Abstract
Leukotriene (LT) B4 is a powerful chemotactic and immune modulating agent that signals via two receptors denoted BLT1 and BLT2. Here we report that BLT1 and BLT2 are expressed at low levels in an apparently silent state in human umbilical vein endothelial cells (HUVEC). However, treatment with LPS leads to a >10 fold increase in the levels of BLT1 mRNA without any significant effects on BLT2 mRNA. In parallel, LPS also increases the amounts of BLT1 protein. Tumor necrosis factor-α (TNF-α) increases the expression of BLT2 mRNA ≈6 times above basal levels with only a modest increase in BLT1 mRNA. Interleukin-1β causes variable and parallel increases of both BLT1 and BLT2 mRNA. The natural ligand LTB4 also increases BLT1, but not BLT2, mRNA and protein expression. Along with the induction of BLT1 and/or BLT2, HUVEC acquire the capacity to respond to LTB4 with increased levels of intracellular calcium and these signals can be blocked by isotype selective BLT antagonists, CP-105696 and LY-255283. In addition, treatment of HUVEC with LTB4 causes increased release of both nitrite, presumably reflecting nitric oxide (NO), and monocyte chemoattractant protein-1. Our data indicate that expression of functional BLT receptors may occur at the surface of endothelial cells in response to LPS, cytokines, and ligand, which in turn may have functional consequences during the early vascular responses to inflammation. Moreover, the results point to BLT receptors as potential targets for pharmacological intervention in LT-dependent inflammatory diseases such as asthma, rheumatoid arthritis, and arteriosclerosis.
Keywords: inflammation, arteriosclerosis, rheumatoid arthritis, asthma, nitric oxide
The leukotrienes (LTs) are a family of lipid mediators that play important roles in a variety of allergic and inflammatory reactions (1, 2). These molecules are divided into two classes, the spasmogenic cysteinyl-LTs (cys-LT) and LTB4, which is a classical nM chemotactic agent produced by neutrophils, macrophages, and mast cells. Thus, LTB4 is a potent chemoattractant for polymorphonuclear leukocytes, comparable to complement peptide C5a and fMet-Leu-Phe (3, 4). Recent data also indicate that LTB4 is a strong chemoattractant for T cells, creating a functional link between early innate and late adaptive immune responses (5–7). Because of these biological effects, LTB4 is regarded as an important chemical mediator in a variety of acute and chronic inflammatory diseases and only recently, genetic and biochemical evidence strongly implicate LTB4 as a mediator of vascular inflammation and arteriosclerosis (8–10). LTB4, is synthesized from arachidonic acid via the concerted action of 5-lipoxygenase, assisted by 5-lipoxygenase-activating protein and the terminal LTA4 hydrolase (11), usually in a single cell or via transcellular routes; this mechanism has been shown to occur in vivo (12, 13).
LTB4 signals primarily via a specific, high-affinity, G protein-coupled seven-transmembrane receptor, termed BLT1 (14). The BLT1 gene is located on the human chromosome 14q11.2-q12 and SP1 binding to CpG regions appears to be important for basal transcription (15). In the promoter region of the BLT1 gene, an ORF encoding a highly homologous GPCR was found, which was subsequently identified as the second, low-affinity, receptor for LTB4 and termed BLT2 (16). BLT1 and BLT2 signal through three classes of G proteins, namely Gi, Gq-like, and Gz, and display different ligand affinity and specificity for LTB4 and structurally related molecules (16, 17). Northern blot analyses revealed BLT1 mRNA expression almost exclusively in leukocytes (14), activated macrophages (18), and eosinophils (19), whereas BLT2 has a different expression profile with mRNA present in spleen, liver, ovary, and leukocytes. In addition, low levels of BLT2 mRNA are also present in almost all human tissues (16).
Endothelial cells are strategically located at the interface between the blood and parenchymal cells and take active part in many physiological and pathological processes, including inflammation. LTB4 is very active in the microcirculation and promotes adhesion of leukocytes to the endothelium, followed by diapedesis and migration into surrounding tissues. Although it has been reported that LTB4 can induce CD54 (or ICAM-1) expression in endothelial cells (20, 21), the prevailing notion is that adhesive and migratory effects of LTB4 primarily originate from its action on the leukocyte, where chemoattractants induce up-regulation of cell-adhesion molecules that can interact with their cognate receptors on endothelial cells (22, 23).
Here, we report that LPS, TNF-α, IL-1β, and LTB4 itself, differentially increase the expression of BLT1 and/or BLT2 on HUVEC, which in turn allows LTB4 mediated Ca2+ signaling. We also show that LTB4 can increase the release of monocyte chemoattractant protein-1 (MCP-1) and generation of nitrite (presumably from NO) from these cells. Taken together, our results indicate that endothelial cells are directly involved in the vascular response to LTB4-dependent inflammatory processes and calls for a reappraisal of the signal transduction mechanisms of the hyperadhesiveness for neutrophils induced by this lipid mediator.
