Abstract
Lung surfactant (LS), a lipid-protein mixture, forms films at the lung air-water interface and prevents alveolar collapse at end expiration. In lung disease and injury, the surface activity of LS is inhibited by leakage of serum proteins such as albumin into the alveolar hypophase. Multilamellar vesicular dispersions of a clinically used replacement, bovine lipid extract surfactant (BLES), to which (2% by weight) chain-perdeuterated dipalmitoylphosphatidycholine (DPPG mixtures-d62) had been added, were studied using deuterium-NMR spectroscopy (2H-NMR) and differential scanning calorimetry (DSC). DSC scans of BLES showed a broad gel to liquid-crystalline phase transition between 10–35°C, with a temperature of maximum heat flow (Tmax) around 27°C. Incorporation of the DPPC-d62 into BLES-reconstituted vesicles did not alter the Tmax or the transition range as observed by DSC or the hydrocarbon stretching modes of the lipids observed using infrared spectroscopy. Transition enthalpy change and 2H-NMR order parameter profiles were not significantly altered by addition of calcium and cholesterol to BLES. 2H-NMR spectra of the DPPC-d62 probes in these samples were characteristic of a single average lipid environment at all temperatures. This suggested either continuous ordering of the bilayer through the transition during cooling or averaging of the DPPC-d62 environment by rapid diffusion between small domains on a short timescale relative to that characteristic of the 2H-NMR experiment. Addition of 10% by weight of soluble bovine serum albumin (1:0.1, BLES/albumin, dry wt/wt) broadened the transition slightly and resulted in the superposition of 2H-NMR spectral features characteristic of coexisting fluid and ordered phases. This suggests the persistence of phase-separated domains throughout the transition regime (5–35°C) of BLES with albumin. The study suggests albumin can cause segregation of protein bound-lipid domains in surfactant at NMR timescales (10−5 s). Persistent phase separation at physiological temperature may provide for a basis for loss of surface activity of surfactant in dysfunction and disease.
INTRODUCTION
Pulmonary or lung surfactant (LS) is a lipid-protein complex secreted at the air-alveolar interface by type II pneumocytes of the alveoli (1). The material forms a putative surface-active monolayer with underlying multilayered structures to reduce the surface tension of the fluid coating of the terminal alveolar airspace at low lung volumes (1–3). LS is also found in airways, such as bronchioles, and likely prevents fluid retention in the narrow capillaries (4,5). In lung injuries, including acute respiratory distress syndromes (ARDS), the surface activity of the material is inhibited due to interactions with plasma protein that has leaked into the alveolar airspace (6,7). The surface activity properties of LS extracted from ARDS lungs and the effects of individual serum proteins (SP) such as albumin, hemoglobin, C-reactive protein, and phospholipases on LS have been studied extensively (8). Despite this effort, the physical characteristics of LS-SP systems as well as the specific modes of interactions of these soluble proteins with LS lipids are not yet clear. Most previous studies on interactions of SP with LS by measurement of surface activity have used relatively large amounts of SPs at ratios of 1:10 and 1:20 (surfactant lipids/protein, wt/wt) to show maximal inhibitions of LS (8). Estimates of such proteins from washings of diseased or injured lungs are rarely as high (6,7,9). In this study we have examined the interaction of a soluble inhibitory protein, bovine serum albumin (BSA), at a small protein/lipid ratio (1:0.1, surfactant lipids/albumin), with an exogenous bovine lipid extract surfactant (BLES) used in the clinical treatment of ARDS patients (10,11).
Most mammalian LS contains the disaturated lipid dipalmitoylphosphatidycholine (DPPC) in large amounts, plus monounsaturated phosphatidycholine (PC), phosphatidylglycerol (PG), and neutral lipids in significant amounts and some associated proteins (10% by weight of lipids (3,10,11)). The surfactant proteins SP-A and SP-D are large water-soluble lectins, which are removed from most semisynthetic and reconstituted LS used in clinical therapy. However the smaller hydrophobic proteins, SP-B and SP-C, known to enhance the surface activity of LS lipids as tested in vitro, are present in most clinically used LS preparations (11). These hydrophobic proteins, although present in fairly small amounts (collectively comprising 2–3% by weight relative to LS lipids), are known to critically enhance the surface activity of LS at an air-water interface, as well as reduce inhibition of surfactant by SPs (8,12–14). BLES is a clinically used LS preparation from cow lung lavage, which contains all the phospholipid and protein components of LS, except the soluble surfactant proteins SP-A and SP-D and neutral lipids (i.e., cholesterol, triglycerides) (10). The composition as well as the physical properties of BLES have been quite extensively studied (10,15–18). This allows interactions between various LS lipids and SP contaminants to be studied to model dysfunctional LS. Also by addition or subtraction of materials from BLES, specific interactions involving other LS components (such as cholesterol, calcium ions, and SP-A) may be studied (15,17,19).
Albumin is an SP responsible for binding and transport of fatty acids in the blood (20). BSA is a 65-kDa protein having hydrophobic pockets through which it binds to fatty acids (21,22). BSA has 17 negatively charged amino acid residues and is overall negatively charged at pH 7 (20). BSA is structurally characteristic of globular-soluble proteins, which are normally not found to interact with model or cellular membranes (23,24). However albumin may interact with LS bilayers at discontinuities, due to the coexistence of gel and fluid environments (18,25), or with the positively charged SP-B. A number of previous studies show that rapid adsorption of LS to an air-water interface to form surface-active films is decreased by albumin and that films formed in the presence of albumin cannot reach low surface tension upon compression (8,12,17,26,27). In ARDS the level of this SP is found to reach higher levels in lung lavage compared to other SPs, likely reflecting its high abundance in plasma (7,9).
In previous studies it was shown that a number of mammalian LS as well as their hydrophobic extracts in hydrated bilayer dispersions undergo broad calorimetrically measurable phase transitions. This phase change, possibly liquid-crystalline to gel, has been normally detected between 10–40°C, with transition midpoints and temperatures of maximal heat flow (Tmax) around 25–30°C, close to that of BLES at ∼27°C. (28–31). In recent studies it was found that imaging of LS bilayers in the liquid-crystalline to gel transition range can show macroscopic coexistence of gel and fluid domains. These domains in LS bilayers were detected by fluorescence microscopy using fluorescence probes in free-standing giant unilamellar (GUV) or multilamellar (MLV) vesicles (25) as well as in monolayers (32–35). The gel domains in bilayers are present up to high temperatures (18,33,35). A recent study has also shown the presence of liquid-ordered (Lo) and liquid-disordered phases in LS bilayers that contained a slight increase of cholesterol (25). These phases are found normally in cell membrane “lipid rafts” (25,34). Knowledge of how interactions of specific additives influence hydrocarbon chain order in LS within the phase coexistence regime can provide information on the structural basis for their ability to modify surfactant function. Some studies have suggested that the domain structural organization of LS film is highly susceptible to small amounts of additives such as cholesterol, calcium ions, and some serum constituents, as well as through pH change (33,36).
Deuterium NMR (2H-NMR) is a valuable tool for studying interactions of various lipid and protein components of membranes (37–39) and has also been applied to models of LS (40,41). Although 2H-NMR has been used extensively for studies of model membrane systems, there have also been some applications of it to material extracted from natural membranes with small amounts of perdeuterated lipids (PDLs) inserted as probe (42–45). Lipid diffusion during the 2H-NMR experiment normally may preclude the resolving of signals from protein-perturbed and -unperturbed lipid populations. There have also been some reports of strong complexation between protein and specific lipids giving rise to slow exchange between free and bound PDL that lead to resolution of different populations of deuterated lipids (46,47). These observations have been discussed in terms of formation of domains which exist over 10–100-ms timescales. Observation of boundary phases of the domains has also been reported (47).
