Abstract
Transmission of human immunodeficiency virus type 1 (HIV-1) occurs primarily via the mucosal route, suggesting that HIV-1 vaccines may need to elicit mucosal immune responses. Here, we investigated the immunogenicity and relative efficacy of systemic immunization with two human ALVAC-HIV-1 recombinant vaccines expressing Gag, Pol, and gp120 (vCP250) or Gag, Pol, and gp160 (vCP1420) in a prime-boost protocol with their homologous vaccine native Env proteins. The relative efficacy was measured against a high-dose mucosal exposure to the pathogenic neutralization-resistant variant SHIVKU2 (simian-human immunodeficiency virus). Systemic immunization with both vaccine regimens decreased viral load levels not only in blood but unexpectedly also in mucosal sites and protected macaques from peripheral CD4+ T-cell loss. This protective effect was stronger when the gp120 antigen was included in the vaccine. Inclusion of recombinant Tat protein in the boosting phase along with the Env protein did not contribute further to the preservation of CD4+ T cells. Thus, systemic immunization with ALVAC-HIV-1 vaccine candidates elicits anti-HIV-1 immune responses able to contain virus replication also at mucosal sites in macaques.
Due to the alarming spread of human immunodeficiency virus type 1 (HIV-1) infection worldwide, the development of a prophylactic vaccine is critical. Vaccine strategies for AIDS have included the use of structural and nonstructural HIV subunit proteins or peptides, naked DNA, bacterial and viral live vectors, or combinations of the above. Most of the vaccines developed thus far induce T-cell responses but are unable to induce neutralizing antibodies to the Env of primary HIV-1 isolates. The fact that a decrease in plasma viremia (9, 41) is concomitant with the appearance of virus-specific cytotoxic T lymphocytes (CTLs) and depletion of CD8+ T cells in simian immunodeficiency virus (SIV)-infected macaques or HIV-infected chimpanzees suggests that CD8+ T cells are able to partially control HIV-1/SIV replication (15, 36, 43, 52). Indeed, several “T-cell vaccines” based on DNA and live vectors confer protection from high-level replication of challenge viruses in rhesus macaques (2, 4, 8, 30, 31, 48). The extent of the decrease in viral replication induced by these vaccines is variable in nonhuman primates, and protection from disease appears to be dependent on the virulence of the virus used in the challenge experiments (28).
The relative efficacy of poxvirus-based vaccine candidates with various degrees of attenuation has been extensively studied in rhesus macaques after challenge with SIV, SHIV, and HIV-2 isolates (1, 3, 8, 24, 25, 29-31, 48). These vaccine modalities elicit variable levels of cell-mediated immune response and prevent infection after challenge exposure to viruses with low virulence, such as HIV-2 (3, 25), and to other somewhat-attenuated SIV isolates (34, 35). Importantly, these vaccine modalities were also able to significantly reduce virus load after challenge with highly pathogenic SIV isolates (8, 29, 32, 48). An ALVAC-SIV vaccine encoding the gag, pol, and env genes of the SIVmac251 isolate was able to reduce plasma virus load during primary infection and conferred protection from CD4+ loss during both acute and chronic phases of infection after rectal exposure to a highly pathogenic SIVmac251 isolate (50).
Among the pox vector-based vaccines, several ALVAC-based HIV-1 vaccines have been tested in phase I and II clinical trials and have been shown to be safe and immunogenic in humans (10, 14, 18, 21, 27). Whether the immunogenicity of these vaccine candidates will be sufficient to protect humans from HIV-1 remains unknown (5, 11, 44). The ongoing human phase III trial in Thailand will provide key information in this regard. Here, we designed a study to assess whether systemic immunization with recombinant canarypox expressing either HIV-1 gp120 or gp160 followed by a boost with either purified gp120 or gp140 proteins would confer protection after mucosal challenge with the pathogenic SHIVKU2. We also investigated whether the addition of the Tat protein of HIV-1 as an immunogen could provide better protection, since a few studies have demonstrated that immunization of macaques with Tat protein either alone or as a Nef-Tat fusion protein in a multicomponent subunit vaccine confers an advantage in a SHIV89.6P model (12, 13, 56) but not in other studies (53). Here, we found that immunization of macaques with this type of ALVAC-based vaccine formulation elicited both antibody and cellular responses and significantly decreased plasma viremia and CD4+ T-cell loss after rectal exposure to the SHIVKU2 isolate. Tat protein immunization had no additive effect on the reduction of virus load in vaccinated rhesus macaques, as also observed by others (42, 46, 53).
MATERIALS AND METHODS
Vaccines and immunization protocol.
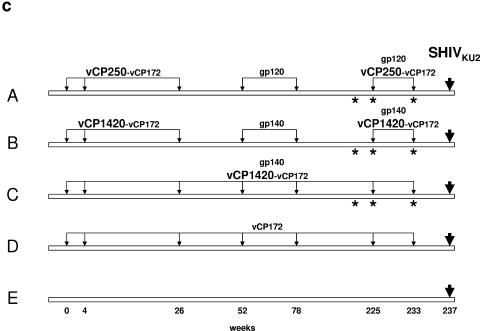
Thirty-three rhesus macaques of Indian origin were used in the present study. The care and use of the animals were in compliance with all relevant institutional guidelines. The ALVAC-based SIV and HIV-1 vaccines used in the present study are presented in Table 1. vCP250 expressing the HIV-1IIIB gag-pro-gp120 TM nef-pol genes epitope, vCP1420 expressing the HIV-1IIIB gag-pro-gp160 nef-pol genes epitope, and vCP172 expressing the SIVmac251 (K6W) gag and pol genes (22) were generated as previously described (50). The HIV-1 gp120 and gp140 used as protein boost were purified from the serum-free culture supernatant of HUT78 cells chronically infected with HIV-1IIIB by immunoaffinity chromatography using anti-gp120 and anti-gp41 monoclonal antibodies as described previously (40). Recombinant Tat protein from the HIV-1IIIB isolate was purified from bacterial culture as described elsewhere (16). Both ALVAC-derived vaccines and the subunit glycoprotein and Tat protein boost were delivered via the intramuscular route. Env and Tat proteins were administered in QS-21 adjuvant (provided by Antigenics, Inc., Framingham, MA). Animals in group A (six macaques) received 108 PFU of ALVAC-gp120 (vCP250) and ALVAC-SIV-gag-pol (vCP172) at weeks 0, 4, 26, 225, and 233 and gp120 protein boost on weeks 52, 78, 225, and 233; group B animals (six macaques) were inoculated with 108 PFU of ALVAC-gp160 (vCP1420) and vCP172 at weeks 0, 4, 26, 225, and 233 and oligomeric gp140 protein boost on weeks 52, 78, 225, and 233. Animals in group C (six macaques) were inoculated with 108 PFU of vCP1420 and vCP172 at weeks 0, 4, 26, 52, 78, 225, and 233 and simultaneously received oligomeric gp140 protein boost on weeks 4, 26, 52, 78, 225, and 233. Three animals per group from groups A, B, and C also received Tat protein (50 μg) by the intramuscular route on weeks 221, 225, and 233 adjuvanted in QS-21. Group D animals (six macaques) received 108 PFU of vCP172 at weeks 0, 4, 26, 52, 78, 225, and 233, whereas animals from group E (nine macaques) were kept as naive controls. All animals from groups A, B, C, and E and three animals from group D were challenged intrarectally with SHIVKU2 4 weeks after the last immunization.
TABLE 1.
Nomenclature of ALVAC recombinant vaccines used for immunization
| Immunogen | Vaccine |
|---|---|
| ALVAC-SIVmac251-gag-pol | vCP172 |
| ALVAC-HIV-1IIIB-gag-pol-gp120-env | vCP250 |
| ALVAC-HIV-1IIIB-gag-pol-gp160-env | vCP1420 |
Immunoprecipitation of gp120 from chicken embryo fibroblasts infected with ALVAC-HIV-1-gag-pol-env (gpe).