Results
Human umbilical vein endothelial cells (HUVEC), cultured as described in Methods, express both BLT1 and BLT2 mRNA as well as BLT1 receptor protein (BLT2 receptor protein not analyzed), although their signaling capacity appear to be low or absent (see below).
Effects of LPS on the Expression of BLT Receptors.
Treatment of HUVEC with LPS (100 ng/ml) led to a rapid (within 60 min) increase in the levels of BLT1 mRNA to amounts that were ≈12 times (11.8 ± 1.3, n = 4) above baseline (Fig. 1). This increase persisted at the same level after 120 min of incubation (11.6 ± 1.8, n = 4). In contrast, LPS did not have any significant impact on the BLT2 mRNA levels, which were 1.4 ± 0.3 and 1.8 ± 0.3 times basal values after 60 and 120 min, respectively. Western blot analysis detected an immunoreactive band migrating as a protein with a molecular mass of ≈38 kDa, which is in good agreement with the calculated molecular mass of 37.6 kDa for BLT1 (Fig. 1). The specificity of the immunoreactivity was verified by using antisera preadsorbed with BLT1 blocking peptide, which failed to detect the BLT1 protein (data not shown). The amounts of immunoreactive protein corresponded well to the increase in mRNA. Thus, it increased significantly after 1 h of LPS treatment and remained elevated during at least 6 h (Fig. 1). In a series of separate Western blots, the amounts of immunoreactive BLT1 protein increased 2.8 ± 0.6 times (n = 5) relative to the untreated control.
Fig. 1.
Increase in BLT1 mRNA and protein in HUVEC stimulated with LPS. HUVEC were incubated with LPS (100 ng/ml) and harvested at different time points to prepare total RNA and protein extracts. mRNA and protein levels were analyzed by semiquantitative RT-PCR and Western blot, as described in Methods. (Upper) The rapid (within 60 min), almost 12-fold, increase in the levels of BLT1 mRNA (filled circles) induced by treatment of HUVEC with LPS. In contrast, only a weak effect on the levels of BLT2 mRNA is observed (open circles). (Lower) The increased levels in BLT1 mRNA corresponded to elevated amounts of immunoreactive BLT1 protein.
Effects of TNF-α and IL-1β on the Expression of BLT Receptors.
Treatment of HUVEC with 100 ng/ml TNF-α led to a rapid increase in the amounts of BLT2 mRNA, which were 4.9 ± 1.3 times the control after 30 min and increased further to 6.2 ± 0.8 times after 1 h. Thereafter, the effect leveled off, and after 120 min, BLT2 mRNA was ≈6 times (6.4 ± 1.3) above the levels in untreated cells (Fig. 2). Treatment with TNF-α also led to a weak increase in BLT1 mRNA, which peaked after 60 min (2.1 ± 0.7 times). This slight increase in mRNA did not lead to a significant increase in the amounts of immunoreactive BLT1 protein as assessed by Western blot (1.0 ± 0.2 times, n = 5). On the other hand, treatment of HUVEC with 5 units/ml IL-1β increased the levels of both BLT1 and BLT2 receptor mRNA in a variable fashion, both with respect to degree of increase and time course. Thus, over a large series of experiments (n = 10), treatment with IL-1β increased mRNA levels of BLT1 and BLT2 ≈2.5–7 and 4–12 times, respectively (data not shown). The mRNA levels peaked after ≈10–60 min, but very early responses were also observed (within minutes) in some experiments. In Western blot analysis, the levels of immunoreactive BLT1 protein were either unaltered or increased significantly after 60 and 120 min (data not shown).
Fig. 2.
Increase in BLT2 mRNA in HUVEC stimulated with TNF-α. HUVEC were incubated with TNF-α (100 ng/ml) and harvested at different time points to prepare total RNA. mRNA levels were analyzed by semiquantitative RT-PCR, as described in Methods. The time course for the increase in BLT1 (filled circles) and BLT2 (open circles) mRNA levels in HUVEC upon treatment with TNF-α is shown.
Effects of Ligand Binding on the Expression of BLT Receptors.
The natural ligand LTB4 also induced BLT1, but not BLT2, mRNA and protein expression in a time-dependent manner. After stimulation of HUVEC with 100 nM LTB4, BLT1 mRNA increased rapidly and stayed elevated for at least 2 h (Fig. 3). Thus, after 30 min of incubation, the levels of BLT1 mRNA had increased by 3.15 ± 0.7 times (n = 5) and then gradually diminished to 2.9 ± 0.8 and 2.1 ± 0.3 times the control levels, after 60 and 120 min, respectively. In contrast, the amounts of BLT2 mRNA gradually decreased to levels corresponding to 0.4 ± 0.2, 0.55 ± 0.15, and 0.2 ± 0.1 (n = 3) times the control values, after 30, 60, and 120 min, respectively. The effects of LTB4 on BLT1 mRNA expression appeared specific, because LTC4, LTD4, and 5(S)-hydroxy-8,11,14-cis-6-trans-eicosatetraenoic acid (5-HETE) were without significant effects (data not shown). In addition, Western blot was performed on 2 × 105 cells from HUVEC cultures stimulated with 100 nM LTB4 (0, 1, 3, and 6 h). As observed after LPS stimulation, BLT1 immunoreactivity increased significantly, particularly after 3 and 6 h (Fig. 3).
