Summary
Learning in shape identification led to global changes in activation across the entire visual pathway, as revealed with whole-brain fMRI. Following extensive training in a shape identification task, brain activity associated with trained shapes relative to the untrained shapes showed: (1) an increased level of activity in retinotopic cortex (RC), (2) a decrease in activation of the lateral occipital cortex (LO), and (3) a decrease in the dorsal attentional network. In addition, RC activations became more correlated (and LO activation, less correlated) with performance. When comparing target-present and target-absent trials within the trained condition, we observed a similar decrease in the dorsal attentional network but not in the visual cortices. These findings indicate a large-scale reorganization of activity in the visual pathway as a result of learning, with the RC becoming more involved (and the LO, less involved) and that these changes are triggered in a top-down manner depending on the perceptual task performed.
Introduction
The traditional view of the representation of form along the visual pathway is that at early stages neurons are filters for simple stimulus characteristics, such as local orientation (Hubel and Wiesel, 1962), and subsequent stages execute a process of “complexification,” linking simple stimulus elements into complex shapes (Fujita et al., 1992; Hubel and Wiesel, 1965; Kobatake and Tanaka, 1994; Lerner et al., 2001; Riesenhuber and Poggio, 1999; Sheinberg and Logothetis, 1997; Tanaka, 1996). Along with the selectivity for stimuli of increased complexity, there is a transition from a high degree of retinotopic order and small receptive fields to larger receptive fields and less specificity for stimulus position (Hubel and Wiesel, 1965; Logothetis et al., 1995; Tanaka, 1996).
Ideas about the cortical locus of perceptual learning have relied on this organizational principle. The specificity of perceptual learning, in terms of visuotopic location or stimulus orientation, leads to inferences about the cortical level at which the learned information is represented (Ball and Sekuler, 1982; Berardi and Fiorentini, 1987; Fahle and Edelman, 1993; Karni and Sagi, 1991; Maffei and Fiorentini, 1976; McKee and Westheimer, 1978)
Single-unit studies in behaving monkeys and fMRI in human subjects have supported the idea that learning can involve changes in the functional properties of neurons in the primary visual cortex (Crist et al., 2001; Furmanski et al., 2004; Li et al., 2004; Schoups et al., 2001; Schwartz et al., 2002). The representation of learned shapes, on the other hand, has been shown to involve later stages in the visual pathway, mainly the inferior temporal cortex (IT) in monkeys (Chelazzi et al., 1993; Kobatake et al., 1998; Logothetis et al., 1995) and the lateral occipital complex (LO) in humans (Grill-Spector et al., 2001; Grill-Spector et al., 2000; Kourtzi and Kanwisher, 2000; Kourtzi and Kanwisher, 2001).
More recently, however, even primary visual cortex has been implicated in the representation of complex learned shapes, particularly when object recognition has to be performed rapidly and in parallel with multiple distractors, though at a “cost” of establishing multiple representations of the same object across a visuotopic map (Gilbert et al., 2001; Li et al., 2004). Basic visual features can be found rapidly regardless of the number of distractors, and it has been proposed that this can be achieved because these features are unique within a retinotopic map in early visual cortices. Conversely, the ability to find complex stimuli, presumably represented in higher cortices as combinations of basic features, diminishes with the number of distractors (Treisman, 1998; Treisman and Gelade, 1980).
We previously showed that training can change the pop-out properties of complex stimuli and, further, that this learning process occurs sequentially at different locations of the visual field (Sigman and Gilbert, 2000). This finding implies that highly trained stimuli, which prior to training are represented as combinations of basic features, may, as a consequence of training, shift their representation toward earlier, retinotopically mapped, cortical areas. This form of learning would therefore be associated not only with changes within a specific visual area, but also with a reorganization of the representation of the object across the visual pathway. This process is accompanied by a release from the dependence of performance on attentional control, leading to an automatization of the task (Schneider and Shiffrin, 1977; Shiffrin and Schneider, 1977). Thus, it is expected that in addition to local changes within the visual cortex, this form of perceptual learning should involve an overall change in activation across the attentional network, which is predominantly distributed across the parietal and frontal cortices (Corbetta et al., 1998; Duncan and Owen, 2000; Hopfinger et al., 2000).
To measure the changes resulting from shape training in human observers, we performed whole brain fMRI (3T) after training subjects extensively on a search task in which a cued shape was embedded in an array of distractors.
Results
Behavior: Robustness of the Learning
The subjects’ task was to identify a cued shape within an array of distractors. The array, which was presented for 150 ms, consisted of an annular arrangement of 12 Ts at four orthogonal orientations, placed at 3 degrees of eccentricity and excluding the meridianal positions (Figure 1A), and was presented for 150 milliseconds. A fixation cross remained at the center of the screen throughout the entire experiment. Subjects were instructed to respond whether the cued target was present or not.
Figure 1. The Behavioral Task and the Paradigm Design.
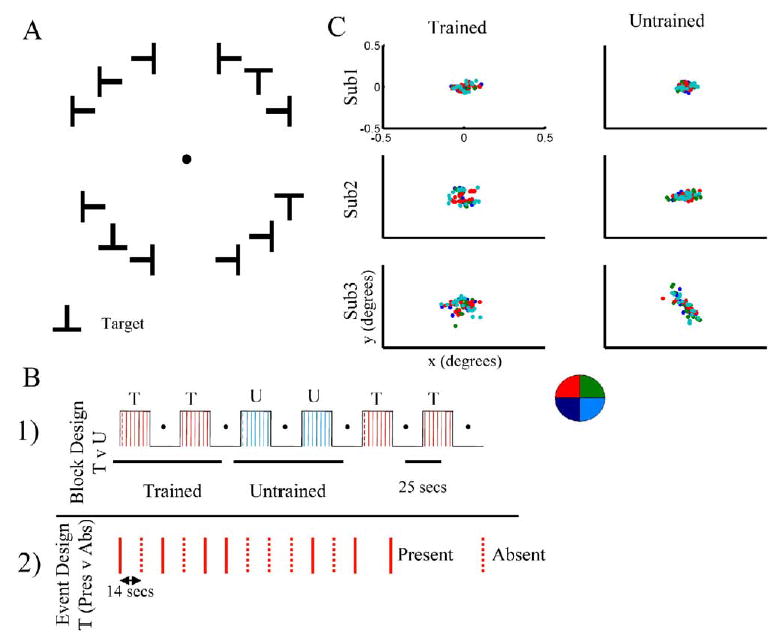
(A) The stimulus consisted of an array of 12 Ts at four possible orientations (Up, Down, Left, or Right) arranged on an annulus. A fixation spot was presented in the center, and the 12 targets were arranged in the four quadrants, avoiding the vertical and horizontal meridians. One T was chosen to be the target (with the exception of the upright T, to avoid the confound of prior familiarity with the target). The distractors were chosen randomly among the remaining orientations and changed on each presentation. The targets and distractors were presented at an eccentricity of 3 degrees and for a duration of 150 ms.
(B) After extensive training on one target, subjects performed the task in the scanner. Two different groups of subjects were tested on two different protocols: (1) Block design: Each session was divided into six periods. At the beginning of the period, a cue was presented in the center of the screen, replacing the fixation spot for 2 s (indicated with a dotted colored lined in the figure). During this period of trials, a new array was presented every 4 s, and on any given presentation the target could be present at any location within the array or not at all. (Red represents blocks in which the target was the trained shape and blue, a period in which the cued target was an untrained shape. The order of trained and untrained blocks was shuffled in different sessions). (2) Event design: Subjects performed the task for the trained target, and the intertrial interval was 14 s. The target was present in 50% of the trials.
(C) Subjects performed the task outside of the scanner while we measured their eye movements. Subjects performed the trained and untrained tasks, and for each condition, trials were classified according to the quadrant in which the target was present (labeled in colors according to the legend). The positions for the different quadrants were overlapping (p > 0.35 for both x and y values), indicating that there was no bias to direct eye movements to the target.
Subjects were trained extensively on one of the targets until they reached a threshold level of performance, which we arbitrarily set at 80%. The training lasted 3 to 6 days, after which the subjects were scanned while conducting the search task, either on the trained or the untrained shapes.
