Abstract
The precise role of each of the seven individual CD11c+ dendritic cell subsets (DCs) identified to date in the response to viral infections is not known. DCs serve as critical links between the innate and adaptive immune responses against many pathogens, including herpes simplex virus type 1 (HSV-1). The role of DCs as mediators of resistance to HSV-1 infection was investigated using CD11c-diphtheria toxin (DT) receptor-green fluorescent protein transgenic mice, in which DCs can be transiently depleted in vivo by treatment with low doses of DT. We show that ablation of DCs led to enhanced susceptibility to HSV-1 infection in the highly resistant C57BL/6 mouse strain. Specifically, we showed that the depletion of DCs led to increased viral spread into the nervous system, resulting in an increased rate of morbidity and mortality. Furthermore, we showed that ablation of DCs impaired the optimal activation of NK cells and CD4+ and CD8+ T cells in response to HSV-1. These data demonstrated that DCs were essential not only in the optimal activation of the acquired T-cell response to HSV-1 but also that DCs were crucial for innate resistance to HSV-1 infection.
Based on serological evidence, it is estimated that 60 to 80% of the adult population is infected with herpes simplex virus type 1 (HSV-1) (46). Most infected individuals remain asymptomatic. Of symptomatic individuals, clinical presentation ranges from mild illness, such as the development of orofacial vesicular lesions, all the way to life-threatening systemic complications, such as hepatitis and encephalitis (39). The outcome of infection is known to be influenced by both specific and nonspecific genetically linked host defense mechanisms (32). The immune system is particularly important in controlling HSV-1 infection in both the periphery and the nervous system, although the events that initiate this immunity in humans are not very well understood. Underlying immunosuppression does not appear to explain the distinct outcomes of host-virus interaction. Rather, the observed range in clinical outcomes appears to reflect differences in intrinsic resistance to infection.
Studies using inbred strains of mice have revealed critical insights into the immunological basis for resistance to HSV-1 (20). Specifically, a range in innate resistance to systemic, lethal HSV-1 infection exists and has subsequently led to the grouping of inbred mice into resistant, moderately susceptible, and susceptible categories based upon the levels of virus required to cause death. In all cases examined, mice of the C57 background (C57BL/6 and C57BL/10) are most resistant and best able to effectively control HSV-1 infection (35).
Human and murine studies suggest that the innate immune response, specifically the ability to produce elevated levels of type I interferon (IFN) at early time points, provides a threshold of resistance to acute HSV-1 infection (44). Although the precise mechanisms by which type I IFNs function during HSV-1 infection are not fully understood in humans, murine studies have shown IFN-α/β to inhibit the onset of immediate-early gene expression (2, 22), limit viral spread into the nervous system (13), and activate host defenses such as NK cells (19). Recent studies further suggest a role for type I IFNs in the development of an optimal, virus-specific CD8+ T-cell response (18) known to be important in the effective control of HSV-1 infection (37).
The innate and acquired immune responses are ultimately linked together during the antigen presentation process, wherein internalized and processed viral antigens are presented to naïve T cells amid the inflammatory cytokine milieu generated during the innate immune response in the draining lymph nodes (1). Professional antigen-presenting cells (APCs) that express both major histocompatibility complex (MHC) classes I and II, along with the appropriate costimulatory signals, are considered the main initiators of the cellular immune response outlined above (6).
Of professional APCs, dendritic cells (DCs) are thought to be the most potent in stimulating T-cell proliferation (6). At least seven phenotypically distinct murine DC populations have been identified to date, all of which are CD11c+ (16, 33). Tissue-resident DCs are predominantly of myeloid origin and include epidermal Langerhans cells (LCs). Although LCs were initially thought to mediate CD4+ and CD8+ T-cell stimulation, recent studies suggests that “conventional” CD11c+ CD8α+ DCs (cDC) are the principal APC during an acute HSV-1 infection, and that this may be a function of their exceptional ability to cross-present exogenous antigens to CD8+ T cells (3). Another DC subset, the “plasmacytoid” CD11c+ B220+ dendritic cell (pDC), has been shown to secrete large amounts of type I IFN in vitro after exposure to HSV-1 in both humans and mice (21). This DC subset appears to be particularly important in providing help for lymph node DCs in optimally inducing anti-HSV cytotoxic T lymphocytes (CTLs) during the primary immune responses (47).
Given the myriad roles that DCs play during both the innate and acquired immune responses to HSV-1, we hypothesized that DCs are key mediators of intrinsic resistance to HSV-1. To test this hypothesis, we used CD11c-diphtheria toxin (DT) receptor-green fluorescent protein (GFP)-transgenic (Tg) mice, generated on a C57BL/6 (B6) background, in which DCs can be transiently depleted in vivo by treatment with low doses of DT (15). Ablation of DCs leads to enhanced susceptibility to HSV-1 infection in the highly resistant B6 mouse strain and to increased viral spread into the nervous system, resulting in paralysis and mortality. Furthermore ablation of DCs impaired the optimal activation of NK cells in vivo in addition to both the CD4+ and CD8+ T-cell responses to HSV-1. Collectively, these studies suggest that DCs are crucial for innate resistance to HSV-1 infection.
MATERIALS AND METHODS
Mice.
A breeding pair of B6.FVB-Tg Itgax-DTR/EGFP 57Lan/J (DTR) mice was purchased from Jackson Laboratories, Bar Harbor, ME, at 4 to 5 weeks of age. Mice were bred in house and were genotyped from tail DNA using published primer sequences (15). Age- and sex-matched mice were used between 5 to 12 weeks of age. Male B6 (H-2b) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 4 to 5 weeks of age. Animals were used between 6 and 12 weeks of age.
Virus.
HSV-1 strain Patton, obtained from R. Tenser, Pennsylvania State University College of Medicine, Hershey, PA, was plaque purified four times on Vero cell monolayers and established as a stock by infection of CV-1 cells at a multiplicity of infection of 0.01.
Diphtheria toxin treatment and HSV-1 immunization of DTR mice.