Fig. 3.
Increase in BLT1 mRNA and protein expression in HUVEC stimulated with LTB4. HUVEC were incubated with LTB4 (100 nM) and harvested at different time points to prepare total RNA and protein extracts. mRNA and protein levels were analyzed by semiquantitative RT-PCR and Western blot as described in Methods. (Upper) Time course for the increase in BLT1 mRNA (filled circles) and decrease in BLT2 mRNA (open circles) in HUVEC treated with the natural agonist LTB4. (Lower) Levels of immunoreactive BLT1 protein in HUVEC treated with LTB4.
Assessment of BLT Receptor Signaling.
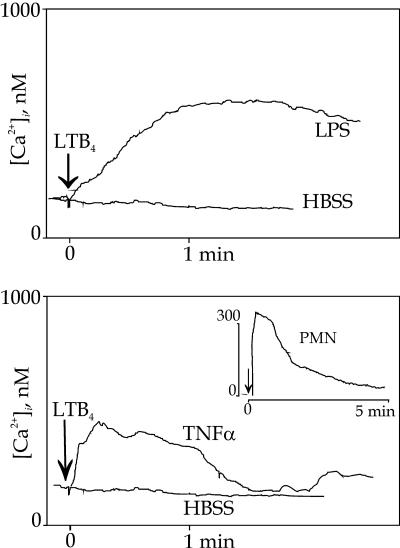
When quiescent HUVEC were stimulated with LTB4 (100 nM) we observed no or minimal cytosolic calcium ([Ca2+]i) changes, the mean rise above the basal level was 26 ± 9 nM (Fig. 4), which is in line with a previous report (21). However, when HUVEC had been exposed to LPS, IL-1β, or TNF-α for 2–4 h, clear increases of [Ca2+]i were seen, where 3 h of incubation conferred maximal responses; therefore, all experiments were performed with 3 h of LPS or cytokine treatment. At this time point, LPS treatment (100 ng/ml) augmented the LTB4-induced [Ca2+]i response 6.7-fold, or with 175 ± 28 nM (n = 20) above basal levels, where the onset of the response was gradual, reached a plateau after ≈1 min, and then slowly declined (Fig. 4 Upper). In contrast, when LPS was substituted by TNF-α (100 ng/ml) LTB4 induced a rapid and 4.3-fold enhanced [Ca2+]i response (112 ± 24 nM; n = 9), peaking within 15–20 s and then declining to basal levels after 1–1.5 min (Fig. 4 Lower). Likewise, a 3 h IL-1β exposure caused a 4.6-fold rise of the LTB4 response to 120 ± 24 nM, with kinetics similar to TNF-α (n = 6; data not shown). These responses of HUVEC to LTB4 differed kinetically from that induced by LTB4 in quiescent neutrophils (Fig. 4 Inset). As a control for the specificity of the response, we substituted LTB4 with thrombin as the stimulus for [Ca2+]i transients, but saw no enhancement of the response by means of previous LPS treatment (data not shown). IFN-γ exposure for 3 h was associated with no rise of [Ca2+]i upon LTB4 stimulation.
Fig. 4.
LTB4-induced Ca2+ mobilization in HUVEC after treatment with LPS or TNF-α. LTB4 induced Ca2+ responses when HUVEC had been treated with LPS (Upper) or TNF-α (Lower) for 3 h but only marginally when treated with HBSS. The kinetics of the TNF-α responses were consistently more rapidly emerging and transient, whereas LPS responses were slower in onset but persistent. (Inset) The Ca2+ response of neutrophils to LTB4 alone.
The LTB4-induced Ca2+ responses appearing after treatment with LPS or TNF-α were completely blocked by pretreatment with the BLT1 inhibitor CP-105696 (1 μM) for 20 min (data not shown; n = 4). The weaker BLT1 antagonist U75302 (1 μM; 20 min) influenced the LTB4 response marginally in LPS treated cells (87% of the control, i.e., 153 ± 13 nM) and TNF-α treated cells. U75302 also acted as an agonist (300 nM) on TNF-α or LPS treated endothelial cells with small but sustained Ca2+ responses: 53 ± 19 (n = 6) and 112 ± 48 nM (n = 6), respectively.