As we had seen in our previous psychophysical experiments, there was a dramatic difference in performance when subjects were searching for the trained in comparison with the untrained figure. Inside the scanner, performance levels were 91.3% ± 6% for the trained target and 21.7% ± 7% for the untrained target (seven subjects; p < 0.001). This showed that the learning effects that we observed, while extremely specific to the trained target compared with the distractors (Sigman and Gilbert, 2000), are robust to the task context, such as body position, distance to the screen, luminosity, and ambient noise.
Subjects were instructed to fixate on the screen, and the presentation time (150 ms) is not long enough to allow a saccade. However, to be sure that they did not perform eye movements, we tracked eye position of the subjects while they were performing the task. We did so for three randomly chosen subjects, and we tracked eye movements, outside of the scanner, while they were performing the Trained and Untrained conditions. Trials were divided according to which quadrant contained the target, and we calculated the mean eye position (x and y values) every 32 ms from trial onset to 300 ms after the trial presentation. We observed that there was no significant difference in the mean eye positions for the T and U conditions (p > 0.5, paired Student’s t test), and, moreover, within each condition, the eye positions when the target was placed in the different quadrants were overlapping (Figure 1C), indicating that there was no bias of eye movements directed toward the target. Note that the scale of Figure 1C (0.5 degrees) is six times smaller than the eccentricity of the targets.
Task-Specific Activations
We first identified the regions activated by the visual search task (contrast T+U, Table 1) and the regions differentially activated in the two conditions (contrast T - U, Figure 2A, Table 2). Upon presentation of the task, we observed both activations and deactivations with respect to baseline. Both networks (of activations and deactivations) were extensive. Among the regions activated (Table 1) were the visual areas in occipital cortex and the dorsal network including parietal, prefrontal, and frontal cortices. Also subcortical regions, including the striatum, were active upon task presentation. The difference maps (T - U, Figure 2) indicate a shift in the level of involvement of these areas in the task. The regions that were more active during the Untrained condition comprise an extended network that includes the lateral occipital, parietal, striatal, and prefrontal regions, while the retinotopic cortex (RC) was the only region that was more activated in the Trained than in the Untrained condition. In particular, we observed differential activations for both signs across the different visual areas (Figure 2B). In the Trained condition, the early visual areas (RC) were more active and higher-level visual areas (LO) were less active. In addition, an extended search, which includes bilateral posterior parietal cortex (PP) and the supplementary motor area (SMA), was strongly activated upon the presentation of the task and showed decreased activation with training.
Table 1.
T + U SPM Result Summary
| Side | Area | Talairach (MNI Space) Coordinates X, Y, Z (mm) | Peak Z Value | P Value (uncorrected) | P Value (corrected) | Cluster Size (cm3) |
|---|---|---|---|---|---|---|
| R | BA17 (V1/RC) | 26, −96, 6 | 3.18 | 0.001 | 0.012 | 0.040 |
| L | BA17 (V1/RC) | −2, −98, −10 | 3.47 | <0.001 | 0.002 | 0.072 |
| R | BA18 (V2/RC) | 32, −90, 20 | 3.50 | <0.001 | 0.012 | 1.512 |
| L | BA18(V2/RC) | −4, −98, −12 | 3.71 | <0.0001 | 0.002 | 0.912 |
| R | BA19 | 36, −86, 22 | 3.97 | <0.0001 | 0.022 | 0.792 |
| L | BA19 | −32, −84, 18 | 3.87 | <0.0001 | 0.029 | 0.672 |
| L | BA39 | −32, −74, 16 | 4.16 | <0.0001 | 0.021 | 0.296 |
| R | LO | 34, −88, 8 | 3.39 | <0.001 | 0.009 | 0.792 |
| L | LO | −46, −70, 2 | 3.37 | <0.001 | 0.003 | 0.256 |
| R | BA7 (PP) | 32, −62, 52 | 3.99 | <0.0001 | 0.030 | 4.776 |
| L | BA7 (PP) | −18, −78, 56 | 4.10 | <0.0001 | 0.019 | 4.024 |
| R | BA6 (SMA) | 8, 2, 56 | 3.87 | <0.0001 | 0.017 | 4.928 |
| L | BA6 (SMA) | −8, −8, 58 | 3.95 | <0.0001 | 0.014 | 5.720 |
| L | BA40 (somatosensory) | −32, −42, 44 | 4.28 | <0.0001 | 0.018 | 7.080 |
| R | BA47 (association) | 32, 26, 6 | 3.17 | 0.001 | 0.005 | 0.104 |
| L | BA47 (association) | −30, 18, −2 | 3.34 | <0.0001 | 0.035 | 0.904 |
| R | Cingulate gyrus | 6, 14, 44 | 3.81 | <0.001 | 0.019 | 3.280 |
| L | Cingulate gyrus | −10, 2, 50 | 3.55 | <0.001 | 0.037 | 1.208 |
| R | Caudate | 14, 0, 12 | 3.59 | <0.001 | 0.024 | 1.192 |
| R | Putamen | 24, 10, −8 | 3.54 | <0.001 | 0.027 | 2.904 |
| L | Putamen | −24, −2, 0 | 3.75 | <0.0001 | 0.016 | 6.360 |
| R | BA1,2,3,4 | 44, −26, 56 | −3.34 | <0.001 | 0.009 | 0.904 |
| L | BA39 | −44, −74, 34 | −3.18 | 0.001 | 0.006 | 0.272 |
p < 0.001; extent threshold > 0.25 cm3.
Figure 2. Differential Activation between the Trained and Untrained Conditions.
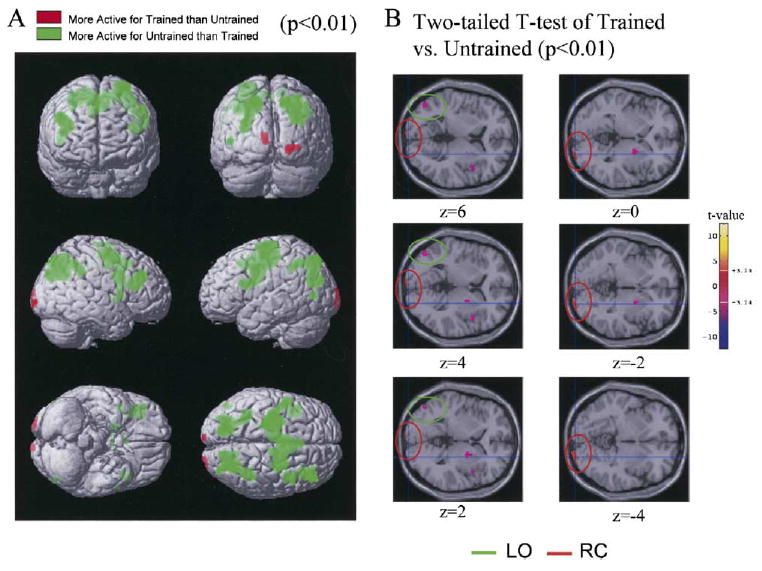
(A) The resulting T-statistic map based on the group random effects analysis of seven subjects is thresholded at p < 0.01 and rendered in the MNI 152 average brain (Evans et al., 1994; Talairach and Tournoux, 1988) (Table 2). The first row shows a view of the front and the back of the brain, the second row, the left and right brain, and the third row, the ventral and dorsal (bottom and top, respectively) views. Areas in which the response to the Trained (T) condition was larger than that to the Untrained (U) condition (T > U) are represented in red, and those where U > T are shown in green. The Untrained condition was more active over an extended network that mainly involved the parietal and frontal cortices and lateral occipital cortices; this difference was more pronounced in the left hemisphere. The Trained condition was more active than the Untrained condition in the middle occipital cortex, corresponding to early visual regions in retinotopic cortex (RC).
(B) A series of 2 mm-thick axial slices (from z = −4 to z = 6), indicating the T-statistic map of the Trained versus the Untrained contrast, thresholded at p < 0.01 (two-tailed Student’s t test). Positive t values (the bar on the right color codes the t values) correspond to the regions which are more active in the T condition (RC). Negative t-values correspond to regions that are more active in the U condition (LO).
Table 2.
