Abstract
Glycoprotein C (gC) of herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) binds complement component C3b and protects virus from complement-mediated neutralization. Differences in complement interacting domains exist between gC of HSV-1 (gC1) and HSV-2 (gC2), since the amino terminus of gC1 blocks complement C5 from binding to C3b, while gC2 fails to interfere with this activity. We previously reported that neutralization of HSV-1 gC-null virus by HSV antibody-negative human serum requires activation of C5 but not of downstream components of the classical complement pathway. In this report, we evaluated whether activation of C5 is sufficient to neutralize HSV-2 gC-null virus, or whether formation of the membrane attack complex by C6 to C9 is required for neutralization. We found that activation of the classical complement pathway up to C5 was sufficient to neutralize HSV-2 gC-null virus by HSV antibody-negative human serum. We evaluated the mechanisms by which complement activation occurred in seronegative human serum. Interestingly, natural immunoglobulin M antibodies bound to virus, which triggered activation of C1q and the classical complement pathway. HSV antibody-negative sera obtained from four individuals differed over an approximately 10-fold range in their potency for complement-mediated virus neutralization. These findings indicate that humans differ in the ability of their innate immune systems to neutralize HSV-1 or HSV-2 gC-null virus and that a critical function of gC1 and gC2 is to prevent C5 activation.
Viruses employ a variety of mechanisms to evade both innate and adaptive immunity. Herpes simplex virus type 1 (HSV-1) establishes latency within the sensory ganglia of the peripheral nervous system, and interferes with the induction of interferon and immunity mediated by antibody and complement (5, 35, 39). HSV-1 also blocks cytotoxic T-lymphocyte activation by preventing antigen presentation by the major histocompatibility complex class I (15, 22, 51). HSV-1 encodes the immediate-early protein ICP47, which prevents the transport of antigenic peptides into the endoplasmic reticulum and subsequent loading onto major histocompatibility complex class I molecules.
HSV-1 glycoproteins E (gE) and I (gI) together form a high-affinity Fc receptor (2, 4, 6, 10, 25, 26). This receptor binds the Fc region of HSV-specific immunoglobulin G (IgG) antibodies in a process called antibody bipolar bridging (10). Antibody bipolar bridging blocks functions mediated by IgG, including antibody-dependent complement neutralization, antibody-dependent cellular cytotoxicity, and phagocytosis (7, 10, 40, 49). In a murine flank model of infection, antibody is significantly more effective at protecting animals against disease caused by an HSV-1 mutant deficient in Fc receptor activity than by wild-type virus (40).
HSV-1 glycoprotein C binds complement component C3b and inhibits the interaction of C5 and properdin (P) with C3b, blocking activation of both the classical and alternative complement pathways (11, 23, 32). HSV-1 gC prevents complement-mediated neutralization of cell-free virus, inhibits complement-mediated lysis of infected cells, and contributes to virulence in vivo, as viruses deficient in binding C3b or blocking C5 and P from interacting with C3b are more attenuated than wild-type virus in a murine flank model of infection (12, 16, 19, 23, 32, 34, 36, 38).
Antibody and complement may interfere with the initial stages of virus infection through several mechanisms, including coating virus to prevent attachment, fusion, and entry into host cells, or inducing aggregation, lysis, and clearance by phagocytic cells (33). Neutralization of virus in the naïve host represents an innate immune response that occurs in the absence of specific antibodies. We previously reported that unlike wild-type virus, HSV-1 deficient in gC is rapidly neutralized by HSV-1- and HSV-2-negative human serum, consistent with conditions during primary infection (12, 13, 34). An examination of the mechanisms by which complement neutralizes HSV-1 gC-null virus indicated that while complement component C5 is required, complement neither blocks attachment to cells nor aggregates virus (13). Activation of the lytic pathway is also not required, since neutralization occurred in the absence of C6 and C8, two components of the membrane attack complex (13). These findings suggest that gC1 protects the virus from complement-mediated neutralization by interfering with C5 or complement components upstream of C5.
Studies evaluating the interaction of gC1 and gC2 with complement are consistent with this hypothesis. HSV gC1 and gC2 bind noncovalently to C3 and its activation products C3b, iC3b, and C3c, and this interaction reduces antibody-independent complement neutralization (12, 16, 19, 32, 38, 48). The domains on gC1 and gC2 that interact with C3b are well conserved in both glycoproteins (24). In addition, gC1 contains a C5- and P-interacting domain located at the amino terminus of the protein (23, 24, 32). This domain accelerates the decay of the alternative complement pathway C3 convertase by preventing P from interacting with C3b, an interaction that normally stabilizes the convertase (14, 32). The C5- and P-interacting domain also prevents C5 from binding C3b. This domain is important in modulating complement activity, since HSV-1 lacking this domain is more readily neutralized by complement alone, and is significantly less virulent than wild-type virus in vivo (34). Interestingly, the C5- and P-interacting domain is absent in gC2, suggesting that the mechanism by which gC2 evades complement-mediated innate immunity may be distinct from that of gC1 (24, 32).
Despite the significance of HSV-2 in human disease, relatively few studies address the complement evasion strategies of this virus (8, 16, 38). Additional studies are therefore warranted to compare the mechanisms by which gC1 and gC2 protect the virus from the effects of complement. We demonstrate that natural IgM antibody in nonimmune human serum binds to HSV-1 and HSV-2 gC-null viruses. Neutralization requires C1q to activate the classical complement pathway and involves both C3 and C5 but not the formation of the membrane attack complex, since neutralization occurs in the absence of C6.
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney cells (Vero) were grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum, 10 mM HEPES (pH 7.3), 2 mM l-glutamine, 20 μg/ml gentamicin, and 1 μg/ml Fungizone (Life Technologies, Rockville, MD). Purified virus pools were prepared by infecting Vero cells at a multiplicity of infection of 0.005. Supernatant fluids at 48 h postinfection were harvested for cell-free virus and centrifuged onto a 5% to 70% sucrose gradient (12).