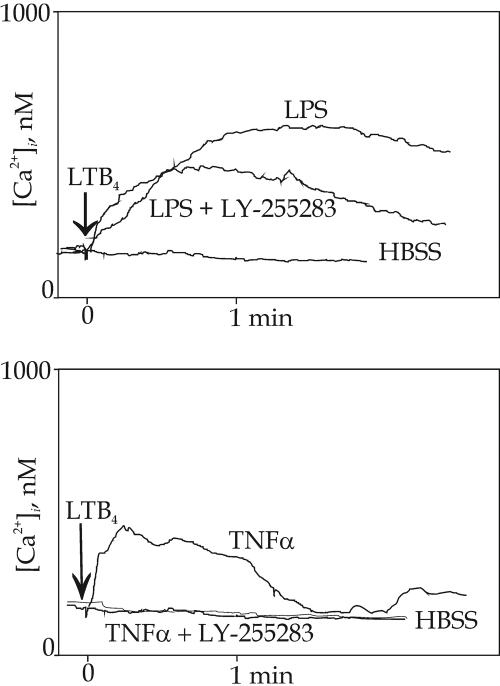
A BLT2 antagonist, LY-255283 (1 μM for 20 min before addition of LTB4) had different effects on the LTB4 response in LPS or TNF-α treated cells. In the former, only a small part of the response was inhibited (Fig. 5Upper), but in the latter the response was blocked, indistinguishable from that in HBSS treated HUVEC (Fig. 5 Lower).
Fig. 5.
Modulation of Ca2+ responses, elicited by LTB4 in cytokine-treated HUVEC, by the antagonist LY-255283. When HUVEC had been treated with LPS for 3 h, followed by LY-255283 (1 μM) for 20 min, most of the Ca2+ response elicited by addition of LTB4 remained (Upper). When the same procedure was repeated and LPS was substituted by TNF-α, no LTB4-induced Ca2+ responses could be observed (Lower).
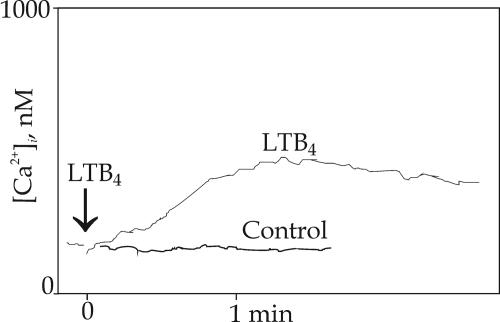
We also tested the effect of a preincubation with LTB4 itself and exposed HUVEC to the ligand for 3–6 h. Upon a second stimulation with LTB4, enhanced [Ca2+]i responses were observed (Fig. 6). The maximum rise above basal levels, 217 ± 35 nM (n = 4), occurred at 4 h and declined to 55 nM after a 6-h exposure. The kinetics of the response was intermediated between LPS and TNF-α, in that many cells reacted with a peak within 10–20 s and responses then declined slowly, whereas other cells reacted more slowly, with a peak after ≈1 min (Fig. 6). In a control experiment with a similar exposure to LY-255283, the endothelial cells did not respond to subsequent LTB4 stimulation.
Fig. 6.
Ca2+ responses elicited by LTB4 in LTB4-treated HUVEC. When HUVEC had been treated with LTB4 for 4 h, followed by activation by LTB4, a major response that was 8.3-fold higher than that of HUVEC pretreated with HBSS alone was noted. When LTB4 was substituted by LY-255283 (control) as pretreatment, no Ca2+ response was observed.
Effects of LTB4 on Nitrite Generation and MCP-1 Release from HUVEC.
In these experiments, we focused on other physiological effects of the up-regulation of BLT1 by LPS or LTB4. To this end, we treated HUVEC for 3 h with either LPS (at 100 ng/ml), LTB4 (250 nM), or Hanks’ balanced salt solution (HBSS) alone, then removed media and added new media and stimulus (i.e., LTB4) or HBSS. Supernatants were removed after incubation for 15 min and assayed for nitrite or MCP-1. Controls (i.e., 100%) are samples treated with HBSS for 3 h and then exposed to HBSS alone for 15 min; they contained 2.3 ± 0.4 μM of nitrite and 656 ± 221 pg/ml of MCP-1 (n = 18).
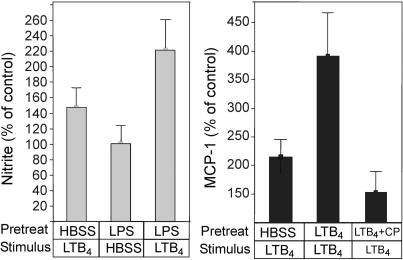
As shown in Fig. 7Left, nitrite release (supposedly reflecting NO generation or non-nitric oxide synthase associated nitrite production) increased in HBSS-treated samples that were stimulated by LTB4 for 15 min to 148%. In good agreement with the results of the Ca2+ experiments described above, treatment with LPS for 3 h, followed by LTB4, caused a significant (P = 0.04; n = 10) up-regulation of nitrite release to 227%, which is a 2.4-fold increase compared to samples treated similarly with LPS but exposed to HBSS alone for the 15-min period. In this assay, the antagonist CP-105696 displayed an agonistic action hampering evaluation of the involvement of BLT1 (n = 6).
