Abstract
The Trim5α protein from several primates restricts retroviruses in a capsid (CA)-dependent manner. In owl monkeys, the B30.2 domain of Trim5 has been replaced by cyclophilin A (CypA) following a retrotransposition. Restriction of human immunodeficiency virus type 1 (HIV-1) by the resulting Trim5-CypA fusion protein depends on CA binding to CypA, suggesting both that the B30.2 domain might be involved in CA binding and that the tripartite RING motif, B-BOX, and coiled coil (RBCC) motif domain can function independently of the B30.2 domain in restriction. To investigate the potential of RBCCs from other Trims to participate in restricting HIV-1, CypA was fused to the RBCC of Trim1, Trim18, and Trim19 and tested for restriction. Despite low identity within the RBCC domain, all fusion proteins were found to restrict HIV-1 but not the nonbinding G89V mutant, indicating that the overall structure of RBCC and not its primary sequence was important for the restriction function. The critical interaction between CA and Trim-CypA appears to take place soon after viral entry. Quantitative PCR analysis of viral reverse transcriptase products revealed that the different fusion proteins block HIV-1 at two distinct stages of its life cycle, either prior to reverse transcription or just before integration. With Trim1 and Trim18, this timing is dependent on the length of the Trim component of the fusion protein. These observations suggest that restriction factor binding can have different mechanistic consequences.
Retroviruses are restricted by a number of host cellular proteins. Some of these factors act at an early stage of the life cycle in a capsid (CA)-dependent manner (36). The prototypic example is the murine gene Fv1, which blocks the replication of murine leukemia virus (MLV) (23). It was found to act postentry but preintegration, with the products of reverse transcription from the restricted virus being detected in infected cells. There are two major alleles of Fv1: Fv1n, which restricts B-tropic virus, and Fv1b, which prevents infection by N-tropic virus. A single amino acid change in residue 110 of the MLV CA can alter MLV from N-tropic to B-tropic and vice versa (22). Similar blocks were subsequently described in a number of primate cells that restrict N-MLV but not B-MLV, suggesting a CA-mediated interaction (38). However, in contrast to Fv1, this restriction factor, Ref1, prevented reverse transcription of N-MLV RNA.
Human immunodeficiency virus type 1 (HIV-1) was also found to be restricted in cells from Old World monkeys and the owl monkey (2, 10, 14). Replacing the HIV-1 CA with that from simian immunodeficiency virus could alleviate this block, indicating CA dependence. This restriction factor, which was termed Lv1, also prevented the initiation of reverse transcription. Similarities between the mode of action of Ref1 and Lv1 prompted experiments showing that preincubation of African green monkey cells with N-MLV, which was restricted in these cells, could abolish the block to subsequent HIV-1 infection, thereby suggesting that Ref1 and Lv1 might be identical (12).
Functional cloning from a rhesus monkey cDNA library led to the identification of Trim5α as a gene that restricted HIV-1 in a CA-specific manner (37). Trim5α is a member of the Trim family of proteins, named after the tripartite motif that they possess, comprising a RING motif, B-BOX, and coiled coil (RBCC) (32). Like many, but not all, members of the Trim family, it also possesses a B30.2 domain downstream of the RBCC (28). Subsequently, Trim5α from African green monkeys was also found to restrict HIV-1 and restriction of N-MLV was shown by the Trim5α from humans and rhesus and African green monkeys (13, 21, 31, 41). These observations, together with the increase of N-MLV titers in cells treated with small interfering RNA to Trim5α, implied that Trim5α was the major contributor to Ref1 activity in primates. However, Trim1 was also shown to restrict N-MLV but not B-MLV, suggesting that other Trim proteins could have antiretroviral activity (41).
In owl monkeys, the Trim5 gene contains a retrotransposed insertion of cyclophilin A (CypA) between exons 7 and 8 (27, 33). One of the mRNA isoforms produced from this locus, V4, is translated into a Trim5-CypA (T5C) fusion protein (27). This protein restricts HIV-1 but not the G89V CA mutant, which does not bind CypA. Only the isoform that contained the intact CypA domain could bind and restrict HIV-1, suggesting that the restriction factor acts by binding HIV-1 CA via the CypA domain (27). Since Trim5α and T5C contain similar RBCC motifs, the results also implied that the B30.2 domain of Trim5α could have the same role as CypA in restriction.
Despite sharing more than 80% homology with the rhesus Trim5α, the human protein shows little restriction of HIV-1. Replacing the B30.2 domain in human Trim5α with the corresponding region from the rhesus protein was sufficient to confer the ability to restrict HIV-1. Exchanging the B30.2 domain of Trim18, which does not restrict N-MLV, with that from Trim1, which does, results in a chimeric protein capable of restriction, confirming the involvement of the B30.2 domain in CA recognition (42). This result also suggested that the RBCC from Trims that do not normally restrict retroviruses could have the potential to do so when combined with a CA-binding domain.
An investigation into the potential of the RBCC from other Trim proteins in restricting HIV-1 could shed light on the role played by the tripartite motif in restriction. In addition, since the Trim proteins have been reported to define different cellular compartments (32), restricting Trim proteins could act as beacons for tracking the path taken by the virus to get to the nucleus. Hence, we set out to test the ability of the RBCCs from Trim1, Trim18, and Trim19 to restrict HIV-1 in fusion with CypA. These studies form the basis of this report.
MATERIALS AND METHODS
Cells and viruses.