DTR transgenic mice were injected intraperitoneally (i.p.) with diphtheria toxin (Sigma-Aldrich, St. Louis, MO) at a concentration of 9 ng/g of body weight suspended in phosphate-buffered saline (PBS), or they were given PBS alone. Prior to undertaking the experiments described herein, the efficacy of DC depletion was examined using flow cytometry in a littermate of noninfected, DTR-Tg mice at 12, 24, 36, and 48 h post-DT treatment (data not shown). The results of these studies fell in line with previously published reports regarding the kinetics of DC depletion (15, 30, 31, 41) and will therefore not be discussed here. Twelve hours posttreatment with DT or PBS, mice were anesthetized by i.p. injection of 60 mg/kg of sodium pentobarbital (Butler, Columbus, OH). Mice were then given 5 × 106 PFU HSV-1 in 50 μl of Iscove’s modified Dulbecco’s medium (IMDM) in each hind footpad using a modification of the previously described multiple-puncture injection method (37). Briefly, 50 μl of virus was delivered subcutaneously into the hind footpad, which was then repeatedly punctured with a 27-gauge needle for ∼15 s. On day 4 postinfection, mice were killed and the draining popliteal lymph nodes (PLN) and spleens were harvested for phenotypic analysis using fluorescence-activated cell sorting. Individual footpads and spinal cord tissues were harvested for PFU assays using Vero cells.
Quantitation of HSV-1 in footpad and spinal cord tissues.
The level of infectious HSV-1 in the footpad and spinal cord was determined as described previously (37). Briefly, individual footpads and the spinal cord were removed and stored in complete IMDM, 10% fetal calf serum (FCS) at −80°C. Tissues were disrupted by homogenization in 1-ml ground glass grinders (Wheaton, Millerville, NJ) and centrifuged at high speed (400 × g). The resultant cell-free homogenate was assayed at various dilutions on Vero cell monolayers in 12-well tissue culture plates overlaid with 0.5% methylcellulose. Plaques were visualized following fixation of the monolayers with 10% buffered formalin and staining with 0.5% crystal violet.
Treatment with clodronate liposomes.
Liposome-encapsulated clodronate was prepared as described previously (43). Clodronate was a kind gift of Roche Diagnostics GmbH, Mannheim, Germany. Mice were injected i.p. with 500 μl of clodronate liposomes and subcutaneously with 100 μl of clodronate liposomes per footpad 2 days prior to HSV-1 infection. This treatment regimen has previously been shown to enable the selective depletion of marginal-zone and metallophilic macrophages from the spleen (42) and their sinusoidal counterparts from the lymph nodes (11). Alternatively, as a control, mice were injected i.p. with 500 μl of empty liposomes and subcutaneously with 100 μl per footpad. Two days following treatment, mice were infected with HSV-1 as previously described and monitored over a 10-day period for survival curve analysis. For flow cytometric analysis, spleen and lymph nodes were harvested from mice on day 5 postinfection with HSV-1.
Antibodies and reagents for surface staining.
The following panel of monoclonal antibodies was used for phenotypic analysis of cell populations in the PLN and spleen: phycoerythrin (PE) anti-CD8a (clone 53-6.7; eBioscience), PE anti-CD4 (clone GK1.5; eBioscience), allophycocyanin anti-CD25 (clone PC61; eBioscience), allophycocyanin anti-B220 (clone RA3-6B2; eBioscience), biotin anti-CD11c (clone N418; eBioscience), biotin anti-NK1.1 (clone PK136; eBioscience), streptavidin activated peridinin-chlorophyll-protein complex (PerCP) (BD Biosciences), and streptavidin PE-Texas Red (BD Biosciences). To visualize glycoprotein B (gB)-specific CD8 T cells, lymphocytes were incubated at 4°C for 1 h with an allophycocyanin-conjugated MHC class I tetramer containing the HSV-1 gB (498SSIEFARL505) peptide. The gB-containing tetramer was obtained from the Tetramer Facility at the National Institutes of Allergy and Infectious Diseases, Atlanta, GA. Flow cytometric analysis was performed on a FACSCalibur instrument (Becton Dickinson, San Jose, CA) by the Core Facility for Flow Cytometry at Louisiana State University Health Sciences Center at Shreveport. The data were analyzed using Flow Jo software (Tree Star, Ashland, OR) and Cell Quest (BD Biosciences).
Intracellular IFN-γ staining.
Lymph node or splenic lymphocytes were cultured for 5 h in 96-well U-bottom microtiter plates (Costar, Cambridge, MA) at a concentration of 1 × 106 cells/well in 0.2 ml complete medium with 1 μl/ml brefeldin A (GolgiPlug; PharMingen). Spleen cells pulsed with vesicular stomatitis virus (VSV) nucleoprotein (52RGYGYQGL59) or HSV-1 glycoprotein B (498SSIEFARL505) peptide were used as stimulators (27). The stimulators were prepared by incubating 1 × 106 spleen cells in 500 μl IMDM-10% FCS containing 10−2 M VSV or HSV-1 peptides at a final concentration of 50 μM for 3 h at 37°C. A total of 3 × 104 stimulators was added per well containing 1 × 106 LN cells. After 5 h of culture, the cells were spun down and surface stained in PBS and supplemented with 2% FCS and 0.5% sodium azide with the above-listed panel of antibodies. After washing the unbound antibody, cells were subjected to intracellular cytokine stain using the Cytofix/Cytoperm kit (PharMingen) according to manufacturer's instructions. For intracellular IFN-γ staining, the allophycocyanin-conjugated monoclonal rat anti-mouse IFN-γ antibody (clone XMG1.2; eBioscience) or its isotype control antibody (rat immunoglobulin G1) was used. Flow cytometric analysis was performed on a FACSCalibur instrument (Becton Dickinson, San Jose, CA) by the Core Facility for Flow Cytometry at Louisiana State University Health Sciences Center at Shreveport. The data were analyzed using Flow Jo software (Tree Star, Ashland, OR) and Cell Quest (BD Biosciences).
