Abstract
Leukocyte production of reactive oxygen species (ROS) is an essential component of the antimicrobial armament mounted during host defense, but when released to the extracellular milieu ROS can also injure host tissues and provoke inflammation. Polyisoprenyl phosphates (PIPPs) are constituents of human leukocyte membranes that regulate pivotal intracellular enzymes, such as phospholipase D (PLD). We prepared new PIPP mimetics and studied their impact in vivo on leukocyte activation, including ROS generation, in acute inflammation.
In a stereospecific and concentration-dependent manner, the PIPP mimetics directly regulated Streptomyces chromofuscus phospholipase D (sPLD) action. The IC50 for a (Z)-isomer of endogenous presqualene diphosphate (PSDP) was 100 nM.
Structure–activity relationships were also determined for PIPP mimetic inhibition of recombinant human PLD1b, a prominent isoform in human leukocytes. The PIPP mimetic rank order for PLD1b inhibition differed from sPLD, although the (Z)-PSDP isomer remained the most potent PIPP mimetic for inhibition of both enzymes. Truncation of PLD1b to its catalytic core uncovered potential regulatory roles for both PSDP's isoprenoid and diphosphate moieties.
The (Z)-PSDP isomer reduced ROS production by activated human leukocytes and decreased murine neutrophil accumulation (65.6%) and ROS production (38.5%) in vivo during zymosan A-initiated peritonitis. When administered intraperitoneally 2 h after zymosan A, the (Z)-PSDP isomer decreased in vivo neutrophil accumulation (72.5%) and ROS generation (74.4%) 6 h later in peritoneal exudates.
Together, these results provide new means to protect and control unchecked inflammatory responses that characterize many human diseases.
Keywords: Lipid mediators, neutrophils, inflammation, reactive oxygen species
Introduction
Innate immune responses to pathogens must be rapid to prevent infection and reversible to avoid unintended tissue injury (Nathan, 2002). Recent evidence has identified several, structurally distinct classes of membrane-derived lipid mediators as autacoid regulators of leukocyte functional responses in acute inflammation (Gilroy et al., 2004). One of these involves isoprenoid metabolism, which in addition to its fundamental role in cholesterol biosynthesis, are also lipid mediators with an important role in the regulation of host inflammatory responses. These previously unappreciated roles of isoprenoids are underscored by the hereditary periodic fever syndromes, as many result from low isoprenoid levels secondary to defective mevalonate kinase activity (Drenth & van der Meer, 2001; Takada et al., 2003). Recently, polyisoprenyl phosphate (PIPP) signaling, and specifically, the mevalonate-derived product presqualene diphosphate (PSDP) was found to directly prevent unwanted superoxide anion generation in human polymophonuclear leukocytes (PMN) (Levy et al., 1997). Present in leukocyte membranes, PSDP levels rapidly decline within seconds of cell activation, unlocking regulatory enzymes, such as phospholipase D (PLD), involved in PMN intracellular signaling and cell activation as in NADPH oxidase assembly for reactive oxygen species (ROS) generation (Levy et al., 1999a).
During host defense and inflammation, superoxide anions, ozone, and other ROS are essential components in microbial killing, yet also display potent cytotoxicity for host tissues (Wentworth et al., 2002; Babior et al., 2003). When uncontrolled, ROS such as ozone can be violently toxic to the host and appears to be linked to the pathobiology of subacute inflammatory disorders, including a reversed passive Arthus reaction in rat skin and human atherosclerosis (Wentworth et al., 2002; 2003). Also, excess superoxide anions and singlet oxygen radicals can have adverse consequences in the lung and compromise the lung's bactericidal activity (Goldstein et al., 1971). Hence, rapid containment and enzymatic regulation of endogenous ROS is required to prevent oxidant-mediated damage to host tissues (Del Maestro et al., 1980).
Biochemical events in PSDP signaling in human PMN raise the possibility that PIPPs can serve as potent candidates that can limit unwanted ROS production. To this end, novel synthetic PIPP mimetics were prepared and assessed for their potential impact in human leukocyte activation and murine acute inflammation.
Methods
All experiments with mice (Charles River Laboratories, Wilmington, MA, U.S.A.) were performed in accordance with Institutional Guidelines for the Proper Care and Handling of Experimental Animals. For zymosan A-initiated peritonitis, mice were 6–8 weeks old, 20–25 g, FVB, male animals. Mice were maintained on a light–dark cycle with light from 07:00 to 20:00 hours at 25°C and fed with a standard laboratory diet and water ad libitum.