All cell lines were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and antibiotics. Viruses were prepared as previously described (35) by transfection of 293T cells with 7 μg each of vesicular stomatitis virus G, Gag-Pol, or vector plasmids. For making viruses to deliver the restriction factors, pHIT60 was used for expressing the Moloney MLV Gag-Pol (35). MLV tester viruses were made with pLNCG (41) and pCIGN (N-tropic) or pCIGB (B-tropic) (3). HIV-1 tester viruses were made with pCSGW (enhanced green fluorescent protein [EGFP] vector) (1) and p8.91 (wild type) (45) or pG89V (CA mutant) (41).
Construction of fusion proteins.
Chimeric proteins are denoted T (for Trim), a number (identifying the Trim), C (cyclophilin A), and S, M, or L (short, medium, or long form). Fusion proteins were made by overlapping PCR using Pfu Ultra (Stratagene). Primers are listed in Table 1. RBCCs were amplified from Trim1α (GenBank AY625005), Trim18α (AF230976), or Trim19 (S50913) cloned in previously described vectors, while CypA was amplified from OMKTrim5CypA V4 (27, 41). Primer pairs T1F-T1CS2 and T1CS3-CR were used for T1CS, while T1F-T1CL2 and T1CL3-CR were used for T1CL. Primer pairs T1F-T1CM2 and T1CM3-CR were used to construct T1CM. Primer pairs T18F-T18CS2 and T18CS3-CR were used for T18CS, while T18F-T18CL2 and T18CL3-CR were used for T18CL. Finally, primer pairs T19F-T19C2 and T19C3-CR were used for T19C.
TABLE 1.
Primers used in this study
| Primer | Sequencea |
|---|---|
| T1F | CACCATGGAAACACTGGAGTC |
| T1CS2 | CGGCGGCGTCGATGAGGACTGTTGACCGTTC |
| T1CS3 | AGTCCTCATCGACGCCGCCGCCTGGGACCTTG |
| CR | TTAAAGTTGTCCACAGTCCAC |
| T1CL2 | CGGCGGCGTCTCGGGTAGGTTCACTGTTCC |
| T1CL3 | ACCTACCCGAGACGCCGCCGCCTGGGACCTTG |
| T1CM2 | CGGCGGCGTCGGCTGTTAAATAATCTAACCCC |
| T1CM3 | TTTAACAGCCGACGCCGCCGCCTGGGACCTTG |
| T18F | CACCATGGAAACACTGGAGTCAG |
| T18CS2 | CGGCGGCGTCGATGAGTGATGCTGACCGCTC |
| T18CS3 | ATCACTCATCGACGCCGCCGCCTGGGACCTTG |
| T18CL2 | CGGCGGCGTCCTTCCCAGGCTCACTGCTGC |
| T18CL3 | GCCTGGGAAGGACGCCGCCGCCTGGGACCTTG |
| T19F | CACCATGGAGCCTGCACCCGCCCG |
| T19C2 | CGGCGGCGTCTTTCCCCTGGGTGATGCAAGAG |
| T19C3 | CCAGGGGAAAGACGCCGCCGCCTGGGACCTTG |
| CHAR | CTCGAGTTAAGCGTAATCTGGAACATCGTATG GGTATTCGAGTTGTCCACAGTC |
| NeoF | ATGATTGAACAAGATGGATTG |
| NeoR | TGACAGCCGGAACACGGCGG |
| HIVEF | TCTGGCTAACTAGGGAACCCA |
| HIVER | CTGACTAAAAGGGTCTGAGG |
| 2LTRF | AACTAGGGAACCCACTGCTTAAG |
| 2LTRR | TTGTCTTCGTTGGGAGTGAATTAG |
| AluR | TGCTGGGATTACAGGCGTGAG |
| Alu probe | (FAM)ACACTACTTGAAGCACTCAAGGCAAGCT TT(TAMRA) |
FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine.
The two PCR products were gel purified using a QIAquick gel extraction kit (QIAGEN) and joined in a second PCR using Trim1F and CR for T1CS, T1CM, and T1CL; T18F and CR for T18CS and T18CL; and T19F and CR for T19C. The C-terminal hemagglutinin (HA)-tagged fusion proteins were made by PCR using the forward primers for the respective Trims together with the reverse primer CHAR, which contains the sequence encoding amino acid residues 99 to 107 of the influenza virus hemagglutinin. All PCR products were cloned into pENTR-Topo and transferred into pLGatewayIY carrying the enhanced yellow fluorescent protein (YFP) gene for restriction assay or pLGatewaySN (M. Yap, unpublished data) for microscopy. The structures of all plasmids were verified by DNA sequencing.
Restriction assay.
Assays in HT1080 cells were performed by two-color fluorescence-activated cell sorting (FACS) analysis as previously described (14, 17).
Quantitative PCR of reverse transcription products, 2-LTR circles, and proviruses.
Virus stocks were treated with RQ1 DNase (Promega) at 10 units/ml for 1 h at room temperature to remove any DNA that could be carried over from transfection during viral production. Cells that had been previously transduced with the restriction factors at a multiplicity of infection (MOI) of >10 were infected with the DNase-treated virus at an MOI of 1. Total genomic DNA of the infected cells was extracted using the DNeasy tissue kit (QIAGEN) at 7, 24, and 48 h postinfection for the detection of reverse transcription products, circles formed by apparent end ligation of unintegrated linear viral DNA (2-LTR circles), and integrated proviruses, respectively.