The interactions of various surfactant lipid-protein components have been studied using 2H-NMR in simplified models of LS (48). Since some recent studies have shown coexistence of gel and fluid domains in BLES at or near mammalian physiological temperatures of 37°C (18,25), an attempt is made here to investigate the phase transition process in this complex LS extract, BLES, using 2H-NMR. The effects of cholesterol, calcium, and the inhibitory protein albumin on phase separation, as seen on the 2H-NMR timescale (10−6–10−4 s), are studied. After adding a small amount of the PDL, DPPC-d62 (2% by weight) to the vesicular system of BLES, such systems are investigated using 2H-NMR. The 2H-NMR observations are correlated with DSC heat flow profiles. The results suggest that although adding cholesterol and calcium had only minor effects on the gel and liquid-crystal properties of BLES, the presence of albumin altered the phase distributions and the lipid order parameters. This indicated possible albumin-induced formation of gel and liquid-crystal domains that persist at least on microsecond timescales over a transition range where no distinct phase separation is obvious in BLES alone. Aspects of LS dysfunction likely reflect perturbation of surfactant bilayer organization by SPs such as albumin.
MATERIALS AND METHODS
Materials
The clinically used BLES in saline dispersion (27 mg/ml) was a generous gift from BLES Biochemicals (London, Canada). Delipidated BSA, cholesterol, and calcium chloride were purchased from Sigma Chemicals (St. Louis, MO). DPPC-d62 was purchased from Avanti Polar Lipids (Birmingham, AL) and was used as received. High-performance liquid chromotography grade chloroform and methanol were purchased from Sigma Chemicals. All experiments were performed using doubly glass-distilled deionized water, the second distillation performed using dilute potassium permanganate (33).
DSC and FTIR
The hydrophobic constituents of BLES were extracted from the supplied saline dispersion using a modified method (49) of Bligh and Dyer (50). Briefly, the BLES dispersion was mixed with solvents to give a suspension of chloroform/methanol/water, 2:1:0.1 (v/v), and the resulting chloroform-rich phase was extracted. A second chloroform extract was performed on the hydrophilic layer, and the chloroform-rich phases were combined (49). Phosphorus was estimated from the organic extract by a modified method (49) of Bartlett (51). Some of the organic extracts of BLES were analyzed for a general molecular composition using electrospray ionization mass spectrometry (ESI-MS) in the positive mode in a mass range of 400–1000 Da (16). The hydrophobic proteins were estimated using matrix-assisted laser desorption-ionization time-of-flight (MALDI-TOF) MS. Both these methods as applied to LS are discussed in detail by others (52–54). To the best of our knowledge, the detection of SP-B and SP-C directly from LS extracts has not been done previously. However, MALDI-TOF has been used to identify various forms and modified versions of the purified SP-B and SP-C after isolation from the LS lipids (34,54).
This organic extract was then dried under a stream of nitrogen and left overnight in vacuum in the dark to remove trace remnants of the solvents. The dried extract was resuspended in saline to make samples for DSC and FTIR by addition of water containing either 2 mM calcium chloride or 10% by weight of albumin (1:0.1, BLES/albumin, dry wt/wt). The lipid-protein system was incubated at 40°C (above the phase transition of BLES) and vortexed vigorously for 5 min. In certain cases, 8% by weight cholesterol was added to the organic extract of BLES before drying and resuspension. Transmission electron microscopy using methods discussed elsewhere (19) suggested that these procedures yielded mostly MLVs in the dispersions, which were similar to those seen in the originally supplied BLES dispersion. The samples were diluted appropriately to concentrations of 2 mg/ml in water for DSC and 6 mg/ml for FTIR. For DSC, the dispersions were scanned at 60°C/h in a DSC-2 differential scanning calorimeter (Microcal, MA) over a range of 10–45°C in three successive cycles by methods discussed elsewhere (49). Samples were initially heated to 45°C before cooling to start the first scan. Doubly distilled water was used as a reference. No appreciable differences between the three cycles were noticed for BLES, and only the third scans were used to compare calorimetric properties between samples. For Fourier transform infrared (FTIR) spectrometry a liquid cell containing ∼50 μl (6 mg/ml) of sample was used in an FTIR spectrometer (Bruker Tensor 27, Bruke, Billerica, MA) in the absorbance mode. The intensity-wavenumber spectra were compared to those obtained from pure water and the data displayed after subtracting the water spectrum from those of the samples (55). The samples were scanned at a rate of 1 cm−1/s, and 160 scans were integrated per sample at a temperature of 26°C. The vibrational frequency shift (in wavenumber) in the CH2 and CH3 symmetric and asymmetric stretching modes of the acyl chains and the phosphate and carboxyl stretching modes of the headgroup region were monitored using methods previously developed by others (55–57).
2H-NMR
The samples for 2H-NMR were prepared from organic extracts, using a higher concentration of BLES dissolved in chloroform/methanol. About 250 mg/ml of BLES lipids in chloroform/methanol was mixed with 7 mg of DPPC-d62 (2.5% weight of BLES), and in certain cases 8% by weight cholesterol was added to such mixtures. These organic extracts were dried and then resuspended in water by methods similar to those used for preparation of dispersions for DSC and FTIR. In some cases 10% albumin (1:0.1, BLES/albumin, dry wt/wt) was dissolved in the water used for resuspension of the dispersion. There was no appreciable difference in the calorimetrically detectable broad transition of the DPPC-d62-BLES samples compared with those measured for pure BLES. FTIR could detect the signal from the carbon-deuterium (C-D) bond vibrations of the fatty acyl chains of the DPPC-d62 (∼2100 cm−1), at a separate wavenumber range from the carbon-hydrogen or CH2 (2800 cm−1) bonds. Also, no significant differences from BLES alone were caused by incorporation of DPPC-d62 in the small amount used.
Wideline 2H-NMR observations were made using a locally assembled spectrometer operating in conjunction with a 9.4 T superconducting solenoid. Spectra were obtained using a quadrupole echo pulse sequence (39) with π/2 pulse lengths of 4–5.5 μs, a pulse separation of 35 μs, and a repetition time of 0.9 s. Depending on the sample, 16,000–48,000 transients were averaged for each spectrum (40,41). Transients were oversampled (58) and digitized with effective dwell times of 4 μs for spectra in the liquid-crystalline phase and 2 μs for spectra displaying gel spectral features. Pulse lengths and spectrometer frequency were carefully adjusted before acquisition to minimize signal in the imaginary channel. To facilitate calculation of first moments, spectra were then symmetrized by zeroing residual noise in the imaginary channel before FTIR (40,41). No line broadening was applied.
RESULTS
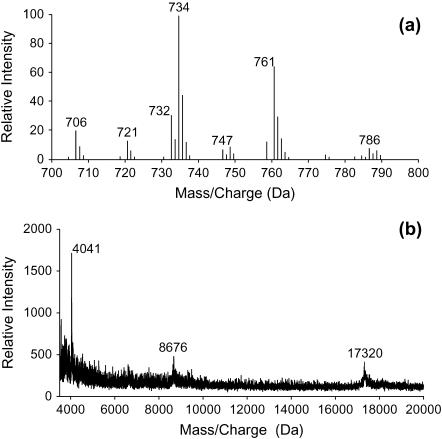
The ESI-MS spectra of a), phospholipids, and b), MALDI-MS spectra of proteins present in BLES are shown in Fig. 1. The ESI-MS spectrum in positive ion mode shows the relative distribution of positively charged, mainly phosphocholine ions detected at varying mass/charge (m/z) ratios, where z is +1. The main disaturated chain PCs found were dipalmitoyl phosphatidylcholine or 16:0/16:0 PC (m/z = 734); palmitoyl-myrisitoyl- or 16:0/14:0-PC (or myristoyl-palmitoyl, 14:0/16:0) PC (m/z = 721). Other mono- and diunsaturated species were mainly palmitoyl-palmitoleoyl- or 16:0/16:1-PC (m/z = 732), palmitoyl-oleoyl- or 16:0/18:1-PC (m/z = 761) or their positional isomers, and dioleoyl or 18:1/18:1-PC (m/z = 786) and minor amounts of other mixed chain phospholipids. The two small peaks on the right side of each major peak indicate phospholipid species which contain one and two carbon-13 atoms (DPPC-734, -735, and -736) as previously seen in ESI-MS used to identify singly or doubly charged species in human surfactant (52,53). The acyl chain distribution in BLES suggests that the mixture is mainly composed of species which would give rise to fluid and gel phase polymorphism in a typical bilayer between 20–30°C. The species in bovine surfactant are also in close approximation to those previously found in human (53), calf, porcine, and some other mammalian LS extracts (32,53,59). The MALDI-TOF spectrum in Fig. 1 b suggests the relative amounts of the hydrophobic proteins present in BLES, with a higher amount of SP-C (4041) and smaller amounts of SP-B monomer (8676) and dimer (17,320) being seen. The molecular weights of SP-C and SP-B are in close approximation to those estimated using amino acid analysis (60) and in isolated proteins from surfactants of other species (54).