T - U SPM Result Summary
| Side | Area | Talairach (MNI Space) Coordinates X, Y, Z (mm) | Peak Z Value | P Value (uncorrected) | P Value (corrected) | Cluster Size (cm3) |
|---|---|---|---|---|---|---|
| R | BA17 (V1/RC) | 20, −102, −4 | 2.67 | 0.004 | 0.030 | 0.144 |
| L | BA17 (V1/RC) | −8, −102, 10 | 2.52 | 0.006 | 0.026 | 0.056 |
| R | BA18 (V2/RC) | 32, −98, −2 | 2.49 | 0.006 | 0.030 | 0.040 |
| L | LO¹ | −46, −72, 4 | −2.95 | 0.002 | 0.012 | 0.344 |
| R | BA7 (PP) | 32, −70, 42 | −4.33 | <0.0001 | 0.008 | 6.320 |
| L | BA7 (PP) | −20, −76, 50 | −4.00 | <0.0001 | 0.014 | 4.000 |
| R | BA6 (SMA) | 8, −8, 56 | −3.80 | <0.0001 | 0.041 | 7.136 |
| L | BA6 (SMA) | −50, 2, 40 | −3.88 | <0.0001 | 0.041 | 11.112 |
| R | BA40 (somatosensory) | 42, −40, 36 | −3.47 | <0.0001 | 0.008 | 1.776 |
| L | BA40 (somatosensory) | −36, −48, 42 | −2.68 | 0.004 | 0.014 | 1.400 |
| R | BA13 (executive function) | 46, 8, 6 | −4.10 | <0.0001 | 0.003 | 0.224 |
| L | BA13 (executive function) | −42, 10, 14 | −2.43 | 0.008 | 0.041 | 0.016 |
| R | Putamen | 22, 0, 0 | −2.59 | 0.005 | 0.029 | 0.296 |
| L | Cingulate gyrus | −2, −2, 48 | −4.02 | <0.0001 | 0.041 | 1.584 |
p < 0.01; extent threshold > 0.25 cm3.
Correlations between fMRI Activation and Behavioral Performance
While the previous results suggest that there may be a reorganization of visual activity after training on an object identification task, we wanted to investigate this issue further by studying the correlations between the levels of activation and the subjects’ accuracy in performing the task. We did not perform this analysis for all regions showing a difference between the T and U condition. Instead, we analyzed only RC, LO, and PP, the regions for which we had predicted changes to occur on the basis of our previous theory of reorganization resulting from perceptual learning (Gilbert et al., 2001).
The regions showing the strongest correlations are the best predictors of performance and are therefore the most likely ones performing the computations to determine the presence or absence of the object. In this regard, we wished to determine whether the most predictive regions changed with learning. Because of the binary nature of the task (seen versus not seen), one cannot parametrically correlate performance with activation on a trial-by-trial basis, so, instead, we established the correlation between performance and BOLD activity on a block-by-block basis, with blocks consisting of 36 trials of each condition.
We observed a negative correlation between activity in RC and performance, both for the Untrained (t = −3.7, p < 0.001) and the Trained condition (t = −6.8, p < 0.001) (Figure 3), although the correlation was significantly more negative (t = −3.1, p < 0.01) in the Trained condition (Figure 3). It is important to compare the strength of the correlation itself, which provides the measure of predictability for any brain region, between trained and untrained conditions. After learning, the correlation between RC activation and performance was tighter, (i.e., showed an even stronger negative correlation), indicating that it became a better predictor of behavioral performance and supporting the idea that the visual “computations” to solve this search task might involve the primary visual cortex. Further support for this comes from the fact that the reverse trend was observed in LO, where we found a significant correlation between performance and LO activation in the Untrained condition (t = 4.1, p < 0.001) and virtually no such correlation in the Trained condition (t = 0.23, p > 0.5). The difference in the correlations between LO activity and performance for the Trained and Untrained conditions was significant (t = 2.8, p < 0.01). Correlations between performance and activity in the posterior parietal cortex also decreased with training, although this difference was not significant: (untrained [U]: t = 4.8, p < 0.001; trained [T]: t = 3.4, p < 0.001; and U - T: t = 1.3, p > 0.1). Each subject performed six different blocks; performance is plotted in the scatter graph, in which data from sessions taken from different subjects are indicated by different colors. Most of the variability resulted from intersubject differences, while data obtained from the same subject showed fairly reliable measures across trial blocks.
Figure 3. Correlation between BOLD Activity and Behavioral Performance for the Three Regions of Interest—RC, LO and PP—in the Trained and Untrained Conditions.
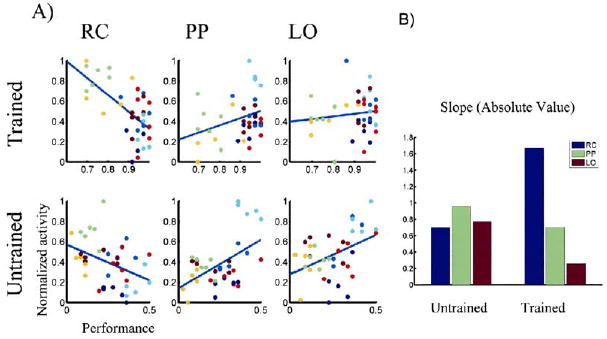
(A) For the regions where we had predicted a change, RC, LO, and PP, (Gilbert et al., 2001) we studied the correlation between BOLD activity and behavioral performance. This analysis was based on the voxel that elicited the biggest difference between the Trained and Untrained conditions within the nearest cluster from previously defined anatomical coordinates (Grill-Spector et al., 1999; Lerner et al., 2001). At each voxel, we calculated the level of BOLD activity for each session and for each condition (37 total) and correlated this with the behavioral performance. For the Trained condition, performance varied within the 60%–100% range, and for the Untrained condition, within the 0%–50% range. The graphs on the left show RC activations (Talairach coordinates [20,−102,−4]), the middle graphs show PP activations (Talairach coordinates [32, −70, 42]) and the graphs on the right show LO activation (Talairach coordinates [−46, −72, 4]). The top row corresponds to the Trained condition and the second row, to the Untrained condition. RC activation is negatively correlated with behavioral performance, and the correlation becomes significantly stronger in the Trained condition. LO and PP are positively correlated, and this correlation becomes substantially weaker in the Trained condition.
(B) To measure how “informative” different regions are about subjects’ performance, we calculated the absolute value of the slope at each voxel. After training, the RC becomes significantly more correlated with; and the LO becomes decorrelated from; performance. Blue bars indicate the RC, green bars indicate the PP, and red bars indicate the LO.
Automatization: Solving the Task upon Smaller Perturbations from the Default State
In this study, we examined changes occurring during two distinct test conditions (searching for a trained or untrained object), as well as the changes for each condition with respect to baseline measurements (collected during periods of passive fixation of equal duration to those of searching, Figure 1B). In different brain regions, these changes could be either positive or negative, with regions activating (Figure 4A) or deactivating (Figure 4B) upon task presentation. In the previous section, we showed that an extensive dorsal network, which is generally active during a large variety of tasks (Duncan and Owen, 2000), becomes less active with training. Here we show that regions known to deactivate upon presentation of a large variety of tasks became less inactive with training.
Figure 4. Analysis of Deactivations: Perturbation from the Default State.
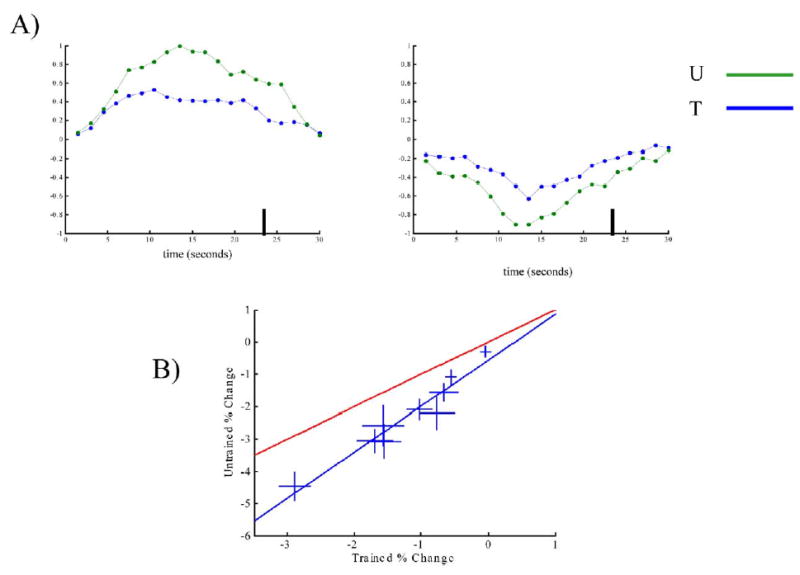
Upon task presentations, we observed regions that activated and others that deactivated in comparison with the resting state (baseline). (A) Top left panel: An example, from a single subject, of an activated voxel in the posterior parietal cortex (Talairach coordinates [32, −62, 52]). The vertical bar at 24 s indicates the end of the stimulus presentation and the beginning of the rest period. There is a significant decrease in activation resulting from training (although it is still significantly active when compared to baseline). Top right panel: An example of a deactivated voxel in the posterior cingulate (Talairach coordinates [2, −82, 22]). The deactivation is more prominent in the Untrained than in the Trained condition. This exemplifies a fairly general rule: upon training, the task is performed upon a smaller perturbation of the default state.