The HSV-1 gC deletion mutant NS-gCnull was derived from strain NS and is referred to as NS-gC1null (12). This mutant virus has the gC1 protein-coding region replaced with a β-galactosidase expression cassette under the control of the HSV-1 infected-cell protein 6 (ICP6) early promoter (12, 18). Wild-type HSV-2 strains include HSV-2(G), HSV-2(333), and HSV-2.12, a low-passage HSV-2 isolate obtained by one of the investigators (H.M.F.) from a genital lesion of an 18-year-old female. The HSV-2 gC deletion mutants were derived from HSV-2 strain G and strain 333 and are referred to as G-gC2null and 333-gC2Δ, respectively (16, 27). The G-gC2null virus contains the β-galactosidase gene in place of gC2, while the 333-gC2Δ virus contains a 130-base-pair deletion in gC2, corresponding to 0.613 to 0.626 map units, that results in no gC2 protein expression (16, 27). A gC2-null strain was constructed from HSV-2.12 by cotransfecting Vero cells with HSV-2.12 DNA and a flanking sequence vector expressing an ICP6::lacZ expression cassette flanked by 848 bp on the 5′ end and 738 bp on the 3′ end of gC2 sequence that replaced most of the gC2 protein-coding region starting −1 bp prior to the start site and extending to 16 bp prior to the stop site (18). Recombinant viruses were generated and 2.12-gC2null was isolated by selection of blue plaques and triple plaque purified prior to use (12).
Complement reagents.
The source of complement was HSV-1- and HSV-2-nonimmune human serum (referred to as normal human serum [NHS]) that was obtained from four healthy adult volunteers. Blood was clotted at room temperature for 20 min and overnight at 4°C, and serum was then separated, aliquoted, and frozen at −80°C. The absence of HSV-1- and HSV-2-specific IgG antibodies was verified by HSV enzyme-linked immunosorbent assay (ELISA) performed by the Clinical Virology Laboratory at the Children's Hospital of Philadelphia, and by virus neutralization assays as described in this paper. Serum from each donor had normal concentrations of immunoglobulins as measured by the Clinical Immunology Laboratory at the Hospital of the University of Pennsylvania. For donor 1, IgA was 131 mg/dl (normal, 50 to 500 mg/dl); IgG was 984 mg/dl (normal, 650 to 2,000 mg/dl); and IgM was 55 mg/dl (normal, 40 to 270 mg/dl). For donor 2, IgA was 147 mg/dl; IgG was 1023 mg/dl; and IgM was 205 mg/dl. For donor 3, IgA was 170 mg/dl; IgG was 905 mg/dl; and IgM 237 mg/dl. For donor 4, IgA was 253 mg/dl; IgG was 1,140 mg/dl; and IgM was 155 mg/dl.
In some experiments, NHS was heated to 56°C for 30 min to inactivate complement. To identify the complement pathways responsible for virus neutralization, NHS was treated with 10 mM EDTA to inactivate the classical, mannan-binding lectin, and alternative pathways; 8 mM EGTA and 2 mM Mg2+ to inactivate the classical and mannan-binding lectin pathways (20); and 100 mM d-mannose to interfere with activation of the mannan-binding lectin pathway (9, 28). To interfere with C3 activation, NHS was treated with the small synthetic peptide compstatin (4W9A) at a concentration of 40 μM, while 40 μM linear compstatin was used as an inactive control (29, 30, 45).
Depletion of IgM and complement components from NHS.
NHS was IgM depleted by adding 30 mM EDTA and passing the serum over an anti-human IgM column as instructed by the manufacturer (Sigma, St. Louis, MO). The IgM purification was repeated twice to remove 90 to 95% of IgM. Serum was then dialyzed against phosphate-buffered saline (PBS) containing 1 mM EDTA to reduce the EDTA concentrations and supplemented with 1 mM Mg2+ and 2 mM Ca2+ prior to use in neutralization experiments.
To deplete NHS of complement components C1q, C5, and C6, immunoadsorbant columns were prepared by coupling IgG fractions of sheep or goat antiserum prepared against C1q, C5, or C6 to cyanogen bromide-Sepharose (Amersham Pharmacia Biotech, Piscataway, NJ) at a final concentration of 10 mg/ml IgG (13). The isolated protein fractions were concentrated and dialyzed against PBS containing 0.1 mM EDTA to reduce EDTA concentrations and to remove sodium azide. The original volume of serum was restored with dialysis buffer and supplemented with 1 mM Mg2+ and 2 mM Ca2+ prior to use. Complement-depleted NHS was reconstituted with 550 μg/ml IgM, 100 μg/ml C1q, 75 μg/ml C5, or 60 μg/ml C6 (Sigma, St. Louis, MO) to restore physiologic concentrations (13).
Purified IgM.
IgM was purified from NHS using an anti-IgM column according to the manufacturer's instructions (Sigma, St. Louis, MO). Protein-containing fractions were pooled, dialyzed against PBS at 4°C, concentrated by ultracentrifugation using membranes with a 50-kDa cutoff, and stored in aliquots at −80°C.
Neutralization assay.
Purified virus was incubated with the NHS or with heat- or EDTA-inactivated serum or PBS as controls for 1 h at 37°C (12, 13). Viral titers were determined by plaque assay on Vero cells.
Assay for classical complement pathway hemolytic activity.
The total hemolytic complement activity (CH50) of NHS or complement component-depleted serum was determined by incubating serial twofold dilutions of serum with antibody-sensitized sheep erythrocytes (EA) (Sigma, St. Louis, MO) for 45 min at 37°C in 96-well microtiter plates (12, 13, 31). The intact EA were removed by centrifugation for 3 min at 120 × g, the supernatant fluids were transferred to a new 96-well plate, and the percentage of EA lysed was determined by spectrophotometry at 405 nm.
ELISA to measure IgM binding to G-gC2null virus.
Sucrose gradient-purified G-gC2null virus was added to 96-well High Binding Costar microtiter plates (Corning Incorporated, Corning, NY) at 2 × 106 PFU/well in Dulbecco's PBS (pH 7.1), incubated for 2 h at room temperature, and blocked overnight at 4°C with 5% (wt/vol) nonfat milk. Serial twofold dilutions of heat-inactivated NHS diluted in PBS/0.05% Tween 20 were added for 1 h at 37°C to virus-coated wells or to control wells coated with nonfat milk in PBS-Tween. Bound IgM was detected at an optical density of 405 nm using horseradish peroxidase-conjugated goat F(ab′)2 IgG anti-human IgM μ-chain (Sigma, St. Louis, MO). The endpoint titer was the serum dilution resulting in an optical density greater than 0.1 and at least twice the optical density of control wells.
Western blot analysis.
Infected-cell extracts were run on 4 to 15% sodium dodecyl sulfate (SDS)- polyacrylamide gel electrophoresis (PAGE), transferred to Immobilon-P transfer membranes (Millipore Corp., Bedford, MA), and reacted with rabbit anti-gC2 antibody R81 and rabbit anti-VP5 antibody.
Statistical analysis.