FIGURE 1.
ESI-MS (a) and MALDI-TOF (b) spectra of BLES in organic solvent obtained in the positive ion mode. The major phosphatidylcholine species in panel a are detected as a singly charged species and represent the major PC present in the lipid extract, and the 734 peak represents the 1,2, dipalmitoylphosphatidylcholine (16:0/16:0 PC). The other species detected with different acyl chain distribution of PC species at m/z are 706 (14:0/16:0); 721 (16:0-sphigomyelin); 732 (16:0/16:1); 747-18:1-sphinogomyelin; 761- (16:0/18:1); 786- (18:2/18:2) phospholipids. In panel b the MALDI-TOF spectra of BLES in organic solvent, the singly charged monomeric species of bovine SP-C at 4041 Da, and SP-B monomers at 8.6 kDa and dimers at 17.3 kDa are detected.
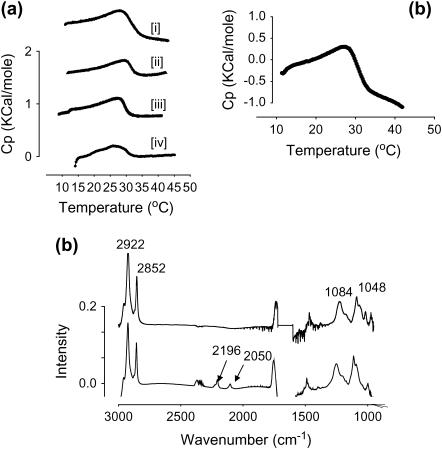
DSC endotherms of vesicular dispersions of i), BLES, ii), BLES with 8% by weight cholesterol, iii), BLES/cholesterol + 2 mM calcium, and iv), BLES + 10 wt % albumin (1:0.1, BLES/albumin, dry wt/wt) are shown in Fig. 2 a. The endotherms show that the BLES dispersions undergo a broad and diffuse phase transition between 10 and 35°C, with a Tmax around 27°C. Addition of cholesterol to the BLES (ii) and calcium ions in the subphase (iii) did not change the endotherms significantly, whereas a slight broadening of the transition was observed for the samples containing albumin (iv). The broadening suggests that albumin slightly affected the chain packing of the phospholipids, consistent with previous DSC studies on interaction of several water-soluble proteins with phospholipid bilayers (24). However, in the previous studies (24), the broadening of the transitions were induced by ∼100- to 1000-fold greater amounts of the soluble proteins (at a ratio of 1:10, lipid/protein, wt/wt or greater) than those used here.
FIGURE 2.
(a) DSC endotherms of i), BLES, ii), BLES with cholesterol, iii), BLES with cholesterol and calcium, and iv), BLES with cholesterol, calcium, and albumin (1:0.1, wt/wt). (b) DSC endotherm for BLES-DPPC-d62. (c) The FTIR spectra of BLES (top) and BLES-DPPC-d62 (bottom). In panel c the C-D2 symmetric and asymmetric stretching modes at 2195 cm−1 and 2090 cm−1 appear as a separate pair of peaks due to the DPPC-d62 present in BLES; however, the relative distribution of the CH2 vibrations (2922 and 2852 cm−1) are not altered in either sample.
The DSC (b) and FTIR (c) profiles of BLES-DPPC-d62 dispersions used for 2H-NMR experiments are also shown in Fig. 2. The FTIR spectra were obtained at 26°C, slightly lower than the Tmax of the transition of BLES around 27°C. The FTIR spectra displayed were produced by subtracting the spectra of pure doubly distilled water from the samples spectra to suppress the effect of the intense broadbands between 3000–2000 cm−1 and 1700–1500 cm−1 from water (55,56). The upper FTIR spectrum in Fig. 2 c is for BLES, and the bottom one is for BLES+DPPC-d62. The main peaks between wave numbers 3000–2000 cm−1 represent the CH2 (2922 cm−1) and CH3 symmetric (Vs) and asymmetric (Vas) vibrations, and those in the range of 1500–1000 cm−1 are for phosphate vibrational modes. The dispersions were highly concentrated (250 mg/ml) to obtain appreciable NMR signals from the small amounts of DPPC-d62, and they were diluted to a concentration of 2–6 mg/ml to obtain the DSC and FTIR scans. The two small additional peaks in the BLES-DPPC-d62 FTIR spectra are from the Vs (2195 cm−1) and Vas (2050 cm−1) modes of the C-D bonds of the DPPC-d62 incorporated in the samples. There was no appreciable difference in either the Tmax of the phase transition in DSC (Fig. 2 b) or the vibrational modes (Vs and Vas) of the acyl chains between samples of BLES and those containing the added DPPC-d62, suggesting that the deuterated phospholipid did not alter the packing states of BLES bilayers significantly. The spectrum of a sample of BLES + albumin (data not shown) did not show any shift of wavenumbers or appearance of new peaks, and it was almost superimposable with those in Fig. 2 c.
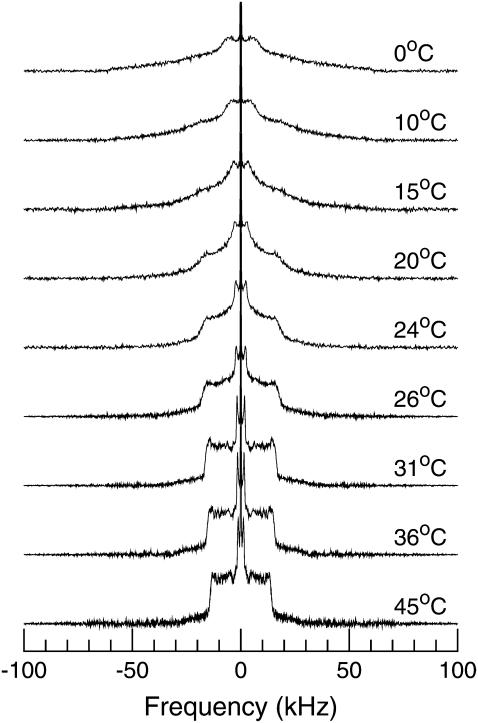
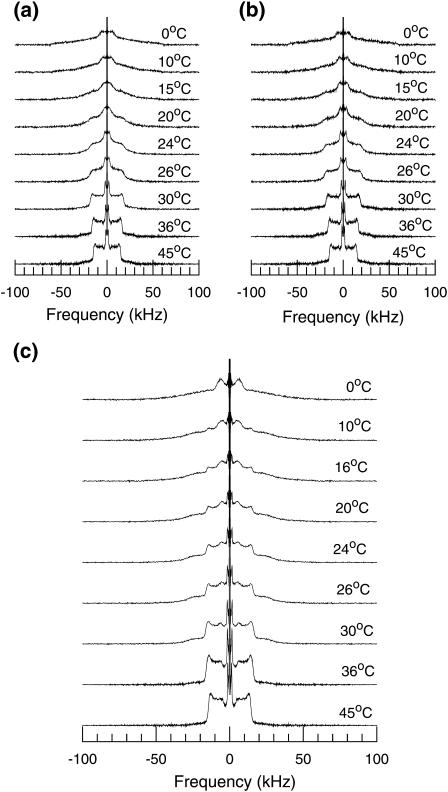
Fig. 3 shows 2H-NMR spectra at selected temperatures for DPPC-d62 incorporated into bilayers of BLES in water. Fig. 4 shows the spectra for (Fig. 4 a) BLES plus cholesterol hydrated in water containing no Ca2+; (Fig. 4 b) BLES plus cholesterol hydrated in water containing 2 mM Ca2+; and (Fig. 4 c) BLES plus cholesterol hydrated in water containing 2 mM Ca2+ and serum albumin at a concentration chosen to give a ratio of BLES/serum albumin of 1:0.1, BLES/albumin (wt/wt). All of the spectra for 45°C in Figs. 3 and 4 are superpositions of Pake doublets characteristic of the fast, axially symmetric reorientation of C-D bonds typically observed in bilayer liquid-crystalline phases. The spectra at 0°C are characteristic of intermediate rate reorientation on the timescale of the experiment and are typical of bilayer gel phases.