MLV late reverse transcriptase (RT) products were detected using primers directed against the neomycin resistance gene in the vector (NeoF and NeoR; see Table 1). HIV-1 early RT products were detected using primers that recognized the RU5 sequence (HIVEF and HIVER), while 2-LTR circles were detected using the primer pair 2LTRF and 2LTRR. Integrated proviruses were detected as previously described (8) using primer pair 2LTRF and AluR together with an Alu probe. Quantitative PCR for detection of reverse transcription products and 2-LTR circles was performed using the ABI Prism 7000 sequence detection system from Applied Biosystems with either 150 ng (reverse transcription products) or 250 ng (2-LTR circles) of total DNA, 70 nM of each primer, and 1× SYBR Green mix (ABgene) in a reaction volume of 25 μl. Quantitative PCR for integrated provirus was performed in a reaction volume of 25 μl containing 250 ng of total DNA, 40 μM of each primer, and 1× Absolute QPCR mix (Abgene). The program consisted of an initial incubation at 50°C for 2 min, followed by 95°C for 15 min before 40 cycles of 95°C for 15 seconds and 60°C for 1 min. The results were normalized against actin positive controls.
Western blot analysis.
TE671 cells were transduced with MLV-based vectors expressing various Trim-CypA fusions in 12-well plates. The volume of virus added was adjusted so that 70% of the cells were transduced with each construct as determined by FACS analyses. Cells were reseeded into 5-cm dishes and grown to confluence before lysing with 0.5 ml of lysis buffer (1% NP-40, 150 mM NaCl, and 50 mM Tris-HCl, pH 8). The lysate was cleared by centrifugation and 25 μg of protein was boiled in 1× sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) loading buffer containing 2.5 M urea. SDS-PAGE was performed with 10% denaturing gels containing 2.5 M urea, and the separated bands were transferred onto polyvinylidene difluoride membranes (Millipore). The membranes were blocked overnight in phosphate-buffered saline containing 0.1% Tween 20 and 5% milk and probed with monoclonal anti-GFP (Santa Cruz Biotechnology) or monoclonal anti-HA (Sigma-Aldrich). Detection was performed using horseradish peroxidase-conjugated anti-mouse antibody (Pierce) and an ECL kit (Amersham).
RESULTS
RBCC-CypA chimeras restrict HIV-1.
We have previously shown that the B30.2 domain of Trim5α was involved in specificity determination during HIV-1 restriction (42). In addition, we found that Trim1 but not Trim18 restricted N-MLV and the specificity of this restriction also mapped to the B30.2 domain. Substituting CypA for the B30.2 domain of human, rhesus, and African green monkey Trim5α resulted in chimeric proteins that restrict HIV-1 in a CypA-binding dependent manner, suggesting that the RBCC and the B30.2 were acting as two different functional domains (42). Thus, it seems possible that the RBCC of Trim1 and other members of the Trim family could replace that of Trim5α to restrict HIV-1 when fused to CypA. To investigate this possibility, a series of chimeric genes were prepared by fusing the CypA-containing exon of owl monkey T5C to the C terminus of the RBCC from African green monkey Trim1α and Trim18α, as well as human Trim19 (the promyelocytic leukemia gene) and testing for restriction.
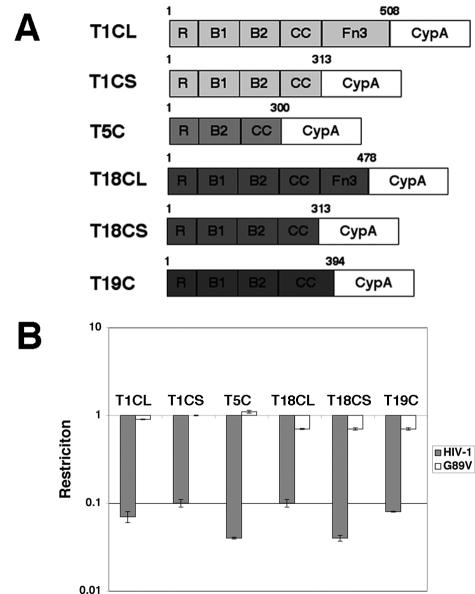
Two CypA chimeras were initially made for both Trim1 and Trim18 (Fig. 1). The first pair consisted of CypA joined to the end of the coiled-coil motif (T1CS and T18CS), while in the second, CypA replaced the B30.2 domains of these proteins (T1CL and T18CL). Trim19 lacks a B30.2 domain and does not restrict HIV-1 when overexpressed in cells (data not shown). It becomes oncogenic when the RBCC region is fused to retinoic acid receptor α at either residue 394 or 552 (17). This suggested that the minimum size of Trim19 RBCC required for activity was at least 394 residues. Hence, a fusion protein was made by joining CypA to the first 394 amino acids of Trim19 (T19C).
FIG. 1.
Restriction of HIV-1 by Trim-CypA fusion proteins. (A) A schematic representation of the fusion proteins is shown with the last amino acid position from the parental Trim indicated on top of each construct at the point of fusion. (B) Bar graph showing restriction. HT1080 cells were transduced with MLV-based vectors carrying the Trim-CypA gene as well as the YFP marker at an MOI of 1. Two days posttransduction, the cells were challenged with HIV-1 or the G89V CA mutant carrying the GFP marker. After 2 further days restriction was measured by FACS analyses. Restriction is measured as a ratio of the percentage of GFP-positive YFP-positive cells to that of GFP-positive YFP-negative cells. A ratio that was less than 0.3 was taken as positive restriction, while a ratio of 0.7 or more showed no restriction.