CFSE-based in vivo cytolytic assay.
To prepare target cells to detect in vivo cytotoxic activity, erythrocytes were removed from naive C57BL/6 spleen and lymph node cell suspensions by osmotic lysis. The cells were then washed and split into two populations. One population was pulsed with 10−6 M gB peptide (498SSIEFARL505), incubated at 37°C for 45 min, and labeled with a high concentration of 5-(6)-carboxy-fluorescein succinimidyl ester (CFSE) (2.5 μM) (CFSEhigh cells). The second control target population was pulsed with a nonspecific control VSV peptide (52RGYGYQGL59) and was labeled with a low concentration of CFSE (0.25 μM) (CFSElow cells). For intravenous injection, equal numbers of cells from each population were mixed together, such that each mouse received a total of 20 × 106 cells in 150 μl of PBS. Cells were injected into mice that had previously been infected with HSV-1 a number of days earlier as described above, and 4 h later they were sacrificed for their lymph nodes and spleens. Cell suspensions were analyzed by flow cytometry, and each population was detected by its differential CFSE fluorescence intensities. Up to 1 × 104 CFSE-positive cells were collected for analysis. To calculate specific lysis, the following formula was used: ratio = (percentage CFSElow/percentage CFSEhigh). Percentage specific lysis = [1 − (ratio unprimed/ratio primed)] × 100 (31).
Statistical analysis.
Statistical analyses were made with the nonparametric Wilcoxon sum of ranks test using GraphPad Prism 3.0 software (San Diego, CA). The P value of significant differences is reported. Unless otherwise stated, plotted data represent means ± standard deviations (SD).
RESULTS
The effect of in vivo ablation of DCs on the course of acute cutaneous HSV-1 infection.
To determine the immune response to HSV-1 in mice lacking DC populations, we employed a novel CD11c-DTR-GFP transgenic mouse model (15), which utilizes a diphtheria toxin (DT)-based system that allows for the inducible, transient ablation of CD11c+ dendritic cells following treatment in vivo with DT. Because murine CD11c expression is largely restricted to the DC compartment—with the exception of some subsets of NK, T, and B cells that express low levels of the antigen, and CD11cnegative/low epidermal langerhans cells, which upregulate CD11c upon maturation—DT treatment of DTR-Tg animals enable the transient ablation of all DC subsets without significantly affecting the number and localization of other cell populations (15). Virtually all DCs are depleted around 18 h after DT injection and return to normal levels around 144 h post-DT injection (30).
In our studies, DTR-Tg mice were infected in each hind footpad with 5 × 106 PFU low-virulence HSV-1 Patton strain 12 to 24 h after i.p. administration of DT or PBS vehicle control. DT-treated DTR-Tg animals infected with HSV-1 exhibited signs of virus-induced morbidity within 3 days of infection. Specifically, unlike the PBS and nontreated groups, 100% of DT-treated DTR-Tg mice became visibly hunched and developed hind limb paralysis between days 3 and 4 following HSV-1 infection (data not shown). Furthermore, DT-treated DTR-Tg mice lost, on average, 12.9% of their total body weight (Fig. 1A). The mortality rate for DT-treated DTR-Tg mice by day 4 was 40%, versus 0% for the PBS and nontreated groups (Fig. 1B). A recent report has shown that DT administration, in addition to depleting CD11c+ DCs from DTR-Tg mice, leads to the depletion of marginal-zone and metallophilic macrophages from the spleen and their sinusoidal counterparts from the lymph nodes (31). To examine the contribution of marginal-zone, metallophilic, and sinusoidal macrophages in the observed increase in susceptibility of B6 mice to HSV-1 Patton, clodronate-containing liposomes were used. Treatment with clodronate-enclosed liposomes has been shown to enable the selective depletion of marginal-zone macrophages from the spleen (42) and sinusoidal macrophages from the lymph nodes (11) while leaving DC populations intact. Treatment with clodronate-containing liposomes appeared to be effective up to day 5 following HSV-1 infection, where differences in levels of macrophages, but not dendritic cells, could still be detected in the lymph nodes and spleen of HSV-1-infected mice (Fig. 1C and D). No difference could be detected in the survival rate of mice treated with clodranate-containing liposomes and infected with HSV-1 compared to their DT-treated, HSV-1-infected counterparts (Fig. 1A), nor was there a difference in morbidity and weight loss (Fig. 1B). These data suggest that resistance to HSV-1 does not involve marginal-zone or sinusoidal macrophages and that the decreased resistance observed in DT-treated DTR-Tg mice is primarily a function of DC ablation.
FIG. 1.
Impact of DC depletion and clodronate treatment on survival and weight loss of HSV-1-infected mice. Groups of five DTR-Tg mice were treated with 9 ng of diphtheria toxin per gram of body weight or PBS vehicle control 12 to 24 h prior to hind footpad infection with HSV-1 at 5 × 106 PFU per footpad. Liposome (Lip.)-treated mice were injected i.p. and subcutaneously with 500 μl and 100 μl, respectively, of clodranate (clod.)-enclosed liposomes or PBS-enclosed liposomes 48 h prior to HSV-1 infection. (A) Survival was calculated using Kaplan-Meier survival probability analysis. The survival rate of the different groups is shown. (B) To calculate weight loss, mice were weighed before DT, PBS, or liposome treatment and again on day 4 following HSV-1 infection. Values reported represent the mean percent change in initial body weight on day 4 ± SD. The efficacy of macrophage depletion in the draining lymph nodes of HSV-1-infected mice was examined by flow cytometry on day 5 postinfection. Lymphocytes were harvested and stained with CD11b (clone M1/70; eBiosciences) and CD11c (clone N418; eBiosciences) cell-specific antibodies. (C and D) Representative histograms depicting the frequency of macrophages (C) and dendritic cells (D) are shown.