The area under the curve (AUC) was used to compare percent virus neutralization. Student's t test (Microsoft Excel software) was used to determine P values. Results were considered significant at a probability (P) of <0.05.
RESULTS
gC2 from multiple strains protects HSV-2 from complement-mediated neutralization by NHS.
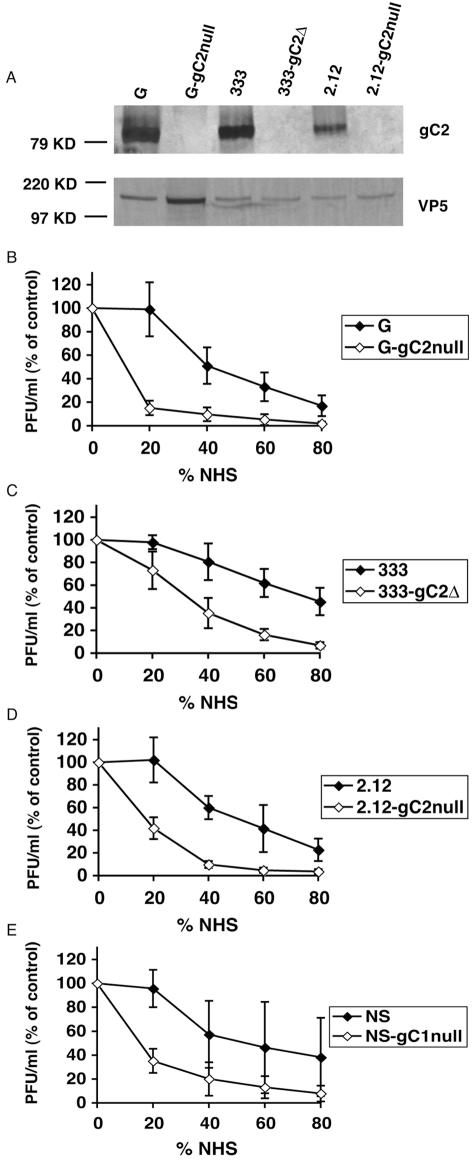
We examined the protective effects conferred by gC2 in multiple HSV-2 strains, including strains G, 333, and 2.12. Western blots confirmed expression of gC2 in wild-type- but not in gC2-null-infected cells (Fig. 1A). Neutralization assays were performed by incubating each virus with increasing concentrations of NHS as the source of complement for 1 h at 37°C. Virus incubated with PBS served as the control. All three wild-type HSV-2 viruses were more resistant to complement-mediated neutralization than the gC2-null viruses (Fig. 1B to D). Similar results were obtained with HSV-1 wild-type and gC1-null viruses (Fig. 1E).
FIG. 1.
(A) Western blot analysis examining gC2 expression in HSV-2 wild-type- and gC2-null-infected cell extracts. The blot was probed with rabbit anti-gC2 antibody R81 or rabbit anti-VP5 as a loading control. (B to E) Neutralization of HSV-1 (NS) and HSV-2 (G, 333, and 2.12) wild-type and gC-null strains by NHS. Viruses were incubated with PBS as a control or NHS as the source of complement at the concentrations indicated for 1 h at 37°C. Results are expressed as PFU/ml (% of control) and were calculated as follows: (PFU with NHS/PFU with PBS) × 100. Results represent the mean titers ± standard deviations of four separate experiments for strain G and of three experiments for strains 333, 2.12, and NS. AUC comparing wild-type and gC-null viruses: P < 0.0001 for strain G, P < 0.002 for strain 333, P < 0.001 for strain 2.12, and P < 0.05 for strain NS.
Although little or no neutralization of HSV-2 wild-type viruses occurred at a concentration of 20% NHS, the titers of 333-gC2Δ, 2.12-gC2null, and G-gC2null were reduced approximately 25%, 75%, and 85%, respectively (Fig. 1B to D). The increased susceptibility to complement neutralization of gC2-null viruses persisted over the range of complement concentrations evaluated. Generally, fourfold or greater concentrations of NHS were required to achieve similar levels of neutralization of wild-type virus compared with gC2-null virus. The results indicate that gC2 protects the virus from complement-mediated neutralization, confirming previous results with the G strain, and extending this observation to two additional HSV-2 gC-null strains (16).
Activation of the alternative complement pathway is not required for neutralization of gC2-null virus.
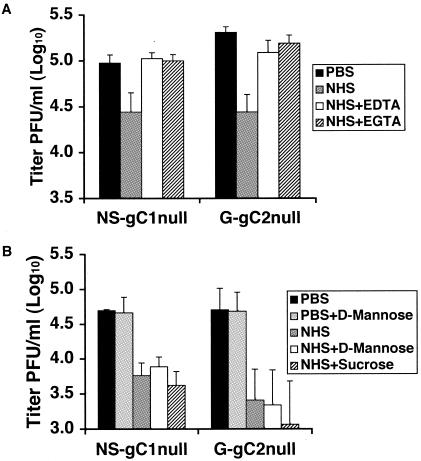
Wild-type HSV-2 was significantly more resistant to complement-mediated neutralization than virus lacking gC2. We evaluated G-gC2null to evaluate the mechanism by which gC2 protects against complement-mediated neutralization and included NS-gC1null for comparison. Purified gC-null viruses were incubated for 1 h at 37°C with 50% NHS or 50% NHS treated with 8 mM EGTA and 2 mM Mg2+, which inactivates both the classical and mannan-binding lectin pathways while leaving the alternative complement pathway intact (19). As controls, viruses were incubated with 50% NHS treated with 10 mM EDTA to inactivate all complement pathways or left untreated (PBS). G-gC2null and NS-gC1null were neutralized by NHS but not by EDTA- or EGTA-treated NHS (Fig. 2A). The results indicate that activation of the alternative complement pathway does not mediate neutralization of the gC2-null viruses.
FIG. 2.
(A) Complement-mediated neutralization of HSV-1 and HSV-2 gC-null viruses does not require activation of the alternative complement pathway. The gC1-null and gC2-null viruses were incubated with 50% NHS or NHS treated with EDTA (NHS+EDTA) or EGTA (NHS+EGTA). Virus treated with PBS served as the control. Results shown represent mean titers ± standard deviations of three independent experiments. P < 0.02, NHS with PBS for NS-gC1null or G-gC2null. P values were not significant for EDTA- or EGTA-treated NHS versus PBS for NS-gC1null or G-gC2null. (B) Complement-mediated neutralization of the gC1-null and gC2-null viruses is not dependent upon activation of the mannan-binding lectin complement pathway. Neutralization assays were performed on gC1-null and gC2-null viruses incubated with PBS, PBS containing d-mannose, 50% NHS, or NHS treated with either d-mannose or sucrose. Results shown represent mean titers ± standard deviations of three independent experiments. P < 0.02, comparing virus incubated with PBS or PBS containing d-mannose with virus incubated with NHS or NHS treated with d-mannose or sucrose.