(B) Quantitative estimation of the effect of training on deactivated regions. Regions of interest (ROIs) were determined after examining the literature for brain areas known to deactivate under a variety of cognitive tasks (Shulman et al., 1997). Within these regions, we compared the activation of the T and U conditions with respect to baseline (Table 3). The scatter graph shows the mean level of activation (or deactivation) for each region. The error bars represent the mean ± SEM. The blue line (slope = 1.42 ± 0.3, intercept = −56 ± 44) is obtained from a linear regression of all of the data, while the red line represents, for comparison, the line y = x. The fact that the experimental data and the blue line fall under the line of equality indicates that, in general, training decreases the level of deactivation of deactivated regions.
To test this, we performed an analysis across regions of interest (ROIs) listed in Table 3. These ROIs were obtained from previous studies that have identified a series of brain areas that exhibit reductions in activity during the performance of a variety of cognitive tasks (Shulman et al., 1997). We found a task-specific deactivation (i.e., both T and U < Rest) in nine out of the thirteen ROIs identified previously. The remaining regions, labeled with a dash in Table 3, were not included for this analysis. For the nine regions where we found deactivation under both conditions, we compared the level of deactivation in the T and the U conditions (Figure 4B, Table 3) and found consistently that training resulted in a decrease in the level of deactivation (and thus, a positive value for the T - U difference). In Table 3, the p values obtained from paired Student’s t tests are reported for each region. For most of these brain areas, the T - U difference did not reach significance, but all regions showed the same trend (T > U). To measure the general trend, we analyzed all of the data together, using an ANOVA with the different regions designated as random variables and T/U as the main factor. The difference was highly significant (p < 0.001), indicating that overall within this network a significant reduction of deactivation results from training.
Table 3.
Analysis of Deactivations
| Area | X | Y | Z | P (T + U < 0) | (T − U) (%) | P (U > T) |
|---|---|---|---|---|---|---|
| M 31/7 | −5 | −49 | 40 | 0.004 | 1.57 | 0.0678 |
| L 40 | −53 | −39 | 42 | — | — | — |
| L 39/19 | −45 | −67 | 36 | 0.0056 | 1.51 | 0.1124 |
| R 40 | 45 | −57 | 34 | — | — | — |
| L lateral 8 | −27 | 27 | 40 | 0.0050 | 1.35 | 0.0182 |
| L 8/9 | −11 | 41 | 42 | 0.0022 | 1.06 | 0.1115 |
| R 8/9 | 5 | 49 | 36 | 0.0387 | 1.42 | 0.0853 |
| L9 | −15 | 55 | 26 | 0.0119 | 0.89 | 0.1081 |
| L10 | −19 | 57 | 8 | — | — | — |
| M10 | −1 | 47 | −4 | 0.0344 | 1.02 | 0.1369 |
| L10/47 | −33 | 45 | −6 | — | — | — |
| M32 | 3 | 31 | −10 | 0.1953 | 0.25 | 0.2076 |
| L20 | −49 | −19 | −18 | 0.0137 | 0.53 | 0.0905 |
Taken together with our finding of a decrease in the activation of the dorsal network, the observed reduction in deactivation suggests a general trend resulting from learning, namely: activated voxels became less active and deactivated voxels, less inactive. In view of this general trend, the fact that the opposite effect was seen in early visual cortex (in which an active region became more activated) becomes even more striking, indicating that the increased activation in RC is not just related to increased task difficulty but rather to a specific reorganization process that occurs in the visual cortex.
Changes within the Trained Condition between Target-Present and Target-Absent Trials
To address whether the changes that we observed were related to attending to and searching for a trained shape or to the fact that the cued shape was seen more frequently in the Trained condition, we designed, using the same task, a slow event-related experiment in which 50% of the trials contained the target (see Experimental Procedures). This allowed for a balanced comparison enabling us to distinguish, within the Trained condition, the trials in which the target was presented and seen (seen, >88% of the trials in which the target was present) and the trials in which the target was not presented (absent). The results of the difference maps seen - absent reveal the following findings (Figure 5): (1) An extended dorsal network, including posterior parietal and premotor regions, was more active in the absent condition. This network is similar (although more markedly left-lateralized) to the one found in the Untrained - Trained contrast and thus very likely reflects the process of attentional shifts made to ensure that the target is not there. The differential involvement of the dorsal network within different trials of the Trained condition suggests that attention is still involved in pop-out search. (2) None of the changes in the visual regions where we saw an increased activation in the T - U comparison were differentially activated here. While we had observed an increase in RC and a decrease in LO resulting from training, we did not observe this difference in the seen-absent differential maps. This is not a matter of statistical power, since even when we lower the threshold (to p < 0.05), we did not observe the changes in RC and LO. Thus, the modulation of the visual cortices in this task did not result from the fact that in the Trained condition the target had been identified but rather from a differential involvement of these regions when subjects performed the task searching for trained, as opposed to untrained, shapes. (3) A cluster of sites in the medial frontal cortex (x = −3, y = 69, z = −15, BA11), were more active in the seen than in the absent condition. This region, which has been shown to be deactivated in a variety of visual tasks (Raichle et al., 2001) was actually deactivated in both of our experimental conditions and was more inactive in the absent than in the seen condition.
Figure 5. Task Dependency of the Effect: Comparison of Seen and Absent Trials within the Trained Condition.
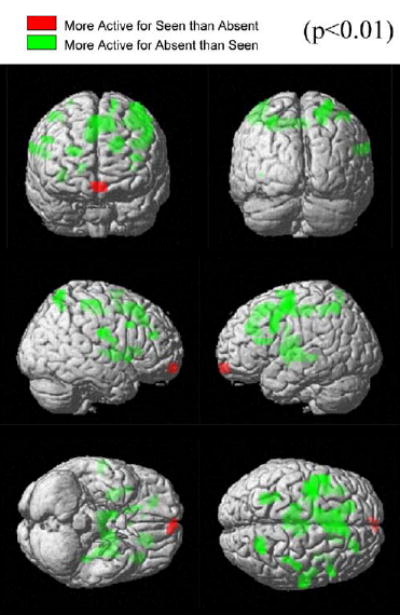
To distinguish the changes resulting from task engagement and the difference arising from different perceptual states (seeing or not seeing the target), we analyzed the activations of the differential map (absent - seen) trials, within the Trained condition. The resulting T-statistic map based on the group random effects analysis of seven subjects is thresholded at p < 0.01 and rendered in the MNI 152 average brain (Evans et al., 1994; Talairach and Tournoux, 1988); see Table 4). Positive t values (green) represent regions in which absent > seen; negative t values (red) represent regions in which seen > absent. The dorsal network, including the posterior parietal, premotor, and prefrontal regions and the striatum were more active in the absent than in the seen condition. We did not see a significant difference in the visual cortex. A region in the medial frontal cortex (Talairach Coordinates [−3, 65, −15], BA 11), which was deactivated in both conditions, was significantly more deactivated in the absent condition and appears as the sole significant brain area in the seen > absent map.