Fig. 7.
LTB4 increases the generation of nitrite and release of MCP-1 from HUVEC. Cultured HUVEC were incubated as described in Methods. Stimulation of cultured HUVEC with LTB4 for 15 min leads to generation of nitrite (Left), as assessed by the Griess reaction for nitrite. Pretreatment of HUVEC with LPS for 3 h followed by stimulation with LTB4 increases the nitrite release further, indicating up-regulation of BLT1. Results are expressed as percentage of nitrite generation by control cells (stimulated with HBSS alone); mean value was 2.3 ± 0.4 μM, n = 28. Preincubation of HUVEC with LTB4 (250 nM) for 3 h increases the response of a second 15-min challenge with LTB4 (Right). Addition of the BLT1 antagonist CP-105696 (CP) during the preincubation period reduces most of the response. Results are expressed as percentage of untreated control HUVEC; mean value was 656 ± 221 pg/ml, n = 18.
In similarly designed experiments on MCP-1 release (Fig. 7 Right), we found that LPS causes a strong MCP-1 release; thus, we used LTB4 treatment for 3 h to up-regulate BLT1, as described above. Compared to controls first treated with HBSS alone and then with LTB4 (which increased MCP-1 release 2.15-fold), samples treated with LTB4 during both time periods showed 3.92-fold enhanced cytokine release (n = 17; P = 0.02), suggesting that priming with LTB4 enhanced the subsequent LTB4 response (Fig. 7). When CP-105696 was present during the 3-h pretreatment period, most of the subsequent response to a second LTB4 challenge was abolished.
Discussion
LTB4 is a classical chemoattracting agent that also promotes neutrophil adhesion and diapedesis through the endothelial cell barrier, a key sequence of events during vascular inflammatory responses and host defense. However, the molecular mechanisms of LTB4-induced transendothelial migration of neutrophils is not fully understood, but seems to involve soluble factors as well as activation of both leukocyte and endothelial cell adhesion molecules (23). Direct effects of LTB4 on endothelial cells have also been discussed, although these cells have appeared only weakly, if at all, reactive to this mediator (24, 25). In this study, we sought to identify factors that might turn on BLT receptor expression and function in these cells.
LPS and TNF-α Differentially Up-Regulate BLT Receptor Expression and Render HUVEC Responsive to LTB4.
LPS treatment of HUVEC led to a rapid increase in BLT1 mRNA, which was also reflected in significantly enhanced levels of BLT1 immunoreactive protein (Fig. 1). Interestingly, from an almost insensitive state in quiescent cells, LPS treated cells responded with a robust increase in [Ca2+]i upon stimulation with LTB4 (Fig. 4) that could be blocked with CP-105696, a selective BLT1 antagonist, but not with the BLT2-selective LY-255283. Moreover, the Ca2+ response in HUVEC exhibited a different kinetic pattern as compared to that in human neutrophils, suggesting that other intracellular signaling pathways, or a different BLT1 receptor complex, are involved.
TNF-α had an almost opposite effect on the expression of BLT receptors with a rapid increase in the levels of BLT2 mRNA and only modest and partially reversible effects on BLT1 mRNA amounts (Fig. 2). As observed with LPS, treatment of HUVEC with TNF-α allowed LTB4 induced Ca2+ signaling (Fig. 4), presumably via BLT2 because TNF-α did not significantly increase BLT1 protein and the LTB4 signal could be blocked with LY-255283.
Considering the unusual gene structure of BLT1 and BLT2, with the ORF of the BLT2 gene located in the promoter of the BLT1 gene (15), it is interesting that signaling via TNF-α or LPS can selectively increase mRNA levels of only one gene at a time. Further work will hopefully elucidate the molecular mechanisms involved.
The Natural BLT Ligand LTB4 Increases the Expression of BLT1 in HUVEC.
Although LTB4 does not evoke significant Ca2+ transients in quiescent HUVEC, the ligand can still influence gene expression. Thus, incubations of HUVEC with LTB4 led to significant increases in the levels of BLT1 mRNA and protein (Fig. 3). Pretreatment of HUVEC with LTB4 also turned on their ability to signal with [Ca2+]i transients in response to ligand activation, presumably via BLT1. Apparently, the expression and functional state of BLT1 receptors in HUVEC seem to be regulated via a signaling pathway involving binding of the receptor’s own primary ligand. It may be noted that modulation of BLT1 expression by LTB4 has been observed in human neutrophils, thus corroborating our results in HUVEC (26).
LTB4 Promotes Nitrite/NO Generation and MCP-1 Release From HUVEC.