The fusion genes were cloned into vector pENTR-Topo and transferred into a retroviral vector containing EYFP, pLGatewayIY, by recombination. They were then introduced into human HT1080 cells by transduction and assayed for restriction by challenging the transduced cells with either HIV-1 or the G89V CA mutant carrying the EGFP marker. Both CypA fusion proteins of Trim1 and Trim18 restricted HIV-1 but not the nonbinding mutant G89V (Fig. 1). T19C also restricted HIV-1 in a CypA-dependent manner. This indicated that the RBCCs of members of the Trim family other than Trim5 also had the potential to contribute to the restriction of HIV-1 if they could bind CA by means of CypA.
Trim-CypA fusion proteins act soon after viral entry.
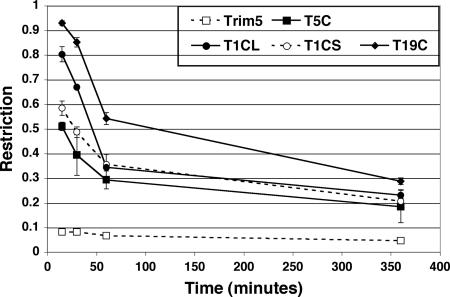
The Trim proteins have been shown to localize to different compartments of the cell (32). This suggests that incoming virions might interact with different Trim-CypA fusion proteins at different stages during their transit of the cell. One way of following the interaction between virus and restriction factor would be to determine the time at which this process occurs after entry. To achieve that, we made use of the ability of the immunosuppressive drug cyclosporine (CsA) to disrupt the binding of CypA to HIV-1 CA (25). Virus entry into cells previously transduced with the various Trim-CypA fusion genes was synchronized by infecting at 4°C, which allows virus binding but prevents entry into the cell. After 1 h, cells were washed with cold phosphate-buffered saline to remove unbound viruses and the temperature was raised to 37°C, allowing fusion to proceed. CsA was then added at various times and the effect on restriction was determined.
As expected, CsA did not affect the ability of Trim5α to restrict HIV-1 (Fig. 2). Addition of CsA in the first 15 min following entry resulted in a significant decrease in restriction, from less than 0.1 (Fig. 1) to greater than 0.5, by T5C, T1CL, T1CS, and T19C. The effect on restriction was less pronounced when CsA was added after 1 h and was essentially abolished when it was added after 6 h. Similar observations were made for T18CS and T18CL (data not shown). These results suggested that the saturating levels of restriction factors bind to incoming virions within 1 hour of viral entry. They also implied that the outcome of the interaction between the restriction factor and the virus was essentially irreversible, since late CsA addition did not affect restriction.
FIG. 2.
Abolition of restriction by CsA. HT1080 cells were transduced with MLV-based vectors carrying the Trim-CypA fusion gene and the YFP marker, and CsA (3 μg/ml) was added at different times following infection. Restriction was measured 2 days later by FACS analysis.
Trim-CypA fusion proteins block HIV-1 at different stages of its life cycle.
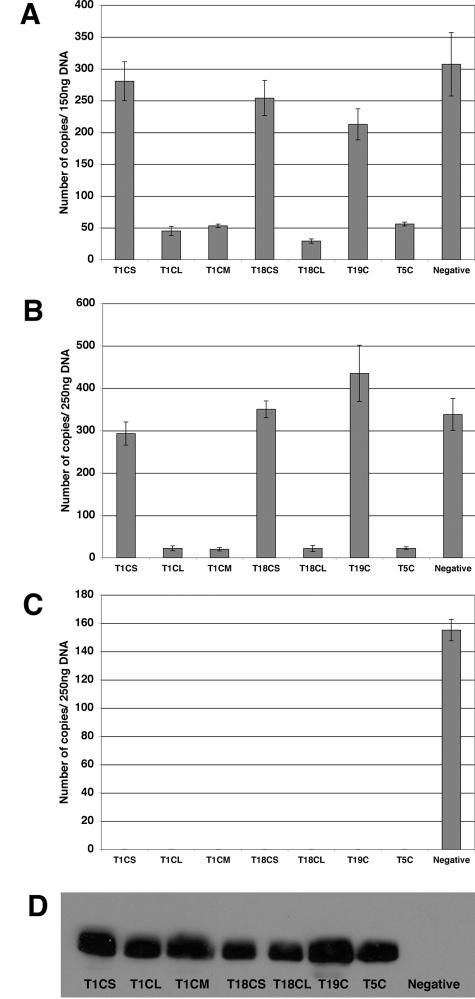
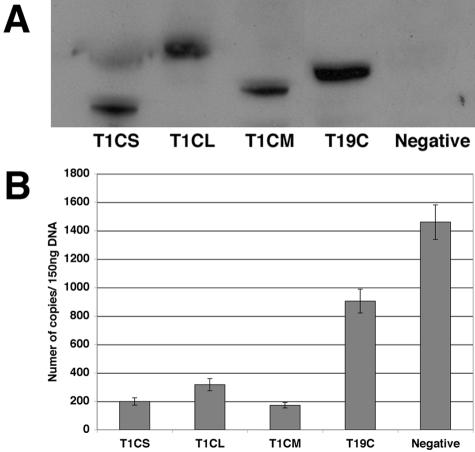
To investigate the nature of the restriction by the different Trim-CypA fusion proteins, we next sought to determine the stage of the viral life cycle that was blocked by the restriction factors. Human TE671 cells were transduced with the HIV-1 restriction genes at high MOIs (>10). The cells were then challenged with equal amounts of N-MLV or HIV-1 2 days posttransduction. Total genomic DNA was extracted from the infected cells either 7, 24, or 48 h postinfection and quantitative PCR was performed to detect early products of reverse transcription, 2-LTR circles, and integrated proviruses, respectively.
