Abstract
It is known that Lassa virus Z protein is sufficient for the release of virus-like particles (VLPs) and that it has two L domains, PTAP and PPPY, in its C terminus. However, little is known about the cellular factor for Lassa virus budding. We examined which cellular factors are used in Lassa virus Z budding. We demonstrated that Lassa Z protein efficiently produces VLPs and uses cellular factors, Vps4A, Vps4B, and Tsg101, in budding, suggesting that Lassa virus budding uses the multivesicular body pathway functionally. Our data may provide a clue to develop an effective antiviral strategy for Lassa virus.
Lassa virus belongs to the family Arenaviridae, which includes Lymphocytic choriomeningitis virus (LCMV), Guanarito virus, Junin virus, and Machupo virus. Lassa virus is the etiological agent of a hemorrhagic fever, Lassa fever, that is endemic in West Africa.
Arenaviruses are enveloped viruses with a bisegmented negative-strand RNA genome. Lassa virus has two genomic RNA segments designated L and S, with approximate sizes of 7.2 and 3.4 kb, respectively. Each RNA segment directs the synthesis of two proteins in opposite orientations, separated by an intergenic region. The S segment directs synthesis of the nucleoprotein and the two virion glycoproteins, GP1 and GP2, which are derived by posttranslational cleavage of a precursor polypeptide, GP-C. The oligomeric structures of GP1 and GP2 make up the spikes on the virion envelope and mediate virus interaction with the host cell surface receptor (2). The L segment codes for the virus RNA-dependent RNA polymerase (L) and a small RING finger protein (Z), which is a viral matrix protein.
Recent studies have revealed that viral matrix proteins play critical roles during a late stage of virus budding in many enveloped RNA viruses, including retroviruses, rhabdoviruses, filoviruses, and orthomyxoviruses; when expressed alone in cells, they are released in the form of virus-like particles (VLPs). These viral proteins possess a so-called L domain containing PT/SAP, PPXY, and YPXL, which are critical motifs for efficient budding (6, 9, 10, 12, 17, 19, 26-28, 31). Lassa virus Z protein is sufficient for the release of VLPs and contains PTAP and PPXY motifs near its carboxy terminus (18, 24).
The PTAP motif was first identified in human immunodeficiency virus (HIV) p6gag and has been reported to bind Tsg101, which is a ubiquitin-conjugating E2 variant with a component of the vesicular protein-sorting machinery. The interaction between p6gag and Tsg101 is required for HIV budding, and Tsg101 appears to facilitate this budding by linking the p6 late domain to the vacuolar protein-sorting (Vps) pathway (5). Recent reports have shown that targeting of Tsg101 by RNA interference causes a strong reduction in Z-mediated LCMV budding and that the Z protein is colocalized with Tsg101 (18).
Another L-domain motif, PPXY, has also been determined to be the principal sequence that binds to the WW domain, consisting of 38 to 40 amino acids containing two widely spaced tryptophans. In fact, it has been shown that the viral PPXY sequences interact with the WW domains of the cellular Nedd4-like ubiquitin ligases, such as Nedd4 and BUL1 (1, 8, 13, 21, 25, 29, 30).
It has been reported that many viruses containing these L-domain motifs functionally interact with the class E proteins that function in the multivesicular body (MVB) pathway, including AIP1, Vps4A, and Vps4B, suggesting that budding of the viruses possessing L domains and MVB vesicle formation might be analogous processes. Class E proteins are cytoplasmic multidomain proteins that transiently attach to the endosomal membrane, where the inward invagination of cargo-laden vesicles takes place.
In this work, to examine whether Lassa virus imitates the MVB pathway to produce its progeny virus particles and, if so, which class E protein is involved in Lassa virus budding, we analyzed Lassa Z VLP formation using dominant-negative (DN) mutants and the small interfering RNAs (siRNAs) of class E proteins participating in the MVB pathway. Our data showed that Vps4A, Vps4B, and Tsg101 are involved in Lassa VLP budding, suggesting that Lassa virus budding mimics the MVB pathway and that these class E proteins might be the targets of anti-Lassa virus therapy.
The DN forms of Vps4A and Vps4B inhibit the budding of Z VLPs.