FIGURE 3.
2H-NMR spectra at selected temperatures for BLES-DPPC-d62 dispersions. Samples were cooled from 45 to 0°C.
FIGURE 4.
2H-NMR spectra at selected temperatures for BLES-DPPC-d62 dispersions with (a) cholesterol, (b) cholesterol plus calcium, and (c) cholesterol plus calcium plus albumin. Samples were cooled from 45 to 0°C.
For the three samples containing no serum albumin (Figs. 3 and 4, a and b), evolution of the spectral shape from liquid-crystal to gel-like proceeded continuously over a broad temperature range as the sample was cooled. This could indicate that all of the DPPC-d62 incorporated into the sample existed in a uniform environment in which the lipid experienced a continuous slowing of its reorientation and continuous increase in chain order on cooling through the phase change. An alternate possibility is that any coexisting domains of ordered and fluid lipid were small enough so that the DPPC-d62 could sample and average all coexisting bilayer environments during the 70-μs duration of each experiment. These are not mutually exclusive possibilities and it is not surprising, given the complexity of the BLES composition, that the average environment sampled by each lipid during an experiment might change continuously on cooling.
For a given temperature above the transition midpoint, the spectra of samples containing cholesterol and cholesterol plus calcium displayed slightly larger splittings, primarily reflecting the chain-ordering effect of cholesterol, but were otherwise qualitatively similar to those of the BLES sample alone. The prominent doublet with the smallest splitting in each spectrum arises from the terminal methyl group of DPPC-d62. It is interesting that from 20 to 30°C, this splitting was slightly larger in spectra from the sample containing calcium and cholesterol (Fig. 4 b) than the spectra of BLES plus cholesterol without calcium (Fig. 4 a). Even though the BLES transition is very broad, this may reflect a slight calcium-induced increase in the transition Tmax due to the interactions of calcium with anionic lipids in the surfactant extract (40,41,56). If such calcium-induced ordering of ionic phospholipids (such as PG) in BLES occurred, it was not apparent in other spectral features that were less sensitive to gradual changes in the broad transition.
Spectra of the sample containing serum albumin (Fig. 4 c) display qualitatively different behavior on cooling through the temperature range over which the DPPC-d62 lipid probe orders. Between 36 and 16°C, the spectra for this sample were superpositions of spectral components characteristic of both gel and liquid-crystalline bilayer phases. A feature with a splitting characteristic of gel phase DPPC-d62 methyl deuterons became visible at 30°C, and the prominent edge characteristic of acyl chain plateau deuterons in the liquid-crystalline phase remained visible even below 16°C. This implies that over this temperature range, DPPC-d62 was partitioned between ordered and fluid domains that were large enough to preclude averaging by diffusion of the DPPC-d62 molecules across domain boundaries during the 70-μs duration of the echo experiment. Using a diffusion constant typical of lipids in liquid-crystalline phases, the area sampled by a lipid in liquid-crystalline domains during acquisition of a 2H-NMR signal has been estimated to be at least 2.5 × 104 Å2 (38). The persistence of a distinct liquid-crystalline spectral component presumably indicates domains of significantly larger area. Given that the liquid-crystalline spectral component was not significantly broadened in comparison to samples without serum albumin, it seems unlikely that the observation of distinct spectral components reflects a protein-induced constraint on DPPC-d62 diffusion. It is more likely that the presence of serum albumin in the aqueous media contributed to the stabilization of relatively large coexisting ordered and fluid bilayer domains in the BLES/cholesterol/Ca2+ mixture. Coexistence of ordered and fluid domains over a broad temperature range for this sample was confirmed through a similar set of observations of a second independent sample of similar composition.
It is interesting that the fluid spectral component observed in the presence of serum albumin in Fig. 4 b persisted down to 16°C, which is ∼22°C below the pure DPPC-d62 bilayer transition. This suggests that the concentration of DPPC in the fluid phase was likely small compared to that of other, presumably unsaturated lipids. It is also interesting that the splitting displayed by the fluid spectral component did not increase significantly as the temperature was lowered through the broad two-phase coexistence region. Spectra characteristic of coexisting ordered and fluid phases were still seen when the sample was thermally cycled back through the phase transition, suggesting reversibility of the phase separation induced by the albumin-BLES interaction.
For a spectrum f(ω), the first spectral moment is defined as
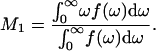 |
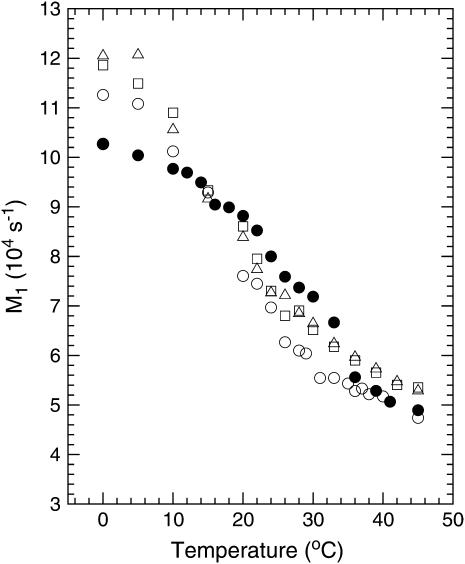
The first moment is a measure of the average, over all deuterated segments, of chain orientational order. Fig. 5 shows the temperature dependence of M1 for spectra of DPPC-d62 from the four samples represented in Figs. 3 and 4. The gradual increase in M1 as the BLES-DPPC-d62 sample was cooled from ∼31 to ∼10°C reflects the very broad phase change expected for a mixture containing saturated and unsaturated phospholipids as well as small concentrations of the hydrophobic surfactant proteins SP-B and SP-C (40). The high-temperature and low-temperature values of M1 are typical of liquid-crystal and gel phase bilayers containing lipid with perdeuterated saturated chains. Addition of cholesterol increased chain order slightly for all temperatures. This is consistent with the cholesterol-induced ordering of simpler bilayers containing DPPC-d62 (37). The presence of Ca2+ in the aqueous phase has little additional effect on the average chain order of the BLES-DPPC-d62-cholesterol sample.
FIGURE 5.
Temperature dependence of 2H-NMR first spectral moments (M1) for (○) BLES, (□) BLES-cholesterol, (▵) BLES-cholesterol-calcium, and (•) BLES-cholesterol-calcium-albumin samples containing DPPC-d62.
The presence of serum albumin in the aqueous phase did alter the temperature dependence of the first spectral moments somewhat as seen in Fig. 5 (solid circles). Above the broad phase change, the values of M1 for BLES-DPPC-d62 plus cholesterol in the presence of serum albumin were closer to those of BLES-DPPC-d62 than to those of BLES-DPPC-d62 plus cholesterol. The influence of serum albumin, along with Ca2+, appears to counteract some of the cholesterol-induced ordering. At low temperatures, the first moments of spectra for the sample containing serum albumin were lower than those for BLES-DPPC-d62 as well as those for BLES-DPPC-d62 plus cholesterol. This is perhaps not surprising given the observation that serum albumin promotes the persistence of a fluid spectral component to lower temperatures. It is striking, however, that despite the presence of a fluid spectral component, the first spectral moments for the sample containing serum albumin (Fig. 5, closed circles) were slightly higher for the intermediate temperatures corresponding to the midrange of the broad transition. This is consistent with the small step in first spectral moment between 36 and 33°C near the onset of the transition. Presumably this indicates the rapid initial growth of ordered domains followed by the slow transfer of remaining fluid material to ordered phase domains over the rest of the broad transition temperature range.
