours postinfection, and the light intensity progressively increased to the highest level 36 h postinfection (data not shown).
FIG. 1.
Construction and replication of the recombinant IHNV. (A) Diagram of the rIHNVLUC and rIHNVLUC-ΔNV genomes. An additional cistron encoding the Renilla luciferase (LUC) was inserted between the M and G genes of the wild-type-like IHNV (rIHNV) genome or of the rIHNV-ΔNV/GFP genome (4), leading to rIHNVLUC and rIHNVLUC-ΔNV, respectively. (B) Cumulative mortality induced in virus-infected trout. Juvenile trout (75 fish/tank) were infected by bath immersion with 5 × 104 PFU/ml of each virus. Mortalities were recorded every day and expressed as a percentage of cumulative mortality. d.p.i., day postinfection; IHNV 32-87, wild-type IHNV; rIHNV, wild-type-like recombinant IHNV (4); rIHNVLUC, recombinant IHNV expressing the LUC gene; mock, mock-infected trout. Experimental fish infections were done in triplicate.
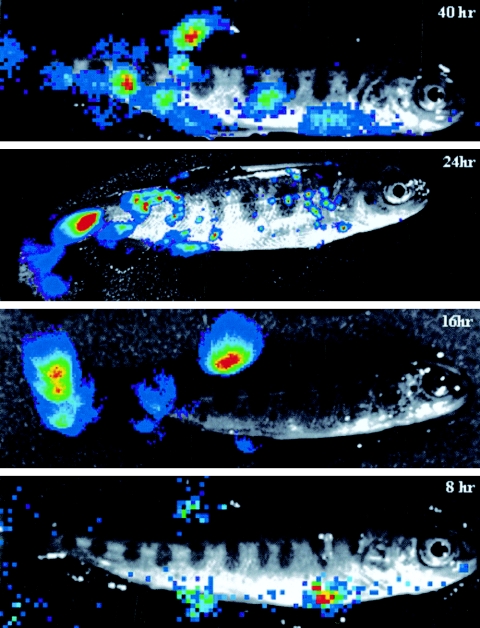
In order to test whether the insertion of the luciferase expression cassette into the IHNV genome had led to a modification in the pathogenic potential of the virus, juvenile trout (n = 75; mean weight, 0.5 g) were infected, in three separate experiments, by bath immersion with the viral isolate IHNV 32-87 and either the wild-type (wt) rIHNV or rIHNVLUC (virus titer, 5 × 104 PFU/ml), and mortalities were recorded for 20 days. All three viruses, rIHNVLUC, wt rIHNV, and IHNV 32-87, proved to be highly pathogenic in yearling trout (Fig. 1B). Experimental infection was repeated in order to sample infected fish during the first 12 days of infection. Various fish organs (spleen, kidney, heart, and liver) were taken every day. Organ samples were homogenized, and luciferase activity was monitored. The most affected organs exhibiting active virus replication appear to be the spleen and kidney at day 3. In the very early stages of infection (days 0 to 2), no significant luciferase activity was detectable in the fish organs (data not shown). In order to follow the spread of the virus in a noninvasive way, we first investigated the possibility of direct visualization of virus replication in the fish by infecting trout and adding the EnduRen live cell substrate (Promega) into the water tank. As a first try, infected fish were taken at 4 days postinfection, a time at which extensive virus replication is expected. Anesthetized fish were subjected to imaging using a CCD camera (Fig. 2A). As visualized in Fig. 2B, active replication of rIHNVLUC was observed most abundantly in the oral cavity, the esophagus/cardiac stomach region, the pyloric caeca, the kidney, and the spleen, but also in the dorsal fin. One of the infected fish was sacrificed, and the skin was removed. The autopsied fish was subjected to CCD imaging to demonstrate that the detected light was readily emitted from inside the body, indicating that imaging can be achieved through the mucus and the skin on live fish (data not shown). The kinetics of virus replication following waterborne exposure was analyzed starting from 8 h postinfection. To do that, the fish were exposed to virus infection by bath immersion (5 × 104 PFU/ml), and at the different time points, the fish were sampled and transferred in a tank containing the water-soluble luciferase substrate. The results of the different observation periods (Fig. 3) were unexpected, since at the longer times post-virus exposure, 40 and 24 h, replication of the virus was seen in various fish organs but virus replication was also evidenced at the fin bases. It was even more demonstrative at 16 and 8 h, times at which viral replication was readily restricted to the fins. No virus replication could be observed in the gills at the times considered.