PLD enzymatic activity
PLD activity was monitored by choline release from phosphatidyl choline (PC) as described earlier (Levy et al., 1999a). Briefly, PC (25 mg ml−1) was brought to dryness under a gentle stream of N2, and liposomes were prepared in 50 mM Tris, 36 mM CaCl2, pH 7.4 by sonication. After warming (5 min, 30°C), PC (final concentration 2 mM) was incubated (10 min, 30°C) with Streptomyces chromofuscus PLD (sPLD) (2 units per reaction, Sigma Chemical Co.) in the presence of authentic PSDP, structurally related mimetics in a concentration rage of 1 nM to 1 μM or vehicle (<0.1% ethanol). Reactions were stopped with 1 M Tris, 50 mM EDTA, pH 8.0, and free choline oxidized with horseradish peroxidase for detection by spectrophotometer (Model 8453, Agilent) (Levy et al., 1999a).
For assessing recombinant human PLD1b activity, Sf9 insect cells confluent in a T175 flask were virally transfected with full-length or truncated human PLD1b (Sung et al., 1999). After 48 h, recombinant PLD was partially purified as described earlier (Sung et al., 1999). Cells were pelleted (500 g, 10 min, 4°C) and resuspended in iced 50 mM Tris, 36 mM CaCl2, pH 7.4 prior to membrane disruption (Sonic Dismembrator, Fisher Scientific). Intracellular membranes were pelleted (14,000 × g, 10 min, 0°C) and resuspended to 250–500 μg protein μl−1 to determine PLD activity as described earlier (Levy et al., 1999a; Sung et al., 1999).
Generation of ROS by human leukocytes
For human PMN, peripheral venous blood (∼120 ml) was obtained by venipuncture from healthy volunteers who denied taking any medication for at least 2 weeks and had given written informed consent to a protocol approved by Brigham and Women's Hospital's Human Research Committee. PMN were freshly isolated from whole blood as described earlier (Levy et al., 1999b) and suspended (7.5 × 106 PMN ml−1, 37°C) in phosphate-buffered saline (pH 7.45) with CaCl2 and MgCl2 (1 mM). ROS generation in vitro was determined by continuously monitoring oxidation of indigo carmine (50 μM) to isatin sulfonic acid at 610 nm at 5 s intervals while immersed in a continuously flowing water bath-jacketed cassette (37°C) or after timed incubations (10 min, 37°C) in the presence of an agonist (i.e., 0.1 μM PMA) or vehicle (<0.1% ethanol).
Zymosan A-initiated peritonitis
PSDP or structurally related compounds (0–5 nmol in 0.1 ml 0.9% sterile saline) were delivered intraperitoneally (i.p.) within the same syringe, after warming to 37°C, with zymosan A (0.9 mg in 0.9 ml 0.9% sterile saline). After 2 h, animals were anesthetized with isoflurane (Abbott Laboratories, North Chicago, IL, U.S.A.) and, while still alive, indigo carmine (5 ml of 50 μM solution in phosphate-buffered saline) was injected (i.p.). Elicited leukocytes in the peritoneal lavage were collected. Total and differential leukocytes were enumerated in the exudate cell pellets (Bannenberg et al., 2005). Superoxide and particularly ROS and ozone formation in vivo in the peritoneal exudates were determined in the cell-free supernatant (800 g, 5 min) by the conversion of indigo carmine to isatin sulfonic acid that led to decrements in absorbance at 610 nm relative to that obtained with the initial materials (Wentworth et al., 2002; Kettle et al., 2004).
Molecular modeling of PSDP and PLD
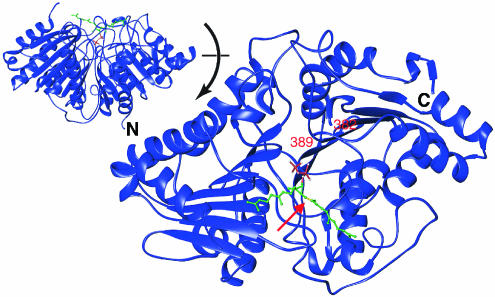
Modeling was performed and carried out with manual positioning using O (Jones, version 8.0, Uppsala, Sweden) for the complex of PSDP and PLD from Streptomyces sp. Strain PMF (PDB accession #1F0I) (Leiros et al., 2000). Residues numbered 382–389 (labeled in Figure 4) are not visible in the crystal structure.
Figure 4.
Interactions between PSDP and PLD. Modeling of native PSDP in the active site of PLD (PDB accession # 1F0I) is based on structural alignment of phosphate within the crystal structure and the beta-phosphate of PSDP (see Methods). Hypothetical position of isoprenoid moiety is based on the corresponding hydrophobic regions of PLD interaction with the hydrophobic regions of PSDP. The red arrow marks the alkene bond (in yellow) of PSDP synthesized as (Z) or (E) for the PSDP mimetics used in the present experiments. The inset shows the complex rotated 90 degrees. The amino terminus (N) and carboxy terminus (C) are indicated. For PSDP, carbon atoms are green, phosphate atoms red, and oxygen atoms white.