In agreement with previous reports (33, 37), much less early HIV-1 RT products were detected in cells transduced with T5C than in cells that were not transduced (Fig. 3A). T1CL and T18CL also seemed to block reverse transcription, with a significant reduction in early RT products compared with the untransduced control. By contrast, the levels of early RT products that were found in cells transduced by T1CS, T18CS and T19C were comparable to those found in the control. Similar results were obtained with the late products of RT (data not shown). This implied that the block by these restriction factors occurred at a post-reverse transcription stage.
FIG. 3.
Trim-CypA fusion proteins block HIV-1 at different stages following entry. Cells were transduced with MLV-based vectors carrying the restricting gene. Two days posttransduction, the cells were challenged with HIV-1 that had been pretreated with DNase. Total DNA was isolated 7, 24, or 48 h following infection and early RT products, 2-LTR circles, and integrated proviruses were quantified, respectively. (A) Quantification of early HIV-1 RT products in TE671 cells. (B) Quantification of late HIV-1 2-LTR circles in TE671 cells. (C) Quantification of integrated proviruses in TE671 cells. (D) Western blot analysis of YFP expressed in Trim-CypA-containing cells. Total cellular protein was extracted from the cells, and 25 μg was separated on a 10% denaturing gel containing 2.5 M urea and blotted. Detection was performed using a monoclonal anti-GFP antibody.
To investigate the effects of the restriction factors on events occurring beyond reverse transcription, the amounts of 2-LTR circles were quantified 24 h postinfection. Although the 2-LTR circles are the dead-end products of the viral life cycle, they are thought to be an indication of productive nuclear entry (6). As expected, the fusion proteins that blocked at reverse transcription (T1CL, T18CL, and T5C) also resulted in a decrease in 2-LTR circles compared to that of the negative control (Fig. 3B). However, cells containing T1CS, T18CS, and T19C, which did not affect reverse transcription, were found to have similar levels of 2-LTR circles as in the negative control, suggesting that the block occurred much later in the infection process, most probably following nuclear entry. It seems unlikely that this late block can be explained by a nuclear localization of these various fusion proteins, since they appear to bind incoming virions very soon after entry. In addition, the nuclear localization signal of T19 (17) is not present in our T19C construct.
In order to assess the effect on integration, the amounts of proviruses in the infected cells were quantitated 48 h postinfection. We could not detect any proviruses in all cells expressing the restriction factors (Fig. 3C). This suggested that T1CS, T18CS, and T19C could impair integration. Hence, although the various restriction factors all appear to bind incoming virions at the same time after viral entry, restriction is manifested at different stages in the viral life cycle.
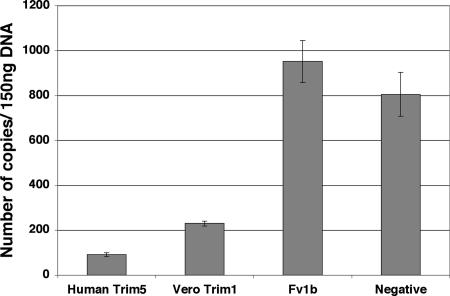
In this context it appeared important to characterize the restriction properties of Trim1 directed against N-MLV (41). Mus dunni cells were transduced with human Trim5α, Vero Trim 1, or the Fv1b gene (MOI > 10) and infected with N-MLV, and the products of reverse transcription were quantified. There was significantly less late MLV RT product in M. dunni cells expressing Vero Trim 1 than in the nontransduced cells, although the reduction was not as great as seen with cells expressing human Trim5α (Fig. 4). This appears consistent with previous observations that Trim5α was the main contributor to the Ref1 activity in human cells (41). Transduced Fv1b, like natural Fv1b in BALB-3T3 cells (19, 40), does not affect reverse transcription.
FIG. 4.
Trim1 blocks N-MLV at reverse transcription. M. dunni cells were transduced with MLV-based vectors carrying the restricting gene. Two days after transduction, the cells were challenged with N-MLV that had been pretreated with DNase. Total DNA was isolated 7 h following infection and late RT products were quantified using a primer pair directed against the neomycin resistance gene carried by the challenge virus.
Although T1CS and T1CL both contained the same RBCC and the same CA binding domain, they seem to block HIV-1 at different stages of the life cycle. One difference between T1CS and T1CL is the presence of a fibronectin type 3 repeat (34) in the latter molecule. To test any involvement of this domain in specifying the stage of restriction, it was removed from T1CL to form T1CM, which contains amino acids 1 to 381 of Trim1 fused to CypA. Despite deletion of the fibronectin repeat, T1CM was found to resemble T1CL in restriction phenotype (Fig. 3), indicating that this domain does not play a role in determining the stage of restriction.
An alternative explanation for the differences in stage of blocking could be due to the levels of fusion proteins present in the cells. To explore this possibility, Western blot analyses were performed. Since our vector expressed both Trim-Cyp and YFP from the same message using an internal ribosome entry site (IRES), we reasoned that the amount of YFP present in these cells would give an indication of the expression levels of the Trim-Cyp fusions. Probing with anti-YFP indicated that the levels of expression were comparable in the cells containing the different Trim-Cyp fusions (Fig. 3D), suggesting that the differences in restriction phenotype observed did not result from differences in expression level. However, we still could not rule out the possibility that the different fusion proteins might have different stabilities in the cell once they were translated.