Discussion
We utilized functional brain imaging to study the large-scale aspects of reorganization of activation upon training in a visual search task. Our hypothesis, based on earlier psychophysical studies, was that early visual retinotopic cortices retain the capacity to reorganize and become responsive to and selective for complex shapes. Though at a cost of having to establish multiple representations of the trained object over a retinotopically mapped region, the benefit would be to optimize rapidity and to shift toward a parallel operation of the recognition process. Different levels of analysis provided support for this hypothesis: (1) A differential change in activation upon training at higher stages of the visual pathway, with earlier stages (RC) becoming more active with training and later stages (LO) becoming less active with training and (2) a change, over different visual cortical areas, in the pattern of correlation between performance and activation, with RC activation becoming more correlated and LO activation becoming less correlated with performance.
Here, we map brain regions based on a system of Talairach coordinates, which does not have sufficient resolution to ascertain whether changes are occurring in V1 or V2, although it does clearly distinguish the retinotopic cortex (RC), which includes V1 and V2 in the medial occipital cortex, from the lateral occipital cortex (LO). Here we will concentrate on this difference on the assumption that this reorganization between higher (nonretinotopic) and lower (retinotopic) cortices provides an important principle. Future higher-resolution studies will be needed to elucidate the precise involvement of each cortical region and, moreover, the retinotopic changes within each region.
The change in the level of activity across the visual pathway is the first indication of reorganization. The direction of the changes has often been difficult to interpret in fMRI data. First, the link between neuronal activation, and the balance of activation of excitatory or inhibitory neurons, and BOLD activity is not well understood (Heeger and Rees, 2002). Second, changes with learning could involve either an increase in activation, if more neurons are involved in the task, or a decrease in activation, if their tuning curves narrow and cells become more selective (Gilbert et al., 2001). What may be more indicative than the sign of change within each region is the observation that the opposite change occurs in LO versus the RC, which argues for large-scale reorganization across much of the visual pathway. The involvement of the RC reinforces results from earlier studies that showed its involvement in perceptual learning (Crist et al., 2001; Furmanski et al., 2004; Li et al., 2004; Schwartz et al., 2002). Though the paradigm in these studies involved training in a single visual field location and therefore a retinotopically specific change, our training paradigm involves presenting the target at different retinotopic locations. Even so, in this task we have described a point-by-point improvement in performance, suggesting a process that is specific for each locus, though incrementally growing to include all loci (Sigman and Gilbert, 2000). Since the target in our study appeared randomly from trial to trial at any location within the array, changes in retinotopically mapped cortices are relatively more difficult to see. A change associated with the trained target in a cortical region representing a particular visuotopic location would only be seen when the target appears at that location and is averaged along with trials in which untrained targets appear at that location. Perhaps for that reason, the greatest change was seen in the cortical areas representing the fovea, which might show a change in activation whenever the target is nearby, which was common to all trials. Also related to this, the observed changes are likely a low estimate, since target-present trials include all target locations, and activation measured within any voxel within the RC represents an average of trials in which the target appeared at the retinotopic locations represented by that voxel and at other locations.
An important argument for the involvement of a cortical area in the task is how much of the variance in the data can be explained in terms of the changes in activity of this cortical region. Thus, the change in the correlation between activity and behavior provided more compelling evidence supporting the idea of reorganization of the cortical representation of the trained shape. After training, the covariation of RC activity with performance became more pronounced, while the correlation between performance and LO activity after training essentially vanished. As with the reversal in the overall levels of activation, here again we see the reverse trend occurring in the pattern of correlations between LO and RC with learning. These changes are mostly explained by intersubject variability, partly because performance fluctuations within each subject were negligible. This population effect indicates that a functional cortical reorganization is associated with the ability to master a perceptual task. Before training, performance in the task is fairly independent of activation in the medial occipital regions of the subjects. After training, subjects who show the weakest RC activation are those who perform the task better. In addition, the session-to-session consistency across each subject shows the reliability of our results. Although the RC becomes more explicative of behavioral variance with training, the correlation between performance and activation is (before and after training) negative. This intriguing finding may be a consequence of the redistribution of activity that results from attention. As previously shown, directing attention to a specific target leads to an increase in the levels of activity in the attended location, and, at the same time, to reductions of activation outside of the field of attention (Duncan et al., 1997; Hopf et al., 2004; Tootell et al., 1998).
The fact that the correlation between performance and activation was negative serves to discount a possible alternative interpretation of our results. It is known that the subjective perceptual state (having seen or not seen a target) modulates activity in V1 (Polonsky et al., 2000). Since one of the consequences of training is that subjects see the target in many more trials, this could potentially explain our observed increase in RC activation as a result of learning. However, if this were the case, we should have found a positive correlation between RC activation and performance. Thus, the finding of a negative correlation between performance and RC activity discards this possibility, and, furthermore, it suggests that learning establishes a discontinuity—a two-state system. While activity decreased monotonically with performance within each condition, learning induced a state change associated with both the improvement in performance and an increase in activity. This change is therefore specific to learning itself.
More direct evidence indicating that the modulation that we observed was not due merely to the number of seen trials (hits) comes from the second experiment comparing activation in target-seen versus that in target-absent trials. Subjects were tested exclusively in the trained condition with a slow event-related design, which permitted us to compare, within the condition in which subjects were looking for the trained target, the activations in which the target was present and those in which the target was absent. We observed a difference in the dorsal network, which was more active for the target-absent trials, probably reflecting an increased attentional engagement, but we did not observe changes in visual cortex between the two different conditions. This suggests that the reorganization in visual cortex activity resulting from learning is associated with the cue that directs the subject to find the trained target, and therefore reflects the top-down influence of searching for the trained target rather than a bottom-up indication of the presence of the trained visual stimulus itself. This is consistent with findings from single-cell recordings in V1. Top-down modulation of V1 responses has been identified with single-cell studies. When monkeys were trained in different visual tasks (bisection or vernier discrimination) involving the same stimulus, the responses of V1 neurons depended on the task being performed (Crist et al., 2001; Li et al., 2004). In these experiments, the animals were cued to the task before stimulus presentation, and the task-related activity began from the first spikes of the recorded neurons, indicative of a top-down signal reflecting the animal’s cognitive state, rather than the nature of the stimulus. Top-down interactions have also been suggested to play a role in figure-ground modulations, in which the information is not conveyed by a task instruction but rather by the stimulus (Lamme, 1995; Roelfsema et al., 1998). The rationale for this conclusion derives from the delay in activity related to the texture discontinuity, but timing alone may not be a reliable indication of the source of contextual influences (Hupe et al., 2001).
In the current fMRI study, we see a source of modulation of the RC which is task dependent and which does not depend on the presence of the target. This does not imply, of course, that a modulation specific to the appearance of the trained target is not present, but, as described before, due to the coarse nature of our measure and to a balance between excitation and inhibition, particularly relevant in a cluttered field, we may not be able to see such a difference here. A higher-resolution fMRI, which would allow mapping of the distribution of activity at different retinotopic locations, is needed to address this trial-specific modulation. Similarly, in the current study and with our present resolution, we cannot be absolutely confident that the changes that we observed in the lateral occipital lie completely within the region LO. The lateral occipital complex involves a series of object-selective regions in the ventral stream, which include the LO, the inferior temporal gyrus, and the fusiform gyrus. The anatomical coordinates of the cluster in the lateral occipital cortex showing a differential activation between the T and U conditions (−46, −72, 4) are very close to those described in the literature for the LO, which correspond to the more posterior regions of the LOC (Grill-Spector, 2003; Grill-Spector et al., 1999; Lerner et al., 2001).
The visual pathway has been traditionally conceived as a feed-forward hierarchical network in which, as one progresses from one layer to the next, each layer becomes more responsive to more complex stimuli (Riesenhuber and Poggio, 1999; Tanaka, 1996). More recently it has been shown that the earlier stages of processing are modulated by top-down influences such that they are capable of providing different information to later stages, depending on the behavioral context (Crist et al., 2001; Gilbert et al., 2001; Lee et al., 2002). In addition, it has been shown that even in the adult brain, V1 is highly plastic to long-term consistent changes, both in response to lesions and to behaviorally relevant and persistent changes in the visual environment (Gilbert et al., 2001). Here we provide evidence indicating that perceptual learning results in changes in the RC that covary with changes in LO, suggesting that plasticity in the early cortex is part of a global remapping of activity over the visual cortical network. This further extends the idea that visual modules do not have fixed functional properties and enhances the importance of large-scale network changes resulting from learning (Ahissar and Hochstein, 1997; Buchel et al., 1999; McIntosh, 2000; McIntosh and Gonzalez Lima, 1998; Ullman, 1996).