Activation of the mannan-binding lectin complement pathway is not required for neutralization of gC1-null or gC2-null virus.
Mannan-binding protein interacts with high-mannose glycans present on some viruses to activate the lectin complement pathway and inhibit viral infection (1, 9, 20). To determine whether neutralization of HSV occurs through activation of the mannan-binding lectin pathway, NS-gC1null and G-gC2null were incubated for 1 h at 37°C with 50% NHS or 50% NHS treated with d-mannose, which prevents activation of the lectin pathway while leaving the classical and alternative pathways intact (9, 28). As controls, the viruses were incubated with PBS, d-mannose in the absence of NHS, or 50% NHS treated with sucrose. Neutralization of viruses was similar for NHS treated with d-mannose or sucrose (Fig. 2B). No neutralization occurred when viruses were treated with d-mannose in the absence of NHS. The results indicate that the mannan-binding lectin pathway is not required for neutralization. Since neither the alternative nor mannan-binding lectin complement pathway is involved, the classical complement pathway is likely responsible.
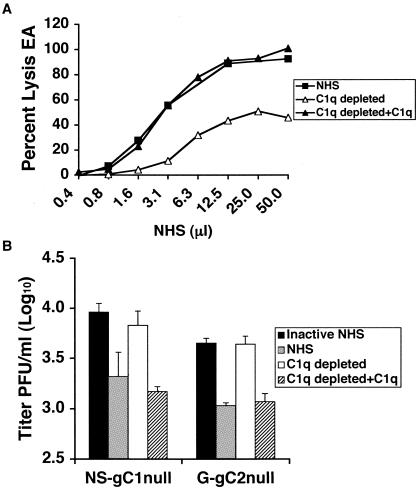
Neutralization of the gC-null viruses is C1q dependent.
We examined whether C1q, the first component of the classical complement pathway, is required to neutralize HSV-1 or HSV-2 gC-null virus. C1q was depleted from NHS, which resulted in a reduction of total hemolytic complement activity that was restored upon reconstitution with C1q (Fig. 3A). C1q-depleted serum showed some residual activity despite being depleted by greater than 95%, indicating that relatively small C1q concentrations are sufficient to initiate the classical complement cascade and lyse antibody-coated erythrocytes. Neutralization experiments were performed to compare 20% NHS, C1q-depleted NHS, and C1q-depleted NHS reconstituted with C1q. As a control, virus was incubated with 20% NHS that had been heat inactivated. C1q-depleted serum failed to neutralize NS-gC1null or G-gC2null, while serum reconstituted with C1q restored neutralization (Fig. 3B). The results indicate that neutralization of the gC-null viruses requires C1q and occurs through activation of the classical complement pathway.
FIG. 3.
(A) Total hemolytic complement activity of NHS, NHS depleted of C1q (C1q depleted), and C1q-depleted serum reconstituted with C1q (C1q depleted+C1q). EA were incubated with serum and the percentage of EA lysed was determined. (B) gC1-null and gC2-null viruses are neutralized by the classical complement pathway. Neutralization experiments were performed with 20% NHS that was C1q depleted or reconstituted. Results shown represent mean titers ± standard deviations of three independent experiments. P < 0.02, comparing heat-inactivated NHS and either NHS or C1q-restored serum for NS-gC1null and G-gC2null. In contrast, values are not significant, P = 0.23 and 0.86, comparing heat-inactivated NHS with C1q-depleted serum for NS-gC1null and G-gC2null, respectively.
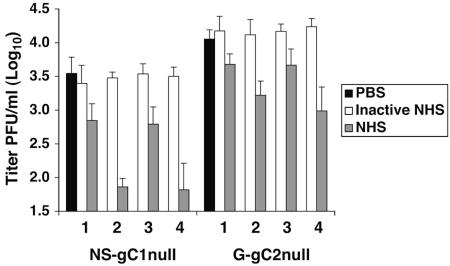
NHS from multiple donors neutralizes gC-null viruses.
We evaluated complement neutralization of NS-gC1null and G-gC2null using four HSV-1 and HSV-2 seronegative human donors to determine whether the extent of NHS neutralization varies among subjects. All samples neutralized the gC-null viruses at 20% NHS, while heat-inactivated NHS failed to neutralize, suggesting that neutralization of the gC-null viruses is mediated by complement (Fig. 4). Interestingly, the extent of neutralization varied up to 10-fold among donors, suggesting differences among subjects in innate immune responses to HSV-1 and HSV-2.
FIG. 4.
Neutralization of HSV-1 and HSV-2 gC-null viruses by NHS from four donors occurs in the absence of specific antibodies against HSV. Neutralization experiments were performed on virus incubated with PBS, heat-inactivated NHS (inactive NHS), or 20% NHS. The four serum samples are labeled 1 to 4. Results shown represent the mean titers ± standard deviations of three separate experiments. P was <0.001 for all four sera, comparing PBS with NHS for NS-gC1null, and P ranged from 0.006 to <0.001 for G-gC2null viruses. In contrast, values are not significant for PBS versus heat-inactivated NHS.
Neutralization of gC-null viruses requires activation of C3.
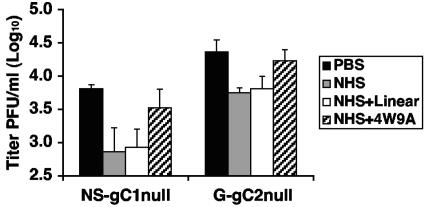
C3 is an integral component of all three complement pathways. High concentrations of C3 present in NHS make it difficult to deplete; therefore, we used compstatin to inhibit C3 activation and determine whether C3 is necessary to neutralize gC-null viruses. Compstatin (4W9A) is a small synthetic peptide that interferes with complement at low concentrations by binding C3, preventing its activation (29, 30, 45). Experiments were performed to examine neutralization following treatment with 20% NHS or 20% NHS treated with either active (4W9A) or inactive (linear) compstatin. As a control, virus was left untreated. The NS-gC1null and G-gC2null viruses were neutralized following treatment with NHS or NHS treated with inactive compstatin (Fig. 5). NHS treated with active compstatin failed to reduce viral titers, which indicates that neutralization of the gC-null viruses requires C3 activation.