DISCUSSION
LS has an unusual lipid-protein composition compared to most biological membranes (1). It has been suggested that the individual lipids and hydrophobic proteins contribute to its surface activity in specific ways. The disaturated lipids such as DPPC mainly enable tight packing of the lipids in films (1,2). Because DPPC films can be compressed to high surface pressures (70 mN/m), surface tension at an air-water interface containing a DPPC monolayer can approach values close to 0 mN/m (2). However DPPC adsorbs and respreads slowly at an air-water interface. Studies of model surfactant materials and materials based on surfactant lung washings and extracts suggest that the presence of more fluid lipids, such as 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC), and of hydrophobic proteins contribute to the rapid adsorption and respreading of the native LS films (3). The ESI mass spectra of BLES suggest the presence of almost equivalent amounts of saturated as well as unsaturated acyl chain phosphatidylcholines in the surfactant (Fig. 1). Although DPPC has been consistently suggested to be the main surfactant component with a sufficiently high gel-fluid transition temperature of 41oC to allow for optimal surface activity, other disaturated components such as 1-palmitoyl-2-myristoyl phosphatidylcholine (16:0/14:0-PC) or 1-myristoyl-2-palmitoyl phosphatidylcholine (14:0/16:0-PC), and monounsaturated species, most likely 1-palmitoyl-2-palmitoleyl-phosphatidycholine (PPPC) (16:0/16:1-PC) and POPC (16:0/18:1-PC), are also present in significant amounts (52,53). Recently a study has shown that surfactants from some mammalian species such as dunnart and squirrel contain higher amounts of PPPC than DPPC (59). However these LS do not have compromised surface activity since higher amounts of cholesterol are present in surfactant of some of these animals (59). It appears that fluidity is tightly controlled is most mammalian surfactant (3). Recent work by Hall and colleagues has also suggested that under sufficiently fast compression, films that have mixtures of fluid and rigid lipids could sustain high surface pressures with corresponding low surface tensions (35,61).
Many 2H-NMR studies are done with synthetic perdeuterated phospholipids as the major component of mixed lipid model systems (39–41). However introduction of any probe to a more natural system such as BLES may cause perturbations. Comparative studies of BLES with those containing the DPPC-d62 probe were done using DSC and FTIR. The DSC profiles of the BLES-DPPC-d62 (Fig. 2 b), as well as the FTIR spectra (Fig. 2 c), suggested that incorporation of the DPPC-d62 did not alter either the midpoint of the transition or the standard methylene and methyl symmetric vibrational modes of BLES phospholipids. The CH2 symmetric vibrational modes of phospholipids are highly susceptible to levels of trans-gauche isomerization (57). The FTIR spectra of the C-D vibrational modes from the DPPC-d62 incorporated in BLES show a similar distribution of the C-D modes or C-H modes in BLES alone, suggesting that the probe's fatty acyl chains were in similar conformations as those of native phospholipids present in BLES. Our study suggests that natural systems such as membrane extracts and LS can be studied using 2H-NMR, although with certain constraints such as the difficulty of detecting signal from the small amounts of “deuterated probe” molecules.
The heterogeneity of the lipid chain distribution in surfactant material raises the likelihood of phase separation in both monolayer and bilayer environments. Monolayer studies have shown that such phase segregation is observed as the coexistence of gel-like (condensed) and fluid (expanded) domains in porcine (32,33), calf (35), and rat (62) surfactant extracts. These domains were also observed in giant unilamellar bilayer of BLES (18) and MLVs of porcine LS (25) close to the physiological temperature of 37°C. From imaging methods, the upper limit for gel or condensed phase in LS extract monolayers has been estimated to be 25–30%, which is close to the total amounts of DPPC detectable in these surfactants (33–35,62). Previous studies have suggested that although small amounts of hydrophobic proteins SP-B and SP-C significantly enhance surfactant lipid surface activity in model lipid mixtures, their concentration in native surfactant (2–3 wt % of lipids) may not significantly change the gel-fluid status of the material (Fig. 1 b) (3).
Previous studies of GUV of BLES have shown the presence of gel and liquid-crystalline domains between 37–10°C during cooling (18). Using two-photon fluorescence microscopy, the gel domains were found to be coupled between the hemilayers of the BLES bilayers, as seen also in other synthetic gel-fluid lipid mixtures and membrane lipid raft models (63). The DPPC-d62 spectra shown in Fig. 3 are typical of a liquid-crystalline phase at higher temperatures and of a gel-like phase at lower temperatures. The spectra at intermediate temperatures do not show any superposition of spectral features that would indicate coexistence of large liquid-crystalline and gel domains. Although such a continuous change of spectral shape could simply indicate a gradual ordering and slowing of chain motions without phase coexistence, it could also result from the coexistence of domains that are small enough to allow all diffusing lipids to sample equivalent average environments over the microsecond timescale of the 2H-NMR timescale of the experiment.
Cholesterol is an important constituent of most eukaryotic cell membranes, and its effects on biological membrane properties have been extensively studied. However its role in LS is not clear (1,30,64–66). Removal of cholesterol during the preparation of BLES is thought to enhance its surface efficacy as a clinical replacement surfactant (10). Reintroducing cholesterol to BLES provides a way to study the effects of this neutral lipid on phospholipid chain conformation in surfactant (25,30,35,66). Some have suggested that cholesterol is not an integral component of surfactant but is introduced into the surfactant pool from lipoproteins (64). It is further suggested that cholesterol contributes minimally to surfactant activity at the levels present in bovine surfactant (64–66). However a recent article by Serna et al. (25) suggests that the phase transition process in LS, as reflected by formation of macroscopic domains in giant vesicles, is altered significantly by addition and removal of small amounts of cholesterol. In these vesicles of porcine lavage surfactant (containing all components of surfactant including SP-A and SP-D), Lo and liquid-crystalline domains are observed to coexist at 37°C. Removal of cholesterol using cyclodextrin results in a change toward coexistence of gel and liquid-crystalline domains, as also seen in our study with BLES which is devoid of cholesterol.
Numerous studies have shown that for concentrations below 10%, cholesterol generally leads to ordering of phospholipids in the fluid or liquid-crystalline phase while enhancing fluidity in the gel phase. 2H-NMR studies suggest that in disaturated lipids like DPPC, cholesterol orders the chains in the gel as well as liquid-crystalline phase (37,67). Like the DPPC-d62 spectra obtained from BLES alone, the DPPC-d62 spectra from BLES with cholesterol are not superpositions of distinct liquid-crystal and gel-like components. As such, they do not provide clear evidence for coexisting gel and liquid-crystal-like domains. The DSC profiles of BLES with cholesterol (Fig. 2 a), however, do show a slight increase (2–3°C) in the Tmax in comparison to those of BLES alone. Fig. 5 shows that addition of cholesterol to BLES results in consistently higher first spectral moments, and thus average order parameters, throughout the temperature range studied.
Addition of calcium ions does not seem to change either the spectral shape or the average orientational order of the probe DPPC-d62 chains. The slight increase in methyl group quadrupole splitting near the transition midpoint may reflect a small calcium-induced condensation of the bilayer. Some previous studies have shown calcium ions to have additional ordering effects on various surfactant lipid extracts in monolayers as well as bilayers (33,36,56). BLES contains negatively charged phospholipids such as PG, and most previous studies have suggested that divalent cations interact strongly with such charged headgroups (65,68–70). This is consistent with earlier 2H-NMR studies that showed a calcium-induced increase in chain order in DPPC/DPPG mixtures (40–42). It has also been observed that the effect of calcium on the ordering of C-D methylene bonds is more pronounced at the C2–C7 positions than in the bilayer core CH3 group (67,68). The effect was seen as a lateral “area compression” of the bilayers (68,70). Other studies showed that such calcium-induced lateral area compressions occur in monolayers of DPPC:PG (69–71) and also in LS films as seen by a 10% increase in the total amount of condensed (gel-like) phase with calcium in the hypophase (33).
Addition of serum albumin to the BLES bilayers resulted in a slight broadening of the DSC profiles, although no change of transition midpoint is noticeable (Fig. 2 a). This is consistent with the effect of water-soluble proteins on simple lipid bilayers as monitored using DSC (24). The most striking effect of adding serum albumin was to give rise to distinct gel and liquid-crystalline DPPC-d62 spectral components over a temperature range of 20–30°C. The most likely explanation for this observation is that the presence of the serum albumin promoted the growth of coexisting domains to a size such that diffusion across domain boundaries was insufficient to result in all DPPC-d62 experiencing the same average environment on the ∼10−5 s timescale of the 2H-NMR experiment. It is interesting to note here that, at least in this BLES-serum albumin mixture where there is a ratio of 10:1, lipid/protein (wt/wt), albumin seems to affect the phospholipid environment to promote persistence of domains which are large enough to not allow full diffusion of the DPPC probe across domain boundaries at NMR timescales.