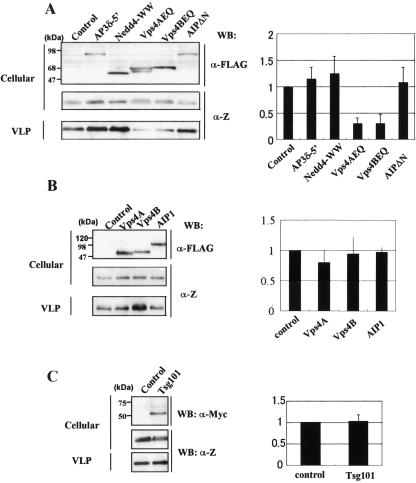
Vps4A and -4B are ATPases, and each is one of the final effectors in the MVB sorting pathway in cells. Recent studies have shown that the enzymatic activity of Vps4A is required for efficient budding of HIV type 1, murine leukemia virus, equine infectious anemia virus, Mason-Pfizer monkey virus, simian virus 5, and Ebola virus, using DN Vps4A (5, 7, 11, 14, 22, 23). It has been reported that Lassa virus Z protein is a matrix protein sufficient for the release of VLPs (18, 24). To examine the involvement of class E proteins in the egress of Lassa virus, we analyzed the effects of overexpression of DN mutants of class E proteins on Lassa Z-induced VLP budding. COS-7 cells (2 × 105) were cotransfected with 1 μg of pCLV-Z, which is pCAGGS encoding Lassa Z, and 4 μg of the expression plasmid for each DN mutant of Nedd4, Vps4A, Vps4B, AIP1, and AP3δ: pNedd4-WW (218 to 533), pVps4AEQ (E228Q), pVps4BEQ (E235Q), pAIPΔN (177 to 868), and pAP3δ-5′ (1 to 742), respectively (3, 5, 15, 30). Previous studies have shown DN effects of these mutants on the budding of other viruses. All DN mutants were expressed as proteins containing a FLAG tag at their N termini. At 48 h after transfection, the supernatant of the cells was clarified from cell debris by centrifugation (13,000 × g; 10 min), and then VLPs were pelleted through a 20% sucrose cushion by ultracentrifugation (195,000 × g; 60 min at 4°C). Cells and VLPs were lysed with lysis A buffer (1% Triton X-100, 25 mM Tris-HCl, pH 8.0, 50 mM NaCl, and 10% Na-deoxycholate) and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by Western blotting with rabbit anti-Z polyclonal antibody to detect Z. As shown in Fig. 1A, a large amount of Z was detected in the VLP fraction pelleted by ultracentrifugation. VLPs released from the cells expressing Z alone were also observed by electron microscopy (EM). VLPs were morphologically indistinguishable from Lassa viruses (Fig. 2B and C) and were never observed in control cells without Z expression (Fig. 2A). This result was consistent with a previous report (4). Lassa Z-induced VLP production was significantly reduced by the overexpression of Vps4AEQ or Vps4BEQ, while AP3δ-5′, Nedd4-WW, and AIPΔN did not reduce VLP production (Fig. 1A). We also observed that the overexpression of a DN mutant of BUL1 did not reduce VLP production (data not shown). These results suggest that Lassa virus also utilizes the MVB sorting pathway in its budding, and the enzymatic activities of Vps4A and Vps4B are clearly required for efficient budding of Lassa virus.
FIG. 1.
Effects of dominant-negative or wild-type host cell factors. (A) COS-7 cells were cotransfected with pCLV-Z and the empty vector as a control (Control) or with the expression plasmid for AP3δ-5′, Nedd4-WW, Vps4AEQ, Vps4BEQ, or AIPΔN. Extracellular VLPs were pelleted from the culture fluids. VLP-associated or cell-associated Z was detected by Western blotting (WB) using rabbit anti-Z antiserum. WB using anti-Flag monoclonal antibody was also used to examine the expression of Flag-tagged AP3δ-5′, Nedd4-WW, Vps4AEQ, Vps4BEQ, or AIPΔN in cells (top). (B and C) COS-7 cells were cotransfected with pCLV-Z and the empty vector (Control) or the expression plasmid for Vps4A, Vps4B, AIP1, or Tsg101. VLP-associated or cell-associated Z and FLAG-tagged proteins were detected as described for panel A. The expression of Myc-tagged Tsg101 was examined by WB using an anti-Myc monoclonal antibody. On the right are shown the intensities of the bands for cell and VLP-associated Z (left) that were quantified using a LAS3000 imaging system (Fuji Film). The efficiency of Z-induced VLP budding in cells cotransfected with pCLV-Z and the control vector (VLP/cellular) was set to 1.0. The data are shown as averages and standard deviations of three independent experiments.