FIG. 5.
Neutralization of HSV-1 and HSV-2 gC-null viruses is dependent upon C3 activation. Neutralization experiments were performed on virus treated with PBS, 20% NHS, or NHS treated with either inactive compstatin (NHS+Linear) or active compstatin (NHS+4W9A). Results represent mean titers ± standard deviations of four independent experiments for NS-gC1null and three independent experiments for G-gC2null. For both viruses, differences were significant between PBS and NHS (P < 0.01) and PBS and NHS treated with inactive compstatin (P < 0.05). However, no significant differences were detected between PBS and NHS treated with active compstatin.
Neutralization of G-gC2null virus is C5 dependent.
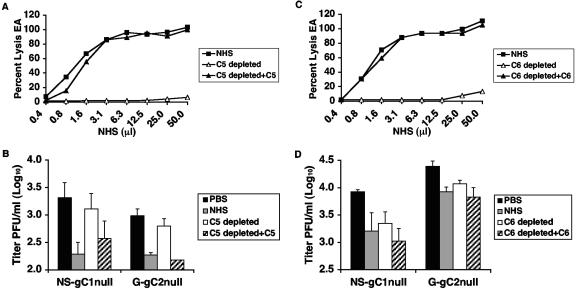
C5 is required for antibody-independent complement neutralization of NS-gC1null; therefore, we evaluated whether G-gC2null is neutralized by a similar mechanism (13). Depletion of C5 from NHS reduced the total hemolytic activity, which was restored when C5 was reconstituted (Fig. 6A). NS-gC1null and G-gC2null were incubated with 20% NHS, serum depleted of C5, and C5-reconstituted serum. C5-depleted serum failed to neutralize the viruses while NHS and reconstituted serum did (Fig. 6B). The results indicate that G-gC2null is similar to NS-gC1null in that neutralization requires C5.
FIG. 6.
(A) Total hemolytic complement activity of NHS, NHS depleted of C5 (C5 depleted), and C5-restored serum (C5 depleted+C5). (B) Neutralization of gC1-null and gC2-null viruses requires the presence of C5. Virus was incubated with PBS, 20% NHS, 20% NHS depleted of C5 (C5 depleted), or C5-restored serum (C5 depleted+C5). Results are expressed as the mean titers ± standard deviations of four independent experiments for NS-gC1null and two for G-gC2null. P < 0.005 for both viruses, comparing NHS and C5-restored NHS with PBS. No significant differences were detected between PBS and C5-depleted NHS. (C) Total hemolytic complement activity of NHS, NHS depleted of C6 (C6 depleted), and C6-restored serum (C6 depleted+C6). (D) Neutralization of gC1-null or gC2-null virus is not dependent on the presence of C6. Neutralization assays were performed on virus treated with PBS, 20% NHS, C6-depleted NHS (C6 depleted), and C6-restored serum (C6 depleted+C6). The results represent the mean titers ± standard deviations of three independent experiments for NS-gC1null and seven experiments for G-gC2null. P < 0.01 for both viruses, comparing PBS with NHS, C6-depleted, and C6-reconstituted serum.
Neutralization of G-gC2null virus does not require C6.
Since neutralization of NS-gC1null is not dependent upon the presence of C6, we examined whether C6 is required for neutralization of G-gC2null (13). C6 depletion resulted in reduced total hemolytic activity, which was restored with reconstituted C6 (Fig. 6C). NS-gC1null and G-gC2null were neutralized to similar levels by 20% NHS, C6-reconstituted, or C6-depleted serum (Fig. 6D). Thus, similar to NS-gC1null, C6 is not required for complement neutralization of G-gC2null.
Natural IgM antibody is required to neutralize gC-null viruses.
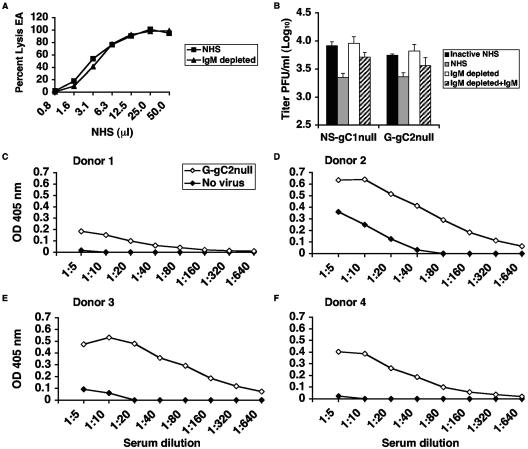
Activation of the classical complement pathway occurs when C1q binds to IgM or two IgG molecules on the virion surface. Activation can also occur when C1q binds directly to viral membrane proteins, as reported for human cytomegalovirus and human T-cell lymphotropic virus (3). Since neutralization of gC1-null and gC2-null viruses occurs in the absence of specific antibody, we evaluated the role of natural IgM antibody in mediating complement neutralization. NHS was depleted of IgM, which resulted in no loss of total hemolytic complement activity, indicating that classical complement pathway components were not depleted along with the IgM (Fig. 7A). Experiments were performed comparing neutralization after treatment with 20% NHS, 20% NHS depleted of IgM, and IgM-restored NHS. IgM-depleted serum failed to neutralize NS-gC1null and G-gC2null, while NHS or IgM-reconstituted NHS neutralized these viruses (Fig. 7B). Therefore, active complement in the absence of natural IgM antibodies is not sufficient to neutralize gC-null viruses; rather, both IgM and complement are required.
FIG. 7.
(A) Total hemolytic complement activity using NHS and NHS depleted of IgM (IgM depleted). Hemolytic assays were performed using antibody-coated sheep erythrocytes to demonstrate an intact classical complement pathway in IgM-depleted serum. (B) Natural IgM antibody is required for neutralization of HSV-1 and HSV-2 gC-null viruses. The gC-null viruses were incubated with 20% NHS, heat-inactivated NHS (inactive NHS), 20% NHS depleted of IgM (IgM depleted), and IgM-restored serum (IgM depleted+IgM). The results shown represent the mean titers ± standard deviations of four independent experiments. P < 0.05, comparing IgM-depleted with reconstituted sera for NS-gC1null and G-gC2null. In contrast, values are not significant when comparing PBS with IgM-depleted serum for NS-gC1null and G-gC2null, P = 0.83 and 0.31, respectively. (C to F) ELISA detects natural IgM antibody binding to gC2-null virus. Heat-inactivated 20% NHS from four donors was serially diluted and added to microtiter wells coated with G-gC2null or control wells. The experiment was performed twice with similar results. The results of one experiment are shown.