FIG. 2.
Bioluminescence imaging on live infected trout. (A) Fish were infected by bath immersion with rIHNVLUC. At 4 days postinfection, fish were subjected to a bath immersion with the EnduRen live cell substrate (Promega) and submitted to CCD imaging after being anesthetized. The luciferase activity is depicted with a pseudocolor scale, using red as the highest and blue as the lowest level of photon flux. Bioluminescence signals are displayed in pseudocolors and superimposed on the grayscale image Several controls were included under the same conditions as above: fish 1 was mock infected with no luciferase substrate bath, fish 2 was mock infected with luciferase substrate bath, fish 3 was rIHNV infected with a luciferase substrate bath, and fish 4 was rIHNVLUC infected with no luciferase substrate bath. On the right, the color code is presented. (B) A closer look at an rIHNVLUC-infected fish. Infected organs are indicated.
FIG.3.
Kinetics of IHNV infection in trout. Fish were infected by bath immersion as for Fig. 1B. At the indicated time postinfection (8 h to 40 h), the fish were sampled and anesthetized before CCD imaging.
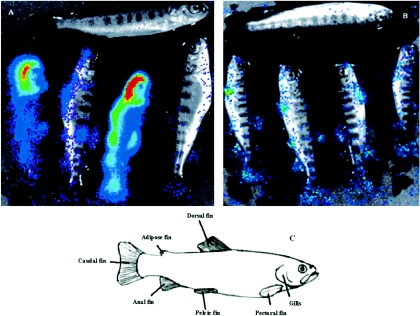
IHNV produces an acute, lethal infection in rainbow trout. However, between 10 and 20% of infected trout do survive the infection (14, 16). In order to investigate the spread of the virus after 3 weeks, we subjected four trout that survived the infection and one mock-infected trout to BLI (Fig. 4A). Contrary to the findings at the earlier time point (when all the analyzed trout showed similar patterns of luciferase activity), we noticed at this late time point a large heterogeneity among the infected trout both in the level and in the distribution of the luciferase activity. Some trout showed a systemic dissemination of the virus, while the ones that succeeded in containing the viral infection demonstrated a localized bioluminescence signal in the fins, which supports the idea of particularly permissive cells for IHNV being localized in the fins.
FIG. 4.
Bioluminescence imaging on surviving trout at 3 weeks postinfection. The fish were infected by bath immersion with rIHNVLUC (A) or the attenuated virus rIHNVLUC- ΔNV (B) and left for 3 weeks. The trout on the top of each panel (A and B) was mock infected. The trout were sampled and subjected to CCD imaging. (C) Schematic representation indicating the fins.
↓
We previously described a recombinant rIHNV-ΔNV in which the NV gene encoding a nonstructural protein of unknown role was deleted and replaced by the reporter green fluorescent protein (GFP) gene (4). This virus is nonpathogenic for trout (28). rIHNV-ΔNV was further engineered to add the luciferase expression cassette between the M and G genes, rIHNVLUC-ΔNV (Fig. 1A). The fish were infected by bath immersion and left for 3 weeks, with no signs of disease and no mortalities. Bioluminescence assays at 3 weeks postinfection on rIHNVLUC-ΔNV-infected fish showed a limited viral replication exclusively localized in the fin bases (Fig. 4B), reminiscent of the observations at the early times (8 h) of infection with rIHNVLUC (Fig. 3).