Previous studies of ARDS and LS dysfunction models have suggested that serum albumin can inhibit the surface activity of various surfactant extracts as well as model lipid-surfactant protein systems (reviewed in Greise (6), Holm (8), and McLean and Lewis (13)). However in some of these studies it was also noted that inhibition of surface activity as monitored by decrease in adsorption to form films and inability of such films to reach low surface tension required large amounts of the inhibitory proteins (1:10 and 1:20, LS-lipid/protein, wt/wt) (6). In some studies, by increasing the concentration of surfactant to inhibitory protein from 1:10 to 1:1, this inhibition could be abolished (8,12,72,73). However in cell-free LS or large aggregate LS from lung washings, lipid/soluble protein ratios are generally estimated to be ∼3:1 (wt/wt) (6–9) and in the case of ARDS or injured lungs, these ratios hardly exceed ∼1:1 (7,9,62). It is not clear why LS from such diseased lung washings show extremely poor surface activity, reminiscent of the activity shown in the laboratory with nonphysiological or nonpathological amounts of soluble proteins. A recent study using minor amounts of albumin in preformed model LS films has suggested that there are dramatic changes in the monolayer collapse behavior and monolayer viscosity (26). In that study, it was suggested that albumin possibly altered the phase transition profiles of the model surfactant lipids and thus decreased the rate of respreading of the material when such films were cycled at an air-water interface (26,74). Our study of surfactant bilayers could support this interpretation. Other studies have suggested that the effect of albumin can be reversed by either using high amounts of SP-A (17) or cationic polymers (36). Comparing Langmuir films of large aggregate LS from hyperventilation-injured lungs with those from normal lungs, large proteinaceous domains were detected in the films from injured lungs using atomic force microscopy (32,62). Also, a significant decrease of condensed or gel-like domains (30%–3%) was noted in the LS from injured lungs compared to the normal (32). Caution is required when comparing LS bilayer studies with those from monolayers. However in light of some correlated studies which have shown similarity in structural organization of LS lipids in gel and fluid domains in monolayers as well as bilayers (25), it is reasonable to assume that albumin which perturbs monolayer packing (36) would also affect the LS in bilayers. Another study of BLES, performed in a capillary surfactometer, shows a sharp change of airway opening profile in the gel to fluid phase transition (20–30°C) range of BLES (75). The temperature dependence of airway opening is similar to the temperature dependence of 2H-NMR first spectral moments for BLES containing DPPC-d62 (Fig. 5), as well as the DSC heat-flow profiles of BLES as detailed elsewhere (34). It is interesting to note that in the upper airway model study (75), albumin altered the airway opening profile quite dramatically, making the sharp changes of airway opening more diffuse than those obtained from BLES alone (75). In that study, the change of LS by albumin was suggested to have some implication for the understanding of exercise-induced asthma (75). These studies and ours suggest that albumin perturbs the packing of lipids and thereby the molecular lipid motions related to the transition of LS (26,32,36,62,75). Some previous studies have suggested that this sort of disruption of lipid rearrangement can eventually lead to gradual loss of functionality in LS (14,26,34,36) as well as in other membranous systems (29,76).
This study suggests that a gel to liquid-crystalline phase change occurs in BLES over a broad temperature range and that it can be monitored by complementary techniques such as DSC and 2H-NMR. Also, incorporation of a small amount of the PDL (DPPC-d62) as a probe in such systems does not significantly alter the transition characteristics. Calcium and cholesterol increase the order parameter of lipid chains as reported by chains of the DPPC-d62 probe molecules. Introduction of albumin in such systems seems to lead to possible induction of specific lipid-soluble protein domain formation which persists over a limited temperature range. Such an albumin-induced change in the distribution and motion of lipids in LS could contribute to the negative effect of albumin on biophysical activities of the material in pathophysiological conditions.
Acknowledgments
We thank R. Devraj, S. Vidyashankar, M. Fritzen-Garcia, and J. Stewart (Biochemistry, MUN) for technical assistance with DSC and FTIR, and L. Lee (Biology, MUN) with the transmission electron microscopy.
The study was supported by a New Investigator award from the Canadian Institute of Health Research (CIHR) to K.N., and operating grants from Health Canada and CIHR to K.M.W.K. and from the Natural Sciences and Engineering Research Council of Canada (NSERC) to M.R.M.
References
- 1.Goerke, J. 1998. Pulmonary surfactant: function and molecular composition. Biochim. Biophys. Acta. 1408:79–89. [DOI] [PubMed] [Google Scholar]
- 2.Schürch, S., S. J. Goerke, and J. A. Clements. 1976. Direct determination of surface tension in the lungs. Proc. Natl. Acad. Sci. USA. 73:4698–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batenburg, J. J., and H. P. Haagsman. 1998. The lipids of pulmonary surfactant: dynamics and interactions with proteins. Prog. Lipid. Res. 37:235–276. [DOI] [PubMed] [Google Scholar]
- 4.Enhorning, G. 1996. Pulmonary surfactant function in alveoli and conducting air-ways. Can. Respir. J. 3:21–27. [Google Scholar]
- 5.Gaver III, D. P., O. E. Jensen, and D. Halpern. 2005. Surfactant and air-way liquid flows. In Lung Surfactant Function and Disorder. K. Nag, editor. CRC Press, Boca Raton, FL. 191–227.
- 6.Greise, M. 1999. Pulmonary surfactant in health and human lung disease: state of the art. Eur. Respir. J. 13:1455–1456. [DOI] [PubMed] [Google Scholar]
- 7.Gregory, T. J., W. J. Longmore, M. A. Moxley, J. A. Whitsett, C. R. Reed, A. A. Fowler III, L. D. Hudson, R. J. Maunder, C. Crim, and T. M. Hyers. 1991. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J. Clin. Invest. 88:1976–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holm, B. 1992. Surfactant inactivation in adult respiratory distress syndrome. In Pulmonary Surfactant: From Molecular Biology to Clinical Practice. B. Robertson, L. M. J. Van Golde, and J. J. Batenburg, editors. Elsevier Science, Amsterdam, The Netherlands. 665–684.