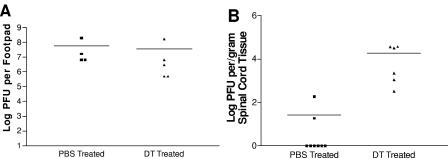
Analysis of HSV-1 replication revealed no significant differences in the levels of virus in the footpads of ablated versus nonablated DT-Tg mice (Fig. 2A) on day 4 p.i. However, there was a significant increase in virus levels detectable in the spinal cord tissue of the DC-ablated group (Fig. 2B; P ≤ 0.01). These data suggest that DCs play a role in limiting the spread of virus into the peripheral, and subsequently central, nervous systems following HSV-1 infection.
FIG. 2.
Ablation of DCs enhances viral spread into the nervous system. On day 4 post-HSV-1 infection, footpads and the spinal cord were harvested from DT- and PBS-treated DTR-Tg mice. Lysates were then prepared and assayed for HSV-1 PFU on permissive Vero cells. (A) Each data point is representative of the mean of both footpads from a single mouse. (B) Each data point is representative of the mean log PFU per/gram of spinal cord from an individual mouse. Statistical analysis using the Wilcoxon sum of ranks test revealed no significant differences in HSV-1 PFU levels in the footpads of DT- versus PBS-treated DTR-Tg mice (P > 0.05). Significant differences (P ≤ 0.01) were detectable in HSV-1 PFU levels in the spinal cords of DT- versus PBS-treated DTR-Tg mice.
DC depletion impairs NK cell activation.
Several recent reports indicate that interaction between NK cells and DCs is vital in the generation of antiviral immunity at early times postinfection (4). NK cells are known to play a role in intrinsic resistance to HSV-1 (10). To determine whether ablation of DCs had a direct impact on the magnitude of NK cell activation following infection, the expression levels of the activation marker B220 on NK cell populations was examined. In vivo expression of the B220 activation marker has been shown to correlate with NK cytolytic activity and may therefore serve as a marker of MHC-nonrestricted killers (5). Because secondary lymphoid organs have been shown to be primary sites of NK cell activation and maturation following viral infection (12), the spleens of mice were examined 24 h p.i. Significant differences were observed in the frequencies of activated NK cells between DT-treated and PBS-treated mice (Fig. 3A and B; P ≤ 0.001). No significant differences in the frequency of activated NK cells could be detected between naïve and DT-treated mice, suggesting that NK cell activation is significantly impaired in the absence of DCs.
FIG. 3.
DC depletion impairs NK cell activation. Spleens were harvested from DT-treated, PBS-treated, and naïve DTR-Tg mice 24 h post-HSV-1 infection. Individual spleens were then mechanically dissociated into a single suspension and surface stained for expression of NK1.1 and B220. (A) Representative histograms depicting the general trend observed from the different mouse groups examined. Numbers in the upper right quadrant represent the frequency of activated NK1.1 cells. Numbers in the lower quadrant represent the frequency of NK1.1+ cells only. (B) The bars represent the mean frequency observed of activated NK1.1+ B220+ in naïve, DT-treated, and PBS-treated mice. Each bar represents the mean value obtained from a minimum of two experiments using a minimum of three mice per treatment. Statistical analysis using the Wilcoxon sum of ranks test revealed significant differences in levels of NK cell activation between naïve and PBS-treated (P ≤ 0.001) mice and DT-treated versus PBS-treated (P ≤ 0.001) mice. No significant differences were detected in the naïve versus DT-treated mice.
DC depletion impedes HSV-1-specific CD8+ T-cell activation and function.
Four days after HSV-1 infection, total CD11c+ cell numbers in peripheral lymph nodes remained relatively low in DT-treated DTR-Tg mice compared to PBS-treated and nontreated DTR-Tg animals (Fig. 4A). This was true for all of the DC subsets that were examined, including the plasmacytoid and conventional DCs (Fig. 4C). The same pattern in total CD11c+ cell numbers was also observed in the spleen (Fig. 4B and D). To gauge the effects of DC depletion on CD8+ T-cell activation and subsequent commitment to the immune response to HSV-1, class I MHC tetramer analysis was performed. Staining with H-2Kb/gB peptide tetrameric reagents is a highly sensitive technique enabling the enumeration and characterization of HSV-1-specific CD8+ T cells during infection (23). Using this approach, we found significantly reduced levels of gB+ CD8+ T cells on day 4 postinfection in both the draining popliteal lymph nodes (Fig. 5A and C; P ≤ 0.05) and spleens (Fig. 5B and D; P ≤ 0.05) of DT-treated DTR-Tg mice. Similarly, the frequency of HSV-1-specific IFN-γ-producing CD8+ T cells within the draining lymph nodes of HSV-1-infected animals was reduced (Fig. 5E) in DT-treated DTR-Tg mice.
FIG. 4.
Total DC cell numbers in the draining popliteal lymph nodes and spleens of HSV-1-infected animals. PLN and splenocyte cells were harvested from mice on day 4 post-HSV-1 infection and surface stained for expression of CD11c, CD8a, and B220. Representative histograms depicting the typical frequency of CD11c+ cells observed on day 4 post-HSV-1 infection in the PLNs and spleen of PBS-treated, DT-treated, and nontreated DTR-Tg mice are depicted in panels A and B, respectively. (C and D) Total cell numbers were calculated by multiplying the total percentage of CD11c+ cells by the number of total cells per PLN or spleen. pDC numbers were calculated by multiplying the total percentage of CD11c+ B220+ cells by the number of total cells per PLN. cDC numbers were calculated by multiplying the total percentage of CD11c+ CD8a+ cells by the total number of cells per PLN. Each bar represents the mean value obtained from a minimum of two experiments using a minimum of three mice per treatment. The error bars represent the standard deviation from the mean.
FIG. 5.