In addition to the reorganization of the visual regions that are involved in shape representation, we observed a change that is more related to the nature of the task, i.e., whether it involved effortful serial search or reduced effort and automatization. These changes include the reduction of activation in parietal, premotor, and prefrontal regions, which have been associated with attentional control (Corbetta et al., 1998; Corbetta and Shulman, 1998). It is important to note that while less active, these regions are still active during the trained condition, in agreement with positron emission tomography studies of training in an orientation discrimination task (Schiltz et al., 1999). Similarly, while the correlation between PP and behavior decreases with training, the correlation does not vanish (while the correlation with LO and performance does vanish). This reduction, but not extinction, of the attentional network supports the idea that all vision is attentive to a degree and that parallel search may reflect a very fast serial process (Joseph et al., 1997). This idea is consistent with our finding that even in the trained condition, the parietal cortices are active, and their activity is increased in target-absent trials. In addition to decreases in the activation of the dorsal network, the negative amplification of activations and deactivations relative to baseline provides another neuronal correlate of automaticity. Recent findings have emphasized that the default state of the brain, i.e., the resting state, is far from being inactive and quiescent, but rather involves a network of active nodes that are systematically inactivated in goal-directed behavior (Gusnard and Raichle, 2001; Raichle et al., 2001; Shulman et al., 1997). This network overlaps extensively with many of the areas that we observed deactivating with the search task, and the general trend was that deactivations decreased with training. This may result from the ability to perform the task without interrupting the internal thought processes, which is supported by reports of our experimental subjects after performing the task. It has been suggested that learning results in a systematic decrease in activity, reflecting a progressive optimization of neuronal responses elicited by the task (Barlow and Foldiak, 1989; Buchel et al., 1999; Gilbert et al., 2001; Squire et al., 1992). In agreement with this general idea, we find that, except for early visual cortex, learning is associated with a decrease in the level of activation of areas that are activated by the task and a decrease in inactivation of the areas inactivated by the task. The resultant effect is therefore not a minimization of activation but rather a minimization of the departure from the default state.
In summary, learning a shape identification task results both in specific and nonspecific global changes in brain activity. Nonspecific changes relate to task complexity, imply a larger departure from the resting state (including activations and deactivations), and appear both in the Trained versus the Untrained difference and also, within the Trained condition, in the difference between the target-absent and target-present trials. Changes that are specific to the task (Trained versus Untrained conditions) involve visual cortices, with a decrease in LO and a significantly increased activation only in the RC, indicating a unique functional role acquired with this learning process. Along with this change, RC becomes increasingly correlated with the behavior and therefore a better predictor of performance. Together, these findings suggest a redistribution in the functional contributions of different cortical areas in executing an object identification task and a powerful top-down influence of stimulus expectation extending down to the earliest stages of visual cortical processing.
Experimental Procedures
Stimulus Design and Behavioral Protocol
Block Design Experiments
We studied seven healthy volunteers (five males and two females; five, right-handed and two, left-handed; age range, 22–29 years). The stimulus consisted of an array of 12 Ts at four possible orientations (Up, Down, Left, or Right) arranged on an annulus of 3 degrees radius. A fixation spot was presented in the center, and 12 targets were arranged along the four quadrants, avoiding the vertical and horizontal meridian. One T was chosen to be the target (with the exception of the upright T to avoid prior familiarity with the target). The distractors were chosen randomly among the remaining orientations and changed on each presentation. The screen flashed for 150 ms, and subjects were instructed to indicate whether or not a target shape was present by pressing the appropriate button of the mouse. In 80% of the trials, the target could appear randomly in any of the array locations; in the remaining 20% of the trials, the target was not present. To compensate for guessing, we measured the false positive rate (fp) (i.e., trials in which the response indicated that the target was seen although the target was absent) for each individual subject. The value of fp was used to adjust the value of positive responses (p) according to the formula:
The false-positive rate was < 2% for all subjects.
Before scanning, subjects were trained extensively in daily sessions. Each training session consisted of 40 blocks, and each block contained 45 trials, for a total of 1800 trials per session. Subjects continued training until their level of performance was above 80% for ten consecutive blocks. The number of sessions required to reach threshold varied from three to six. During training, stimuli were presented at an observation distance of 150 cm on a NEC monitor 5FGp refreshed at a rate of 60 Hz. Observation was binocular, and subjects were instructed not to move their heads.
After subjects had been trained extensively on one target, they performed an imaging session in a 3T scanner (see below for imaging details). Subjects performed six sessions. Each session began with a 13 s period in which the subject had to fixate a cross in the center of the screen; this was followed by a series of six periods, each composed of two blocks of stimulus presentations (Figure 1). At the beginning of each period, a cue stimulus was presented in the center of the screen, replacing the fixation spot for 2 s (indicated by a dotted colored lined, Figure 1). The cue represented the search target for the period. During each period, the target would either be presented in any location within the array or be omitted entirely, and arrays were presented on an average of every 4 s (jittered with a normal distribution from 3.5 s to 4.5 s). Stimulus presentations are indicated in Figure 1 by the colored solid line (red representing a trained period and blue, an untrained period). Each period lasted 75 s, and included 13 trials (12 with the target presented in each of the 12 possible locations and one trial in which the target was not present). In the middle of the period, after the first six trials, we incorporated a resting (fixation) time of 25 s during which only the fixation cross appeared. After each period, we allowed 25 s of fixation, then presented a new target, and began again. Each 25 s epoch, either of fixation or of a target condition (trained or untrained) is referred to as a block. The sequence of blocks was pseudorandomly balanced in the different sessions to control for potential order effects, but each one contained six Trained blocks, six Untrained blocks, and twelve fixation blocks. Although in this study we mainly focus on the differences between the Trained and Untrained conditions, fixation blocks are important to address the sign and strength of activation of each condition with respect to baseline. This becomes fundamental for the analysis of the changes in deactivations with learning.
Event Design Experiment
In a second experiment, we tested eight subjects (five males and three females; all, right-handed; age range, 23–29 years) only in the trained orientation, in a slow event-related design. Training, stimulus design, and imaging details were identical to those in the previous experiment. In this design, subjects performed a total of six sessions, during which they performed the task only in the trained orientations. A fixation spot was present in the center of the screen throughout the experiment, and 500 ms before stimulus presentation, we provided a cue by dimming the fixation spot. The stimulus was presented for 150 ms, and the intertrial interval was 14 s. In 50% of the trials, the target was present in a random location within the array.
Imaging
All experiments were performed with a GE-Sigma 3-Tesla MRI scanner (maximum gradient strength, 40 mT/m; maximum gradient slew rate, 150 T/m/s) with head-coil at the Weill Medical College of Cornell University in New York. Contiguous, multi-slice T2* weighted gradient-echo echoplanar images (EPIs) (TE = 35 ms; 64 × 64 pixels; field of view = 24 cm) were obtained in an orientation parallel to the anterior commissure-posterior commissure (AC-PC) plane. This sequence enhances blood oxygenation level-dependent (BOLD) contrast (Ogawa et al., 1990). The volume acquired covered the whole brain (20 slices with a 6 mm slice thickness, giving a 12.0 cm vertical field of view). The sampling rate is given by the effective repetition time (TR), which was 1.5 s/volume. An anatomical scan was acquired using a standard three-dimensional T1 weighted sequence (0.9766 × 0.9766 × 13 mm voxel size, 180 slices).
Image Processing and Statistical Analysis
Image processing and statistical analysis were carried out using SPM99 (Frackowiack et al., 2004; Friston et al., 1995) (see also http://www.fil.ion.ucl.ac.uk/spm) in the MATLAB (MathWorks, Inc., Sherborn, MA) environment. The standard image processing procedures featured in SPM99 were performed: the functional EPI images were realigned to correct for slight head movement between consecutive scans. The functional images were coregistered to corresponding anatomical images for individual subjects. All images were stereotactically transformed to the standardized coordinate space of Talairach and Tournoux ([Montreal Neurological Institute] MNI 152 average brain) to spatially normalize for individual differences in brain morphology. The normalized functional images were spatially smoothed with an isotropic Gaussian kernel (FWHM = 7.5 mm) to increase the signal-to-noise ratio. The global signal estimated as the mean intensity of the brain region within each volume was removed through proportional scaling.