Freshly isolated chicken embryo fibroblast cells (Charles River SPAFAS, Wilmington, MA) were cultured in complete RPMI medium containing 10% fetal calf serum overnight. The monolayer was washed with RPMI medium and infected with 5 ml of either vCP250 or vCP1420 (multiplicity of infection = 5) at 37°C for 2 h. Residual virus inoculum was removed by washing the monolayer with 10 ml of complete medium, and the cells were cultured with 20 ml of complete RPMI medium for 72 h. After the culture revealed a cytopathic effect, cells were washed with phosphate-buffered saline (PBS) and labeled with [35S]methionine (50 μCi/ml) in methionine-free RPMI medium containing 2% dialyzed fetal calf serum for 18 h. A flask of uninfected fibroblasts was also labeled with [35S]methionine (50 μCi/ml) as a negative control. Both cells and supernatant were harvested, solubilized, and immunoprecipitated with serum from an HIV-1-infected individual as described elsewhere (49).
Protein immunogens.
HIV-1 envelope glycoproteins gp120 and gp140 were purified from the culture supernatant of HUT78 cells lines by immunoaffinity chromatography as described elsewhere (39, 40). Briefly, a clone of a chronically infected HUT78 cell line was adapted to grow in serum-free conditioned medium. The culture supernatant was harvested when the cells were cultured to the log phase, and the glycoproteins were purified by immunoaffinity chromatography using anti-gp120 and anti-gp41 murine monoclonal antibodies. Recombinant Tat protein was expressed in bacteria and purified as described before (16).
Challenge virus stock.
The SHIVKU2 challenge stock was prepared by culturing phytohemagglutinin (PHA)-activated peripheral blood mononuclear cells (PBMC) from an infected macaque that was inoculated intravenously with SHIVKU2. SHIVKU2 was derived from in vivo animal passage of SHIVHXB2 (38), and the Env proteins of both viruses had significant sequence homology. However, SHIVKU2 was shown to have characteristics similar to primary isolates and behaved as a biological variant of SHIVHXB2 in terms of neutralization properties (19). Moreover, our preliminary titration experiments clearly demonstrated SHIVKU2 transmits efficiently via the mucosal route (data not shown). The titer of the SHIVKU2 challenge stock was determined in vivo in rhesus macaques by inoculating rectally six animals with different dilutions of virus stock (data not shown). Since six of six animals inoculated with 1 ml of undiluted virus stock became infected, as evidenced by high plasma viremia, this dose of virus was selected for all challenge studies.
Binding and neutralizing antibody assays.
Serum samples were tested for HIV- and SIV-specific antibody responses by using an enzyme-linked immunosorbent assay (ELISA) described elsewhere (50). Briefly, polystyrene plates (Greiner Bio-One, Longwood, FL) were coated with HIV-1IIIB gp140 protein (50 ng/well) in 100 μl of phosphate buffer (pH 7.2) at 4°C overnight. The solution was aspirated, the wells were washed with wash buffer (PBS containing 0.5% Tween 20) four times, and each well was treated with 200 μl of blocking buffer (water containing 2.5% bovine serum albumin and 1.25% dry milk) for 1 h at 37°C. After a wash with wash buffer, 100 μl of serum serially diluted in Dilsim II (bioMerieux, Durham, NC) was added to each well, followed by incubation for 1 h at 37°C. Plates were again washed with wash buffer, and each well was incubated for 1 h at 37°C with 100 μl of 1:5,000 dilution of goat anti-human horseradish peroxidase-labeled immunoglobulin G (KPL Labs, Gaithersburg, MD) in conjugate buffer (PBS containing 10% normal goat serum, 0.1% Tween 20, and 0.2% bovine serum albumin). Plates were then thoroughly washed with wash buffer, and each well was reacted with 100 μl of Enhanced K-Blue Substrate (Neogen, Lexington, KY) at room temperature for 30 min. Reaction was terminated by adding 100 μl of 2N H2SO4 to each well, and the optical density was read in an ELISA plate reader at 450 nm. Antibody titers were defined as the highest dilution of immune serum yielding an optical density greater than two times the optical density detected with a corresponding dilution of preimmune serum. To assess SHIV-specific serum-neutralizing activity, two types of assays were conducted with sera from the vaccinated animals. In the first assay, sera were tested for their ability to neutralize a T-cell-line-adapted stock of SHIVHXB2 assayed in MT2 cells as described previously (45). In the second assay, neutralization of the challenge stock of SHIVKU2 was examined in PHA-activated human PBMC by measuring a reduction in viral p27 Gag antigen synthesis (45).
CTL assay using recombinant vaccinia virus.
PBMC (107) from the monkeys were cultivated in vitro with paraformaldehyde-fixed, autologous B-LCL infected with either vaccinia-HIV-1BH10 Env or vaccinia-SIVmac251 Gag. On day 3 of culture, 20 U of recombinant human interleukin-2/ml was added to the cultures. On day 12 of culture, the lymphocytes were centrifuged over a Ficoll-diatrizoate gradient and assessed as effector cells in a 51Cr release cytotoxicity assay. Target cells were autologous B-LCL (106) cultured overnight with vaccinia virus-SIVmac251 Gag, HIV-1BH10 Env, or vaccinia virus expressing herpes equine virus (control) at a multiplicity of infection of 10 PFU/cell. Target cells were then washed and labeled with 100 μCi of sodium 51Cr for 1.5 h. After the washing step, 104 target cells were added per well in 96-well U-bottom plates in 100-μl volumes. Effector cells were added in another 100-μl volume at various concentrations to give effector/target ratios of 20:1, 10:1, 5:1, and 2.5:1. Plates were incubated at 37°C for 4 h. Then, 50 μl of supernatant was transferred to counting plates, and 200 μl of scintillation fluid was added and analyzed in a 1450 Microbeta liquid scintillation counter. Specific release was calculated as follows: [(experimental release − spontaneous release)/(100% release − spontaneous release)] × 100. Only cytotoxic activities above 10% were considered positive.
Virological assays.
Animals were bled periodically after challenge, and the viral load in plasma was assessed by using a nucleic acid sequence-based amplification assay (NASBA) to quantify SIV RNA (51). In addition, PBMC collected from animals 21 days after virus challenge were subjected to quantitative virus isolation by coculture with PHA-activated human PBMC to confirm virus transmission. The CD4+ T-cell count in the PBMC of challenged animals was determined by standard flow cytometric analysis.
For viral load quantification in tissues, total RNA was extracted from homogenized tissues by a method described previously (17). Briefly, a small portion of the tissue was excised and lysed by homogenization in a tube homogenizer with sodium citrate lysis buffer (pH 7.0) containing guanidine thiocyanate, sarcosyl, and β-mercaptoethanol. The lysed sample was treated with sodium acetate, extracted with phenol-chloroform (5:1), and RNA was precipitated with isopropanol. Extracted RNA was further purified by dissolving the pellet in sodium citrate lysis buffer and by reprecipitation with isopropanol. After a wash with 70% ethanol, the RNA pellet was air dried and dissolved in water, and the RNA content was quantitated by measuring the optical density at 260 nm. Quantitation of viral RNA was performed in a small aliquot (0.5 to 1 μg) of total RNA extract by NASBA as described elsewhere (51). This assay has a lower limit sensitivity of 500 copies of RNA, and the SIV RNA load was expressed as viral RNA copies per μg of total tissue RNA.
Statistical analysis.
Analyses of the area under the curve (AUC) and the peak of plasma viral RNA loads were performed by using the Wilcoxon rank sum test and Wilcoxon-Gehan tests, respectively. CD4+ T-cell counts, adjusted for baseline counts, were square-root transformed before analysis and were compared by using the Kruskal-Wallis test and repeated measures analysis of variance. The statistical analysis included CD4+ T-cell values at days 28, 42, 58, 83, and 119. Viral RNA levels were tested by the Wilcoxon-Gehan test in three tissues where sufficient detectable levels were found and by the Fisher exact test in two tissues where vaccinated animals had undetectable levels.
RESULTS
Vaccines and study design.