IgM antibody binds to G-gC2null virus.
An ELISA was used to measure binding of IgM in heat-inactivated NHS to G-gC2null. Serial twofold dilutions of serum starting with 20% NHS were incubated with G-gC2null or control wells. IgM in NHS from each of four donors was detected bound to G-gC2null (Fig. 7C to F). Interestingly, the endpoint titers varied among subjects (donor 1, 1:20; donors 2 and 3, 1:320; and donor 4, 1:80), which correlates with the IgM concentrations in the sera of the four donors (donor 1, 55 mg/dl; donor 2, 205 mg/dl; donor 3, 237 mg/dl; and donor 4, 155 mg/dl). The results indicate that IgM binds to virus and suggest that the natural IgM antibody titer may depend on serum IgM concentrations.
DISCUSSION
HSV-1 and HSV-2 gC-null viruses were used to evaluate the mechanisms of complement-mediated neutralization. Neutralization was inhibited by the addition of EDTA or EGTA to NHS, but not by d-mannose, and was dependent upon the presence of C1q. These results indicate that the gC-null viruses are neutralized through activation of the classical complement pathway. Neutralization of both gC-null strains required C3 and C5 activation but not C6, indicating that assembly of the membrane attack complex is not necessary for neutralization. We previously reported that activated C5 but not C6 is required for neutralization of HSV-1 gC-null, and now observe similar findings for HSV-2 gC-null virus (13). The wild-type HSV-1 and HSV-2 parental strains resisted complement neutralization, which supports similar mechanisms of action for gC1 and gC2 in protecting against complement neutralization.
The finding that C5 is necessary for neutralization offers insight into the importance of gC1 and gC2 binding to C3b. Activation of C5 requires the C5 convertase, which consists of C4bC2aC3b; therefore, HSV-1 and HSV-2 likely prevent C5 activation by binding C3b. While gC1 and gC2 share highly conserved C3b binding regions, only gC1 contains a domain at the amino terminus that interferes with C5 binding to C3b (23, 24, 32, 46). Thus, gC1 contains two complement-interacting domains that function to prevent activation of the classical complement pathway, while gC2 contains only one. HSV-2 gC has a higher affinity for C3b than HSV-1 gC, which may render a C5-interacting domain on gC2 unnecessary (44).
The mechanism by which activated C5 mediates virus neutralization remains unknown. We previously reported that complement fails to block virus attachment, does not induce aggregation, and occurs in the absence of phagocytic cells, excluding virus opsonization as the mechanism (13). Since C6 is not required, neutralization is not mediated by lysis of viral particles. Complement prevents HSV infection prior to early viral gene expression (13). Therefore, C5 neutralization occurs after virus attachment and prevents one of the following steps in replication: entry, uncoating, transport of viral DNA to the nucleus, or initiation of immediate-early or early viral gene expression (13). Studies are planned to address these processes, with a particular emphasis on virus entry.
We evaluated the mechanisms by which gC-null viruses activate the classical complement pathway in nonimmune human serum. The possibilities that we considered included binding of natural IgM antibody to virus and C1q binding in an antibody-independent manner. We dismissed natural IgG antibodies from consideration since we previously reported that neutralization of gC1-null virus occurs in IgG-depleted serum (12). We also thought that cross-reactive antibodies were unlikely to be involved based on our prior studies that demonstrated neutralization of gC1-null virus using serum from a child seronegative for HSV-1, HSV-2, human cytomegalovirus, Epstein-Barr virus, and varicella-zoster virus (12). Moreover, antibodies that cross-react with antigens on the cells used to grow the virus were not implicated since neutralization persisted following adsorption of serum against Vero cells or when virus was purified from human Hep-2 cells (12). Our results now demonstrate that activation of the classical complement pathway is triggered by natural IgM antibody binding to virus. C1q was required for neutralization; however, we exclude direct binding of C1q to virus as the mechanism for initiating complement activation since in the absence of IgM, complement neutralization does not occur.
The extent of neutralization by NHS varied among donors. Most experiments were performed using serum from donor 1. This serum neutralized approximately 0.7 to 1.0 log10 of HSV-1 and HSV-2 gC-null viruses. The other NHS samples had greater neutralizing activity. Possible explanations for this variation include differences in the levels of particular complement components, the concentration of IgM in serum, the ability of IgM to bind viral antigens, and the avidity of the IgM, since the binding avidities of natural antibodies vary widely, from 5 × 10−3 to 5 × 10−11 (42). Germ line-variable genes that have not undergone somatic hypermutation encode natural antibodies. Antigen- and germ-free mice produce natural antibodies that bind specific bacterial and viral antigens; however, the neutralizing antibody titers are dependent on the strain of mice (17, 41, 42). Thus, genetic factors also likely contribute to the ability of natural antibodies to bind virus (42). Additional studies that include a larger number of seronegative human donors will be required to distinguish among the many factors that may contribute to donor variability.
In some experiments NHS neutralized more NS-gC1null than G-gC2null, while in other assays the opposite was noted. However, in general, NHS had similar effects on both viruses. The extent of neutralization by NHS may vary based on the titer of virus added to serum and possibly the number of defective virus particles in the preparation (12). Since these variables were not controlled in our studies, the experiments were not optimal to compare results for NHS neutralization of the two viruses. Rather, the studies were designed to address the components in NHS that mediate virus neutralization, and revealed that IgM, C1q, C3, and C5 of the classical complement pathway are all required.
The molecules on HSV-1 and HSV-2 that are recognized by natural antibodies remain to be determined. For some viruses, including porcine endogenous retrovirus, pseudorabies virus, and lymphocytic choriomeningitis virus, neutralization by nonimmune human serum is triggered by natural antibodies binding to Galα1-3Gal epitopes on virus (21, 37, 43, 47, 50). However, we previously reported that naturally occurring antibodies to Galα1-3Gal are not involved in neutralizing HSV-1 virus (12).
The finding that natural IgM antibodies bind to HSV-1 and HSV-2 raises interesting implications. Human germ line-encoded IgM antibodies may have evolved to control HSV, allowing natural antibodies to function with the complement system to prevent HSV infection shortly after transmission. In turn, HSV has adapted to the human host over millennia by evolving complement-regulatory proteins such as gC1 and gC2 that thwart immunity mediated by antibody and complement (12, 13, 16, 19, 35, 38).