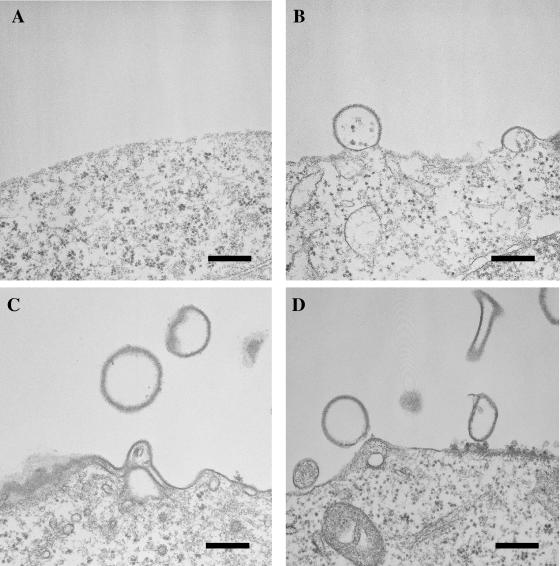
FIG. 2.
Lassa virus-like particles produced by expression of Z. At 24 h posttransfection of COS-7 cells (B and C) or 293T cells (D) with pCLV-Z, pleomorphic particles were seen budding from the plasma membrane. (A) Control COS-7 cells. EM was performed as described previously (16). Bars, 300 nm.
Effects of overexpression of Vps4A and Vps4B on Lassa VLP production.
To further determine the functions of Vps4A and Vps4B, we next cotransfected pVps4A or pVps4B with pCLV-Z in COS-7 cells. As shown in Fig. 1B, overexpression of Vps4A or Vps4B did not promote VLP release. This may indicate that endogenous Vps4A and Vps4B are sufficient for producing VLPs. These results suggest that a functional Vps pathway is required for Lassa virus release.
Functional involvement of Tsg101 in Lassa VLP production.
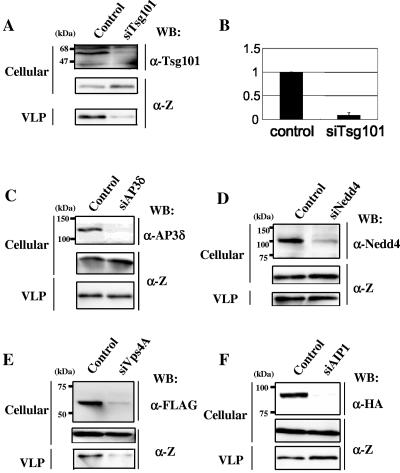
Tsg101 is also the cellular factor which is involved in the budding of viruses containing a PT/SAP motif as an L domain. Therefore, we investigated whether exhaustion of endogenous Tsg101 by siRNA reduced the production of Lassa virus Z-induced VLPs. 293T cells (1 × 105) were pretreated with 100 pmol of siRNA specific for Tsg101 (siTsg101) or control RNA (siRNA control) 1 day before plasmid transfection. The following day, these cells were cotransfected with 100 pmol of siTsg101 or siRNA control and 0.5 μg of pCLV-Z. Cotransfection of siTsg101, but not siRNA control, with pCLV-Z caused a severe reduction in the levels of endogenous Tsg101 expression (Fig. 3A, top). The production of VLPs was significantly reduced when siTsg101 was cotransfected with pCLV-Z (Fig. 3A and B). We used 293T cells in this experiment and also confirmed by EM that VLPs were released from 293T cells, as well as COS-7 cells (Fig. 2D). Although the effect of overexpression of Tsg101 on VLP production was also examined, overexpression of Tsg101 did not promote VLP release (Fig. 1C), suggesting that the amount of endogenous Tsg101 is sufficient for producing VLPs. These results clearly demonstrate that Tsg101 plays a fundamental role in Lassa virus budding and that endogenous Tsg101 is sufficient for producing VLPs. Perez et al. have shown that Tsg101 plays a crucial role in LCMV budding, although LCMV Z contains only PPPY and not PT/SAP (18). This suggests that Tsg101 can recognize a motif other than PT/SAP or that it indirectly interacts with LCMV Z proteins. Upstream of the PPPY motif within the C terminus of LCMV Z is the sequence STAP, which is closely related to the canonical PT/SAP L domain. Moreover, replacement of the first proline residue of the PTAP motif of HIV p6 by alanine resulted in only a moderate reduction in the binding affinity of p6 to Tsg101 (5). Therefore, Tsg101 may recognize this STAP sequence. It is of interest to further analyze how Tsg101 interacts with Lassa virus and LCMV Z proteins.