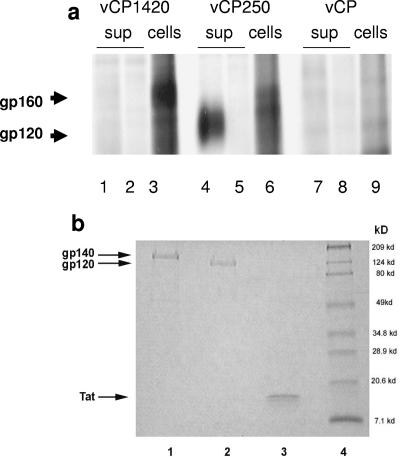
The aim of the present study was to assess the immunogenicity and relative efficacy of multiple immunizations of two ALVAC-based HIV-1 vaccines expressing either the uncleavable Env precursor gp160 (vCP1420) or the gp120-TM fusion protein (vCP250). Expression of Env proteins by these two constructs were assessed in chicken embryo fibroblasts. vCP1420 expressed the Env precursor that remained cell associated, whereas in the case of the vCP250 construct the gp120 was shed in the supernatant (Fig. 1a, lanes 1 and 3 and lanes 4 and 6, respectively). The Env proteins were neither detected by normal human serum (Fig. 1a, lanes 2 and 5) nor by the immune sera in mock-infected cells (Fig. 1a, lanes 7 and 9). All three proteins used as subunit boosts were homogeneous preparations with a high level of purity as shown by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) profiles for HIV-1IIIB gp140 (Fig. 1b, lane 1), gp120 (Fig. 1b, lane 2), and recombinant Tat protein (Fig. 1b, lane 3).
FIG.1.
(a) Expression of HIV-1IIIB Env proteins in chicken embryo fibroblasts infected with vCP 1420 and vCP250 ALVAC-HIV-1 vaccines. Chicken embryo fibroblasts were infected with vCP1420 (lanes 1 to 3), vCP250 (lanes 4 to 6), and mock virus (lanes 7 to 9) and labeled with [35S]methionine overnight. Cells (lanes 3, 6, and 9) and supernatants (lanes 1, 2, 4, 5, 7, and 8) were lysed and immunoprecipitated with normal human serum (lanes 2, 5, and 8) or serum from an HIV-1-infected individual (lanes 1, 3, 4, 6, 7, and 9) and analyzed by SDS-PAGE, as described in Materials and Methods. (b) PAGE profiles of Env and Tat proteins. Proteins were heated at 95°C for 5 min with SDS sample buffer under reducing conditions, electrophoresed on 10 to 20% gradient gel, and stained with Coomassie blue. Lane 1, gp140; lane 2, gp120; lane 3, Tat; lane 4, molecular weight marker. (c) Schematic representation of the study design. The asterisks designate the time of Tat administration in half of the animals in groups A, B, and C.
The experimental vaccination regimen included five groups of animals (A to E) (Fig. 1c). Group A was immunized with vCP250 and boosted with the homologous cleaved gp120 protein. Animals in group B were immunized with vCP1420 and boosted with purified uncleaved gp140. Group C animals received simultaneously both vCP1420 and gp140. Group D in addition to animals from groups A to C were immunized with an ALVAC construct expressing only Gag and Pol of the SIVmac251 isolate (vCP172), whereas group E animals were left naive. A subgroup of macaques from groups A, B, and C was also immunized with Tat protein (see Materials and Methods). Macaques from all groups were challenged with SHIVKU2 rectally 4 weeks after the last immunization. We chose to use multiple doses of recombinant ALVAC because ALVAC-based vaccines have been used up to 12 times with minimal interference of vector-directed immunogenicity in cancer patients (33).
HIV-1-specific immune responses elicited by vaccination.
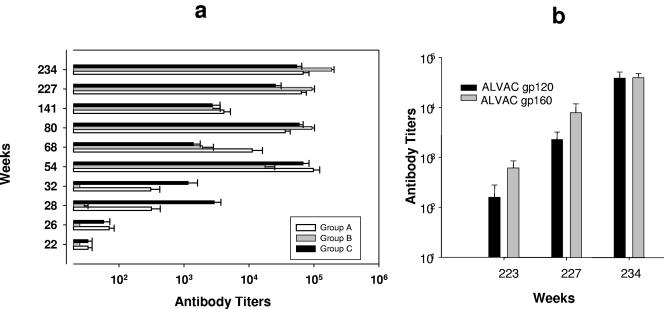
Endpoint serum antibody titers to HIV-1 antigens measured by ELISA demonstrated that, during the ALVAC priming phase until week 52, group A animals receiving ALVAC-gp120 had higher anti-Env antibody titers compared to group B animals primed with ALVAC-gp160 (Fig. 2A). As expected, animals in group C that received both ALVAC-gp160 and gp140 proteins simultaneously during this phase had the highest anti-Env titers, especially after immunization on week 26. Antibody titers to Env were markedly enhanced in all groups of animals after boosting with the Env protein on week 52, with comparable titers in groups A and C and a slightly lower titer in group B. Similar increases in anti-Env titers were noted after Env protein boosts on weeks 78 and 225, and at the end of the immunization regimen the antibody titers were equivalent in all groups (Fig. 2a). Since antibodies to Tat protein have been correlated to protection in some studies (12) but not in others (53), we investigated their possible contribution by immunizing a subgroup of ALVAC-env-immunized animals with Tat protein. Immunization of animals with Tat protein elicited ELISA antibody titers to Tat (Fig. 2b), their titers increased progressively after each inoculation and, at the end of the immunization regimen, all immunized groups had equivalent titers of anti-Tat antibodies.
FIG. 2.
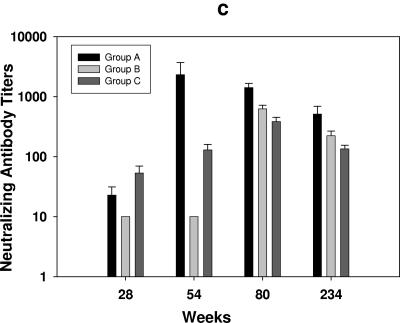
Humoral immune response in vCP 250 and vCP1420 ALVAC-immunized macaques. (a) Antibody titers to HIV-1IIIB gp140 protein were measured by ELISA in group A, group B, and group C animals on designated weeks after immunization on weeks 26, 52, 78, 225, and 233. (b) Anti-Tat antibody titers in the serum were measured on weeks 223, 227, and 234 after Tat protein boost in ALVAC-gp120 and ALVAC-gp160 animals. The minimum dilution of serum tested in the assay was 1:25. Values are expressed as mean antibody titers ± the standard errors from six animals from each group. (c) Neutralization of SHIVHXB2 was measured in MT2 cells in the presence of sera from groups A, B, and C macaques collected on weeks 28, 54, 80, and 234. The lowest dilution of serum tested in the assay was 1:10. The results are expressed as dilution of serum inhibiting 50% of infection in MT2 cells and are expressed as mean titers ± the standard errors from six animals of each group.
Neutralizing antibody titers in sera from all ALVAC-vaccinated animals (groups A to C) collected at weeks 28, 54, 80, and 234 were measured against both the laboratory-adapted SHIVHXB2 in MT2 cells and the primary challenge stock SHIVKU2 in human PBMC. Since binding antibody titers were weak before week 28 (Fig. 2a), no neutralization assay was conducted with sera from earlier time points. As shown in Fig. 2c, neutralizing antibodies to the laboratory-adapted SHIVHXB2 were detected in most animals of groups A and C on week 28 with neutralization titers increasing significantly after subsequent protein boost. Interestingly, sera from group A had slightly higher neutralizing titers compared to the other groups. None of the sera neutralized the SHIVKU2 challenge stock in a human PBMC-based assay (titer < 1:5 as based on 80% reduction in p27 synthesis to be considered positive) (data not shown).
Cell-mediated immune responses induced by vaccination.
Cellular responses induced by vaccination were measured by cytotoxic assays specific for the SIV Gag and the HIV-1 Env in blood of all animals twice during the immunization regimen (weeks 28 to 54). CTL responses to Env were low in all groups and did not differ in animals immunized with the two constructs (group A versus groups B and C) (Table 2). Five Mamu-A*01-positive animals were also included in the study: four in group B and one in group D. Memory response to the dominant Gag p11C epitope was demonstrated in all of these immunized macaques (52).
TABLE 2.