We therefore sought to detect the fusion proteins directly. Unexpectedly, the fusion proteins could not be detected using polyclonal anti-CypA antibodies from two different sources even though both reacted well with cellular CypA. Since antibodies to Trim1 are not readily available, we prepared C-terminally HA-tagged versions of T1CL, T1CS, T1CM, and T19C. These proteins all restricted HIV-1 but not G89V (data not shown) and were present at similar levels in the transduced cells (Fig. 5A). However, to our surprise, HA-tagged T1CS was found to block reverse transcription (Fig. 5B). Similar results were obtained when the proteins were tagged at the N terminus. A possible explanation is that the HA tag altered the conformation of the original fusion protein, causing it to behave differently from the untagged protein. However, T19C-HA still blocked at a late stage despite being present at levels similar to those of the other proteins that blocked early. Hence, it is unlikely that the differences in stage of block result solely from concentration effects of the restriction factors.
FIG. 5.
Expression of Trim-CypA HA-tagged fusion proteins in TE671 cells. Cells were transduced with MLV-based vectors carrying the restricting gene. Two days posttransduction, the cells were challenged with HIV-1 that had been pretreated with DNase. Total DNA was isolated 7 h following infection and early RT products were quantified. (A) Western blot analysis of HA-tagged Trim-CypA. Total cellular protein was extracted from the cells and 25 μg was separated on a 10% denaturing gel containing 2.5 M urea and blotted. Detection was performed using a monoclonal anti-HA antibody. (B) Quantification of early HIV-1 RT products in TE671 cells.
DISCUSSION
In this study we show that fusing CypA to the RBCC domains of Trim1, Trim18, and Trim19 results in the generation of chimeric proteins capable of restricting HIV-1 replication. Thus, despite sharing less than 20% primary sequence identity to the RBCC domain of the naturally occurring restriction factor found in the owl monkey, there is sufficient similarity to allow functional replacement.
Different roles in restriction have been proposed for the RBCC of Trim5α. The RING domain of some proteins has been shown to possess E3 ligase activity, which is involved in the addition of ubiquitin to proteins targeted for degradation (5, 15, 18, 20, 24, 39). Although it is tempting to speculate that restriction could involve ubiquitin-mediated targeted destruction of CA, a recent report has shown that proteosomal degradation is not required for restriction (30). Alanine substitution of the zinc-coordinating cysteines in the Trim5α RING was shown to reduce but not abolish restriction (37). Hence, although the RING is important for restriction activity, it is not the only component of the RBCC that contributes to restriction, as evidenced by the loss of activity that follows B-box deletion (16, 29). Indeed, the B-box and coiled-coil motifs have been implicated in mediating protein-protein interactions, either with other copies of the same protein or with other proteins to form multiprotein complexes (4, 9, 32). Thus, the RBCC domain may act as a scaffold on which other cellular components of the restriction machinery assemble, possibly leading to the sequestering of incoming viral particles. The RBCC has also been implicated in localization of the Trim proteins (32). Hence, another role of the RBCC in restriction could be to bring the restriction factor to the appropriate cellular compartment through which the incoming virus must pass.
Previous work has suggested that controlled disassembly of CA from reverse transcription complexes is essential for optimal HIV-1 replication (11). It therefore seems possible that restriction factor binding might perturb this process. One might envisage that factor binding could destabilize the reverse transcription complexes, resulting in premature dissociation of the cores and failure to initiate reverse transcription. Factors that block after reverse transcription may act by hyperstabilizing the core, preventing subsequent dissociation required for release of the genetic material for integration. This process might be facilitated by homomultimerizing signals present within the RBCC domain (26, 32).
Our studies show that all the various TrimCypA chimeras bind to CA very soon after viral entry, an observation consistent with a very recent report concerning Trim5-CypA (30). However, two different patterns of restriction were observed, before and after the process of reverse transcription. For the later block, the formation of 2-LTR circles, an indicator of nuclear entry, was not affected. A similar post-reverse transcription block of MLV in hamster cells that also does not affect the formation of 2-LTR circles has recently been reported (7). These results stand in contrast to Fv1, which does not block reverse transcription but does reduce the number of 2-LTR circles (19). The restriction of simian immunodeficiency virus by squirrel monkey Trim5 was also reported to block after reverse transcription; it would be interesting to determine whether production of 2-LTR circles was affected (44).
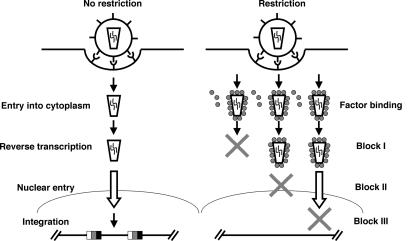
It therefore follows that CA-targeted restriction can result in blocks at three different stages of the viral life cycle: prior to reverse transcription, pre-2-LTR circle, and post-2-LTR circle (Fig. 6). It will be of great interest to compare the CA content of reverse transcription and preintegration complexes isolated from control cells and cells that are restricted at different stages in the viral life cycle.
FIG. 6.
Model of action of restriction factors. Restriction factors bind early after entry but can lead to viral replication arrest at three different stages. Block I occurs prior to reverse transcription and is observed with Trim5α, T5C, T1CL, T1CM, and T18CL. Block II prevents the formation of 2-LTR circles as seen with Fv1 and most likely occurs just before nuclear entry, whereas block III interferes with integration and is observed with T1CS, T18CS, and T19C.