In conclusion, we show here for the first time a bioluminescence assay on live fish that enabled us to follow virus replication in the fish hours and days after natural infection. At the early time, virus replication was only detectable at all the fin bases and, thus, the fins represent the prime portal of entry for rhabdovirus into salmonids. In addition, definitive evidence was provided through the use of a nonpathogenic recombinant virus that is able to target the fin bases but is unable to propagate into the fish body. It should be noted that several other fish pathogens have shown significantly preferred microhabitats in the fins (6, 13, 19, 21, 23-25, 27). Investigations are currently being conducted to identify whether specialized cells on the fin bases are involved in the entry and the spread of IHNV in the fish.
Acknowledgments
A.H. was funded by Intervet International (Boxmeer, Netherland).
Thanks to Promega and Berthold companies, which made freely available to us the EnduRen live cell substrate and the NightOWL BLI system, respectively. The Fish Facility staff (INRA) is gratefully acknowledged for the animal experiments. Thanks to Xenogen company for their IVIS imaging system 100 and their Living Image software. We thank Windy Brand Williams (INRA) for careful reading of the manuscript.
REFERENCES
- 1.Basurco, B., and A. Benmansour. 1995. Distant strains of the fish rhabdovirus VHSV maintain a sixth functional cistron which codes for a nonstructural protein of unknown function. Virology 212:741-745. [DOI] [PubMed] [Google Scholar]
- 2.Bhaumik, S., and S. S. Gambhir. 2002. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc. Natl. Acad. Sci. USA 99:377-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biacchesi, S., M. Bearzotti, E. Bouguyon, and M. Bremont. 2002. Heterologous exchanges of the glycoprotein and the matrix protein in a Novirhabdovirus. J. Virol. 76:2881-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biacchesi, S., M. I. Thoulouze, M. Bearzotti, Y. X. Yu, and M. Bremont. 2000. Recovery of NV knockout infectious hematopoietic necrosis virus expressing foreign genes. J. Virol. 74:11247-11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bremont, M. 2005. Reverse genetics on fish rhabdoviruses: tools to study the pathogenesis of fish rhabdoviruses. Curr. Top. Microbiol. Immunol. 292:119-141. [DOI] [PubMed] [Google Scholar]
- 6.Buchmann, K. 1998. Histochemical characteristics of Gyrodactylus derjavini parasitizing the fins of rainbow trout (Oncorhynchus mykiss). Folia Parasitol. (Prague) 45:312-318. [PubMed] [Google Scholar]
- 7.Burke, J. A., and R. Grischkowsky. 1984. An epizootic caused by infectious hematopoietic necrosis virus in an enhanced population of sockeye salmon, Oncorhyncus nerka (Walbaum), smolts at Hidden Creek, Alaska. J. Fish Dis. 7:421-429. [Google Scholar]
- 8.Chilmonczyk, S., and J. R. Winton. 1994. Involvement of rainbow-trout leukocytes in the pathogenesis of infectious hematopoietic necrosis. Dis. Aquat. Org. 19:89-94. [Google Scholar]
- 9.Contag, C. H., P. R. Contag, J. I. Mullins, S. D. Spilman, D. K. Stevenson, and D. A. Benaron. 1995. Photonic detection of bacterial pathogens in living hosts. Mol. Microbiol. 18:593-603. [DOI] [PubMed] [Google Scholar]
- 10.Contag, C. H., and B. D. Ross. 2002. It's not just about anatomy: in vivo bioluminescence imaging as an eyepiece into biology. J. Magn. Reson. Imaging 16:378-387. [DOI] [PubMed] [Google Scholar]
- 11.Contag, P. R., I. N. Olomu, D. K. Stevenson, and C. H. Contag. 1998. Bioluminescent indicators in living mammals. Nat. Med. 4:245-247. [DOI] [PubMed] [Google Scholar]