To identify voxels activated or deactivated during task execution (as compared to baseline) or voxels more activated in one condition than the other (for example T - U contrast), we followed the standard statistical procedures of SPM. For individual subjects, a whole-brain voxel-by-voxel multiple linear regression model was employed. This general linear model is set to explain the observed BOLD time series from every single voxel by a linear summation of a set of regressors. The weight of each regressor is fitted to minimize the error of the model through a least-squares algorithm. Regressors are referred to as principal regressors (modeling effects of interest, i.e., the studied variables) and regressors modeling effects of no interest (e.g., head movements or global changes across sessions). The principal regressor was set by a model of the neural activation (stimulus onset and duration time) convolved with a prototypical hemodynamic response function (HRF). For the event-related analysis, the neural activity is modeled as an impulse (“event”), and for the block analysis the neural activity is modeled as sustained for the duration of the block (“epoch”). The canonical HRF was chosen, i.e., a mixture of two γ functions with an initial peak at 6 s and a later undershoot peaked at 16 s, with a 6:1 ratio between the early and the late γ functions. The regressors of interest modeled “Trained” and “Untrained” epochs for the block design experiment and “Target-Seen” and “Target-Absent” trials in the event-related experiment. For the latter, an additional regressor modeled trials in which the target was present and not seen. There were not enough of these trials to perform proper comparisons between this and other conditions. In addition, the model included the covariates of no interest, which consisted of the first-order temporal derivative of the regressor of interest, global signal, realignment parameters, and scanning periods, and an AR(1) model that accommodates the intrasubject temporal correlation pattern. All regressors were estimated at every brain voxel, and, based on these weight estimates, a contrast image for each condition based on the principal regressors was generated; these were combined in a series of linear contrasts to assess specific regional group effects.
An example of the temporal course, locked to stimulus onset and averaged over blocks, is provided in Figure 4, showing a characteristic delay in the response of ~6 s for both activations and deactivations and a subsequent undershoot/overshoot against the resting state.
For the second-stage group-level analyses, within-group comparisons were performed using random effects statistical models in the form of one-sample Student’s t tests, which accounted for intersubject variability and allowed population-based inferences to be drawn (Frackowiack et al., 2004). For each subject, the contrast image for each condition was generated (as described in the previous paragraph), and these were combined in a series of linear contrasts to assess group effects. These comparisons generated statistical parametric maps of the t statistic (SPM{t}), which was transformed to a unit normal distribution (SPM{Z}). Voxel-wise inferences at the group level were then drawn according to Gaussian random field theory as implemented in SPM99. When making inferences about statistical maps with a prior hypothesis (LO and RC), the comparisons based on one-tailed Student’s t tests were considered significant if their initial voxel-wise p-values were less than 0.01 and p < 0.05 corrected for multiple comparisons using the small volume correction function of SPM to the nearest cluster. (Friston, 1997) For regions with no a priori hypotheses, the comparisons were considered significant if their initial voxel-wise p values were <0.001 and p < 0.05 corrected for multiple comparisons using the small volume correction within each ROI as defined in MNI space (Tzourio-Mazoyer et al., 2002).
Correlation Analysis
Each subject performed six sessions, and thus we collected data for a total of 42 sessions. Of these 42, five were discarded due to significant movement artifacts. For each session we calculated (using SPM99 as described above) the percent change in activity with respect to baseline for each condition (based on six blocks, which constituted 78 trials), and we calculated subjects’ performance for each session. This analysis was performed at the most activated voxel within each of the studied regions, RC, LO, and PP, defined as the nearest cluster from previously defined anatomical coordinates (Grill-Spector et al., 1999; Lerner et al., 2001). We employed a linear regression model with the measures of the BOLD activation change as the dependent variable and the corresponding measures of performance as the independent variable to estimate the correlation levels (i.e., the slopes or the regression coefficients) between these two measures. We then performed two-sample paired Student’s t tests to address whether the value of the slopes were significantly different from zero for each condition and whether there was a significant difference in the value of the slopes across conditions.
Table 4.
Seen – Absent SPM Result Summary
| Side | Area | Talairach (MNI Space) Coordinates X, Y, Z (mm) | Peak Z Value | P Value (uncorrected) | P Value (corrected) | Cluster Size (cm3) |
|---|---|---|---|---|---|---|
| L | BA11 | −3, 69, −15 | 3.73 | <0.0001 | 0.001 | 0.432 |
| R | BA7 (PP) | 24, −66, 66 | −3.43 | <0.001 | 0.012 | 1.755 |
| R | BA6 (SMA) | 66, 12, 9 | −2.56 | 0.005 | 0.021 | 0.162 |
| L | BA6 (SMA) | −51, 0, 42 | −3.45 | <0.001 | 0.017 | 7.938 |
| R | BA40 (somatosensory) | 39, −36, 54 | −2.69 | 0.004 | 0.034 | 0.189 |
| L | BA40 (somatosensory) | −51, −30, 42 | −2.71 | 0.003 | 0.017 | 0.189 |
| R | BA13 (executive function) | 39, 18, 0 | −2.39 | 0.008 | 0.012 | 0.054 |
| L | BA13 (executive function) | −33, 6, 18 | −2.52 | 0.006 | 0.017 | 0.081 |
| R | putamen | 21, 18, 0 | −2.76 | 0.003 | 0.030 | 0.351 |
| L | cingulate gyrus | −6, −3, 48 | −3.35 | <0.001 | 0.006 | 1.053 |
p < 0.01; extent threshold > 0.25 cm3.
Acknowledgments
We would like to thank Guillermo Cecchi and Lucia Chemes, for their participation in the early stages of this project; Michael Worden, for his help with the design of the stimulus and training subjects; and Michael Posner, for his helpful comments and support. We also thank Keith Hazleton for assistance in imaging sessions. This work was supported by a Human Frontiers Science Program fellowship (M.S.), NIH award EY07968 (C.D.G.), and the DeWitt Wallace Fund of the New York Community Trust.
References
- Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387:401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- Ball K, Sekuler R. A specific and enduring improvement in visual motion discrimination. Science. 1982;218:697–698. doi: 10.1126/science.7134968. [DOI] [PubMed] [Google Scholar]
- Barlow, H.B., Foldiak, P. (1989). Adaptation and decorrelation in the cortex. In The Computing Neuron, RM Durbin, C Miall, and GJ Mitchison, eds (New York: Addison-Wesley), pp. 54–72
- Berardi N, Fiorentini A. Interhemispheric transfer of visual information in humans: spatial characteristics. J Physiol. 1987;384:633–647. doi: 10.1113/jphysiol.1987.sp016474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Coull JT, Friston KJ. The predictive value of changes in effective connectivity for human learning. Science. 1999;283:1538–1541. doi: 10.1126/science.283.5407.1538. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R. A neural basis for visual search in inferior temporal cortex. Nature. 1993;363:345–347. doi: 10.1038/363345a0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Human cortical mechanisms of visual attention during orienting and search. Philos Trans R Soc Lond B Biol Sci. 1998;353:1353–1362. doi: 10.1098/rstb.1998.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Crist RE, Li W, Gilbert CD. Learning to see: experience and attention in primary visual cortex. Nat Neurosci. 2001;4:519–525. doi: 10.1038/87470. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Duncan J, Humphreys G, Ward R. Competitive brain activity in visual attention. Curr Opin Neurobiol. 1997;7:255–261. doi: 10.1016/s0959-4388(97)80014-1. [DOI] [PubMed] [Google Scholar]
- Evans, A.C., Kamber, M., Collins, D.L., Macdonald, D. (1994) An MRI-based probabilistic atlas of neuroanatomy. In Magnetic Resonance Scanning and Epilepsy, S Shorvon, D Fish, F Andermann, G Byder, and H Stefan, eds (New York: Plenum Press), pp. 263–274
- Fahle M, Edelman S. Long-term learning in vernier acuity: effects of stimulus orientation, range and of feedback. Vision Res. 1993;33:397–412. doi: 10.1016/0042-6989(93)90094-d. [DOI] [PubMed] [Google Scholar]
- Frackowiack, R.S.J., Friston, K.J., Frith, C.D., Dolan, R.J., Price, C.J., Ashburner, J., Penny, W., and Zeki, S. (2004). Human Brain Function, Second Edition (London: Elsevier/Academic Press).