Materials
Phosphatidylcholine (PC, C10 : 0) was purchased from Avanti Polar Lipids (Alabaster, AL, U.S.A.), and sPLD, choline oxidase, horseradish peroxidase, indigo carmine, PMA, and zymosan A were obtained from Sigma Chemical Co. (St Louis, MO, U.S.A.). Sf9 cells were from BD Pharmingen (San Jose, CA, U.S.A.). Authentic PSDP, PSMP, and their structural mimetics were prepared by total synthesis in stereochemically pure form and were purified by HPLC. The (E)-isomers, (E)-PS-CO2Me, (E)-PSMP-(nBu)4N+, (E)-PSDP-(nBu)4N+, were prepared as described earlier by Poulter and co-workers (Rogers et al., 1995). The (Z)-isomers were prepared using similar methods. (Z)-PS-CO2Me was separated from its (E)-isomer and its spectroscopic data matched those reported earlier (Rogers et al., 1995). The conversion of isomerically pure (Z)-PS-CO2Me to (Z)-PSMP and (Z)-PSDP was performed similarly to the synthesis of the (E)-isomers as described earlier (Rogers et al., 1995). All compounds were characterized by NMR spectroscopy and gave satisfactory data.
Statistical analysis
Results are expressed as mean±s.e. Statistical significance of differences was assessed by Student's t-test for comparison of two samples. P<0.05 was set as the level of significance.
Results
Structure–activity relationships for PLD inhibition by new PIPP mimetics
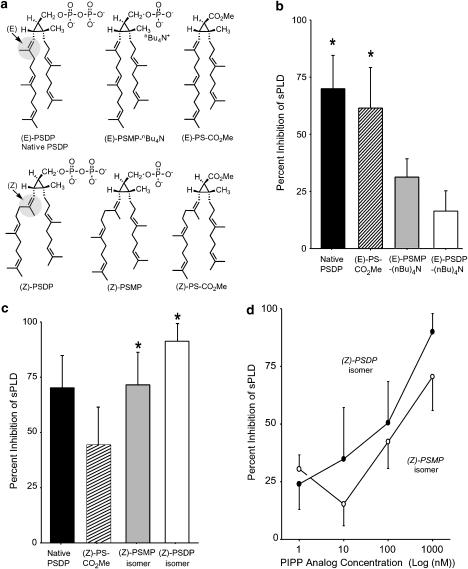
In PMN, PLD action is an essential component in the activation of the NADPH oxidase required for ROS production (Agwu et al., 1991). We synthesized a series of PSDP mimetics (Figure 1a) that preserved distinct structural elements (i.e., isoprenoid backbone, stereochemistry, cyclopropylcarbinyl ring, or phosphates) to identify those features pivotal to PLD inhibition. Purified bacterial PLD from Streptomyces chromofuscus (sPLD) was significantly inhibited by native, (E)-PSDP (Figure 1b). Of interest, a synthetic (E)-PSDP mimetic devoid of phosphates ((E)-PS-CO2Me) displayed similar inhibition (∼60%). In contrast, shielding of either (E)-PSMP or (E)-PSDP by tetra-butyl ammonium groups prevented inhibition of sPLD (Figure 1b). Together, these results suggest that the isoprenoid backbone of PSDP possesses sPLD regulatory activity.
Figure 1.
Structure–activity relationship for new PIPP mimetics and PLD inhibition. (a) Synthetic mimetics of native PSDP were prepared to determine the impact of stereoselectivity on its biological actions. (b) The impact of phosphate removal or shielding (via tetra-butyl ammonium (−(nBu)4N+)) and (c) isoprenoid stereochemistry was determined for inhibition of sPLD (2 units per reaction) by native PSDP or structural isomer (1 μM) (see Methods). Values are the mean±s.e.m. for n=6. *P<0.05 by Student's t-test for (b) isolated sPLD inhibition and (c) difference between (Z)- and (E)-isomers. (d) Concentration-dependent inhibition of sPLD activity by (Z)-PSDP and (Z)-PSMP isomers. Mean±s.e.m. for n=3.
A change in alkene bond geometry from (E) to (Z) (Figure 1a) markedly enhanced inhibition of sPLD by both the monophosphate and diphosphate (Z)-isomers. However, these modifications did not significantly alter the inhibitory capacity of the carboxy methyl ester (Figure 1c). It is noteworthy that endogenous PSDP carries this isoprenoid unit in the (E) configuration (Figure 1a). The inhibition of PLD by (Z)-PSDP and (Z)-PSMP was significantly greater than that observed for either the (E)-PSDP or (E)-PSMP mimetics (n=6, P<0.05). PLD inhibition by (Z)-PSDP and (Z)-PSMP was concentration-dependent in the nM range (Figure 1d). The half maximal sPLD inhibition with PSDP at 100 nM gave an apparent ∼2 : 1 PSDP : sPLD relationship. These results point to the contributions of the isoprenoid chains as well as indicate an additional site of interaction for the diphosphate in the regulation of PLD.