In quiescent HUVEC, LTB4 only elicits weak Ca2+ responses, and not much is known regarding the functional consequences of signaling via BLT receptors. We reasoned that up-regulation of BLT receptors would perhaps uncover previously unknown functional responses of HUVEC to LTB4. However, LPS and cytokines are powerful activators of endothelial cells and gave maximal responses in almost all assays tested, e.g., expression of adhesion molecules and cytokine release. Nonetheless, we could show that LTB4 promotes nitrate generation in HUVEC and that this effect is augmented after pretreatment with LPS, presumably reflecting a functional up-regulation of BLT1. We also found that LTB4 can release MCP-1 from HUVEC, and this response is increased after pretreatment with the ligand. For this bioactivity, we could demonstrate that BLT1 receptors are involved (Fig. 7).
BLT Receptor Expression and Function on HUVEC May Be Modulated by the Profile of LPS and Cytokines.
Our results indicate that endothelial cells respond to LPS, TNF-α, and IL-1β with increased expression of BLT receptors capable of transmitting LTB4 signals and functional responses. Interestingly, each of these agents had different effects on the expression of the respective receptor subtype, BLT1 and BLT2. Thus, depending on the relative abundance of these proinflammatory substances, the receptor composition and function will be tuned at the surface of endothelial cells. Challenge with LPS elicits an innate immune response that mimics bacterial infection and sepsis. Downstream in this pathway, cytokines will be released, including TNF-α and IL-1β. Inasmuch as LTB4 is regarded as an important signaling molecule in the innate immune system, the actions of LPS, TNF-α, and IL-1β on the responsiveness of endothelial cells to this particular mediator appear adequate and functional.
Potential for LTB4–BLT Receptor Interactions During Leukocyte Adhesion.
It has been shown that LTB4 induces hyperadhesiveness of endothelial cells, an effect that appears to be mediated via increased activity of CD54 (20, 21). One can then envisage a scenario in which activated leukocytes release LTB4 that, in turn, acts on endothelial cells that are prepared to transmit the signals and further promote the adhesion and transmigration process. This concept is further supported by our findings that LTB4 stimulation of HUVEC appears to promote generation of NO, a potent vasodilator that can increase blood flow, and increases the release of MCP-1, a powerful chemoattractant for monocytes and T cells (27). MCP-1 could potentially signal back to the adhering leukocytes to further stimulate LTB4 synthesis, as has been shown for macrophages in vitro (28). Therefore, our data support the notion that LTB4 can promote leukocyte adhesion, not only via effects on the leukocyte, but also via direct actions on the endothelium. These actions also include up-regulation of the BLT1 receptor, which presumably will further amplify the functional effects of LTB4. It is also worth noting that endothelial cells contain LTA4 hydrolase, thus allowing synthesis of LTB4 via cell–cell interactions and transcellular biosynthesis.
Several lines of evidence indicate that LTs are involved in vascular inflammation, in particular arteriosclerosis (10). Here, endothelial cells are important players in the pathogenesis and, interestingly, LTB4 has been ascribed a specific role in the disease processes (9). Our results provide clues to understanding the mechanisms of leukocyte adherence and transmigration of endothelial cells. Moreover, they may have implications for the molecular pathology of cardiovascular and rheumatic diseases and point to BLT receptors as potential targets for pharmacological intervention in chronic inflammatory disorders.
Methods
Materials.
LTB4, 5(S)-hydroxy-8,11,14-cis-6-trans-eicosatetraenoic acid, LTC4, LTD4, and U75302 were purchased from Biomol (Plymouth Meeting, PA). Oligonucleotides were from Cybergene (Huddinge, Sweden). LPS from Escherichia coli serotype O55:B5, TNF-α, IL-1β, VCl3, sulfanilamide, N-(1-naphtyl)-ethylene diamine, H3PO4, Fura 2-AM, and Pluronic F127 were from Sigma. Polyclonal antibodies against BLT1 (and BLT2) were purchased from Cayman Chemical (Ann Arbor, MI). LY-255283 and CP-105696 were kind gifts from Lilly Research Laboratories (Indianapolis, IN) and Pfizer, respectively.
Cell Culturing.
HUVEC were obtained from the vessels by collagenase treatment, cultivated, and identified as described (21, 29). HUVEC were trypsinized when confluent, resuspended in medium, and seeded into new culture flasks (for a maximum of two passages, once per week). The cell viability was >95%, as judged by cell morphology, trypan blue exclusion, and analysis of lactate dehydrogenase release. Glass dishes were seeded with 104 HUVEC per well and grown until confluence.
Analysis of mRNA by RT-PCR.
