Abstract
Cytoplasmic hepatitis B virus (HBV) capsids are not enveloped and secreted unless the packaged RNA pregenome is reverse transcribed. The expression of the capsid protein C, together with envelope proteins in the absence of pregenomic RNA, produced normal amounts of intracellular capsids, but the secretion of virion-like particles was greatly reduced. The I97L C protein mutant, allowing immature nucleocapsid envelopment in the background of an HBV genome, did not promote the envelopment of capsids lacking a pregenome, suggesting that this mutation is not sufficient to induce secretion competence independently of the pregenome.
Hepatitis B virus (HBV) particles consist of an icosahedral nucleocapsid (approximately 30 nm diameter) surrounded by an envelope carrying hydrophobic surface proteins (2). For capsid formation, a terminal redundant RNA molecule (pregenome) is bound by the viral P protein and packaged by multiple copies of the C protein. The pregenome is then reverse transcribed by P in the lumen of the particle and finally converted to double-stranded DNA. The DNA-containing nucleocapsid can be enveloped by the viral surface proteins, generating virions. Interestingly, immature (that means RNA-containing) capsids are efficiently excluded from budding, in contrast to mature DNA-containing capsids (3, 6, 9, 12). Apparently, viral DNA synthesis in the lumen of the particle is coupled to a change in the capsid that can be sensed by the envelopment machinery. The nature of this signal, however, is not well defined. In the case of duck hepatitis B virus capsids, maturation may be linked to dephosphorylation of the C protein (10).
C protein self-assembles into icosahedral capsids when expressed in Escherichia coli. In this case, unspecific cellular RNA is packaged (4). The crystal structure of capsids that are built by C-terminally truncated C proteins containing no detectable nucleic acid has been determined (13). It is unclear whether this conformation resembles the one of RNA-containing immature or DNA-containing mature capsids.
In order to address the question of whether recombinant HBV capsids consisting of full-length C protein but lacking HBV-specific genomic nucleic acid will be enveloped, we expressed the wild-type (WT) HBV C protein together with viral envelope proteins from vectors not allowing the synthesis of the pregenome or P protein in human hepatoma cells (Huh7). For this purpose, the cells were transiently transfected in 10-cm dishes with 10 μg of plasmids, and naked capsids and virion-like particles (VLPs) in medium that was harvested after 4 days were separated by isopycnic CsCl gradient centrifugation (3). Capsids were precipitated from 1-ml fractions by adding 1 ml of 1% (vol/vol) mild detergent (IGEPAL CA 630; ICN Biomedicals, Inc., Aurora, Ohio) and 2 ml of 10% (wt/vol) polyethylene glycol 8000-2% (wt/vol) NaCl, incubating overnight at 4°C, and spinning at 4,000 rpm at 4°C for 1 h. The pellet was dissolved and loaded on a 1% agarose-Tris-acetate-EDTA gel. After electrophoresis, capsids were detected by Western blotting by using a rabbit anticapsid antiserum (DAKO, Hamburg, Germany) (5).
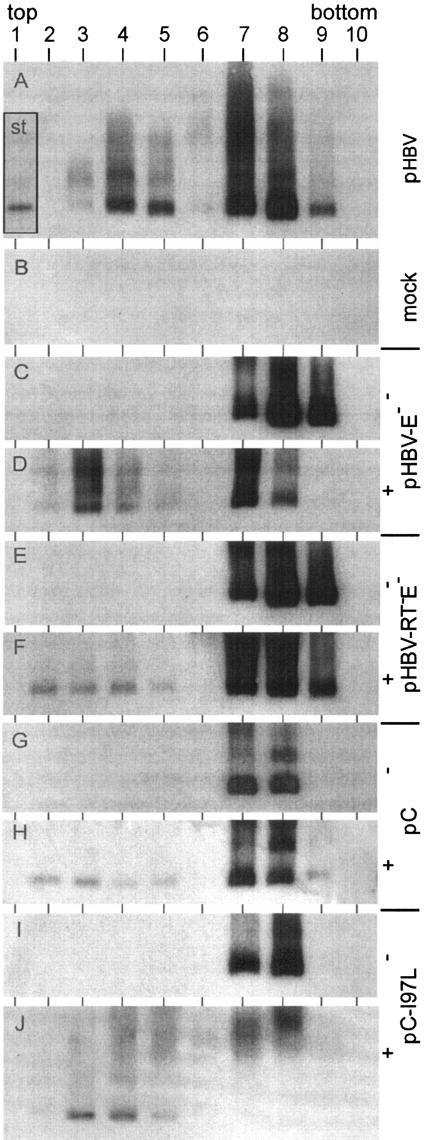
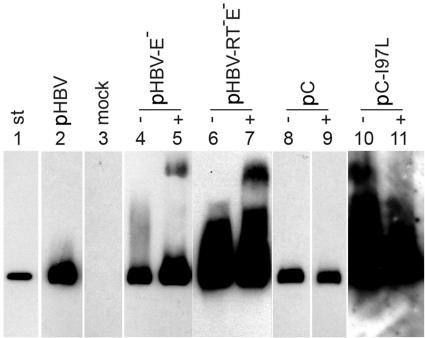