FIG. 3.
Effects of siRNA specific for Tsg101, AP3δ, Nedd4, Vps4A, or AIP1 on Lassa VLP budding. (A, C, D, E, and F) 293T cells were pretreated with siRNA specific for Tsg101 (siTsg101), AP3δ (siAP3δ), Nedd4 (siNedd4), Vps4A (siVps4A), or AIP1 (siAIP1) or with control RNA (Control) 1 day before plasmid transfection. The following day, these cells were cotransfected with pCLV-Z and siRNA or control RNA. Endogenous Tsg101, AP3δ, or Nedd4 was detected by mouse anti-Tsg101 (A, top), anti-adaptin monoclonal antibody (C, top), or rabbit anti-Nedd4 polyclonal antibody (D, top), respectively. FLAG-tagged Vps4A or hemagglutinin (HA)-tagged AIP1 was detected by mouse anti-FLAG (E, top) or anti-HA (F, top) monoclonal antibody, respectively. Cell-associated (middle) and VLP-associated (bottom) Z proteins were detected by Western blotting (WB) using rabbit anti-Z antiserum. (B) The intensity of the band for VLP-associated Z in panel A was quantified as described in the legend to Fig. 1. The extent of Z in the VLPs released from cells cotransfected with pCLV-Z and the control siRNA was set to 1.0. The data are shown as averages and standard deviations of three independent experiments.
Exhaustion of cellular factors by siRNA.
We also examined whether exhaustion of AP3δ, Nedd4, Vps4A, or AIP1 by siRNA had any effect on Lassa VLP budding (Fig. 3C to F). To deplete these cellular proteins, we used 100 pmol of siRNA specific for AP3δ, Nedd4, or Vps4A or 1 μg of the plasmid expressing siRNA specific for AIP1, pBSi-AIP1 (a gift from T. Sakaguchi) (20). Exhaustion of Vps4A by siRNA, as well as Tsg101, greatly reduced the production of Lassa VLPs, while exhaustion of AP3δ, Nedd4, and AIP1 had no effect on VLP budding. These results are consistent with the data on DN mutants (Fig. 1A and B), strongly suggesting that Lassa virus Z uses Vps4A, but not AP3δ, Nedd4, or AIP1, for budding.
The mechanisms responsible for Lassa virus budding have not been addressed previously. Previous reports, along with the present study, showed that the Z protein plays a key role in viral budding. Here, we have confirmed that the Z protein is sufficient for VLP budding. Furthermore, in this study, we clarified that Vps4A, Vps4B, and Tsg101 are important for the budding of Lassa virus Z-induced VLPs. This strongly suggests that the budding of Lassa virus, as well as many other enveloped RNA viruses, including retroviruses, rhabdoviruses, and filoviruses, also uses the MVB pathway functionally. On the other hand, AP3δ, Nedd4, and AIP1 appear not to be involved in Lassa VLP budding, indicating that the cellular factors required for virus budding are not quite identical among viruses that utilize the MVB pathway. Strecker et al. recently reported that a Lassa Z mutant with a deletion of PPPY motif was defective in budding (24). This result suggests that the PPPY motif within Z is essential for Lassa virus and that the Nedd4-like E3 ubiquitin ligase containing a WW domain is involved in Lassa virus budding through interaction with the PPPY motif within the Z protein. Although our data showed that Nedd4 and BUL1 appear not to be the cellular factors participating in Lassa virus budding, any other Nedd4-like E3 ubiquitin ligase that has a WW domain may regulate Lassa virus budding. It is very important to further identify the cellular factors needed for virus budding, and the identification may contribute to anti-Lassa virus therapy.