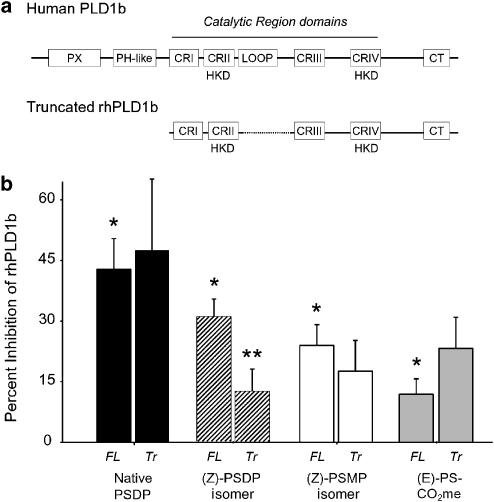
In human PMN, PLD1b is a prominent isoform that accumulates at phagosomal membranes upon cell activation (Cadwallader et al., 2004), and native PSDP is a potent inhibitor of recombinant human PLD1b (Levy et al., 1999a). Thus, we next utilized the new mimetics to assess the potential site(s) of interaction for PSDP within this isolated mammalian PLD. To this end, recombinant full-length human PLD1b as well as a truncated form of hPLD1b were expressed for incubations with the PSDP mimetics (Figure 2a). The truncated hPLD1b (Δ1–325, Δ505–621) carries the carboxy-terminal domain (CT) and four highly conserved domains (CRI-IV) in bacterial and mammalian PLDs essential for catalysis (Sung et al., 1999), but select regulatory sites, such as PX and PH-like domains that can interact with phosphorylated lipids (Stahelin et al., 2004), are not present. Full-length human PLD1b was significantly inhibited by native PSDP (1 μM). At equimolar amounts, the rank-order potency was native (E)-PSDP>(Z)-PSDP>(Z)-PSMP>(E)-PS-CO2Me (Figure 2b). The low activity of (E)-PS-CO2Me for rhPLD1b was distinct from that observed with sPLD (Figure 1c), emphasizing the importance of the diphosphate in PSDP's inhibition of PLD1b. Of note, the activity of (Z)-PSDP was markedly decreased by truncation of PLD1b, but the inhibitory properties of the (E)-containing native PSDP and its carboxy methyl ester for PC hydrolysis were not substantially changed by truncation of the enzyme (Figure 2b). Hence, native, (E)-PSDP can directly inhibit both (Δ1–325, Δ505–621)-hPLD1b and sPLD, which are similar in size and share homologous CR domains. The (Z)-PSDP and (Z)-PSMP isomers each lose activity for (Δ1–325, Δ505–621)-hPLD1b, suggesting the presence of additional regulatory site(s) in hPLD1b that are outside the enzyme's catalytic center.
Figure 2.
Structure–activity relationship for human PLD inhibition by PIPP mimetics. (a) Full-length (FL) and truncated (Tr) PLD1b were expressed in Sf9 cells. The truncated version of PLD1b lacked amino acids 1–325 and 505–621, regions containing PX (83–209), PH-like (222–330), and loop regulatory (505–620) domains. (b) After partial purification, PLD activity was monitored in the presence of select PIPP mimetics (1 μM) (see Methods). Values are the mean±s.e.m. for n=5. *P<0.05 by Student's t-test for PLD inhibition and **P<0.05 for the difference between truncated and full-length rhPLD1b.
Synthetic PIPP mimetics regulate inflammatory ROS
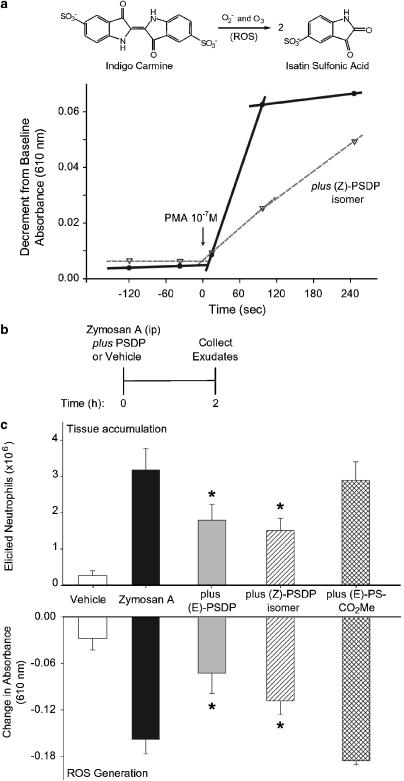
To determine whether the new PSDP mimetics had direct actions on leukocyte ROS generation, we monitored conversion in vitro of indigo carmine to isatin sulfonic acid during human PMN activation (Figure 3a, inset). When freshly isolated human PMN were activated with phorbol myristate acetate (PMA, 10−7 M) in the presence of native, (E)-PSDP or the new (Z)-PSDP isomer (1 μM), ROS production was markedly reduced (Figure 3a). (Z)-PSDP decreased the initial rate (20–90 s) of ROS generation from 33.8 to 10.0 pmol s−1 (Figure 3a), suggesting inhibition of NADPH oxidase assembly.
Figure 3.