- 9.Gunther, A., C. Seibert, R. Schmidt, S. Zeigler, F. Grimminger, M. Yabut, B. Temmesfeld, D. Walmrath, H. Mort, and W. Seeger. 1996. Surfactant alteration in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am. J. Respir. Crit. Care Med. 153:176–184. [DOI] [PubMed] [Google Scholar]
- 10.Yu, S., P. G. Harding, and F. Possmayer. 1983. Bovine pulmonary surfactant: chemical composition and physical properties. Lipids. 18:522–529. [DOI] [PubMed] [Google Scholar]
- 11.Kahn, M. C., G. J. Anderson, W. R. Anyan, and S. B. Hall. 1995. Phosphatidylcholine molecular-species of calf lung surfactant. Am. J. Physiol. 18:L567–L573. [DOI] [PubMed] [Google Scholar]
- 12.Holm, B., G. Enhorning, and R. H. Notter. 1988. A biophysical mechanism by which plasma proteins inhibit lung surfactant activity. Chem. Phys. Lipids. 49:49–55. [DOI] [PubMed] [Google Scholar]
- 13.McLean, M., and J. E. Lewis. 1995. Biomimectic pulmonary surfactants. Life Sci. 56:363–378. [DOI] [PubMed] [Google Scholar]
- 14.Hawgood, S., and J. A. Clements. 1990. Pulmonary surfactant and its apoproteins. J. Clin. Invest. 86:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panda, A. K., K. Nag, R. R. Harbottle, F. Possmayer, and N. O. Petersen. 2004. Thermodynamic studies of mixed molecular Langmuir films. Part. 2. Mutual mixing of DPPC and bovine lung surfactant extract with long-chain fatty acids. Colloids Surf. A. Physiochem. Eng. Aspects. 247:9–17. [Google Scholar]
- 16.Harbottle, R. R., K. Nag, N. Stewart-McIntyre, F. Possmayer, and N. O. Petersen. 2003. Molecular organization revealed by time-of-flight secondary ion mass spectrometry of a clinically used extracted pulmonary surfactant. Langmuir. 19:3698–3704. [Google Scholar]
- 17.Cockshutt, A. M., J. Weitz, and F. Possmayer. 1990. Pulmonary surfactant-associated protein A enhances the surface activity of lipid extract surfactant and reverses inhibition by blood proteins in vitro. Biochemistry. 29:8424–8429. [DOI] [PubMed] [Google Scholar]
- 18.Nag, K., J. S. Pao, R. R. Harbottle, F. Possmayer, N. O. Petersen, and L. A. Bagtolli. 2002. Segregation of saturated chain lipids in pulmonary surfactant films and bilayers. Biophys. J. 82:2041–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nag, K., K. Rodriguez-Capote, A. K. Panda, L. Frederick, S. A. Hearn, N. O. Petersen, S. Schürch, and F. Possmayer. 2004. Disparate effects of two phosphatidylcholine binding proteins, C-reactive protein and surfactant protein A, on pulmonary surfactant structure and function. Am. J. Physiol. 287:L1145–L1153. [DOI] [PubMed] [Google Scholar]
- 20.Peters, T. Jr. 1985. Serum albumin. Adv. Protein. Chem. 37:161–245. [DOI] [PubMed] [Google Scholar]
- 21.Figge, J., T. H. Rossing, and V. Fencl. 1991. The role of serum-proteins in acid base equilibria. J. Lab. Clin. Med. 117:453–467. [PubMed] [Google Scholar]
- 22.Estronca, L. M. B. B., M. J. Moreno, J. A. N. Laranjinha, and W. L. C. Vaz. 2005. Kinetics and thermodynamics of lipid amphiphile exchange between lipoproteins and albumin in serum. Biophys. J. 88:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bleustein, B. C., M. Sennett, R. T. V. Kung, D. Felsen, D. P. Poppas, and R. B. Stewart. 2000. Differential scanning calorimetry of albumin shoulders: interspecies differences and fatty acid binding effects on protein denaturation. Lasers Surg. Med. 27:465–470. [DOI] [PubMed] [Google Scholar]
- 24.Bach, D. 1983. Calorimetric studies of model and natural biomembranes. In Biomembrane Structure & Function. D. Chapman, editor. MacMillan Press, London, UK. 1–41.
- 25.Serna, J. B., J. Perez-Gil, A. Smith, A. C. Simonsen, and L. A. Bagtolli. 2004. Cholesterol rules: direct observation of the co-existence of two fluid phases in native pulmonary surfactant membranes at physiological temperatures. J. Biol. Chem. 279:40715–40722. [DOI] [PubMed] [Google Scholar]
- 26.Warriner, H. E., J. Ding, A. J. Waring, and J. A. Zasadzinski. 2002. A concentration-dependent mechanism by which serum albumin inactivates replacement lung surfactant. Biophys. J. 82:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otsubo, E., and T. Takei. 2002. Characterization of the surface activity of a synthetic surfactant with albumin. Biol. Pharm. Bull. 25:1519–1523. [DOI] [PubMed] [Google Scholar]
- 28.Keough, K. M. W., E. Farrel, M. Cox, G. Harrel, and H. W. Taeusch. 1985. Physical, chemical and physiological characteristics of isolates of pulmonary surfactant from adult rabbits. Can. J. Physiol. Pharmacol. 63:1043–1051. [DOI] [PubMed] [Google Scholar]
- 29.Ebel, H., P. Grabitz, and T. Heimburg. 2001. Enthalpy and volume changes in lipid membranes. I. The proportionality of heat and volume changes in lipid melting transition and its implication for the elastic constants. J. Phys. Chem. 105:7353–7360. [Google Scholar]
- 30.Larsson, M., K. Larsson, T. Nylander, and P. Wollmer. 2003. The bilayer melting transition in lung surfactant bilayers: the role of cholesterol. Eur. Biophys. J. 31:633–636. [DOI] [PubMed] [Google Scholar]
- 31.Trauble, H., H. Eibl, and H. Sawada. 1974. Respiration—a critical phenomenon? Lipid phase transitions in lung alveolar surfactant. Naturwissenschaften. 61:344–354. [DOI] [PubMed] [Google Scholar]
- 32.Nag, K., R. R. Harbottle, A. K. Panda, and N. O. Petersen. 2004. Atomic force microscopy of interfacial monomolecular films of pulmonary surfactant. In Methods in Molecular Biology: Atomic Force Microscopy. P. C. Braga and D. Ricci, editors. Humana Press, Totowa, NJ. 242:231–243. [DOI] [PubMed]
- 33.Nag, K., J. Perez-Gil, M. L. F. Ruano, L. A. D. Worthman, J. Stewart, C. Casals, and K. M. W. Keough. 1998. Phase transitions in films of lung surfactant at the air-water interface. Biophys. J. 74:2983–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nag, K., S. Vidyashankar, A. K. Panda, and R. R. Harbottle. 2005. Chain dancing, super-cool surfactant and heavy breathing: membranes, rafts and phase transitions. In Lung Surfactant Function and Disorder. K. Nag, editor. CRC Press, Boca Raton, FL. 145–172.
- 35.Piknova, B., V. Schramm, and S. B. Hall. 2002. Pulmonary surfactant: phase behavior and function. Curr. Opin. Struct. Biol. 12:487–494. [DOI] [PubMed] [Google Scholar]
- 36.Taeusch, H. W., J. B. Serna, J. Perez-Gil, C. Alonso, and J. A. Zasadzinski. 2005. Inactivation of pulmonary surfactant due to serum-inhibited adsorption and reversal by hydrophilic polymers: experimental. Biophys. J. 89:1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vist, M. R., and J. H. Davis. 1990. Phase equilibria of cholesterol/dipalmitoyl-phosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 29:451–464. [DOI] [PubMed] [Google Scholar]
- 38.Davis, J. H. 1983. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim. Biophys. Acta. 737:117–171. [DOI] [PubMed] [Google Scholar]
- 39.Davis, J. H., K. R. Jeffrey, M. Bloom, M. I. Valic, and T. P. Higgs. 1976. Quadrupole echo deuteron magnetic resonance spectroscopy in ordered hydrocarbon chains. Chem. Phys. Lett. 42:390–394. [Google Scholar]
- 40.Dico, A. S., J. Hancock, M. R. Morrow, J. Stewart, S. Harris, and K. M. W. Keough. 1997. Pulmonary surfactant protein SP-B interacts similarly with dipalmitoylphosphatidyglycerol and dipalmitoylphosphatidylcholine in phosphatidylcholine/phosphatidylglycerol mixture. Biochemistry. 36:4172–4177. [DOI] [PubMed] [Google Scholar]
- 41.Morrow, M. R., N. Abu-Libdeh, J. Stewart, and K. M. W. Keough. 2003. Interactions of pulmonary surfactant protein SP-A with DPPC/egg-PG bilayers. Biophys. J. 85:2397–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franzin, C. M., and P. M. Macdonald. 2001. Polylysine-induced 2HNMR-observable domains in phosphatidylserine/phosphatidylcholine lipid bilayers. Biophys. J. 81:3346–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weislander, A., J. Ulmuis, G. Lindblom, and K. Fontell. 1978. Water binding and phase structures for different Acholeplasma laidlawii membrane lipids studied by deuteron nuclear magnetic resonance and x-ray diffraction. Biochim. Biophys. Acta. 512:241–253. [DOI] [PubMed] [Google Scholar]