DC depletion impedes HSV-1-specific CD8+ T-cell activation and function. For tetramer analysis, PLN and splenocyte cells were harvested from day 4-infected animals and surface stained for expression of CD8a. The stained lymphocytes were incubated with the allophycocyanin-conjugated MHC class I tetramer containing the gB peptide for 1 h at 4°C and washed twice prior to flow cytometric analysis. Representative histograms from the PLNs and spleen of nontreated, DT-treated, and PBS-treated mice are depicted in panels A and B, respectively. Numbers in the upper right quadrant represent the frequency of gB+ CD8+ T cells. (C and D) The bars represent the total number of CD8a+ gB+ double-positive cells in the PLNs and spleen on day 4. Each bar represents the mean value obtained from a minimum of two experiments using a minimum of three mice per treatment. The error bars represent the standard deviation from the mean. Statistical analysis using the Wilcoxon sum of ranks test showed a significant difference on day 4 p.i. between PBS plus HSV-1 versus DT plus HSV-1 (P ≤ 0.05) mice and HSV-1 versus DT plus HSV-1 (P ≤ 0.05) DTR-Tg mice. (E) Intracellular staining was used to gauge the total number of HSV-1-specific CD8 T cells producing IFN-γ in the PLNs of DT-treated and PBS-treated DTR-Tg mice on day 4 post-HSV-1 infection. On day 4 p.i., PLNs were harvested and dissociated into a single cell suspension by mechanical dissociation. PLN cells (106) were placed in each well of a 96-well U-bottomed plate and cultured for 6 h in the presence of 5 × 104 gB peptide-pulsed spleen cells used to restimulate HSV-1-specific IFN-γ production. VSV peptide-pulsed spleen cells served as a negative stimulator control. After 6 h, the restimulated PLN cells were surface stained for expression of CD8, fixed, and permeabilized and stained for expression of intracellular IFN-γ. The bars represent the mean number of total CD8 T cells producing IFN-γ per popliteal lymph node. Each bar represents the mean value obtained from a minimum of two experiments using a minimum of three mice per treatment. The error bars represent the standard deviation from the mean.
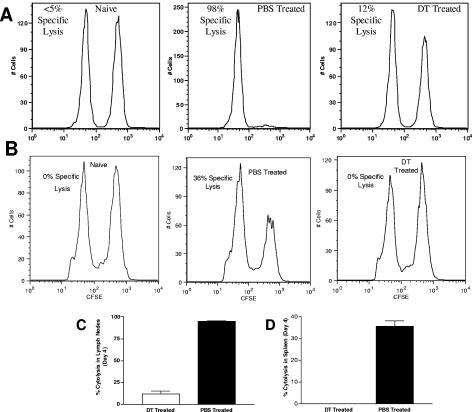
The cytolytic function of HSV-1-specific CD8+ T cells within the draining nodes of DT-treated and PBS-treated DTR-Tg mice on day 4 p.i. was determined using an in vivo cytotoxicity assay to measure the disappearance of CFSE-labeled, HSV-1 gB peptide-pulsed spleen cells as targets (24). Target cell lysis in uninfected control mice was less than 5% (Fig. 6A, left panel) compared to target cells pulsed with an irrelevant, H-2Kb-binding peptide, while greater than 98% lysis of gB targets was observed in HSV-1-infected DTR-Tg mice treated with PBS (Fig. 6A, center panel). Ablation of DC by DT treatment substantially diminished CD8+ CTL function (Fig. 6A, right panel; P ≤ 0.001). The selective loss of peptide-pulsed target cells translated into a ninefold reduction in CD8+ T-cell-mediated specific lysis (Fig. 6C). The same overall pattern of decreased CTL activity was observed in the spleens of the DT-treated DTR-Tg mice (Fig. 6B and D; P ≤ 0.001).
FIG. 6.
DC depletion impairs HSV-1 specific CTL activity. To prepare target cells for detection of in vivo cytolytic activity, erythrocytes were removed from naïve C57Bl/6 spleen suspensions by osmotic lysis. The cells were then washed and split into two populations. One population was pulsed with 10−5 M gB peptide (498SSIEFARL505), incubated at 37°C for 45 min, and labeled with a high concentration of CFSE (2.5 μM) (CFSEhigh cells). The second control target population was pulsed with a nonspecific control VSV peptide (52RGYGYQGL59) and labeled with a low concentration of CFSE (0.25 μM) (CFSElow cells). For i.v. injection, an equal number of cells from each population was mixed together such that each mouse received a total of 20 × 106 cells in 150 μl of PBS. The target cells were then injected into mice that had previously been infected with HSV-1 a number of days earlier, as described in Materials and Methods. Four hours after i.v. injection of target cells, mice were sacrificed and harvested of their popliteal lymph nodes and spleens. Cell suspensions were analyzed by flow cytometry, and each population was detected by their differential CFSE fluorescence intensities. Representative histograms from the PLNs and spleen of naïve, PBS-treated, and DT-treated mice 4 days post-HSV-1 infection are shown in panels A and B, respectively. Up to 1 × 104 CFSE-positive cells were collected for analysis. To calculate specific lysis, the following formula was used (24): ratio = (percentage CFSElow/percentage CFSEhigh). For percentage of specific lysis (C and D), the following formula was used: [1 − (ratio unprimed/ratio primed)] × 100. The bars represent the mean values from one representative experiment of two total, using three mice per treatment. The error bars represent the standard deviation from the mean. Statistical analysis using the Wilcoxon sum of ranks test revealed significant differences in the percentage of HSV-1-specific cytolysis between DT-treated and PBS-treated mice in both the spleen (P ≤ 0.001) and the popliteal lymph nodes (P ≤ 0.001).
DC depletion impairs CD4+ T-cell activation.
The role of CD4+ T-cell help during the primary anti-HSV-1 responses remains a matter of speculation. It is known, however, that activated CD4+ T cells enhance the HSV-1-specific CTL response (32, 39). Because the immunodominant epitopes binding to the class II MHC restriction elements are not well defined, the analysis precluded the use of specific peptide reagents. Instead, the total frequency of CD4+ T cells expressing the panactivation marker CD25 was examined. The analysis revealed a profound decrease in the frequency of CD4+ CD25+ T cells in DT-treated DTR-Tg mice compared to PBS-treated or nontreated mice in both the draining popliteal lymph nodes (Fig. 7A and C; P ≤ 0.05) and spleens (Fig. 7B and D; P ≤ 0.05). Therefore, in vivo ablation of DC also significantly impacted the early HSV-1-specific CD4+ T-cell response.