Cytotoxic activitya in response to Gag and Env in immunized macaques
| Group | Vaccine regimen | No of positive animals/total no. tested
|
|||
|---|---|---|---|---|---|
| Wk 28
|
Wk 54
|
||||
| Env (HIV- 1BH10) | Gag (SIVmac251) | Env (HIV- 1BH10) | Gag (SIVmac251) | ||
| A | vCP250 + vCP172 + gp120 | 1/6 | 0/6 | 0/6 | 0/6 |
| B | vCP1420 + vCP172 + gp140 | 2/6 | 0/6 | 2/6 | 0/6 |
| C | vCP1420 + vCP172 + gp140 simultaneously | 1/6 | 0/6 | 0/6 | 2/6 |
| D | vCP172 | 0/6 | 1/6 | 0/6 | 1/6 |
Only cytotoxic activity 10% above background was scored positive.
ALVAC-HIV-1-gpe vaccines decrease plasma viremia and protect from CD4+ T-cell loss.
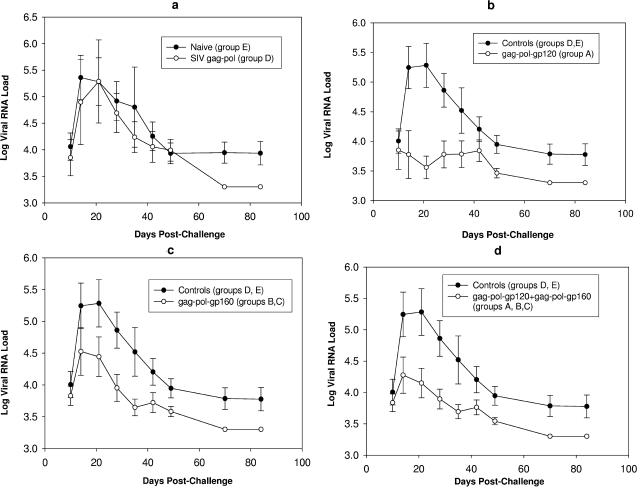
After challenge exposure, the contribution of the various immunization regimens to the containment of viral replication and CD4+ T-cell loss was assessed. Plasma viral RNA loads of all groups are shown in Fig. 3. Eight of nine naive control macaques became viremic after intrarectal challenge exposure to SHIVKU2. Plasma viral RNA levels during primary viremia in the ALVAC-SIV-gag-pol-immunized (group D) animals were lower than in the naive animals from group E, but the difference was not significant (P = 0.73) (Fig. 3a and Table 3), indicating the limitation of a vaccine constituted of only the Gag and Pol product. Similarly, set point viremia did not differ significantly in these two animal groups. Thus, these animal groups were combined in a single control group to increase the statistical power of the analysis.
FIG. 3.
Plasma viral RNA load in ALVAC-immunized macaques challenged with SHIVKU2 by the rectal route. Plasma virus load was compared between naive group E and SIV-gag-pol group D (a), control (groups D and E) and vCP250-immunized/gp120-boosted group A animals (b), control (groups D and E) and vCP1420-immunized/gp140-boosted groups B and C animals (c), and control (groups D and E) and vCP250-immunized/gp120-boosted group A animals plus vCP1420-immunized/gp140-boosted groups B and C animals (d). Values are expressed as log-transformed mean RNA loads ± the standard errors from animals from each group.
TABLE 3.
Statistical analysis of plasma viral RNA load measured in different vaccine groups after SHIVKU2 challenge
| Group comparisona |
P
|
|
|---|---|---|
| AUC | Peak viremia | |
| vCP250/gp120 (group A) vs naive (group E) | 0.049 | 0.026 |
| vCP1420/gp160 (groups B and C) vs naive (group E) | 0.072 | 0.089 |
| vCP172 (group D) vs naive (group E) | 0.73 | 0.73 |
| vCP250/gp120 (group A) and vCP1420/gp160 (groups B and C) vs naive (group E) | 0.027 | 0.025 |
| vCP250/gp120 (group A) vs vCP172 (group D) and naive (group E) | 0.041 | 0.013 |
| vCP1420/gp160 (groups B and C) vs vCP172 (group D) and naive (group E) | 0.062 | 0.099 |
| vCP250/gp120 (group A) and vCP1420/gp160 (groups B and C) vs vCP172 (group D) and naive (group E) | 0.019 | 0.019 |
| vCP250/gp120 (group A) vs vCP1420/gp160 (groups B and C) | 0.29 | 0.26 |
vCP250, ALVAC-HIV-1IIIB-gpe (gp120 Env); vCP1420, ALVAC-HIV-1IIIB-gpe (gp160 Env); vCP172, ALVAC-SIVmac251-gp.
Among vaccinated macaques, two animals each from groups A and B and one animal from group C (a total of 5 of 18) had consistently undetectable levels of plasma viremia (<2,000 RNA copies/100 μl input). However, these animals mounted weak titers of anti-p27 antibodies, suggesting occult infection (data not shown).
Plasma viremia in animals from group A differed significantly from the combined control group (D and E) (P = 0.041 within the first 2 weeks from challenge measured as the total AUC and P = 0.013 for peak viremia) (Fig. 3b and Table 3). This difference also remained statistically significant when plasma viremia of group A was compared only with naive animals in group E (P = 0.049 for AUC and 0.026 for peak viremia) (Table 3). When plasma viremia in groups B and C was compared, no significant difference was noted during primary viremia between the two groups (P = 0.73). These two groups were subsequently combined for assessing the vaccine effect of the vCP1420 construct. Interestingly, the plasma virus level in animals of groups B and C and control groups was not statistically significant (P = 0.062 for AUC and 0.099 for peak viremia) (Fig. 3c). Similarly, no significant difference in plasma viral RNA level in these animals was observed when the comparison included naive animals only (group E) (P = 0.072 for AUC and 0.089 for peak viremia). Comparison of groups A, B, and C combined with the control group (groups D and E) again revealed a significant difference in plasma viremia (P values of 0.019 for both AUC and peak viremia) (Fig. 3d), and this difference remained significant when only naive animals were included in the analysis (P = 0.027 for AUC and 0.025 for peak viremia). Although vCP250 vaccination resulted in a more effective control of plasma virus level than vaccination with vCP1420, the difference in plasma viremia between group A and groups B and C was not statistically significant (P = 0.29 for AUC and 0.26 for peak viremia) (Table 3). Taken together, these results demonstrate that Env protein contributed to the protection against pathogenic SHIV challenge. Further, the form of Env protein presented to the immune system determined the protective efficacy of the vaccine.
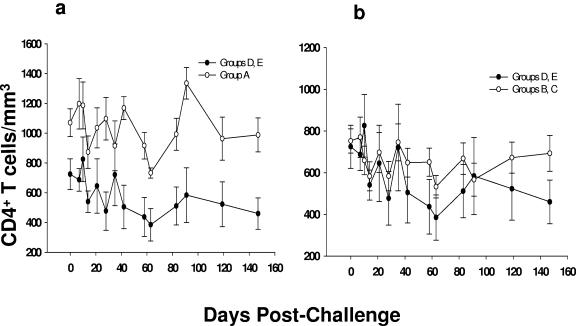
We also assessed the effect of vaccination in protection from CD4+ T-cell loss. For this, CD4+ T-cell counts before virus challenge and at different time points were measured (Fig. 4 and Table 4). Differences in CD4+ T-cell loss among group D and E animals were not significant (P = 0.40), as expected (Table 4). In contrast, there was a significant preservation of CD4+ T cells in macaques from group A compared to naive and ALVAC-SIV-gag-pol-vaccinated macaques (groups D and E) with a P value of 0.014 (Fig. 4A and Table 4). Preservation of CD4+ T cells in group A was also significant compared to naive animals in group E (P = 0.020). CD4+ T cells were also preserved in group B and C animals compared to the control group (groups D and E) (P = 0.039) (Fig. 4b). However, this difference was not significant when a comparison was made among groups B, C, and E (P = 0.081).
FIG. 4.
CD4+ T-cell counts in ALVAC-immunized and control macaques after challenge exposure to SHIVKU2. Mean CD4+ T-cell counts were compared between control groups (groups D and E) and vCP250 group A (a) and vCP1420 groups B and C (b), respectively. Values on day 0 represent average CD4+ T-cell counts from two time points measured before challenge.