- 12.Cook, S. H., and D. E. Griffin. 2003. Luciferase imaging of a neurotropic viral infection in intact animals. J. Virol. 77:5333-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Kinkelin, P., P. Besse, and G. Tuffery. 1968. A recent necrosing disease of fish teguments and fins: larval bucephalosis due to Bucephalus polymorphus (Baer, 1827). Bull. Off Int. Epizoot. 69:1207-1230. [PubMed] [Google Scholar]
- 14.Drolet, B. S., P. P. Chiou, J. Heidel, and J. A. Leong. 1995. Detection of truncated virus particles in a persistent RNA virus infection in vivo. J. Virol. 69:2140-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drolet, B. S., J. S. Rohovec, and J. C. Leong. 1994. The route of entry and progression of infectious hematopoietic necrosis virus in Oncorhynchus-Mykiss (Walbaum)—a sequential immunohistochemical study. J. Fish Dis. 17:337-347. [Google Scholar]
- 16.Kim, C. H., D. M. Dummer, P. P. Chiou, and J. A. Leong. 1999. Truncated particles produced in fish surviving infectious hematopoietic necrosis virus infection: mediators of persistence? J. Virol. 73:843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurath, G., and J. C. Leong. 1985. Characterization of infectious hematopoietic necrosis virus mRNA species reveals a nonvirion rhabdovirus protein. J. Virol. 53:462-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaPatra, S. E., J. S. Rohovec, and J. L. Fryer. 1989. Detection of infectious hematopoietic necrosis virus in fish mucus. Fish Pathol. 24:197-202. [Google Scholar]
- 19.Loot, G., N. Poulet, Y. Reyjol, S. Blanchet, and S. Lek. 2004. The effects of the ectoparasite Tracheliastes polycolpus (Copepoda: Lernaeopodidae) on the fins of rostrum dace (Leuciscus leuciscus burdigalensis). Parasitol. Res. 94:16-23. [DOI] [PubMed] [Google Scholar]
- 20.Lorenz, W. W., R. O. McCann, M. Longiaru, and M. J. Cormier. 1991. Isolation and expression of a cDNA encoding Renilla reniformis luciferase. Proc. Natl. Acad. Sci. USA 88:4438-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez, J. L., A. Casado, and R. Enriquez. 2004. Experimental infection of Flavobacterium psychrophilum in fins of Atlantic salmon Salmo salar revealed by scanning electron microscopy. Dis. Aquat. Org. 59:79-84. [DOI] [PubMed] [Google Scholar]
- 22.Massoud, T. F., and S. S. Gambhir. 2003. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 17:545-580. [DOI] [PubMed] [Google Scholar]
- 23.Molnar, K. 2002. Site preference of Myxosporean spp. on the fins of some Hungarian fish species. Dis. Aquat. Org. 52:123-128. [DOI] [PubMed] [Google Scholar]
- 24.Molnar, K., and C. Szekely. 2003. Infection in the fins of the goldfish Carassius auratus caused by Myxobolus diversus (Myxosporea). Folia Parasitol. (Prague) 50:31-36. [PubMed] [Google Scholar]
- 25.Molnar, K., and C. Szekely. 1997. An unusual location for Ergasilus sieboldi Nordmann (Copepoda, Ergasilidae) on the operculum and base of pectoral fins of the pikeperch (Stizostedion lucioperca L.). Acta Vet. Hung. 45:165-175. [PubMed] [Google Scholar]
- 26.Neukirch, M. 1984. An experimental study of the entry and multiplication of viral hemorrhagic septicaemia virus in rainbow trout, Salmo gairdneri Richardson, after water-borne infection. J. Fish Dis. 7:231-234. [Google Scholar]
- 27.Quillet, E., M. Dorson, G. Aubard, and C. Torhy. 2001. In vitro viral haemorrhagic septicaemia virus replication in excised fins of rainbow trout: correlation with resistance to waterborne challenge and genetic variation. Dis. Aquat. Org. 45:171-182. [DOI] [PubMed] [Google Scholar]
- 28.Thoulouze, M. I., E. Bouguyon, C. Carpentier, and M. Bremont. 2004. Essential role of the NV protein of Novirhabdovirus for pathogenicity in rainbow trout. J. Virol. 78:4098-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto, T., W. N. Batts, and J. R. Winton. 1992. In vitro infection of salmonid epidermal tissues by infectious hematopoietic necrosis virus and viral hemorrhagic septicemia virus. J. Aquat. Animal Health 4:231-239.