When Huh7 cells were transfected with a plasmid carrying the WT HBV genome (genotype A) (Fig. 1, top), the Western blot showed strong signals in fractions 3 to 5 (Fig. 2A), which had buoyant densities between 1.23 and 1.27 g/ml, indicative for virions. This demonstrates that the detection of virions by using the capsid antigen as a marker was well feasible in this experimental approach. In most experiments, a second peak was visible in fractions 7 to 9, with buoyant densities of 1.32 g/ml to 1.37 g/ml being typical for naked capsids containing RNA. For unknown reasons, the signals of this peak differed, ranging from undetectable to very strong, in different transfections using the same plasmids. Naked HBV capsids in the media of transfected cells have also been observed by others (8). The source of this material is unknown. The capsid-positive lanes contained additional antibody-reactive bands, probably corresponding to capsid aggregates, located closer to the gel pocket. As a control, capsids were prepared in parallel from cell lysates and detected in the same way (Fig. 3, lane 2). Cytoplasmic capsids formed no or only small amounts of aggregates. The reason for the different aggregations of cytoplasmic and culture medium-derived capsids in the agarose gel is unknown to us. Material from mock-transfected cells generated no signal on the Western blots (Fig. 2B and 3, lane 3).
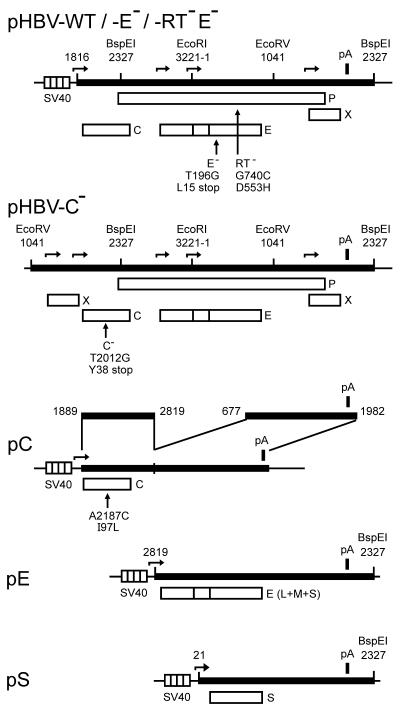
FIG. 1.
Map of plasmids. Plasmid pHBV-WT contains a 1.2-mer overlength copy of the HBV genotype A genome (black bar). Numbering of the 3,221-bp-long genome starts with the deoxycytidine nucleotide of the unique EcoRI site. Some restriction enzyme sites and their positions are indicated for orientation. The transcription of the pregenome starts from the SV40 early promoter (striped box). Transcription start sites from the SV40 and HBV promoters are indicated by horizontal arrows. The HBV polyadenylation signal (pA) is shown as a black box. The four open reading frames C (core), E (envelope), P, and X are depicted as open boxes. Plasmid pHBV-E− carries a point mutation that creates a stop codon in the E frame. This mutation is silent in P. Plasmid pHBV-RT−E− carries an additional point mutation in P that destroys the reverse transcriptase activity of the P protein. Plasmid pHBV-C− carries a 1.4-mer of the HBV genome. The pregenome is expressed from the authentic core promoter. The C gene carries a missense mutation. Plasmid pC expresses only the C protein. The point mutation C-I97L has been introduced into this plasmid. Plasmid pE directs the expression of all three HBV envelope proteins (L, M, and S). Plasmid pS expresses only the small envelope protein S.
FIG. 2.
Detection of virion-like particles and naked capsids in culture medium. Medium of Huh7 cells transiently transfected with the plasmids (Fig. 1) indicated on the right was fractionated by isopycnic CsCl gradient centrifugation. HBV capsids in the fractions were precipitated after the addition of mild detergent, separated through an agarose gel, and depicted by Western blotting. Naked capsids appeared in fractions 7 to 9, and enveloped virions appeared in fractions 3 to 5. −, no cotransfection; +, cotransfection of plasmids pE and pS (Fig. 1) for envelope protein expression; st inserted in panel A, 1 ng of purified capsids expressed in E. coli.