TABLE 4.
Preservation of CD4+ T cells in vaccinated and control macaques
| Group comparison | P |
|---|---|
| vCP250 (group A) vs naive (group E) | 0.020 |
| vCP1420 (groups B and C) vs naive (group E) | 0.081 |
| vCP172 (group D) vs naive (group E) | 0.40 |
| vCP250 vs vCP172 + naive | 0.014 |
| vCP1420 vs vCP172 + naive | 0.039 |
Tat protein did not confer further virological benefit.
Since half of the animals from groups A, B, and C were immunized with Tat protein three times before virus challenge, we sought to determine whether the immunization with Tat would further contribute to containment of viral replication and preservation of CD4+ T cells. The statistical analysis on the plasma virus level in macaques immunized with Tat protein and with those animals that did not receive Tat demonstrated that Tat did not contribute further to protection (P = 0.32 for AUC) (Table 5). Furthermore, the addition of Tat to animals in groups A, B, and C demonstrated no difference compared to non-Tat-immunized animals from the same three groups (P = 0.10 for AUC for group A; P = 0.39 for groups B and C combined). Similarly, Tat protein did not affect virus levels when Tat-immunized animals from groups A, B, and C together were compared to the non-Tat-immunized group (P = 0.67 for AUC).
TABLE 5.
Statistical analysis of the effect of Tat protein on viral RNA load in different vaccine groups
| Group comparison | AUC |
|---|---|
| Tat-immunized vs all non-Tat-immunized | 0.32 |
| Tat-immunized vs non-Tat-immunized vCP1420/gp160 | 0.39 |
| Tat-immunized vs non-Tat-immunized vCP250/gp120 | 0.10 |
| Tat-immunized vs non-Tat-immunized vCP250/gp120 and vCP1420/gp160 | 0.67 |
SIV RNA load in tissues of ALVAC-HIV-1-gpe-vaccinated animals after challenge with SHIVKU2.
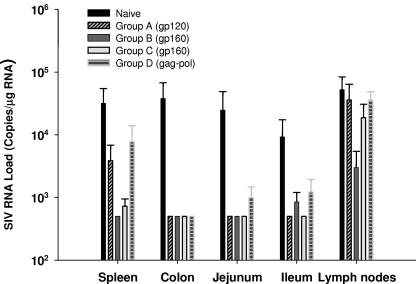
We previously observed using a peptide-based vaccine that mucosal delivery of HIV/SIV antigens resulted in a better control of virus RNA levels in tissue than systemic immunization after mucosal challenge with the same viral strain (SHIVKU2) (7). However, as we have demonstrated that systemic delivery of a recombinant poxvirus vaccine such as NYVAC-SIV results in generation of memory T cells present in mucosal sites as well as systemic sites (54), we sought to determine whether systemic immunization of animals with the ALVAC-SIV/HIV vaccine candidate would affect virus RNA levels in mucosal tissues. To this end, total RNA from lymph nodes and gut-associated tissues of both ALVAC-HIV-1 (groups A to C) and ALVAC-SIV-gag-pol (group D) vaccinated and naive animals (group E) was harvested and SIV RNA was quantitated by NASBA. As shown in Fig. 5, viral RNA levels in several tissues of the vaccinated animals were consistently lower than the level in the tissues of the control animals. Statistical analysis of these results indicated that the RNA load in spleens, colons, and ilea of vaccinated animals was significantly lower than for naive animals (P = 0.0016 for spleen, 0.0041 for colon, and 0.015 for ileum). However, the difference in lymph nodes did not reach statistical significance. Interestingly, the RNA load in colons, jejuna, and ilea of SIV-gag-pol-vaccinated animals (group D) was lower than for the naive animals (group E), although this difference was not noted for plasma viremia. However, no statistical analysis was performed because of the low number of animals enrolled in group D.
FIG. 5.
RNA virus levels in the tissues of macaques in groups A (vCP250), B and C (vCP 1420), and E (naive) at euthanasia. SIV RNA virus levels in tissues were compared between naive animals, vCP250 group A, vCP1420 group B, vCP1420 group C, and vCP172 group D separately.
Postchallenge neutralizing antibody response.
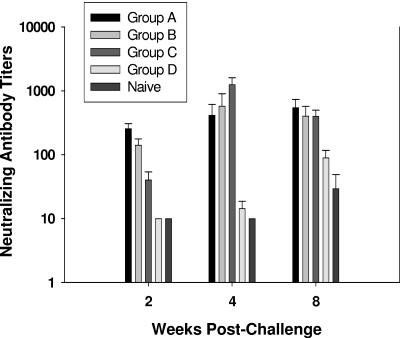
Neutralization of challenge virus as well as SHIVHXB2 was examined with sera collected 2, 4, and 8 weeks postchallenge. None of the serum from the challenged animals neutralized SHIVKU2 (data not shown). However, sera from groups A, B, and C neutralized SHIVHXB2 almost to a similar level, whereas the sera from ALVAC-SIV-gag-pol-immunized animals (group D) and naive group E had no neutralizing activity on weeks 2 and 4 but developed a weak response compared to the immunized groups on week 8 (Fig. 6).
FIG. 6.
SHIVHXB2 neutralizing antibody titers in the sera of infected macaques. Neutralization of SHIVHXB2 was measured in MT2 cells in the presence of sera from groups A, B, C, D, and naive macaques collected on 2, 4, and 8 weeks after rectal inoculation with SHIVKU2. The lowest dilution of serum tested was 1:10. The results are expressed as dilutions of serum inhibiting 50% of infection in MT2 cells and are expressed as mean titers ± the standard errors from animals of each group.
DISCUSSION
Results from several clinical trials have demonstrated that canarypox-based HIV-1 vaccines are safe and immunogenic in humans (6, 14, 18, 20, 37, 47). We previously evaluated the relative efficacy of this vaccine modality in rhesus macaques immunized with a recombinant SIVmac canarypox virus expressing SIV gag, pol, and env genes (ALVAC-SIV-gpe) and exposed mucosally to the pathogenic SIVmac251 strain. The ALVAC-SIV recombinant vaccine elicited both antibodies and CTL responses against SIV Gag and Env antigens in immunized animals. After mucosal challenge exposure, a decrease in SIVmac251 plasma RNA levels was observed during primary infection. In addition, the immunized macaques were protected from CD4 loss during both acute and chronic phases of infection (50) and survived longer than nonimmunized macaques. In that study, however, boosting of ALVAC-SIV-gpe-immunized animals with SIV gp120 did not further contribute to protection (50). Because the Env protein of SIV differs substantially from the Env of HIV, we wanted to assess more directly the contribution of two Env proteins using HIV-1 antigens. Thus, the present study was designed to assess the relative efficacy of an ALVAC-based HIV-1 vaccine constructed in the same way as the vaccine used in the phase III trial in Thailand and compare it with another construct whereby both expression of the canarypox Env and the protein boost differed. The SHIVKU2 used as a challenge virus is a neutralization-resistant phenotype of SHIVHXB2 and has biological characteristics similar to primary virus. The present study clearly demonstrates that immunization of macaques with a prime-boost including ALVAC-HIV-1-gpe encoding the gp120 gene (vCP250) and ALVAC-SIV-gag-pol (vCP174), followed by a gp120 protein boost, was effective in controlling plasma viremia, as well as the loss of CD4 cells in macaques following intrarectal challenge with a pathogenic X4-tropic SHIVKU2 isolate. In contrast, immunization of macaques with ALVAC-HIV-1-gpe encoding the uncleavable gp160 gene (vCP1420) followed by gp140 boost did not significantly affect viral replication or CD4+ T-cell count.
The protective immunological responses induced by these vaccine regimens in this animal model, however, remain unclear. Both vaccines combined with the Env protein boost elicited strong binding antibodies in ALVAC-HIV-1-gpe-primed animals, and these antibodies were able to neutralize the T cell-line-adapted SHIVHXB2 isolate. Similar induction of neutralizing antibodies by protein boost against homologous HIV-1 isolate was reported in ALVAC-HIV-1-primed human volunteers (47). However, we were unable to detect neutralizing antibodies against the challenge virus SHIVKU2. Hence, neutralizing antibodies may not play a major role in the containment of SHIVKU2 in ALVAC-HIV-1-gpe-immunized animals. However, other antibody-dependent immune mechanisms such as antibody-dependent cellular cytotoxicity in sera or other body fluids may play a role in the efficacy of these vaccine candidates, as demonstrated in the use of a replication-competent adenovirus-based vaccine candidate (26).