FIG. 7.
DC depletion impairs CD4+ T-cell activation. PLN and splenocyte cells were harvested from day 4-infected mice and surface stained for expression of CD4 and CD25. Representative histograms depicting the typically observed frequencies of activated CD4 T cells in the PLNs and spleen of PBS-treated, DT-treated, and nontreated DTR-Tg mice on day 4 postinfection are shown in panels A and B, respectively. Numbers in the upper right quadrant represent the percentage of activated T helper cells gated in the rectangle. (C and D) Total cell numbers were calculated by multiplying the percentage of double-positive cells in the rectangular gate by the number of total cells per PLN or spleen. Each bar represents the mean value obtained from a minimum of two experiments using a minimum of three mice per treatment. The error bars represent the standard deviation from the mean. Statistical analysis using the Wilcoxon sum of ranks test showed a significant difference on day 4 p.i. PBS plus HSV-1 versus DT plus HSV-1 (P ≤ 0.05) and HSV-1 versus DT plus HSV-1 (P ≤ 0.05) DTR-Tg mice are shown.
DISCUSSION
Dendritic cells have long been postulated to play a role in mediating resistance to HSV-1. Initially, it was proposed that skin-associated APC, specifically Langerhans cells (LC), were central to the process, as depletion of LC from the skin epidermis of mice lead to the enhancement of HSV-1 virulence and pathogenesis (38). However, the physical skin abrasion depletion technique employed specifically targeted LC and epidermal DC populations (38) and may have overlooked the role of other recently identified DC populations (16). Furthermore, reports showing priming of HSV-specific CTLs after skin infection to be independent of antigen presentation by LCs have led to a revision of the traditional view of Langerhans cells in epidermal immunity (3). As such, the role of DCs in mediating resistance to HSV-1 infections remains elusive.
To examine the role of DCs in resistance to HSV-1 infection, we employed the newly generated CD11c-DTR-GFP transgenic mouse model. This model enables the selective ablation of DCs in vivo and has successfully been used to study the role of DCs in microbial immunity (40) and in the development of airway hypersensitivity (41). Using the CD11c-DTR-GFP transgenic mouse model, we have shown that the transient ablation of DCs from typically resistant B6 mice increases susceptibility to infection. This was specifically manifested in the higher levels of virus detectable in the spinal cords of DC-depleted versus nondepleted animals. Spread of virus into the peripheral and central nervous systems inevitably led to increased levels of morbidity and mortality in DC-depleted mice. Furthermore, the depletion of DCs was found to impede the optimal activation of all immune parameters examined, including the early HSV-1 nonspecific NK cell response and the HSV-1 specific T-cell response.
A potential disadvantage of the CD11c-DTR-GFP transgenic mouse model is the unintentional depletion of marginal-zone and sinusoidal macrophage populations from the spleen and lymph nodes, respectively, following DT administration (31). These macrophage populations have been shown to function in antigen sampling and in the induction of B-cell responses following infections with VSV, lymphocytic choriomeningitis virus, or Listeria monocytogenes (29, 31). In our studies, treatment with clodronate-containing liposomes, whereby these macrophage populations can be selectively depleted while leaving DC populations intact, did not result in increased sensitivity of B6 mice to HSV-1. These results are likely a reflection of the fact that immunity to HSV-1 is not dependent on antibody levels (28) and confirm the notion that DCs play a greater role than marginal-zone and sinusoidal macrophage populations in mediating resistance to HSV-1.
Recent reports have indirectly hinted at the role of DCs in resistance to HSV-1. Injection of neonatal mice with Flt3 ligand, for example, has been shown to trigger a specific increase in the number of DCs, leading to an enhanced ability in these mice to control HSV-1 infection and a subsequent improvement in overall survival rate (45). Flt3 ligand-injected mice have also been shown to have reduced amounts of latent virus within infected neurons, suggesting that the enhanced numbers of DCs potentially play a role in controlling viral spread into the nervous system (36). These results are not necessarily surprising, considering the myriad roles of DCs during both the innate and adaptive immune responses.
During the innate immune response, the ability to produce elevated levels of type I IFN is known to play a significant role in HSV-1 pathogenesis and is thought to provide a threshold of resistance in both humans and mice (8, 13). Although the precise mechanisms by which type I IFNs function during HSV-1 infection are not fully understood in humans, murine studies have specifically shown that IFN-α/β cytokines inhibit the onset of immediate-early gene expression (2, 22), limit viral spread into the nervous system (13), and activate host defenses such as NK cells (19). Recent studies further suggest a role for type I IFNs in the development of an optimal, virus-specific CD8+ T-cell response (18). Plasmayctoid dendritic cells (pDC; CD11c+ B220+) have been shown to secrete large amounts of type I IFN in vitro following exposure to HSV-1 in both humans and mice (9). Studies suggest that pDCs are probably the most potent producers of type I IFNs in response to HSV-1 on a per-cell basis (14). Therefore, the increased susceptibility of DT-treated DTR-Tg mice to HSV-1 infection may in part be a function of the observed decrease in total pDC cell numbers. Specifically, it would be hypothesized that DT treatment leads to decreased pDC cell numbers and a subsequent reduction in total type I IFN produced during infection. This presumed decrease in IFN-α/β levels would explain in part the increased propensity of virus to cross over into the peripheral and central nervous systems in the absence of DCs.