- Friston KJ. Testing for anatomically specified regional effects. Hum Brain Mapp. 1997;5:133–136. doi: 10.1002/(sici)1097-0193(1997)5:2<133::aid-hbm7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Fujita I, Tanaka K, Ito M, Cheng K. Columns for visual features of objects in monkey inferotemporal cortex. Nature. 1992;360:343–346. doi: 10.1038/360343a0. [DOI] [PubMed] [Google Scholar]
- Furmanski CS, Schluppeck D, Engel SA. Learning strengthens the response of primary visual cortex to simple patterns. Curr Biol. 2004;14:573–578. doi: 10.1016/j.cub.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001;31:681–697. doi: 10.1016/s0896-6273(01)00424-x. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K. The neural basis of object perception. Curr Opin Neurobiol. 2003;13:159–166. doi: 10.1016/s0959-4388(03)00040-0. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Hendler T, Malach R. The dynamics of object-selective activation correlate with recognition performance in humans. Nat Neurosci. 2000;3:837–843. doi: 10.1038/77754. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Heeger DJ, Rees G. What does fMRI tell us about neuronal activity? Nat Rev Neurosci. 2002;3:142–151. doi: 10.1038/nrn730. [DOI] [PubMed] [Google Scholar]
- Hopf JM, Noesselt T, Tempelmann C, Braun J, Schoenfeld MA, Heinze HJ. Popout modulates focal attention in the primary visual cortex. Neuroimage. 2004;22:574–582. doi: 10.1016/j.neuroimage.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture in two nonstriate visual areas (18 and 19) of the cat. J Neurophysiol. 1965;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- Hupe JM, James AC, Girard P, Lomber SG, Payne BR, Bullier J. Feedback connections act on the early part of the responses in monkey visual cortex. J Neurophysiol. 2001;85:134–145. doi: 10.1152/jn.2001.85.1.134. [DOI] [PubMed] [Google Scholar]
- Joseph JS, Chun MM, Nakayama K. Attentional requirements in a preattentive feature search task. Nature. 1997;387:805–807. doi: 10.1038/42940. [DOI] [PubMed] [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc Natl Acad Sci USA. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobatake E, Tanaka K. Neuronal selectivities to complex object features in the ventral visual pathway of the macaque cerebral cortex. J Neurophysiol. 1994;71:856–867. doi: 10.1152/jn.1994.71.3.856. [DOI] [PubMed] [Google Scholar]
- Kobatake E, Wang G, Tanaka K. Effects of shape-discrimination training on the selectivity of inferotemporal cells in adult monkeys. J Neurophysiol. 1998;80:324–330. doi: 10.1152/jn.1998.80.1.324. [DOI] [PubMed] [Google Scholar]
- Kourtzi Z, Kanwisher N. Cortical regions involved in perceiving object shape. J Neurosci. 2000;20:3310–3318. doi: 10.1523/JNEUROSCI.20-09-03310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z, Kanwisher N. Representation of perceived object shape by the human lateral occipital complex. Science. 2001;293:1506–1509. doi: 10.1126/science.1061133. [DOI] [PubMed] [Google Scholar]
- Lamme VA. The neurophysiology of figure-ground segregation in primary visual cortex. J Neurosci. 1995;15:1605–1615. doi: 10.1523/JNEUROSCI.15-02-01605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TS, Yang CF, Romero RD, Mumford D. Neural activity in early visual cortex reflects behavioral experience and higher-order perceptual saliency. Nat Neurosci. 2002;5:589–597. doi: 10.1038/nn0602-860. [DOI] [PubMed] [Google Scholar]
- Lerner Y, Hendler T, Ben-Bashat D, Harel M, Malach R. A hierarchical axis of object processing stages in the human visual cortex. Cereb Cortex. 2001;11:287–297. doi: 10.1093/cercor/11.4.287. [DOI] [PubMed] [Google Scholar]
- Li W, Piech V, Gilbert CD. Perceptual learning and top-down influences in primary visual cortex. Nat Neurosci. 2004;7:651–657. doi: 10.1038/nn1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Poggio T. Shape representation in the inferior temporal cortex of monkeys. Curr Biol. 1995;5:552–563. doi: 10.1016/s0960-9822(95)00108-4. [DOI] [PubMed] [Google Scholar]
- Maffei L, Fiorentini A. The unresponsive region of visual cortical receptive fields. Vision Res. 1976;16:1131–1139. doi: 10.1016/0042-6989(76)90253-4. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Towards a network theory of cognition. Neural Netw. 2000;13:861–870. doi: 10.1016/s0893-6080(00)00059-9. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez Lima F. Large-scale functional connectivity in associative learning: interrelations of the rat auditory, visual and limbic systems. J Neurophysiol. 1998;80:3148–3162. doi: 10.1152/jn.1998.80.6.3148. [DOI] [PubMed] [Google Scholar]
- McKee SP, Westheimer G. Improvement in vernier acuity with practice. Percept Psychophys. 1978;24:258–262. doi: 10.3758/bf03206097. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky A, Blake R, Braun J, Heeger DJ. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nat Neurosci. 2000;3:1153–1159. doi: 10.1038/80676. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenhuber M, Poggio T. Hierarchical models of object recognition in cortex. Nat Neurosci. 1999;2:1019–1025. doi: 10.1038/14819. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Lamme VAF, Spekreijse H. Object-based attention in the primary visual cortex of the macaque monkey. Nature. 1998;395:376–381. doi: 10.1038/26475. [DOI] [PubMed] [Google Scholar]
- Schiltz C, Bodart JM, Dubois S, Dejardin S, Michel C, Roucoux A, Crommelinck M, Orban GA. Neuronal mechanisms of perceptual learning: changes in human brain activity with training in orientation discrimination. Neuroimage. 1999;9:46–62. doi: 10.1006/nimg.1998.0394. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing: I. Detection, search and attention. Psychol Rev. 1977;84:1–66. [Google Scholar]
- Schoups AA, Vogels R, Qian N, Orban GA. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Maquet P, Frith C. Neural correlates of perceptual learning: a functional MRI study of visual texture discrimination. Proc Natl Acad Sci USA. 2002;99:17137–17142. doi: 10.1073/pnas.242414599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinberg DL, Logothetis NK. The role of temporal cortical areas in perceptual organization. Proc Natl Acad Sci USA. 1997;94:3408–3413. doi: 10.1073/pnas.94.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffrin RM, Schneider W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending and a general theory. Psychol Rev. 1977;84:127–191. [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin F, Raichle ME, Petersen RS. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Sigman M, Gilbert CD. Learning to find a shape. Nat Neurosci. 2000;3:264–269. doi: 10.1038/72979. [DOI] [PubMed] [Google Scholar]
- Squire LR, Ojemann J, Miezin F, Petersen SE, Vidden TO, Raichle ME. Activation of the hippocampus in normal humans: A functional anatomical study of memory. Proc Natl Acad Sci USA. 1992;89:1837–1841. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach, P., and Tournoux, J. (1988). A Stereotactic Coplanar Atlas of the Human Brain (Stuttgart, Germany: Thieme).
- Tanaka K. Representation of visual features of objects in the inferotemporal cortex. Neural Netw. 1996;9:1459–1475. doi: 10.1016/s0893-6080(96)00045-7. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Hadjikhani N, Hall EK, Marrett S, Vanduffel W, Vaughan JT, Dale AM. The retinotopy of visual spatial attention. Neuron. 1998;21:1409–1422. doi: 10.1016/s0896-6273(00)80659-5. [DOI] [PubMed] [Google Scholar]
- Treisman A. Feature binding, attention and object perception. Philos Trans R Soc Lond B Biol Sci. 1998;353:1295–1306. doi: 10.1098/rstb.1998.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cognit Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ullman, S. (1996). High-Level Vision: Object Recognition and Visual Cognition (Cambridge, MA: The MIT Press).