PSDP regulates neutrophil-initiated ROS generation and inflammation. (a) ROS generation was measured by oxidation of indigo carmine to isatin sulfonic acid (inset) when freshly isolated human PMN (7.5 × 106 ml−1) were activated by exposure to phorbol ester (PMA 10−7 M, 37°C) in the absence or presence of (Z)-PSDP (1 μM). The calculated rate of change in absorbance at 610 nm is shown at baseline (prior to addition of the agonist), for the initial rate (t=20–90 s) and for a subsequent 150 s. Reactions are representative of n=7. (b) Murine peritonitis was initiated by injection of zymosan A (0.9 mg, i.p) and elicited PMN accumulation and in vivo oxidation of indigo carmine (decrements in absorbance at 610 nm) were determined 2 h later in peritoneal exudates. (c) The native (E)-PSDP, (Z)-PSDP, or (E)-PS-CO2Me isomer (1 nmol) or vehicle (0.9% sterile saline) was instilled (i.p.) in the same syringe as zymosan A. Values are for the mean±s.e.m. for n⩾3. *P<0.05 by Student's t-test for a difference with PIPP mimetic.
With decreased ROS generation by activated PMN in the presence of (Z)-PSDP, we next determined if this PSDP mimetic could regulate leukocyte activation in vivo in response to a microbial phagocytic stimulus, yeast zymosan A, that was given (i.p.) to mice to induce acute peritonitis (Figure 3b). Shortly after instillation of zymosan A, activated PMN respond (Bannenberg et al., 2005) and, here, within 2 h after administration of zymosan A, there was substantial PMN accumulation and ROS generation (8.01±0.96 nmol ROS) in peritoneal exudates (mean±s.e., n=6) (Figure 3c). Native PSDP or the (Z)-PSDP isomer, when given (i.p.) with zymosan A, potently inhibited both PMN tissue accumulation and ROS formation. The PIPP mimetic (1 nmol) gave 65.6 and 38.5% inhibition, respectively (n=6, P<0.05) (Figure 3c). For direct comparison between in vitro and in vivo PIPP mimetic activity, the least active mimetic in vitro for rhPLD1b inhibition, (E)-PS-CO2Me, was also administered (i.p.) with zymosan A and peritoneal inflammatory responses were monitored. At concentrations equimolar to native PSDP and (Z)-PSDP, this relatively inactive compound in vitro did not significantly block either ROS generation or leukocyte infiltration in vivo (n=3, Figure 3c). These findings indicate that the in vitro structure–function relationship for PSDP and these PIPP mimetics, (Z)-PSDP and (E)-PS-CO2Me, was retained in vivo.
To further explore the pharmacological effects of PSDP, we administered native PSDP or the (Z)-PSDP isomer (i.p.) after acute peritoneal inflammation was initiated (i.e., 2 h after zymosan A administration). To determine if these compounds dampen the immune response and promote resolution, we monitored leukocyte trafficking into the peritoneal exudates 6 h after PSDP, PIPP isomer, or vehicle administration. Both PSDP and (Z)-PSDP led to decrements of PMN numbers (66.0 and 72.5% inhibition, respectively) without significant change in the number of either macrophages or lymphocytes (Table 1). In these in vivo experiments, the (Z)-PSDP isomer was more potent than native PSDP for inhibition of PMN trafficking and ROS generation (Table 1). These findings indicate that PSDP and a structurally related isomer decreased the acute inflammatory response without accelerating leukocyte class switching at this time point. Together, these results indicate that PSDP and select PIPP mimetics have the capacity to directly regulate PMN activation in vitro and in vivo and modulate the host response to peritoneal microbial challenge.
Table 1.
Delayed addition of polyisoprenyl phosphates dampens acute peritoneal inflammationa
| Total leukocytes | Neutrophils | Macrophages | Lymphocytes | ROS generation(percent inhibition) | |
|---|---|---|---|---|---|
| Zymosan A | 19.7±5.0 | 15.3±4.1 | 2.6±0.5 | 1.3±0.5 | |
| (Z)-PSDP | 7.3±3.8* | 4.2±3.8* | 2.6±0.2 | 0.5±0.1 | 74.4* |
| Native PSDP | 9.5±3.8 | 5.2±3.3 | 3.5±0.7 | 0.8±0.3 | 27.2 |
Native PSDP, (Z)-PSDP, or vehicle was added 2 h after acute peritoneal inflammation was initiated by administration of zymosan A. After 6 h, peritoneal exudates were obtained and leukocyte counts determined (see Methods). Values represent the mean and s.e.m. for n⩾4 animals.
P<0.05 by Student's t-test.