As discussed in Results, it seems unlikely that this difference is due to concentration effects. Certainly, concentration does not affect the phenotype of Fv1- or Trim5-mediated restriction, since cells transduced with factor and expressing at least 10-fold more protein than seen in nontransduced cells block virus replication at the same stage as cells expressing normal levels of factor (19, 38, 43; M. Dodding, unpublished data). The three blocks that have been identified represent three different modes of restriction. However, they need not be mutually exclusive; viruses escaping the earlier blocks could be restricted later in the infection process.
It is tempting to speculate that each block could involve a different mechanism (for example, ubiquitination, modulation of core stability, and sequestering). Each mechanism could be degenerate, and inhibiting one might still lead to restriction by the others. This could account for the current discrepancies in data favoring one mechanism over the other.
Acknowledgments
We thank Viviane Bechtold and Ken Raj for their help and advice on quantitative PCR.
This work was supported by the United Kingdom Medical Research Council.
REFERENCES
- 1.Bainbridge, J. W., C. Stephens, K. Parsley, C. Demaison, A. Halfyard, A. J. Thrasher, and R. R. Ali. 2001. In vivo gene transfer to the mouse eye using an HIV-based lentiviral vector; efficient long-term transduction of corneal endothelium and retinal pigment epithelium. Gene Ther. 8:1665-1668. [DOI] [PubMed] [Google Scholar]
- 2.Besnier, C., Y. Takeuchi, and G. Towers. 2002. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. USA 99:11920-11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bock, M., K. N. Bishop, G. Towers, and J. P. Stoye. 2000. Use of a transient assay for studying the genetic determinants of Fv1 restriction. J. Virol. 74:7422-7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borden, K. L., J. M. Lally, S. R. Martin, N. J. O'Reilly, E. Solomon, and P. S. Freemont. 1996. In vivo and in vitro characterization of the B1 and B2 zinc-binding domains from the acute promyelocytic leukemia protooncoprotein PML. Proc. Natl. Acad. Sci. USA 93:1601-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boutell, C., and R. D. Everett. 2003. The herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 interacts with and ubiquitinates p53. J. Biol. Chem. 278:36596-36602. [DOI] [PubMed] [Google Scholar]
- 6.Brown, P. O. 1997. Integration, p. 161-203. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 7.Bruce, J. W., K. A. Bradley, P. Ahlquist, and J. A. Young. 2005. Isolation of cell lines that show novel, murine leukemia virus-specific blocks to early steps of retroviral replication. J. Virol. 79:12969-12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler, S. L., M. S. T. Hansen, and F. D. Bushman. 2001. A quantitative assay for HIV DNA integration in vivo. Nat. Med. 7:631-634. [DOI] [PubMed] [Google Scholar]
- 9.Cao, T., E. Duprez, K. L. Borden, P. S. Freemont, and L. D. Etkin. 1998. Ret finger protein is a normal component of PML nuclear bodies and interacts directly with PML. J. Cell Sci. 111:1319-1329. [DOI] [PubMed] [Google Scholar]
- 10.Cowan, S., T. Hatziioannou, T. Cunningham, M. A. Muesing, H. G. Gottlinger, and P. D. Bieniasz. 2002. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc. Natl. Acad. Sci. USA 99:11914-11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forshey, B. M., U. von Schwedler, W. I. Sundquist, and C. Aiken. 2002. Formation of a human immunodeficiency virus type 1 core of optimal stability is crucial for viral replication. J. Virol. 76:5667-5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatziioannou, T., S. Cowan, S. P. Goff, P. D. Bieniasz, and G. J. Towers. 2003. Restriction of multiple divergent retroviruses by Lv1 and Ref1. EMBO J. 22:385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatziioannou, T., D. Perez-Caballero, A. Yang, S. Cowan, and P. D. Bieniasz. 2004. Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5alpha. Proc. Natl. Acad. Sci. USA 101:10774-10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann, W., D. Schubert, J. LaBonte, L. Munson, S. Gibson, J. Scammell, P. Ferrigno, and J. Sodroski. 1999. Species-specific, postentry barriers to primate immunodeficiency virus infection. J. Virol. 73:10020-10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang, J. H. 2004. FIGC, a novel FGF-induced ubiquitin-protein ligase in gastric cancers. FEBS Lett. 578:21-25. [DOI] [PubMed] [Google Scholar]
- 16.Javanbakht, H., F. Diaz-Griffero, M. Stremlau, Z. Si, and J. Sodroski. 2005. The contribution of RING and B-box 2 domains to retroviral restriction mediated by monkey TRIM5alpha. J. Biol. Chem. 280:26933-26940. [DOI] [PubMed] [Google Scholar]
- 17.Jensen, K., C. Shiels, and P. S. Freemont. 2001. PML protein isoforms and the RBCC/TRIM motif. Oncogene 20:7223-7233. [DOI] [PubMed] [Google Scholar]
- 18.Joazeiro, C. A., S. S. Wing, H. Huang, J. D. Leverson, T. Hunter, and Y. C. Liu. 1999. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science 286:309-312. [DOI] [PubMed] [Google Scholar]