Acknowledgments
We thank K. Sakurada and H. Motani for their helpful advice. We also thank T. Sakaguchi (Hiroshima University, Japan) for providing the plasmids pCAGGS-HA-AIP1, pBSi-AIP1, and pBSi-NC.
This work was supported by grants from the Japan Society for the Promotion of Science (JSPS), the Japan Science and Technology Agency (JST), and the Nakajima Foundation.
REFERENCES
- 1.Bouamr, F., J. A. Melillo, M. Q. Wang, K. Nagashima, M. de Los Santos, A. Rein, and S. P. Goff. 2003. PPPYVEPTAP motif is the late domain of human T-cell leukemia virus type 1 Gag and mediates its functional interaction with cellular proteins Nedd4 and Tsg101. J. Virol. 77:11882-11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao, W., M. D. Henry, P. Borrow, H. Yamada, J. H. Elder, E. V. Ravkov, S. T. Nichol, R. W. Compans, K. P. Campbell, and M. B. Oldstone. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:2079-2081. [DOI] [PubMed] [Google Scholar]
- 3.Dong, X., H. Li, A. Derdowski, L. Ding, A. Burnett, X. Chen, T. R. Peters, T. S. Dermody, E. Woodruff, J. J. Wang, and P. Spearman. 2005. AP-3 directs the intracellular trafficking of HIV-1 Gag and plays a key role in particle assembly. Cell 120:663-674. [DOI] [PubMed] [Google Scholar]
- 4.Eichler, R., T. Strecker, L. Kolesnikova, J. ter Meulen, W. Weissenhorn, S. Becker, H. D. Klenk, W. Garten, and O. Lenz. 2004. Characterization of the Lassa virus matrix protein Z: electron microscopic study of virus-like particles and interaction with the nucleoprotein (NP). Virus Res. 100:249-255. [DOI] [PubMed] [Google Scholar]
- 5.Garrus, J. E., U. K. von Schwedler, O. W. Pornillos, S. G. Morham, K. H. Zavitz, H. E. Wang, D. A. Wettstein, K. M. Stray, M. Cote, R. L. Rich, D. G. Myszka, and W. I. Sundquist. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55-65. [DOI] [PubMed] [Google Scholar]
- 6.Gottlinger, H. G., T. Dorfman, J. G. Sodroski, and W. A. Haseltine. 1991. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. USA 88:3195-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottwein, E., J. Bodem, B. Muller, A. Schmechel, H. Zentgraf, and H. G. Krausslich. 2003. The Mason-Pfizer monkey virus PPPY and PSAP motifs both contribute to virus release. J. Virol. 77:9474-9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harty, R. N., M. E. Brown, G. Wang, J. Huibregtse, and F. P. Hayes. 2000. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc. Natl. Acad. Sci. USA 97:13871-13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harty, R. N., J. Paragas, M. Sudol, and P. Palese. 1999. A proline-rich motif within the matrix protein of vesicular stomatitis virus and rabies virus interacts with WW domains of cellular proteins: implications for viral budding. J. Virol. 73:2921-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang, M., J. M. Orenstein, M. A. Martin, and E. O. Freed. 1995. p6gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J. Virol. 69:6810-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irie, T., J. M. Licata, J. P. McGettigan, M. J. Schnell, and R. N. Harty. 2004. Budding of PPxY-containing rhabdoviruses is not dependent on host proteins TGS101 and VPS4A. J. Virol. 78:2657-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayakar, H. R., K. G. Murti, and M. A. Whitt. 2000. Mutations in the PPPY motif of vesicular stomatitis virus matrix protein reduce virus budding by inhibiting a late step in virion release. J. Virol. 74:9818-9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kikonyogo, A., F. Bouamr, M. L. Vana, Y. Xiang, A. Aiyar, C. Carter, and J. Leis. 2001. Proteins related to the Nedd4 family of ubiquitin protein ligases interact with the L domain of Rous sarcoma virus and are required for gag budding from cells. Proc. Natl. Acad. Sci. USA 98:11199-11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Licata, J. M., M. Simpson-Holley, N. T. Wright, Z. Han, J. Paragas, and R. N. Harty. 2003. Overlapping motifs (PTAP and PPEY) within the Ebola virus VP40 protein function independently as late budding domains: involvement of host proteins TSG101 and VPS-4. J. Virol. 