Discussion and conclusions
To establish the functional determinants in PSDP's direct interactions with PLD, we designed a series of structurally related compounds to probe the contribution of the isoprenoid backbone, phosphates, and stereochemistry of native PSDP. We assessed the role of the diphosphate in PLD inhibition by replacing it with a carboxy methyl ester group (CO2Me), or the anionic phosphates were shielded with tetra-butyl ammonium groups. The results indicated that in the absence of both phosphates, the isoprenoid backbone alone could also inhibit sPLD, but not recombinant human PLD1b. To further evaluate the role of the isoprenoid backbone, we changed the configuration of the longer isoprenoid chains by switching the geometry of the C=C bond connected to the cyclopropyl ring from the (E)- to the (Z)-orientation. This change in stereochemistry and resulting conformational change markedly enhanced sPLD inhibition to levels observed with native PSDP. Recombinant human PLD1b activity was also blocked by native PSDP and the (Z)-PIPP mimetics. Of interest, truncation of PLD1b to primarily its' catalytic domains markedly altered the structure–activity relationships for the compounds with a loss of potency for (Z)-PSDP as an inhibitor that was not shared by (E)-PSDP. Together, these findings indicate both a direct interaction between PLD and the diphosphate present in PSDP and another interaction domain in PLD present in both the bacterial and mammalian forms of the enzyme for the (Z)-isoprenoid structure, implicating at least two distinct sites of interaction for inhibition of PLD by PSDP.
The PLD superfamily of enzymes shares the ability to bind a phosphodiester moiety in their catalytic center. Streptomyces sp. strain PMF PLD contains duplicated sequence motifs HXK(X)4D required for catalytic activity that are in close contact with the phospholipid phosphate head group (Leiros et al., 2000). Structural comparison of this PLD to other microbial PLDs and human PLD1b is most notable for conservation of the two HKD motifs, which are related by a pseudo-two-fold rotation axis running through the active-site-bound phosphate. The catalytic center is deeply buried in the protein, and the entrance to the substrate-binding pocket appears shielded by two hydrophobic loops that are held to be responsible for PLD's membrane association (Leiros et al., 2000). Figure 4 depicts a structural model for PSDP in relation to the enzyme's catalytic center. PSDP and the related active compounds are of the appropriate size and shape to block entry of PLD's substrate, namely PC. This depiction is consistent with both PSDP being a competitive inhibitor of the enzyme (Levy et al., 1999a) and the presence of a phosphate-coordinating center composed of histidine, lysine, and asparagine residues of the HKD motifs. The (Z)-PSDP isomer also fits in the substrate's catalytic center, thereby providing a reasonable mechanism for inhibition of PLD-initiated formation of ROS during inflammation. Since a complete crystal structure is not yet available for human PLD, the present modeling was carried out with the Streptomyces sp. strain PMF PLD structure (Leiros et al., 2000) that, like hPLD1b, contains HKD motifs. Although sPLD and rhPLD1b have similar catalytic mechanisms, their sequences outside the HKD motifs share little homology, likely accounting for the difference in the structure–activity relationship for PSDP and the PIPP mimetics with these two PLD enzymes (Figures 1c and 2b). Interaction sites with the human enzyme outside the HKD motifs are not excluded because the human enzyme is known to interact with Rho low molecular weight GTP-binding proteins (Bowman et al., 1993) that are subject to potential regulation by isoprenoid compounds.
Human PMN are coated with antibody, and when activated generate substantial amounts of singlet oxygen (Steinbeck et al., 1992) and oxidants with the chemical signature of ozone (Wentworth et al., 2002). Activation of isolated human PMN initiated ROS formation that was blocked by the addition of native, (E)-PSDP as well as the new (Z)-PSDP mimetic in vitro. These results indicate that PMN are one of the direct targets for PSDP's inhibition in its protective impact in peritonitis. By reducing superoxide anion, ozone, and other ROS formation in peritonitis, the PSDP mimetic dampened the amplitude of the acute inflammatory response and lessened risk for ‘bystander' injury of host abdominal tissues.
Acute inflammatory responses to pathogens or injury characterize several prevalent clinical disorders, including peritonitis (Winyard et al., 2003). Bacterial infection or surgical injury during laparatomy commonly results in acute peritoneal inflammation. Abdominal tissues can be subject to unwanted injury by activated leukocytes, leading to fibrinous intestinal adhesions that can entrap the bowel as well as other unwanted sequellae (Ray et al., 1998). To assess whether leukocyte-generated ROS in vivo is regulated by the PSDP mimetics during peritonitis, we administered yeast zymosan A into mouse peritoneum. This microbial cell wall component stimulates leukocyte Toll-like receptor signaling (Beutler, 2002), phagocytosis (Getting et al., 1997), and here triggered peritonitis with leukocyte infiltration and marked in vivo ROS generation. In addition to the pivotal roles that cytokines and chemokines play in inflammation and sepsis, lipid mediators, such as leukotriene B4, also serve critical roles in leukocyte recruitment and activation (Bannenberg et al., 2005). Moreover, leukotriene B4 initiates human PMN PSDP remodeling within seconds in a temporal manner that is concomitant with PLD activation and NADPH oxidase assembly – a process that is inhibitable by lipoxin A4, which blocks PMN PSDP turnover (Levy et al., 1999a). Local administration of a new PSDP mimetic at low levels in vivo blocked zymosan A-initiated leukocyte accumulation and ROS formation. Hence, this new synthetic (Z)-containing isomer of PSDP (denoted (Z)-PSDP) can serve as a potent inhibitor of PMN accumulation and activation, displaying a 50% inhibitory concentration at ∼1 nmol, similar in experimental peritonitis to that of the common nonsteroidal anti-inflammatory drug indomethacin (IC50 ∼0.5 nmol) (Kaplan et al., 1984).