These ALVAC vaccine candidates elicit durable memory responses directed to both dominant and subdominant CTL epitopes (52), and such immune response may account for virus containment after challenge exposure (52). In phase I trials with an ALVAC prime/gp120 boost combination (6, 47), these vaccines induced proliferative responses in most of the immunized people. Interestingly, in the SIVmac251 macaque model, poxvirus-based HIV vaccine candidates induce proliferative response and inversely correlate with the virus level (30, 31, 55).
The results presented here demonstrate that ALVAC-gp120-immunized animals had a significantly lower virus load compared to ALVAC-gp160-immunized animals, suggesting that the form of Env presented during the primary immunization phase may influence the protective immune response. This hypothesis is supported by the finding that priming of macaques with ALVAC expressing gp120 elicited more durable and higher titers of binding antibodies than the ALVAC-gp160-expressing vaccine, leading to a better priming effect (see Fig. 2A). However, boosting of ALVAC-primed animals with either gp120 or gp140 (groups A and B) or simultaneous immunization with ALVAC and gp140 protein (group C) decreased these differences in antibody titers and, at the time of SHIVKU2 challenge, no differences were observed between these groups. Interestingly, the neutralizing antibody response to SHIVHXB2 appeared to be consistently higher in the ALVAC-gp120-immunized group (group A) compared to ALVAC-gp160-immunized animals (groups B and C), suggesting that a gp120-based ALVAC vaccine may elicit a more functionally active antibody response compared to immunization with gp140. This again may be due to a better priming effect observed with the ALVAC-gp120-immunized group than with the ALVAC-gp160-immunized group.
As described here, the SIV RNA load associated with the gut-associated tissues of ALVAC-HIV-1-immunized animals was contained compared to naive animals. Although the RNA load in lymph nodes was lower in immunized animals, such reduction was not statistically significant. This containment of virus replication in tissues following ALVAC-HIV-1 vaccination suggests that tissue-specific cellular responses may contribute to this type of virus containment.
Recent studies have demonstrated that a subunit vaccine composed of Tat protein of HIV-1 may contain viral replication following challenge with pathogenic SHIV isolates (12, 56). However, such a protective effect of Tat protein against SHIV challenge was not reproduced in an independent challenge study (53). We examined here whether the immunization of ALVAC-primed animals with Tat and Env proteins could confer additional protective advantage over boosting with Env protein alone. The results presented here demonstrate that, although immunization of ALVAC-primed animals with purified Tat protein clearly elicited strong anti-Tat antibody response, such antibodies did not enhance the efficacy of the ALVAC vaccine against SHIV challenge. It is not clear why the Tat protein did not exhibit any efficacy against SHIVKU2 challenge. Several factors, such as the functional state of the Tat protein, the animal species, the timing of immunization, and the quality of anti-Tat antibodies elicited by the vaccine, may account for this difference.
A number of clinical studies performed in human volunteers have clearly demonstrated that ALVAC vaccines administered either as a single immunogen or in combination with a subunit protein boost elicit both humoral and cellular immune responses (23). The relevance to humans of our findings in macaques will be assessed in the ongoing phase III trail in Thailand, designed to evaluate the protective efficacy of an ALVAC-based vaccine against HIV infection.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This study was performed under NIAID contract (N01-AI-55271) with Advanced BioScience Laboratories, Inc.
We thank Nancy Miller for helpful discussion. We also thank Steven J. Snodgrass for editorial assistance and Sharon Orndorff and Jim Treece for technical coordination.
REFERENCES
- 1.Abimiku, A. G., G. Franchini, J. Tartaglia, K. Aldrich, M. Myagkikh, P. D. Markham, P. Chong, M. Klein, M. P. Kieny, E. Paoletti, R. C. Gallo, and M. Robert-Guroff. 1995. HIV-1 recombinant poxvirus vaccine induces cross-protection against HIV-2 challenge in rhesus macaques. Nat. Med. 1:321-329. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, S., B. Makitalo, R. Thorstensson, G. Franchini, J. Tartaglia, K. Limbach, E. Paoletti, P. Putkonen, and G. Biberfeld. 1996. Immunogenicity and protective efficacy of a human immunodeficiency virus type 2 recombinant canarypox (ALVAC) vaccine candidate in cynomolgus monkeys. J. Infect. Dis. 174:977-985. [DOI] [PubMed] [Google Scholar]
- 4.Barouch, D. H., A. Craiu, M. J. Kuroda, J. E. Schmitz, X. X. Zheng, S. Santra, J. D. Frost, G. R. Krivulka, M. A. Lifton, C. L. Crabbs, G. Heidecker, H. C. Perry, M. E. Davies, H. Xie, C. E. Nickerson, T. D. Steenbeke, C. I. Lord, D. C. Montefiori, T. B. Strom, J. W. Shiver, M. G. Lewis, and N. L. Letvin. 2000. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc. Natl. Acad. Sci. USA 97:4192-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belshe, R., G. Franchini, M. P. Girard, F. Gotch, P. Kaleebu, M. L. Marthas, M. B. McChesney, R. McCullough, F. Mhalu, D. Salmon-Ceron, R. P. Sekaly, K. van Rompay, B. Verrier, B. Wahren, and M. Weissenbacher. 2004. Support for the RV144 HIV vaccine trial. Science 305:177-180. [DOI] [PubMed] [Google Scholar]
- 6.Belshe, R. B., C. Stevens, G. J. Gorse, S. Buchbinder, K. Weinhold, H. Sheppard, D. Stablein, S. Self, J. McNamara, S. Frey, J. Flores, J. L. Excler, M. Klein, R. E. Habib, A. M. Duliege, C. Harro, L. Corey, M. Keefer, M. Mulligan, P. Wright, C. Celum, F. Judson, K. Mayer, D. McKirnan, M. Marmor, and G. Woody. 2001. Safety and immunogenicity of a canarypox-vectored human immunodeficiency virus type 1 vaccine with or without gp120: a phase 2 study in higher- and lower-risk volunteers. J. Infect. Dis. 183:1343-1352. [DOI] [PubMed] [Google Scholar]
- 7.Belyakov, I. M., Z. Hel, B. Kelsall, V. A. Kuznetsov, J. D. Ahlers, J. Nacsa, D. I. Watkins, T. M. Allen, A. Sette, J. Altman, R. Woodward, P. D. Markham, J. D. Clements, G. Franchini, W. Strober, and J. A. Berzofsky. 2001. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 7:1320-1326. [DOI] [PubMed] [Google Scholar]
- 8.Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. D. Markham, G. M. Shearer, R. C. Gallo, M. P. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic SIVmac251: dependence on route of challenge exposure. J. Virol. 72:4170-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. M. McGrath, J. Tartaglia, P. Caudrelier, R. El Habib, M. Klein, A. Lazzarin, D. M. Stablein, M. Deers, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. 2000. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 16:2019-2035. [DOI] [PubMed] [Google Scholar]
- 11.Burton, D. R., R. C. Desrosiers, R. W. Doms, M. B. Feinberg, R. C. Gallo, B. Hahn, J. A. Hoxie, E. Hunter, B. Korber, A. Landay, M. M. Lederman, J. Lieberman, J. M. McCune, J. P. Moore, N. Nathanson, L. Picker, D. Richman, C. Rinaldo, M. Stevenson, D. I. Watkins, S. M. Wolinksky, and J. A. Zack. 2004. Public health. A sound rationale needed for phase III HIV-1 vaccine trials. Science 303:316. [DOI] [PubMed] [Google Scholar]