77:1812-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin-Serrano, J., A. Yarovoy, D. Perez-Caballero, and P. D. Bieniasz. 2003. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc. Natl. Acad. Sci. USA 100:12414-12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noda, T., H. Sagara, E. Suzuki, A. Takada, H. Kida, and Y. Kawaoka. 2002. Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP. J. Virol. 76:4855-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parent, L. J., R. P. Bennett, R. C. Craven, T. D. Nelle, N. K. Krishna, J. B. Bowzard, C. B. Wilson, B. A. Puffer, R. C. Montelaro, and J. W. Wills. 1995. Positionally independent and exchangeable late budding functions of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J. Virol. 69:5455-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez, M., R. C. Craven, and J. C. de la Torre. 2003. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proc. Natl. Acad. Sci. USA 100:12978-12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puffer, B. A., L. J. Parent, J. W. Wills, and R. C. Montelaro. 1997. Equine infectious anemia virus utilizes a YXXL motif within the late assembly domain of the Gag p9 protein. J. Virol. 71:6541-6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaguchi, T., A. Kato, F. Sugahara, Y. Shimazu, M. Inoue, K. Kiyotani, Y. Nagai, and T. Yoshida. 2005. AIP1/Alix is a binding partner of Sendai virus C protein and facilitates virus budding. J. Virol. 79:8933-8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakurai, A., J. Yasuda, H. Takano, Y. Tanaka, M. Hatakeyama, and H. Shida. 2004. Regulation of human T-cell leukemia virus type 1 (HTLV-1) budding by ubiquitin ligase Nedd4. Microbes Infect. 6:150-156. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt, A. P., G. P. Leser, E. Morita, W. I. Sundquist, and R. A. Lamb. 2005. Evidence for a new viral late-domain core sequence, FPIV, necessary for budding of a paramyxovirus. J. Virol. 79:2988-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shehu-Xhilaga, M., S. Ablan, D. G. Demirov, C. Chen, R. C. Montelaro, and E. O. Freed. 2004. Late domain-dependent inhibition of equine infectious anemia virus budding. J. Virol. 78:724-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strecker, T., R. Eichler, J. Meulen, W. Weissenhorn, H. Dieter Klenk, W. Garten, and O. Lenz. 2003. Lassa virus Z protein is a matrix protein and sufficient for the release of virus-like particles. J. Virol. 77:10700-10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vana, M. L., Y. Tang, A. Chen, G. Medina, C. Carter, and J. Leis. 2004. Role of Nedd4 and ubiquitination of Rous sarcoma virus Gag in budding of virus-like particles from cells. J. Virol. 78:13943-13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wills, J. W., C. E. Cameron, C. B. Wilson, Y. Xiang, R. P. Bennett, and J. Leis. 1994. An assembly domain of the Rous sarcoma virus Gag protein required late in budding. J. Virol. 68:6605-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiang, Y., C. E. Cameron, J. W. Wills, and J. Leis. 1996. Fine mapping and characterization of the Rous sarcoma virus Pr76gag late assembly domain. J. Virol. 70:5695-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasuda, J., and E. Hunter. 1998. A proline-rich motif (PPPY) in the Gag polyprotein of Mason-Pfizer monkey virus plays a maturation-independent role in virion release. J. Virol. 72:4095-4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuda, J., E. Hunter, M. Nakao, and H. Shida. 2002. Functional involvement of a novel Nedd4-like ubiquitin ligase on retrovirus budding. EMBO Rep. 3:636-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasuda, J., M. Nakao, Y. Kawaoka, and H. Shida. 2003. Nedd4 regulates egress of Ebola virus-like particles from host cells. J. Virol. 77:9987-9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan, B., X. Li, and S. P. Goff. 1999. Mutations altering the Moloney murine leukemia virus p12 Gag protein affect virion production and early events of the virus life cycle. EMBO J. 18:4700-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]