- 44.Spohn, K. H., and R. Kimmich. 1983. Characterization of the mobility of various chemical groups in the purple membrane of Halobacterium halobium by 13C, 31P and 2H solid state NMR. Biochem. Biohys. Res. Commun. 114:713–720. [DOI] [PubMed] [Google Scholar]
- 45.Bechinger, B., and M. Welk. 2003. Deuterium solid-state NMR investigation of exchange labeled orientated plasma membranes at different hydration levels. Biophys. J. 85:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roux, M., R. Auzley-Velty, F. Dedaini-Pillard, and B. Perly. 2002. Cyclodextrin-induced lipid lateral separation in DMPC membranes: 2H nuclear magnetic resonance study. Biophys. J. 82:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roux, M., V. Beswick, Y.-M. Coic, T. Huynh Dinh, A. Senson, and J.-M. Neuman. 2000. PMP 118–36, a yeast plasma membrane protein fragment, binds phsophatidylserine from bilayer mixtures with phosphatidylcholine: a H2NMR study. Biophys. J. 79:2624–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nag, K., K. M. W. Keough, and M. R. Morrow. 2004. Biophysical studies of lung surfactant. Physics in Canada. 60:141–149. [Google Scholar]
- 49.Keough, K. M. W., and N. Kariel. 1987. Differential scanning calorimetric studies of aqueous dispersions of phosphatidylcholines containing two polyenoic chains. Biochim. Biophys. Acta. 902:11–18. [DOI] [PubMed] [Google Scholar]
- 50.Bligh, E., and W. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917. [DOI] [PubMed] [Google Scholar]
- 51.Bartlett, G. R. 1959. Phosphorous assay in column chromatography. J. Biol. Chem. 234:466–468. [PubMed] [Google Scholar]
- 52.Postle, T. 2000. The analysis of lung surfactant phospholipid by electrospray ionisation mass spectrometry—applications to disease states. Appl. Cardiopulm. Pathophysiol. 9:286–289. [Google Scholar]
- 53.Postle, A. D., A. Maunder, K. B. M. Reid, J. Y. Wang, S. M. Wright, M. Moustaki, and J. O. Warner. 1999. Deficient hydrophilic lung surfactant proteins A and D with normal surfactant phospholipid molecular species in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 20:90–98. [DOI] [PubMed] [Google Scholar]
- 54.Curstedt, T., J. Johansson, P. Persson, A. Eklund, B. Robertson, and H. Jörnvall. 1990. Hydrophobic surfactant-associated polypeptides. SP-C is a lipopeptide with two palmitoylated cysteine residue, whereas SP-B lacks covalently linked fatty acyl groups. Proc. Natl. Acad. Sci. USA. 87:2985–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mautone, A. J., K. E. Reilly, and R. Mendelsohn. 1980. Fourier transform infrared and differential scanning calorimetric studies of a surface active material from rabbit lung. Biochim. Biophys. Acta. 896:1–10. [DOI] [PubMed] [Google Scholar]
- 56.Ge, Z., C. W. Brown, J. G. Turcotte, Z. Wang, and R. H. Notter. 1995. FTIR studies of calcium-dependent molecular order in lung surfactant and surfactant extract dispersions. J. Colloid. Interface. Sci. 173:471–477. [Google Scholar]
- 57.Dluhy, R. A., K. E. Reilly, R. D. Hunt, M. L. Mitchell, A. J. Mautone, and R. Mendelsohn. 1989. Infrared spectroscopic investigation of pulmonary surfactant. Surface film transition at the air-water interface and bulk phase thermotropism. Biophys. J. 56:1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prosser, R. S., J. H. Davis, F. W. Dahlquist, and M. A. Lindorfer. 1991. 2H nuclear magnetic resonance of the gramicidin A backbone in a phospholipids bilayer. Biochemistry. 30:4687–4696. [DOI] [PubMed] [Google Scholar]
- 59.Lang, C. J., A. D. Postle, S. Orgeig, F. Possmayer, W. Bernhard, A. K. Panda, K. D. Jurgens, W. K. Milsom, K. Nag, and C. B. Daniels. 2005. Dipalmitoylphosphatidylcholine is not the major surfactant phospholipid species in all mammals. Am. J. Physiol. 289:1426–1439. [DOI] [PubMed] [Google Scholar]
- 60.Johansson, J., T. Curstedt, and B. Robertson. 1994. The proteins of the surfactant system. Eur. Respir. J. 7:372–391. [DOI] [PubMed] [Google Scholar]
- 61.Smith, E. C., J. M. Crane, T. G. Laderas, and S. B. Hall. 2003. Metastability of a super compressed fluid monolayer. Biophys. J. 85:3048–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Panda, A. K., K. Nag, R. R. Harbottle, K. Raodriguez-Capote, R. A. W. Veldhuizen, N. O. Petersen, and F. Possmayer. 2004. Effect of acute lung injury on structure and function of pulmonary surfactant films. Am. J. Respir. Cell Mol. Biol. 30:641–650. [DOI] [PubMed] [Google Scholar]
- 63.Keller, S. L. 2003. A closer look at the canonical ‘raft mixture’ in model membrane studies. Biophys. J. 84:725–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haas, M. A., and W. L. Longmore. 1980. Regulation of lung surfactant cholesterol metabolism by serum lipoproteins. Lipids. 15:401–406. [DOI] [PubMed] [Google Scholar]
- 65.Veldhuizen, R. A. W., K. Nag, S. Orgeig, and F. Possmayer. 1998. The role of lipids in pulmonary surfactant. Biochim. Biophys. Acta. 1408:90–108. [DOI] [PubMed] [Google Scholar]
- 66.Gunasekara, L., S. Schürch, W. M. Schoel, K. Nag, Z. Leonenko, M. Haufs, and M. Amrein. 2005. Pulmonary surfactant function is abolished by an elevated proportion of cholesterol. Biochim. Biophys. Acta. 1737:27–35. [DOI] [PubMed] [Google Scholar]
- 67.Keough, K. M. W., M. R. Morrow, and R. Bittman. 1996. NMR studies of phospholipids-cholesterol bilayers: phase diagrams and molecular dynamics. In The Encyclopedia of Nuclear Magnetic Resonance. D. M. Grant and R. K. Harris, editors. S. I. Chan, section editor. John Wiley & Sons, Hoboken, NJ. 3601–3607.
- 68.Huster, D., G. Paasche, U. Deitrich, O. Zschornig, T. Gutberiet, K. Gawrisch, and K. Arnold. 1999. Investigation of phospholipid area compression induced by calcium-mediated dextran sulphate interactions. Biophys. J. 77:879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dluhy, R. A., D. G. Cameron, H. H. Mantsch, and R. Mendelsohn. 1983. Fourier transform infrared spectroscopic studies of the effect of calcium ions on phsophatidylserine. Biochemistry. 22:6318–6325. [Google Scholar]
- 70.Kaznessis, Y. N., S. Kim, and R. G. Larson. 2002. Simulations of zwitterionic and anionic phospholipid monolayers. Biophys. J. 82:1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nag, K., N. H. Rich, and K. M. W. Keough. 1994. Interactions between dipalmitoylphosphatidylglycerol and phosphatidylcholine and calcium. Thin Solid Films. 244:841–844. [Google Scholar]
- 72.Holm, B. A., R. J. Notter, and J. N. Finkelstein. 1985. Surface property changes from interaction of albumin with natural lung surfactant extracts. Chem. Phys. Lipids. 38:287–298. [DOI] [PubMed] [Google Scholar]
- 73.Veldhuizen, R. A. W., B. Welk, R. Harbottle, S. Hearn, K. Nag, N. O. Petersen, and F. Possmayer. 2002. Mechanical ventilation of isolated rat lungs changes the structure and biophysical properties of surfactant. J. Appl. Physiol. 92:1169–1175. [DOI] [PubMed] [Google Scholar]
- 74.Zasadzinski, J. A., J. Ding, H. E. Warriner, F. Bringezu, and A. J. Waring. 2001. The physics and physiology of lung surfactants. Curr. Opin. Colloid Interface Sci. 6:506–513. [Google Scholar]
- 75.Enhorning, G., J. Hohlfeld, N. Krug, G. Lema, and R. C. Welliver. 2000. Surfactant function affected by airway inflammation and cooling: possible impact on exercise induced asthma. Eur. Respir. J. 15:532–538. [DOI] [PubMed] [Google Scholar]
- 76.Vereb, G., J. Szöllősi, J. Matkó, P. Nagy, T. Farkas, L. Vigh, L. Mátyus, T. A. Waldmann, and S. Damjanovich. 2003. Dynamic, yet structured: the cell membrane three decades after the Singer-Nicholson model. Proc. Natl. Acad. Sci. USA. 100:8053–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]