FIG. 3.
Detection of cytoplasmic capsids. Cytoplasmic capsids of Huh7 cells transiently transfected with the indicated plasmids (Fig. 1) were precipitated, separated by agarose gel electrophoresis, and depicted by Western blotting. Ten times the amount of material was loaded for lanes 6, 7, 10, and 11 relative to lanes 2 to 5 and 8 and 9. −, without cotransfection; +, with cotransfection of plasmids pE and pS for envelope protein expression (Fig. 1); st, 1 ng of purified capsids expressed in E. coli.
When the HBV C protein was expressed without P and envelope protein coexpression and in the absence of a pregenome by transfection of the vector pC (Fig. 1), naked capsids could be found in the culture medium in most experiments (Fig. 2G). VLPs could not be detected, as expected, because the HBV envelope proteins are required for nucleocapsid envelopment (1). In order to coexpress a functional set of viral surface proteins, together with C protein, the two expression vectors pE and pS (Fig. 1) were cotransfected with plasmid pC. All three HBV envelope proteins, S, M, and L, were expressed from plasmid pE. Plasmid pS directed the expression of additional S protein. The usage of both plasmids guaranteed a balanced ratio between L and S proteins that was necessary for efficient nucleocapsid envelopment (see below) (11). In the cotransfection experiment, again naked capsids could be found in fractions 7 and 8 (Fig. 2H) and, in addition, weak signals were visible in fractions 2 to 5, having buoyant densities between 1.21 g/ml and 1.26 g/ml (density of peak fraction 3, 1.228 g/ml). In comparison to WT virions (Fig. 2A) (densities of fractions 3 to 5, 1.23 to 1.27 g/ml; density of peak fraction 4, 1.248 g/ml), this VLP peak was reproducibly extended to lower density fractions and much weaker. The amounts of cytoplasmic capsids, however, were similar (Fig. 3, lanes 2 and 9). Apparently, the envelopment and secretion of capsids containing no functional pregenome-P protein complex were inefficient and possibly abnormal. Such mechanisms would assure that such capsids which might be formed in the infected liver (7), e.g., because of an unbalanced C protein versus pregenome-P protein expression, are only inefficiently incorporated into virion-like particles.
The unproductive and aberrant formation of VLPs in the case of C plus envelope protein coexpression was not due to nonfunctional envelope proteins, because a viral genome carrying a stop codon blocking S, M, and L expression (Fig. 1, plasmid pHBV-E−), and therefore unable to form virions (Fig. 2C), could efficiently be transcomplemented by the two vectors (Fig. 2D). An HBV mutant with an additional point mutation inactivating the reverse transcriptase activity of the P protein (Fig. 1, plasmid pHBV-RT−E−) forms only immature, RNA-containing nucleocapsids which are not or only inefficiently enveloped (3). The complementation of the E− mutation of the RT−E− genome by pE/pS cotransfection generated small amounts of VLPs (Fig. 2F) in a manner very similar to that of the coexpression of C and envelope proteins (Fig. 2H). This result demonstrates that capsids containing no pregenome and P protein behaved like immature particles carrying the pregenome in the assay. The fact that small amounts of VLPs were visible in these assays whereas no VLPs could be detected in RNase protection experiments with the envelope-complemented RT−E− mutant (3) may be due to C protein overexpression by the simian virus 40 (SV40) vector that was used in the present work, whereas Gerelsaikhan et al. expressed the C protein from the authentic HBV promoter.
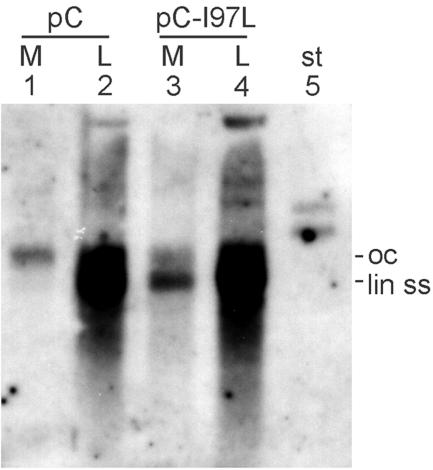
A core protein point mutation (isoleucine 97 changed to leucine, C-I97L) has been described as causing the envelopment and secretion of relatively immature capsids (14) containing predominantly single-stranded DNA, an intermediate of viral genome replication. We introduced this point mutation into the C protein expression vector pC (Fig. 1) and confirmed the phenotype (Fig. 4) by transcomplementation of a C-negative HBV genome (Fig. 1). As shown by Southern blotting, complementation with the WT C gene generated virions containing mainly double-stranded, mature DNA (Fig. 4, lane 1), while cytoplasmic capsids contained the whole set of replicative intermediates (lane 2). This result reflects the selective envelopment and release of mature nucleocapsids. In contrast, complementation with the C-I97L mutant produced virions carrying mainly single-stranded DNA (lane 3), while the pattern of replicative intermediates in cytoplasmic nucleocapsids was unchanged (lane 4). When the C-I97L mutant core protein was coexpressed with envelope proteins in the absence of the pregenome and the P protein, only small amounts of VLPs, similar to those observed with the RT− mutant and with the coexpression of WT C and envelope proteins, could be observed (Fig. 2J). Apparently, the C-I97L mutation was not sufficient to induce a constitutive “mature” status of the capsid independent of the pregenome-P protein. Possibly, this mutation supports an early appearance of the maturation state still dependent on viral DNA synthesis. Therefore it seems not to be possible to unravel the maturation signal by comparing the crystal structures of recombinant capsids composed of the WT and I97L mutant C proteins, respectively.