With respect to the adaptive immune response, it has long been appreciated that the function of T-cell immunity correlates with the outcome of infection (32, 39). Specifically, immunodepletion and adoptive transfer studies have amply demonstrated the role of CTLs in reducing viral replication (42), resolving cutaneous disease (7), and providing overall protection upon challenge (26). CD8+ T cells appear to play a particularly important role in preventing infection of peripheral nerve endings and the reactivation of latent virus from neurons in the dorsal root ganglia of infected mice (17, 25). It is now understood that conventional DCs (cDC; CD11c+ CD8α+) are the principal DC subset involved in initiating CTL immunity to HSV-1 (8). We have shown that DT treatment of DTR-Tg mice significantly reduces levels of cDCs, inevitably leading to a proportional decrease in the cytolytic activity and IFN-γ secretion ability of CTLs and in the activation levels of CD4 T cells.
Our studies suggest that DCs act as a cellular switch triggering various aspects of immunity during an HSV-1 infection. During the innate immune response, DCs play an important role in NK cell activation and aid in keeping viral spread at bay. During the adaptive immune response, DCs activate the potent HSV-1-specific T-cell response known to be important in the effective resolution of acute infection and in limiting HSV-1 spread into the peripheral nervous system (17, 34). The observed early onset of morbidity and mortality, prior to the optimal activation of CTLs, suggests that a critical time frame exists during the immune response to HSV-1 wherein DCs are absolutely required for resistance. Future studies employing a temporally staggered DT treatment regimen of DTR-Tg mice will be undertaken to examine whether such a critical time frame exists.
DCs are a heterogeneous group, with nearly seven unique populations identified to date in mice. Many questions remain to be answered about the role of each individual subset in immunity. Future studies delineating the contribution of individual subsets in resistance to HSV-1 are envisioned. The studies described herein provide a potential mechanism by which the observed spectrum of susceptibility to HSV-1 infection observed in humans can be explained and for which effective therapies can be designed.
Acknowledgments
The authors gratefully acknowledge Deoborah Chervenak and Lijia Yin of the LSUHSC Research Core Facility for their excellent technical assistance with flow cytometric analysis. We also acknowledge the assistance of Shannon Mumphrey. Nico van Rooijen generously provided the authors with clodronate enclosed liposomes.
This work was supported by grant R01-AI-49428 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Ahmed, R., and C. A. Biron. 1999. Immunity to viruses, p. 1295. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 2.Aichele, P., J. Zinke, L. Grode, R. A. Schwendener, S. H. Kaufmann, and P. Seiler. 2003. Macrophages of the splenic marginal zone are essential for trapping of blood-borne particulate antigen but dispensable for induction of specific T cell responses. J. Immunol. 171:1148-1155. [DOI] [PubMed] [Google Scholar]
- 3.Allan, R. S., C. M. Smith, G. T. Belz, A. L. van Lint, L. M. Wakim, W. R. Heath, and F. R. Carbone. 2003. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science 301:1925-1928. [DOI] [PubMed] [Google Scholar]
- 4.Andoniou, C. E., S. L. van Dommelen, V. Voigt, D. M. Andrews, G. Brizard, C. Asselin-Paturel, T. Delale, K. J. Stacey, G. Trinchieri, and M. A. Degli-Esposti. 2005. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat. Immunol. 6:1011-1019. [DOI] [PubMed] [Google Scholar]
- 5.Ballas, Z. K., and W. Rasmussen. 1990. Lymphokine-activated killer (LAK) cells. IV. Characterization of murine LAK effector subpopulations. J. Immunol. 144:386-395. [PubMed] [Google Scholar]
- 6.Belz, G. T., F. R. Carbone, and W. R. Heath. 2002. Cross-presentation of antigens by dendritic cells. Crit. Rev. Immunol. 22:439-448. [PubMed] [Google Scholar]
- 7.Bonneau, R. H., and S. R. Jennings. 1989. Modulation of acute and latent herpes simplex virus infection in C57Bl/6 mice by adoptive transfer of immune lymphocytes with cytolytic activity. J. Virol. 63:1480-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukowsik, J. F., and R. M. Welsh. 1986. The role of natural killer cells and interferon in resistance to acute infection of mice with herpes simplex virus type 1. J. Immunol. 136:3481-3485. [PubMed] [Google Scholar]
- 9.Colonna, M., A. Krug, and M. Cella. 2002. Interferon-producing cells: on the front line in immune responses against pathogens. Curr. Opin. Immunol. 14:373-379. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham, A. L., and Z. Mikloska. 2001. The holy grail: immune control of human herpes simplex virus infection and disease. Herpes 8(Suppl. 1):6A-10A. [PubMed] [Google Scholar]
- 11.Delemarre, F. G., N. Kors, and N. van Rooijen. 1990. Elimination of spleen and of lymph node macrophages and its difference in the effect on the immune response to particulate antigens. Immunobiology 182:70-78. [DOI] [PubMed] [Google Scholar]
- 12.Ferlazzo, G., and C. Münz. 2004. NK cell compartments and their activation by dendritic cells. J. Immunol. 172:1333-1339. [DOI] [PubMed] [Google Scholar]
- 13.Halford, W. P., L. A. Veress, B. M. Gebhardt, and D. J. Carr. 1997. Innate and acquired immunity to herpes simplex virus type 1. Virology 236:328-337. [DOI] [PubMed] [Google Scholar]
- 14.Hochrein, H., B. Schlatter, M. O'Keeffe, C. Wagner, F. Schmitz, M. Shiemann, S. Bauer, M. Suter, and H. Wagner. 2004. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and independent pathways. Proc. Natl. Acad. Sci. USA 101:11416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung, S., D. Unutmaz, P. Wong, G. Sano, K. De los Santos, T. Sparwasser, S. Wu, S. Vuthoori, K. Ko, F. Zavala, E. G. Pamer, D. R. Littman, and R. A. Lang. 2002. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 17:211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelsall, B. L., C. A. Biron, O. Sharma, and P. M. Kaye. 2002. Dendritic cells at the host-pathogen interface. Nat. Immunol. 3:699-702. [DOI] [PubMed] [Google Scholar]