Genetic defects in the PMN NADPH oxidase result in chronic granulomatous disease in which reduced ROS production leads to ineffective microbial clearance (Klebanoff, 1980). While chronic granulomatous disease highlights the importance of this PMN response in infection, there are several other human diseases stemming from overexuberant inflammation (Weiss, 1989) and leukocyte release of anti-microbial effectors, such as ROS, that are unwanted and toxic to host tissues (Wentworth et al., 2003). In addition, aberrant isoprenoid chemical signaling events are linked to unregulated inflammation in several human periodic fever syndromes (Drenth & van der Meer, 2001; Takada et al., 2003). Most of these illnesses are without specific disease-remitting therapy. Here, administration of a PSDP mimetic reduced peritoneal leukocyte recruitment and activation in response to microbial stimuli. The ability of PSDP and structurally related mimetics to dampen leukocyte-driven ROS generation in vivo during inflammation, as demonstrated here, can provide new mechanisms for protection from oxidant-mediated tissue injury and toward controlling unchecked inflammatory responses.
Acknowledgments
We thank Jeffrey vom Saal, Yee-Ping Sun, Eric Tjonahen, and Tamara Baer for technical assistance and Mary Small for assistance in manuscript preparation. This study was supported by NIH Grants HL68669, DE13499, and DE016191.
Abbreviations
- PIPP
polyisoprenyl phosphate
- PLD
phospholipase D
- PMN
polymorphonuclear leukocyte
- PSDP
presqualene diphosphate
- PSMP
presqualene monophosphate
- ROS
reactive oxygen species
References
- AGWU D.E., MCPHAIL L.C., SOZZANI S., BASS D.A., MCCALL C.E. Phosphatidic acid as a second messenger in human polymorphonuclear leukocytes. Effects on activation of NADPH oxidase. J. Clin. Invest. 1991;88:531–539. doi: 10.1172/JCI115336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BABIOR B.M., TAKEUCHI C., RUEDI J., GUTIERREZ A., WENTWORTH P., JR Investigating antibody-catalyzed ozone generation by human neutrophils. Proc. Natl. Acad. Sci. U.S.A. 2003;100:3031–3034. doi: 10.1073/pnas.0530251100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANNENBERG G.L., CHIANG N., ARIEL A., ARITA M., TJONAHEN E., GOTLINGER K.H., HONG S., SERHAN C.N. Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- BEUTLER B. Toll-like receptors: how they work and what they do. Curr. Opin. Hematol. 2002;9:2–10. doi: 10.1097/00062752-200201000-00002. [DOI] [PubMed] [Google Scholar]
- BOWMAN E.P., UHLINGER D.J., LAMBETH J.D. Neutrophil phospholipase D is activated by a membrane-associated Rho family small molecular weight GTP-binding protein. J. Biol. Chem. 1993;268:21509–21512. [PubMed] [Google Scholar]
- CADWALLADER K.A., UDDIN M., CONDLIFFE A.M., COWBURN A.S., WHITE J.F., SKEPPER J.N., KTISTAKIS N.T., CHILVERS E.R. Effect of priming on activation and localization of phospholipase D-1 in human neutrophils. Eur. J. Biochem. 2004;271:2755–2764. doi: 10.1111/j.1432-1033.2004.04204.x. [DOI] [PubMed] [Google Scholar]
- DEL MAESTRO R., THAW H.H., BJORK J., PLANKER M., ARFORS K.E. Free radicals as mediators of tissue injury. Acta Physiol. Scand. Suppl. 1980;492:43–57. [PubMed] [Google Scholar]
- DRENTH J.P.H., VAN DER MEER J.W.M. Hereditary periodic fever. N. Engl. J. Med. 2001;345:1748–1757. doi: 10.1056/NEJMra010200. [DOI] [PubMed] [Google Scholar]
- GETTING S.J., FLOWER R.J., PERRETTI M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br. J. Pharmacol. 1997;120:1075–1082. doi: 10.1038/sj.bjp.0701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILROY D.W., LAWRENCE T., PERRETTI M., ROSSI A.G. Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug Disc. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN E., TYLER W.S., HOEPRICH P.D., EAGLE C. Adverse influence of ozone on pulmonary bactericidal activity of murine lung. Nature. 1971;229:262–263. doi: 10.1038/229262a0. [DOI] [PubMed] [Google Scholar]