- 19.Jolicoeur, P., and E. Rassart. 1980. Effect of Fv-1 gene product on synthesis of linear and supercoiled viral DNA in cells infected with murine leukemia virus. J. Virol. 33:183-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, J. M., and M. Gellert. 2003. Autoubiquitylation of the V(D)J recombinase protein RAG1. Proc. Natl. Acad. Sci. USA 100:15446-15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keckesova, Z., L. M. Ylinen, and G. J. Towers. 2004. The human and African green monkey TRIM5alpha genes encode Ref1 and Lv1 retroviral restriction factor activities. Proc. Natl. Acad. Sci. USA 101:10780-10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak, C. A., and A. Chakraborti. 1996. Single amino acid changes in the murine leukemia virus capsid protein gene define the target for Fv1 resistance. Virology 225:300-306. [DOI] [PubMed] [Google Scholar]
- 23.Lilly, F. 1970. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J. Natl. Cancer Inst. 45:163-169. [PubMed] [Google Scholar]
- 24.Lorick, K. L., J. P. Jensen, S. Fang, A. M. Ong, S. Hatakeyama, and A. M. Weissman. 1999. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc. Natl. Acad. Sci. USA 96:11364-11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luban, J., K. L. Bossolt, E. K. Franke, G. V. Kalpana, and S. P. Goff. 1993. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell 73:1067-1078. [DOI] [PubMed] [Google Scholar]
- 26.Mische, C. C., H. Javanbakht, B. Song, F. Diaz-Griffero, M. Stremlau, B. Strack, Z. Si, and J. Sodroski. 2005. Retroviral restriction factor TRIM5α is a trimer. J. Virol. 79:14446-14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nisole, S., C. Lynch, J. P. Stoye, and M. W. Yap. 2004. A Trim5-cyclophilin A fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc. Natl. Acad. Sci. USA 101:13324-13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nisole, S., J. P. Stoye, and A. Saïb. 2005. Trim family proteins: retroviral restriction and antiviral defence. Nat. Rev. Microbiol. 3:799-808. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Caballero, D., T. Hatziioannou, A. Yang, S. Cowan, and P. D. Bieniasz. 2005. Human tripartite motif 5α domains responsible for retrovirus restriction activity and specificity. J. Virol. 79:8969-8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Caballero, D., T. Hatziioannou, F. Zhang, S. Cowan, and P. D. Bieniasz. 2005. Restriction of human immunodeficiency virus type 1 by Trim-CypA occurs with rapid kinetics and independently of cytoplasmic bodies, ubiquitin, and proteasome activity. J. Virol. 79:15567-15572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perron, M. J., M. Stremlau, B. Song, W. Ulm, R. C. Mulligan, and J. Sodroski. 2004. TRIM5alpha mediates the postentry block to N-tropic murine leukemia viruses in human cells. Proc. Natl. Acad. Sci. USA 101:11827-11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reymond, A., G. Meroni, A. Fantozzi, G. Merla, S. Cairo, L. Luzi, D. Riganelli, E. Zanaria, S. Messali, S. Cainarca, A. Guffanti, S. Minucci, P. G. Pelicci, and A. Ballabio. 2001. The tripartite motif family identifies cell compartments. EMBO J. 20:2140-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sayah, D. M., E. Sokolskaja, L. Berthoux, and J. Luban. 2004. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature 430:569-573. [DOI] [PubMed] [Google Scholar]
- 34.Skorstengaard, K., M. S. Jensen, P. Sahl, T. E. Petersen, and S. Magnusson. 1986. Complete primary structure of bovine plasma fibronectin. Eur. J. Biochem. 161:441-453. [DOI] [PubMed] [Google Scholar]
- 35.Soneoka, Y., P. M. Cannon, E. E. Ramsdale, J. C. Griffiths, G. Romano, S. M. Kingsman, and A. J. Kingsman. 1995. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 23:628-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoye, J. P. 2002. An intracellular block to primate lentivirus replication. Proc. Natl. Acad. Sci. USA 99:11549-11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427:848-853. [DOI] [PubMed] [Google Scholar]
- 38.Towers, G., M. Bock, S. Martin, Y. Takeuchi, J. P. Stoye, and O. Danos. 2000. A conserved mechanism of retrovirus restriction in mammals. Proc. Natl. Acad. Sci. USA 97:12295-12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vichi, A., D. M. Payne, G. Pacheco-Rodriguez, J. Moss, and M. Vaughan. 2005. E3 ubiquitin ligase activity of the trifunctional ARD1 (ADP-ribosylation factor domain protein 1). Proc. Natl. Acad. Sci. USA 102:1945-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, W. K., J. O. Kiggans, D. M. Yang, C. Y. Ou, R. W. Tennant, A. Brown, and R. H. Bassin. 1980. Synthesis and circularization of N- and B-tropic retroviral DNA Fv-1 permissive and restrictive mouse cells. Proc. Natl. Acad. Sci. USA 77:2994-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yap, M. W., S. Nisole, C. Lynch, and J. P. Stoye. 2004. Trim5alpha protein restricts both HIV-1 and murine leukemia virus. Proc. Natl. Acad. Sci. USA 101:10786-10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yap, M. W., S. Nisole, and J. P. Stoye. 2005. A single amino acid change in the SPRY domain of human Trim5alpha leads to HIV-1 restriction. Curr. Biol. 15:73-78. [DOI] [PubMed] [Google Scholar]
- 43.Yap, M. W., and J. P. Stoye. 2003. Intracellular localisation of Fv1. Virology 307:76-89. [DOI] [PubMed] [Google Scholar]
- 44.Ylinen, L. M., Z. Keckesova, S. J. Wilson, S. Ranasinghe, and G. J. Towers. 2005. Differential restriction of human immunodeficiency virus type 2 and simian immunodeficiency virus SIVmac by TRIM5α alleles. J. Virol. 79:11580-11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zufferey, R., D. Nagy, R. J. Mandel, L. Naldini, and D. Trono. 1997. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15:871-875. [DOI] [PubMed] [Google Scholar]