Acknowledgments
We thank John Lambris for providing both active and inactive compstatin, Richard Hodinka for performing the ELISA to detect HSV antibodies in NHS, Anne Crivaro for measuring immunoglobulin concentrations, Betsy Herold for HSV-2(G)-gC2null, David Johnson for HSV-2(333)-gC2null isolate C2-8, and Roselyn Eisenberg and Gary Cohen for anti-gC2 antibody R81 and anti-VP5 antibody.
This work was supported by Public Health Service grants HL28220, A133063, DE14152, and DK35081 and training grants T32-GM07229 and T32-AI07324.
REFERENCES
- 1.Anders, E. M., C. A. Hartley, P. C. Reading, and R. A. Ezekowitz. 1994. Complement-dependent neutralization of influenza virus by a serum mannose-binding lectin. J. Gen. Virol. 75:615-622. [DOI] [PubMed] [Google Scholar]
- 2.Bell, S., M. Cranage, L. Borysiewicz, and T. Minson. 1990. Induction of immunoglobulin G Fc receptors by recombinant vaccinia viruses expressing glycoproteins E and I of herpes simplex virus type 1. J. Virol. 64:2181-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blue, C. E., O. B. Spiller, and D. J. Blackbourn. 2004. The relevance of complement to virus biology. Virology 319:176-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman, T. L., I. You, I. M. Joseph, P. J. Bjorkman, S. L. Morrison, and M. Raghavan. 1999. Characterization of the interaction between the herpes simplex virus type I Fc receptor and immunoglobulin G. J. Biol. Chem. 274:6911-6919. [DOI] [PubMed] [Google Scholar]
- 5.Chee, A. V., and B. Roizman. 2004. Herpes simplex virus 1 gene products occlude the interferon signaling pathway at multiple sites. J. Virol. 78:4185-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubin, G., I. Frank, and H. M. Friedman. 1990. Herpes simplex virus type 1 encodes two Fc receptors which have different binding characteristics for monomeric immunoglobulin G (IgG) and IgG complexes. J. Virol. 64:2725-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubin, G., E. Socolof, I. Frank, and H. M. Friedman. 1991. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J. Virol. 65:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenberg, R. J., M. Ponce de Leon, H. M. Friedman, L. F. Fries, M. M. Frank, J. C. Hastings, and G. H. Cohen. 1987. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb. Pathog. 3:423-435. [DOI] [PubMed] [Google Scholar]
- 9.Ezekowitz, R. A., M. Kuhlman, J. E. Groopman, and R. A. Byrn. 1989. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J. Exp. Med. 169:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank, I., and H. M. Friedman. 1989. A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J. Virol. 63:4479-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman, H. M., G. H. Cohen, R. J. Eisenberg, C. A. Seidel, and D. B. Cines. 1984. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature 309:633-635. [DOI] [PubMed] [Google Scholar]
- 12.Friedman, H. M., L. Wang, N. O. Fishman, J. D. Lambris, R. J. Eisenberg, G. H. Cohen, and J. Lubinski. 1996. Immune evasion properties of herpes simplex virus type 1 glycoprotein gC. J. Virol. 70:4253-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman, H. M., L. Wang, M. K. Pangburn, J. D. Lambris, and J. Lubinski. 2000. Novel mechanism of antibody-independent complement neutralization of herpes simplex virus type 1. J. Immunol. 165:4528-4536. [DOI] [PubMed] [Google Scholar]
- 14.Fries, L. F., H. M. Friedman, G. H. Cohen, R. J. Eisenberg, C. H. Hammer, and M. M. Frank. 1986. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J. Immunol. 137:1636-1641. [PubMed] [Google Scholar]
- 15.Fruh, K., K. Ahn, H. Djaballah, P. Sempe, P. M. van Endert, R. Tampe, P. A. Peterson, and Y. Yang. 1995. A viral inhibitor of peptide transporters for antigen presentation. Nature 375:415-418. [DOI] [PubMed] [Google Scholar]
- 16.Gerber, S. I., B. J. Belval, and B. C. Herold. 1995. Differences in the role of glycoprotein C of HSV-1 and HSV-2 in viral binding may contribute to serotype differences in cell tropism. Virology 214:29-39. [DOI] [PubMed] [Google Scholar]
- 17.Gobet, R., A. Cerny, E. Ruedi, H. Hengartner, and R. M. Zinkernagel. 1988. The role of antibodies in natural and acquired resistance of mice to vesicular stomatitis virus. Exp. Cell Biol. 56:175-180. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein, D. J., and S. K. Weller. 1988. An ICP6::lacZ insertional mutagen is used to demonstrate that the UL52 gene of herpes simplex virus type 1 is required for virus growth and DNA synthesis. J. Virol. 62:2970-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris, S. L., I. Frank, A. Yee, G. H. Cohen, R. J. Eisenberg, and H. M. Friedman. 1990. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J. Infect. Dis. 162:331-337. [DOI] [PubMed] [Google Scholar]
- 20.Haurum, J. S., S. Thiel, I. M. Jones, P. B. Fischer, S. B. Laursen, and J. C. Jensenius. 1993. Complement activation upon binding of mannan-binding protein to HIV envelope glycoproteins. AIDS 7:1307-1313. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi, S., S. Ogawa, Y. Takashima, and H. Otsuka. 2004. The neutralization of pseudorabies virus by anti-alpha-galactocyl natural antibody in normal serum. Virus Res. 99:1-7. [DOI] [PubMed] [Google Scholar]
- 22.Hill, A., P. Jugovic, I. York, G. Russ, J. Bennink, J. Yewdell, H. Ploegh, and D. Johnson. 1995. Herpes simplex virus turns off the TAP to evade host immunity. Nature 375:411-415. [DOI] [PubMed] [Google Scholar]
- 23.Hung, S. L., C. Peng, I. Kostavasili, H. M. Friedman, J. D. Lambris, R. J. Eisenberg, and G. H. Cohen. 1994. The interaction of glycoprotein C of herpes simplex virus types 1 and 2 with the alternative complement pathway. Virology 203:299-312. [DOI] [PubMed] [Google Scholar]