Preparation of total RNA and RT-PCR were essentially as described (30). Briefly, RT-PCR was conducted with a GeneAmp/PerkinElmer RNA PCR kit, using 1 μg of RNA for reverse transcription, followed by two rounds (30 + 20 cycles) of DNA amplification with annealing between 50°C and 60°C and extension at 72°C, using a GeneAmp/PerkinElmerPCR system 2400. The nested PCR primers used were: BLT1: first PCR, 5′-ATGAACACTACATCTTCTGCAGC-3′, 5′-CTAGTTCAGTTCGTTTAACTTGAGAGG-3′; second PCR, 5′-AGGTGTAGAGTTCATCTCTCTGCT-3′, 5′-CTCCAGCAGCTTGGCGACGAAGC-3; BLT2: first PCR, 5′-CATTCTTGTCTTACCCTCTGC-3′, 5′-AGTTCGGAGCTCCATGGTCC-3′; second PCR, 5′-GTGGTAGAGATAGTGACAGC-3′, 5′-AAGGTTGACTGCGTGGTAGG-3′. For identification of the BLT1 and BLT2 mRNA, the corresponding DNA fragments generated by RT-PCR were sequenced by using the DYEnamic ET terminator cycle sequencing premix kit (Amersham Pharmacia). For semiquantitative analysis of PCR products, the fluorescent dye PicoGreen (Molecular Probes) was used as described (31, 32). The fluorescence was measured (λex = 485 nm; λem = 538 nm) in a SpectraMax GeminiXS fluorometer from Molecular Devices. The standard curve for the quantitative analysis was obtained with λ DNA standard in TE buffer supplied by the manufacturer and was linear from 1 to 1,000 ng per well. The results were recalculated relative to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase amplified from each sample and expressed as mean ± SD.
Immunoblotting.
Aliquots of 105 cells were mixed with loading buffer containing SDS, heated for 5 min to 95°C, and chilled on ice. Samples were then electrophoresed through an SDS/polyacrylamide gel (4–15% gradient gel; Bio-Rad) at 120 V for ≈70 min and subsequently electroblotted onto a poly(vinyl-difluoride) membrane (PALL, Fluorotrans Transfer membrane). The efficiency of the protein transfer was controlled by Coomassie staining of the gel. Membranes were then soaked for 2 h in 0.05% T-TBS [20 mM Tris·Cl, pH 7.5, with 0.5 M NaCl and 0.05% (vol/vol) Tween 20] containing 5% (wt/vol) nonfat dry milk. Blots were rinsed and washed twice for 5 min in 0.05% T-TBS and subsequently incubated for 14 h at 4°C with a BLT1 polyclonal antiserum (dilution 1:1,000) in 0.05% T-TBS containing 2% dry milk. After incubation, blots were rinsed and washed twice for 10 min with 0.05% T-TBS followed by a 1-h incubation at 25°C with a donkey anti-rabbit IgG antibody coupled to horseradish peroxidase for detection of BLT1 receptor. Detection of immunoreactive bands was carried out with the enhanced chemiluminescence detection method (ECL PLUS kit; Amersham Pharmacia Life Science) according to the manufacturer’s instructions. Results were quantitated with a LAS-1000 Pro version 2.0 image analyzer with image gauge version 3.46 software (Fuji).
Calcium Mobilization Experiments.
Mobilization of [Ca2+]i was monitored spectrophotometrically by using Fura 2-AM, as described (33). In one single microscopic field, the system generates individual data from 5 to 10 cells and calculates a mean value. The results are given as the ratio of fluorescence between 340 and 380 nm, calibrated and calculated with the miracal (Life Science Resources, Cambridge, U.K.) software, according to the recommendations of the manufacturer.
MCP-1 Analyses.
MCP-1 concentrations in supernatants of HUVEC, incubated in HBSS with 2% FCS with or without stimuli, were analyzed by ELISA according to the manufacturer’s instructions (R & D Systems). Values were normalized to unstimulated controls set to 100%.
Assay of Nitrite.
Formation of NO was analyzed by measurements of nitrite/nitrate, based on a modified Griess reaction (34, 35). In brief, HUVEC were stimulated for indicated time periods with HBSS or LTB4, and supernatants were harvested and centrifuged for 6 min at 1,850 × g. The samples (100 μl), together with nitrite/nitrate standards, were mixed with 100 μl of VCl3 (8 mg/ml) in 1 M HCl and nitrite and then quantified spectrophotometrically at 540 nm by using Griess reagent [0.5% (wt/vol) sulfanilamide/0.05% (wt/vol) N-(1-naphtyl)-ethylene diamine/2.5% H3PO4].
Acknowledgments
This work was supported by Swedish Research Council Grants O3X-10350 and 19X-05991, the AFA Health Foundation, Konung Gustav V:s 80-Year Fund, The Swedish League against Rheumatism, and European Community FP6 LSHM-CT-2004-005033.