- 12.Cafaro, A., A. Caputo, C. Fracasso, M. T. Maggiorella, D. Goletti, S. Baroncelli, M. Pace, L. Sernicola, M. L. Koanga-Mogtomo, M. Betti, A. Borsetti, R. Belli, L. Akerblom, F. Corrias, S. Butto, J. Heeney, P. Verani, F. Titti, and B. Ensoli. 1999. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat. Med. 5:643-650. [DOI] [PubMed] [Google Scholar]
- 13.Cafaro, A., A. Caputo, M. T. Maggiorella, S. Baroncelli, C. Fracasso, M. Pace, A. Borsetti, L. Sernicola, D. R. Negri, P. ten Haaft, M. Betti, Z. Michelini, I. Macchia, E. Fanales-Belasio, R. Belli, F. Corrias, S. Butto, P. Verani, F. Titti, and B. Ensoli. 2000. SHIV89.6P pathogenicity in cynomolgus monkeys and control of viral replication and disease onset by human immunodeficiency virus type 1 Tat vaccine. J. Med. Primatol. 29:193-208. [DOI] [PubMed] [Google Scholar]
- 14.Cao, H., P. Kaleebu, D. Hom, J. Flores, D. Agrawal, N. Jones, J. Serwanga, M. Okello, C. Walker, H. Sheppard, R. El Habib, M. Klein, E. Mbidde, P. Mugyenyi, B. Walker, J. Ellner, and R. Mugerwa. 2003. Immunogenicity of a recombinant human immunodeficiency virus (HIV)-canarypox vaccine in HIV-seronegative Ugandan volunteers: results of the HIV Network for Prevention Trials 007 Vaccine Study. J. Infect. Dis. 187:887-895. [DOI] [PubMed] [Google Scholar]
- 15.Castro, B. A., J. Homsy, E. Lennette, K. K. Murthy, J. W. Eichberg, and J. A. Levy. 1992. HIV-1 expression in chimpanzees can be activated by CD8+ cell depletion or CMV infection. Clin. Immunol. Immunopathol. 65:227-233. [DOI] [PubMed] [Google Scholar]
- 16.Chang, H. C., F. Samaniego, B. C. Nair, L. Buonaguro, and B. Ensoli. 1997. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 11:1421-1431. [DOI] [PubMed] [Google Scholar]
- 17.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 18.Clements-Mann, M. L., K. Weinhold, T. J. Matthews, B. S. Graham, G. J. Gorse, M. C. Keefer, M. J. McElrath, R.-H. Hsieh, J. Mestecky, S. Zolla-Pazner, J. Mascola, D. Schwartz, R. Siliciano, L. Corey, P. F. Wright, R. Belshe, R. Dolin, S. Jackson, S. Xu, P. Fast, M. C. Walker, D. Stablein, J.-L. Excler, J. Tartaglia, A.-M. Duliege, F. Sinangil, and E. Paoletti. 1998. Immune responses to human immunodeficiency virus (HIV) type 1 induced by canarypox expressing HIV-1MN gp120, HIV-1SF2 recombinant gp120, or both vaccines in seronegative adults. NIAID AIDS Vaccine Evaluation Group. J. Infect. Dis. 177:1230-1246. [DOI] [PubMed] [Google Scholar]
- 19.Crawford, J. M., P. L. Earl, B. Moss, K. A. Reimann, M. S. Wyand, K. H. Manson, M. Bilska, J. T. Zhou, C. D. Pauza, P. W. Parren, D. R. Burton, J. G. Sodroski, N. L. Letvin, and D. C. Montefiori. 1999. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J. Virol. 73:10199-10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans, T. G., M. C. Keefer, K. J. Weinhold, M. Wolff, D. Montefiori, G. J. Gorse, B. S. Graham, M. J. McElrath, M. L. Clements-Mann, M. J. Mulligan, P. Fast, M. C. Walker, J. L. Excler, A. M. Duliege, and J. Tartaglia. 1999. A canarypox vaccine expressing multiple human immunodeficiency virus type 1 genes given alone or with rgp120 elicits broad and durable CD8+ cytotoxic T lymphocyte responses in seronegative volunteers. J. Infect. Dis. 180:290-298. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari, G., W. Humphrey, M. J. McElrath, J. L. Excler, A. M. Duliege, M. L. Clements, L. C. Co, D. P. Bolognesi, and K. J. Weinhold. 1997. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc. Natl. Acad. Sci. USA 94:1396-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franchini, G., C. Gurgo, H. G. Guo, R. C. Gallo, E. Collalti, K. A. Fargnoli, L. F. Hall, F. Wong-Staal, and M. S. Reitz, Jr. 1987. Sequence of simian immunodeficiency virus and its relationship to the human immunodeficiency viruses. Nature 328:539-543. [DOI] [PubMed] [Google Scholar]
- 23.Franchini, G., S. Gurunathan, L. Baglyos, S. Plotkin, and J. Tartaglia. 2004. Poxvirus-based vaccine candidates for HIV: two decades of experience with special emphasis on canarypox vectors. Expert Rev. Vaccines 3(Suppl. 1):S75-S88. [DOI] [PubMed] [Google Scholar]
- 24.Franchini, G., P. Markham, E. Gard, K. Fargnoli, S. Keubaruwa, L. Jagodzinski, M. Robert-Guroff, P. Lusso, G. Ford, and F. Wong-Staal. 1990. Persistent infection of rhesus macaques with a molecular clone of human immunodeficiency virus type 2: evidence of minimal genetic drift and low pathogenetic effects. J. Virol. 64:4462-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franchini, G., M. Robert-Guroff, J. Tartaglia, A. Aggarwal, A. G. Abimiku, J. Benson, P. D. Markham, K. Limbach, G. Hurteau, J. Fullen, K. Aldrich, N. Miller, J. Sadoff, E. Paoletti, and R. C. Gallo. 1995. Highly attenuated HIV type 2 recombinant poxviruses, but not HIV-2 recombinant Salmonella vaccines, induce long-lasting protection in rhesus macaques. AIDS Res. Hum. Retrovir. 11:909-920. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Roman, V. R., L. J. Patterson, D. Venzon, D. Liewehr, K. Aldrich, R. Florese, and M. Robert-Guroff. 2005. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J. Immunol. 174:2185-2189. [DOI] [PubMed] [Google Scholar]
- 27.Gupta, K., M. Hudgens, L. Corey, M. J. McElrath, K. Weinhold, D. C. Montefiori, G. J. Gorse, S. E. Frey, M. C. Keefer, T. G. Evans, R. Dolin, D. H. Schwartz, C. Harro, B. Graham, P. W. Spearman, M. Mulligan, and P. Goepfert. 2002. Safety and immunogenicity of a high-titered canarypox vaccine in combination with rgp120 in a diverse population of HIV-1-uninfected adults: AIDS Vaccine Evaluation Group Protocol 022A. J. Acquir. Immune Defic. Syndr. 29:254-261. [DOI] [PubMed] [Google Scholar]
- 28.Haigwood, N. L. 2004. Predictive value of primate models for AIDS. AIDS Rev. 6:187-198. [PubMed] [Google Scholar]
- 29.Hanke, T., R. V. Samuel, T. J. Blanchard, V. C. Neumann, T. M. Allen, J. E. Boyson, S. A. Sharpe, N. Cook, G. L. Smith, D. I. Watkins, M. P. Cranage, and A. J. McMichael. 1999. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 73:7524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hel, Z., J. Nacsa, E. Tryniszewska, W. P. Tsai, R. W. Parks, D. C. Montefiori, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2002. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T-cell responses. J. Immunol. 169:4778-4787. [DOI] [PubMed] [Google Scholar]
- 31.Hel, Z., W. P. Tsai, A. Thornton, J. Nacsa, L. Giuliani, E. Tryniszewska, M. Poudyal, D. Venzon, X. Wang, J. Altman, D. I. Watkins, W. Lu, A. von Gegerfelt, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2001. Potentiation of simian immunodeficiency virus (SIV)-specific CD4+ and CD8+ T cell responses by a DNA-SIV and NYVAC-SIV prime/boost regimen. J. Immunol. 167:7180-7191. [DOI] [PubMed] [Google Scholar]
- 32.Hel, Z., D. Venzon, M. Poudyal, W.-P. Tsai, L. Giuliani, R. Woodward, C. Chougnet, G. M. Shearer, J. D. Altman, D. I. Watkins, N. Bischofberger, A. G. Abimiku, P. D. Markham, J. Tartaglia, and G. Franchini. 2000. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat. Med. 6:1140-1146. [DOI] [PubMed] [Google Scholar]
- 33.Hodge, J. W., J. P. McLaughlin, J. A. Kantor, and J. Schlom. 1997. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine 15:759-768. [DOI] [PubMed] [Google Scholar]
- 34.Hu, S. L., K. Abrams, G. N. Barber, P. Moran, J. M. Zarling, A. J. Langlois, L. Kuller, W. R. Morton, and R. E. Benveniste. 1992. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science 255:456-459. [DOI] [PubMed] [Google Scholar]
- 35.Hu, S.-L. 1994. Is subunit envelope antigen our best bet for an AIDS vaccine?, p. 275-281. In M. Girard and B. Dodet (ed.), Retroviruses of human AIDS and related animal diseases. Marnes-La-Coquette, Paris, France.