FIG. 4.
Phenotype of the C protein I97L mutation. Huh7 cells were transiently cotransfected with plasmid pHBV-C− carrying an HBV genome with a nonfunctional C gene (Fig. 1) together with either plasmid pC or plasmid pC-I97L for C gene complementation. Viral DNA was isolated from cytoplasmic capsids in cell lysates (L) and from culture medium (M) and depicted by Southern blotting. The WT C protein blocked the envelopment and release of capsids carrying immature viral DNA, while the C-I97L mutant released this restriction. st, 10 pg of undigested plasmid pC (5.7 kbp); oc, open circular; lin ss, linear double stranded.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 402, project C2.
REFERENCES
- 1.Bruss, V., and D. Ganem. 1991. The role of envelope proteins in hepatitis B virus assembly. Proc. Natl. Acad. Sci. USA 88:1059-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganem, D., and R. J. Schneider. 2001. Hepadnaviridae: the viruses and their replication, p. 2923-2969. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 3.Gerelsaikhan, T., J. E. Tavis, and V. Bruss. 1996. Hepatitis B virus nucleocapsid envelopment does not occur without genomic DNA synthesis. J. Virol. 70:4269-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kann, M., and W. H. Gerlich. 1994. Effect of core protein phosphorylation by protein kinase C on encapsidation of RNA within core particles of hepatitis B virus. J. Virol. 68:7993-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koschel, M., D. Oed, T. Gerelsaikhan, R. Thomssen, and V. Bruss. 2000. Hepatitis B virus core gene mutations which block nucleocapsid envelopment. J. Virol. 74:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mabit, H., and H. Schaller. 2000. Intracellular hepadnavirus nucleocapsids are selected for secretion by envelope protein-independent membrane binding. J. Virol. 74:11472-11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michalak, T., and A. Nowoslawski. 1982. Crystalline aggregates of hepatitis B core particles in cytoplasm of hepatocytes. Intervirology 17:247-252. [DOI] [PubMed] [Google Scholar]
- 8.Nassal, M. 1992. The arginine-rich domain of the hepatitis B virus core protein is required for pregenome encapsidation and productive viral positive-strand DNA synthesis but not for virus assembly. J. Virol. 66:4107-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perlman, D., and J. Hu. 2003. Duck hepatitis B virus virion secretion requires a double-stranded DNA genome. J. Virol. 77:2287-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perlman, D. H., E. A. Berg, P. B. O'Connor, C. E. Costello, and J. Hu. 2005. Reverse transcription-associated dephosphorylation of hepadnavirus nucleocapsids. Proc. Natl. Acad. Sci. USA 102:9020-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Persing, D. H., H. E. Varmus, and D. Ganem. 1986. Inhibition of secretion of hepatitis B surface antigen by a related presurface polypeptide. Science 234:1388-1391. [DOI] [PubMed] [Google Scholar]
- 12.Wei, Y., J. E. Tavis, and D. Ganem. 1996. Relationship between viral DNA synthesis and virion envelopment in hepatitis B viruses. J. Virol. 70:6455-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wynne, S. A., R. A. Crowther, and A. G. Leslie. 1999. The crystal structure of the human hepatitis B virus capsid. Mol. Cell 3:771-780. [DOI] [PubMed] [Google Scholar]
- 14.Yuan, T. T., G. K. Sahu, W. E. Whitehead, R. Greenberg, and C. Shih. 1999. The mechanism of an immature secretion phenotype of a highly frequent naturally occurring missense mutation at codon 97 of human hepatitis B virus core antigen. J. Virol. 73:5731-5740. [DOI] [PMC free article] [PubMed] [Google Scholar]