- 17.Khanna, K. M., R. H. Bonneau, P. R. Kinchington, and R. L. Hendricks. 2003. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18:593-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D. F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009-1015. [DOI] [PubMed] [Google Scholar]
- 19.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez, C. 1981. Resistance to herpes simplex virus-type 1 (HSV-1). Curr. Top. Microbiol. Immunol. 92:15-23. [DOI] [PubMed] [Google Scholar]
- 21.McKenna, K., A. S. Beignon, and N. Bhardwaj. 2005. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J. Virol. 79:17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miitnacth, S., P. Straub, H. Kirchner, and H. Jacobsen. 1988. Interferon treatment inhibits onset of herpes simplex virus immediate-early transcription. Virology 164:201-210. [DOI] [PubMed] [Google Scholar]
- 23.Mueller, S. N., C. M. Jones, W. Chen, Y. Kawaoka, M. R. Castrucci, W. R. Heath, and F. R. Carbone. 2003. The early expression of glycoprotein B from herpes simplex virus can be detected by antigen-specific CD8+ T cells. J. Virol. 77:2445-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller, S. N., C. M. Jones, C. M. Smith, W. R. Heath, and F. R. Carbone. 2002. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J. Exp. Med. 195:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nash, A. A. 2000. T cells and the regulation of herpes simplex virus latency and reactivation. J. Exp. Med. 191:1455-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nash, A. A., K. N. Leung, and P. Wildy. 1985. The T-cell mediated immune response of mice to herpes simplex virus, p. 87-102. In B. Roizman and C. Lopez (ed.), The herpesviruses, vol. 4. Plenum Publishing Corp., New York, N.Y. [Google Scholar]
- 27.Nugent, C. T., R. M. Wolcott, R. Chervenak, and S. R. Jennings. 1994. Analysis of the cytolytic T-lymphocyte response to herpes simplex virus type 1 glycoprotein B during primary and secondary infection. J. Virol. 68:7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollara, G., D. R. Katz, and B. M. Chain. 2004. The host response to herpes simplex virus infection. Curr. Opin. Infect. Dis. 17:199-203. [DOI] [PubMed] [Google Scholar]
- 29.Preston, C. M. 2000. Repression of viral transcription during herpes simplex virus latency. J. Gen. Virol. 81:1-19. [DOI] [PubMed] [Google Scholar]
- 30.Probst, H. C., and M. Van den Broek. 2005. Priming of CTLs by lymphocytic choriomeningitis virus depends on dendritic cells. J. Immunol. 174:3920-3924. [DOI] [PubMed] [Google Scholar]
- 31.Probst, H. C., K. Tschannen, B. Odermatt, R. Schwendener, R. M. Zinkernagel, and M. Van Den Broek. 2005. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin. Exp. Immunol. 141:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouse, B. T., and M. Gierynska. 2001. Immunity to herpes simplex virus: a hypothesis. Herpes 8(Suppl. 1):2A-5A. [PubMed] [Google Scholar]
- 33.Serbina, N. V., P. T. Salazar-Mather, C. A. Biron, W. A. Kuziel, and E. G. Pamer. 2003. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19:59-70. [DOI] [PubMed] [Google Scholar]
- 34.Simmons, A. 1989. H-2-linked genes influence the severity of herpes simplex virus infection of the peripheral nervous system. J. Exp. Med. 169:1503-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simmons, A., and D. C. Tscharke. 1992. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J. Exp. Med. 175:1337-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, J. R., A. M. Thackray, and R. Bujdoso. 2001. Reduced herpes simplex virus type 1 latency in Flt-3 ligand-treated mice is associated with enhanced numbers of natural killer and dendritic cells. Immunology 102:352-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith, P. M., R. M. Wolcott, R. Chervenak, and S. R. Jennings. 1994. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-gamma (IFN-gamma). Virology 202:76-88. [DOI] [PubMed] [Google Scholar]
- 38.Sprecher, E., and Y. Becker. 1986. Skin Langerhans cells play an essential role in the defense against HSV-1 infection. Arch. Virol. 91:341-349. [DOI] [PubMed] [Google Scholar]
- 39.Stanberry, L. R., A. L. Cunningham, A. Mindell, L. L. Scott, S. L. Spruance, F. Y. Aoki, and C. J. Lacey. 2000. Prospects for control of herpes simplex virus disease through immunization. Clin. Infect. Dis. 30:549-566. [DOI] [PubMed] [Google Scholar]
- 40.Tian, T., J. Woodworth, M. Skold, and S. M. Behar. 2005. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J. Immunol. 175:3268-3272. [DOI] [PubMed] [Google Scholar]
- 41.van Rijt, L. S., S. Jung, A. Kleinjan, N. Vos, M. Willart, C. Duez, H. C. Hoogsteden, and B. N. Lambrecht. 2005. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 201:981-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Rooijen, N., and R. Van Nieuwmegan. 1984. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res. 238:355-358. [DOI] [PubMed] [Google Scholar]
- 43.Van Rooijen, N., and A. Sanders. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174:83-93. [DOI] [PubMed] [Google Scholar]
- 44.Vollstedt, S., S. Arnold, C. Schwerdel, M. Franchini, G. Alber, J. P. Di Santo, M. Ackermann, and M. Suter. 2004. Interplay between alpha/beta and gamma interferons with B, T, and natural killer cells in the defense against herpes simplex virus type 1. J. Virol. 78:3846-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vollstedt, S., M. Franchini, H. P. Hefti, B. Odermatt, M. O'Keeffe, G. Alber, B. Glanzmann, M. Riesen, M. Ackermann, and M. Suter. 2003. Flt3 ligand-treated neonatal mice have increased innate immunity against intracellular pathogens and efficiently control virus infections. J. Exp. Med. 197:575-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitley, R. J., and B. Roziman. 2001. Herpes simplex virus infections. Lancet 357:1513-1518. [DOI] [PubMed] [Google Scholar]
- 47.Yoneyama, H., K. Matsuno, E. Toda, T. Nishiwaki, N. Matsuo, A. Nakano, S. Narumi, B. Lu, C. Gerard, S. Ishikawa, and K. Matsushima. 2005. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J. Exp. Med. 202:425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]