- 24.Hung, S. L., S. Srinivasan, H. M. Friedman, R. J. Eisenberg, and G. H. Cohen. 1992. Structural basis of C3b binding by glycoprotein C of herpes simplex virus. J. Virol. 66:4013-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, D. C., and V. Feenstra. 1987. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J. Virol. 61:2208-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, D. C., M. C. Frame, M. W. Ligas, A. M. Cross, and N. D. Stow. 1988. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J. Virol. 62:1347-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson, D. C., M. R. McDermott, C. Chrisp, and J. C. Glorioso. 1986. Pathogenicity in mice of herpes simplex virus type 2 mutants unable to express glycoprotein C. J. Virol. 58:36-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kase, T., Y. Suzuki, T. Kawai, T. Sakamoto, K. Ohtani, S. Eda, A. Maeda, Y. Okuno, T. Kurimura, and N. Wakamiya. 1999. Human mannan-binding lectin inhibits the infection of influenza A virus without complement. Immunology 97:385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katragadda, M., D. Morikis, and J. D. Lambris. 2004. Thermodynamic studies on the interaction of the third complement component and its inhibitor, compstatin. J. Biol. Chem. 279:54987-54995. [DOI] [PubMed] [Google Scholar]
- 30.Klepeis, J. L., C. A. Floudas, D. Morikis, C. G. Tsokos, E. Argyropoulos, L. Spruce, and J. D. Lambris. 2003. Integrated computational and experimental approach for lead optimization and design of compstatin variants with improved activity. J. Am. Chem. Soc. 125:8422-8423. [DOI] [PubMed] [Google Scholar]
- 31.Klerx, J. P., C. J. Beukelman, H. Van Dijk, and J. M. Willers. 1983. Microassay for colorimetric estimation of complement activity in guinea pig, human and mouse serum. J. Immunol. Methods 63:215-220. [DOI] [PubMed] [Google Scholar]
- 32.Kostavasili, I., A. Sahu, H. M. Friedman, R. J. Eisenberg, G. H. Cohen, and J. D. Lambris. 1997. Mechanism of complement inactivation by glycoprotein C of herpes simplex virus. J. Immunol. 158:1763-1771. [PubMed] [Google Scholar]
- 33.Lachmann, P. J., and A. Davies. 1997. Complement and immunity to viruses. Immunol. Rev. 159:69-77. [DOI] [PubMed] [Google Scholar]
- 34.Lubinski, J., L. Wang, D. Mastellos, A. Sahu, J. D. Lambris, and H. M. Friedman. 1999. In vivo role of complement-interacting domains of herpes simplex virus type 1 glycoprotein gC. J. Exp. Med. 190:1637-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lubinski, J. M., M. Jiang, L. Hook, Y. Chang, C. Sarver, D. Mastellos, J. D. Lambris, G. H. Cohen, R. J. Eisenberg, and H. M. Friedman. 2002. Herpes simplex virus type 1 evades the effects of antibody and complement in vivo. J. Virol. 76:9232-9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lubinski, J. M., L. Wang, A. M. Soulika, R. Burger, R. A. Wetsel, H. Colten, G. H. Cohen, R. J. Eisenberg, J. D. Lambris, and H. M. Friedman. 1998. Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo. J. Virol. 72:8257-8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKane, B. W., S. Ramachandran, J. Yang, X. C. Xu, and T. Mohanakumar. 2003. Xenoreactive anti-Gα(1,3)Gal antibodies prevent porcine endogenous retrovirus infection of human in vivo. Hum. Immunol. 64:708-717. [DOI] [PubMed] [Google Scholar]
- 38.McNearney, T. A., C. Odell, V. M. Holers, P. G. Spear, and J. P. Atkinson. 1987. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J. Exp. Med. 166:1525-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melroe, G. T., N. A. DeLuca, and D. M. Knipe. 2004. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J. Virol. 78:8411-8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagashunmugam, T., J. Lubinski, L. Wang, L. T. Goldstein, B. S. Weeks, P. Sundaresan, E. H. Kang, G. Dubin, and H. M. Friedman. 1998. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 72:5351-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ochsenbein, A. F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R. M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286:2156-2159. [DOI] [PubMed] [Google Scholar]
- 42.Ochsenbein, A. F., and R. M. Zinkernagel. 2000. Natural antibodies and complement link innate and acquired immunity. Immunol. Today 21:624-630. [DOI] [PubMed] [Google Scholar]
- 43.Quinn, G., J. C. Wood, D. J. Ryan, K. M. Suling, K. M. Moran, D. L. Kolber-Simonds, J. L. Greenstein, H. J. Schuurman, R. J. Hawley, and C. Patience. 2004. Porcine endogenous retrovirus transmission characteristics of galactose α1-3 galactose-deficient pig cells. J. Virol. 78:5805-5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rux, A. H., H. Lou, J. D. Lambris, H. M. Friedman, R. J. Eisenberg, and G. H. Cohen. 2002. Kinetic analysis of glycoprotein C of herpes simplex virus types 1 and 2 binding to heparin, heparan sulfate, and complement component C3b. Virology 294:324-332. [DOI] [PubMed] [Google Scholar]
- 45.Sahu, A., B. K. Kay, and J. D. Lambris. 1996. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J. Immunol. 157:884-891. [PubMed] [Google Scholar]
- 46.Seidel-Dugan, C., M. Ponce de Leon, H. M. Friedman, R. J. Eisenberg, and G. H. Cohen. 1990. Identification of C3b-binding regions on herpes simplex virus type 2 glycoprotein C. J. Virol. 64:1897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeuchi, Y., C. D. Porter, K. M. Strahan, A. F. Preece, K. Gustafsson, F. L. Cosset, R. A. Weiss, and M. K. Collins. 1996. Sensitization of cells and retroviruses to human serum by (alpha 1-3) galactosyltransferase. Nature 379:85-88. [DOI] [PubMed] [Google Scholar]
- 48.Tal-Singer, R., C. Seidel-Dugan, L. Fries, H. P. Huemer, R. J. Eisenberg, G. H. Cohen, and H. M. Friedman. 1991. Herpes simplex virus glycoprotein C is a receptor for complement component iC3b. J. Infect. Dis. 164:750-753. [DOI] [PubMed] [Google Scholar]
- 49.Van Vliet, K. E., L. A. De Graaf-Miltenburg, J. Verhoef, and J. A. Van Strijp. 1992. Direct evidence for antibody bipolar bridging on herpes simplex virus-infected cells. Immunology 77:109-115. [PMC free article] [PubMed] [Google Scholar]
- 50.Welsh, R. M., C. L. O'Donnell, D. J. Reed, and R. P. Rother. 1998. Evaluation of the Galα1-3Gal epitope as a host modification factor eliciting natural humoral immunity to enveloped viruses. J. Virol. 72:4650-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.York, I. A., C. Roop, D. W. Andrews, S. R. Riddell, F. L. Graham, and D. C. Johnson. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 77:525-535. [DOI] [PubMed] [Google Scholar]