- 36.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T-cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin, X., M. Ramanathan, Jr., S. Barsoum, G. R. Deschenes, L. Ba, J. Binley, D. Schiller, D. E. Bauer, D. C. Chen, A. Hurley, L. Gebuhrer, R. El Habib, P. Caudrelier, M. Klein, L. Zhang, D. D. Ho, and M. Markowitz. 2002. Safety and immunogenicity of ALVAC vCP1452 and recombinant gp160 in newly human immunodeficiency virus type 1-infected patients treated with prolonged highly active antiretroviral therapy. J. Virol. 76:2206-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joag, S. V., Z. Li, L. Foresman, D. M. Pinson, R. Raghavan, W. Zhuge, I. Adany, C. Wang, F. Jia, D. Sheffer, J. Ranchalis, A. Watson, and O. Narayan. 1997. Characterization of the pathogenic KU-SHIV model of acquired immunodeficiency syndrome in macaques. AIDS Res. Hum. Retrovir. 13:635-645. [DOI] [PubMed] [Google Scholar]
- 39.Kalyanaraman, V. S., R. Pal, R. C. Gallo, and M. G. Sarngadharan. 1988. A unique human immunodeficiency virus culture secreting soluble gp160. AIDS Res. Hum. Retrovir. 4:319-329. [DOI] [PubMed] [Google Scholar]
- 40.Kalyanaraman, V. S., V. Rodriguez, F. Veronese, R. Rahman, P. Lusso, A. L. DeVico, T. Copeland, S. Oroszlan, R. C. Gallo, and M. G. Sarngadharan. 1990. Characterization of the secreted, native gp120 and gp160 of the human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 6:371-380. [DOI] [PubMed] [Google Scholar]
- 41.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrew, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang, X., D. R. Casimiro, W. A. Schleif, F. Wang, M. E. Davies, Z. Q. Zhang, T. M. Fu, A. C. Finnefrock, L. Handt, M. P. Citron, G. Heidecker, A. Tang, M. Chen, K. A. Wilson, L. Gabryelski, M. McElhaugh, A. Carella, C. Moyer, L. Huang, S. Vitelli, D. Patel, J. Lin, E. A. Emini, and J. W. Shiver. 2005. Vectored Gag and Env but not Tat show efficacy against simian-human immunodeficiency virus 89.6P challenge in Mamu-A*01-negative rhesus monkeys. J. Virol. 79:12321-12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matano, T., R. Shibata, C. Siemon, M. Connors, H. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNeil, J. G., M. I. Johnston, D. L. Birx, and E. C. Tramont. 2004. Policy rebuttal: HIV vaccine trial justified. Science 303:961. [DOI] [PubMed] [Google Scholar]
- 45.Montefiori, D. C., R. G. Collman, T. R. Fouts, J. Y. Zhou, M. Bilska, J. A. Hoxie, J. P. Moore, and D. P. Bolognesi. 1998. Evidence that antibody-mediated neutralization of human immunodeficiency virus type 1 by sera from infected individuals is independent of coreceptor usage. J. Virol. 72:1886-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mooij, P., I. G. Nieuwenhuis, C. J. Knoop, R. W. Doms, W. M. Bogers, P. J. Ten Haaft, H. Niphuis, W. Koornstra, K. Bieler, J. Kostler, B. Morein, A. Cafaro, B. Ensoli, R. Wagner, and J. L. Heeney. 2004. Qualitative T-helper responses to multiple viral antigens correlate with vaccine-induced immunity to simian/human immunodeficiency virus infection. J. Virol. 78:3333-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nitayaphan, S., P. Pitisuttithum, C. Karnasuta, C. Eamsila, M. de Souza, P. Morgan, V. Polonis, M. Benenson, T. VanCott, S. Ratto-Kim, J. Kim, D. Thapinta, R. Garner, V. Bussaratid, P. Singharaj, R. El Habib, S. Gurunathan, W. Heyward, D. Birx, J. McNeil, and A. E. Brown. 2004. Safety and immunogenicity of an HIV subtype B and E prime-boost vaccine combination in HIV-negative Thai adults. J. Infect. Dis. 190:702-706. [DOI] [PubMed] [Google Scholar]
- 48.Ourmanov, I., C. R. Brown, B. Moss, M. Carroll, L. Wyatt, L. Pletneva, S. Goldstein, D. Venzon, and V. M. Hirsch. 2000. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 74:2740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pal, R., G. M. Hoke, and M. G. Sarngadharan. 1989. Role of oligosaccharides in the processing and maturation of envelope glycoproteins of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 86:3384-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pal, R., D. Venzon, N. L. Letvin, S. Santra, D. C. Montefiori, N. R. Miller, E. Tryniszewska, M. G. Lewis, T. C. Vancott, V. Hirsch, R. Woodward, A. Gibson, M. Grace, E. Dobratz, P. D. Markham, Z. Hel, J. Nacsa, M. Klein, J. Tartaglia, and G. Franchini. 2002. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romano, J. W., R. N. Shurtliff, E. Dobratz, A. Gibson, K. Hickman, P. Markham, and R. Pal. 2000. Quantitative evaluation of simian immunodeficiency virus infection using NASBA technology. J. Virol. Methods 86:61-70. [DOI] [PubMed] [Google Scholar]
- 52.Santra, S., J. E. Schmitz, M. J. Kuroda, M. A. Lifton, C. E. Nickerson, C. I. Lord, R. Pal, G. Franchini, and N. L. Letvin. 2002. Recombinant canarypox vaccine-elicited CTL specific for dominant and subdominant simian immunodeficiency virus epitopes in rhesus monkeys. J. Immunol. 168:1847-1853. [DOI] [PubMed] [Google Scholar]
- 53.Silvera, P., M. W. Richardson, J. Greenhouse, J. Yalley-Ogunro, N. Shaw, J. Mirchandani, K. Khalili, J. F. Zagury, M. G. Lewis, and J. Rappaport. 2002. Outcome of simian-human immunodeficiency virus strain 89.6p challenge following vaccination of rhesus macaques with human immunodeficiency virus Tat protein. J. Virol. 76:3800-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevceva, L., X. Alvarez, A. A. Lackner, E. Tryniszewska, B. Kelsall, J. Nacsa, J. Tartaglia, W. Strober, and G. Franchini. 2002. Both mucosal and systemic routes of immunization with the live, attenuated NYVAC/simian immunodeficiency virus SIV(gpe) recombinant vaccine result in gag-specific CD8+ T-cell responses in mucosal tissues of macaques. J. Virol. 76:11659-11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Villinger, F., G. T. Brice, A. E. Mayne, P. Bostik, K. Mori, C. H. June, and A. A. Ansari. 2002. Adoptive transfer of simian immunodeficiency virus (SIV) naive autologous CD4+ cells to macaques chronically infected with SIV is sufficient to induce long-term nonprogressor status. Blood 99:590-599. [DOI] [PubMed] [Google Scholar]
- 56.Voss, G., K. Manson, D. Montefiori, D. I. Watkins, J. Heeney, M. Wyand, J. Cohen, and C. Bruck. 2003. Prevention of disease induced by a partially heterologous AIDS virus in rhesus monkeys by using an adjuvanted multicomponent protein vaccine. J. Virol. 77:1049-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]