- KAPLAN H.B., EDELSON H.S., KORCHAK H.M., GIVEN W.P., ABRAMSON S., WEISSMANN G. Effects of non-steroidal anti-inflammatory agents on human neutrophil functions in vitro and in vivo. Biochem. Pharmacol. 1984;33:371–378. doi: 10.1016/0006-2952(84)90228-4. [DOI] [PubMed] [Google Scholar]
- KETTLE A.J., CLARK B.M., WINTERBOURN C.C. Superoxide converts indigo carmine to isatin sulfonic acid: implications for the hypothesis that neutrophils produce ozone. J. Biol. Chem. 2004;279:18521–18525. doi: 10.1074/jbc.M400334200. [DOI] [PubMed] [Google Scholar]
- KLEBANOFF S.J. Oxygen metabolism and the toxic properties of phagocytes. Ann. Int. Med. 1980;93:480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- LEIROS I., SECUNDO F., ZAMBONELLI C., SERVI S., HOUGH E. The first crystal structure of a phospholipase D. Structure. 2000;8:655–667. doi: 10.1016/s0969-2126(00)00150-7. [DOI] [PubMed] [Google Scholar]
- LEVY B.D., FOKIN V.V., CLARK J.M., WAKELAM M.J., PETASIS N.A., SERHAN C.N. Polyisoprenyl phosphate (PIPP) signaling regulates phospholipase D activity: a ‘stop' signaling switch for aspirin-triggered lipoxin A4. FASEB J. 1999a;13:903–911. doi: 10.1096/fasebj.13.8.903. [DOI] [PubMed] [Google Scholar]
- LEVY B.D., GRONERT K., CLISH C., SERHAN C.N.Leukotriene and lipoxin biosynthesis Lipid Second Messengers 1999bBoca Raton, FL: CRC Press LLC; 83–111.ed. Rubin, R.P., pp [Google Scholar]
- LEVY B.D., PETASIS N.A., SERHAN C.N. Polyisoprenyl phosphates in intracellular signalling. Nature. 1997;389:985–990. doi: 10.1038/40180. [DOI] [PubMed] [Google Scholar]
- NATHAN C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- RAY N.F., DENTON W.G., THAMER M., HENDERSON S.C., PERRY S. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J. Am. Coll. Surg. 1998;186:1–9. doi: 10.1016/s1072-7515(97)00127-0. [DOI] [PubMed] [Google Scholar]
- ROGERS D.H., YI E.C., POULTER C.D. Enantioselective synthesis of (+)-presqualene diphosphate. J. Org. Chem. 1995;60:941–945. [Google Scholar]
- STAHELIN R.V., ANANTHANARAYANAN B., BLATNER N.R., SINGH S., BRUZIK K.S., MURRAY D., CHO W. Mechanism of membrane binding of the phospholipase D1 PX domain. J. Biol. Chem. 2004;279:54918–54926. doi: 10.1074/jbc.M407798200. [DOI] [PubMed] [Google Scholar]
- STEINBECK M.J., KHAN A.U., KARNOVSKY M.J. Intracellular singlet oxygen generation by phagocytosing neutrophils in response to particles coated with a chemical trap. J. Biol. Chem. 1992;267:13425–13433. [PubMed] [Google Scholar]
- SUNG T.C., ZHANG Y., MORRIS A.J., FROHMAN M.A. Structural analysis of human phospholipase D1. J. Biol. Chem. 1999;274:3659–3666. doi: 10.1074/jbc.274.6.3659. [DOI] [PubMed] [Google Scholar]
- TAKADA T., AKSENTIJEVICH I., MAHADEVAN V., DEAN J.A., KELLEY R.I., KASTNER D.L. Favorable preliminary experience with etanercept in two patients with the hyperimmunoglobulinemia D and periodic syndrome. Arth. Rheum. 2003;48:2645–2651. doi: 10.1002/art.11218. [DOI] [PubMed] [Google Scholar]
- WEISS S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- WENTWORTH P., JR, MCDUNN J.E., WENTWORTH A.D., TAKEUCHI C., NIEVA J., JONES T., BAUTISTA C., RUEDI J.M., GUTIERREZ A., JANDA K.D., BABIOR B.M., ESCHENMOSER A., LERNER R.A. Evidence for antibody-catalyzed ozone formation in bacterial killing and inflammation. Science. 2002;298:2195–2199. doi: 10.1126/science.1077642. [DOI] [PubMed] [Google Scholar]
- WENTWORTH P., NIEVA J., TAKEUCHI C., GALVE R., WENTWORTH A.D., DILLEY R.B., DELARIA G.A., SAVEN A., BABIOR B.M., JANDA K.D., ESCHENMOSER A., LERNER R.A. Evidence for ozone formation in human athersclerotic arteries. Science. 2003;302:1053–1056. doi: 10.1126/science.1089525. [DOI] [PubMed] [Google Scholar]
- WINYARD P.G., WILLOUGHBY D.A., ROSENGRANT M.W. Inflammation Protocols. Totowa, NJ: Humana Press; 2003. [Google Scholar]