Abbreviations
- LT
leukotriene
- HUVEC
human umbilical vein endothelial cells
- MCP-1
monocyte chemoattractant protein-1
- [Ca2+]i
cytosolic calcium
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Samuelsson B. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 2.Funk C. D. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 3.Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. H. Nature. 1980;286:264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- 4.Malmsten C., Palmblad J., Udén A.-M., Rådmark O., Engstedt L., Samuelsson B. Acta Physiol. Scand. 1980;110:449–451. doi: 10.1111/j.1748-1716.1980.tb06696.x. [DOI] [PubMed] [Google Scholar]
- 5.Goodarzi K., Goodarzi M., Tager A. M., Luster A. D., von Andrian U. H. Nat. Immunol. 2003;4:965–973. doi: 10.1038/ni972. [DOI] [PubMed] [Google Scholar]
- 6.Ott V. L., Cambier J. C., Kappler J., Marrack P., Swanson B. J. Nat. Immunol. 2003;4:974–981. doi: 10.1038/ni971. [DOI] [PubMed] [Google Scholar]
- 7.Tager A. M., Bromley S. K., Medoff B. D., Islam S. A., Bercury S. D., Friedrich E. B., Carafone A. D., Gerszten R. E., Luster A. D. Nat. Immunol. 2003;4:982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- 8.Dwyer J. H., Allayee H., Dwyer K. M., Fan J., Wu H., Mar R., Lusis A. J., Mehrabian M. N. Engl. J. Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 9.Helgadottir A., Manolescu A., Thorleifsson G., Gretarsdottir S., Jonsdottir H., Thorsteinsdottir U., Samani N. J., Gudmundsson G., Grant S. F., Thorgeirsson G., et al. Nat. Genet. 2004;36:233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 10.Funk C. D. Nat. Rev. Drug Discov. 2005;4:664–672. doi: 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]
- 11.Haeggström J. Z. J. Biol. Chem. 2004;279:50639–50642. doi: 10.1074/jbc.R400027200. [DOI] [PubMed] [Google Scholar]
- 12.Brezinski D. A., Nesto R. W., Serhan C. N. Circulation. 1992;86:56–63. doi: 10.1161/01.cir.86.1.56. [DOI] [PubMed] [Google Scholar]
- 13.Fabre J. E., Goulet J. L., Riche E., Nguyen M., Coggins K., Offenbacher S., Koller B. H. J. Clin. Invest. 2002;109:1373–1380. doi: 10.1172/JCI14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokomizo T., Izumi T., Chang K., Takuwa Y., Shimizu T. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 15.Kato K., Yokomizo T., Izumi T., Shimizu T. J. Exp. Med. 2000;192:413–420. doi: 10.1084/jem.192.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokomizo T., Kato K., Terawaki K., Izumi T., Shimizu T. J. Exp. Med. 2000;192:421–431. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokomizo T., Kato K., Hagiya H., Izumi T., Shimizu T. J. Biol. Chem. 2001;276:12454–12459. doi: 10.1074/jbc.M011361200. [DOI] [PubMed] [Google Scholar]
- 18.Toda A., Yokomizo T., Masuda K., Nakao A., Izumi T., Shimizu T. Biochem. Biophys. Res. Commun. 1999;262:806–812. doi: 10.1006/bbrc.1999.1284. [DOI] [PubMed] [Google Scholar]
- 19.Huang W. W., Garcia-Zepeda E. A., Sauty A., Oettgen H. C., Rothenberg M. E., Luster A. D. J. Exp. Med. 1998;188:1063–1074. doi: 10.1084/jem.188.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmblad J. E., Lerner R. Clin. Exp. Immunol. 1992;90:300–304. doi: 10.1111/j.1365-2249.1992.tb07946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmblad J., Lerner R., Larsson S. H. J. Immunol. 1994;152:262–269. [PubMed] [Google Scholar]
- 22.Wedmore C. V., Williams T. J. Nature. 1981;289:646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- 23.Ulbrich H., Eriksson E. E., Lindbom L. Trends Pharmacol. Sci. 2003;24:640–647. doi: 10.1016/j.tips.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Lerner R. J. Lab. Clin. Med. 1994;124:723–729. [PubMed] [Google Scholar]
- 25.Nohgawa M., Sasada M., Maeda A., Asagoe K., Harakawa N., Takano K., Yamamoto K., Okuma M. J. Leukocyte Biol. 1997;62:203–209. doi: 10.1002/jlb.62.2.203. [DOI] [PubMed] [Google Scholar]
- 26.Stankova J., Turcotte S., Harris J., Rola-Pleszczynski M. J. Immunol. 2002;168:3570–3576. doi: 10.4049/jimmunol.168.7.3570. [DOI] [PubMed] [Google Scholar]
- 27.Daly C., Rollins B. J. Microcirculation. 2003;10:247–257. doi: 10.1038/sj.mn.7800190. [DOI] [PubMed] [Google Scholar]
- 28.Matsukawa A., Hogaboam C. M., Lukacs N. W., Lincoln P. M., Strieter R. M., Kunkel S. L. J. Immunol. 1999;163:6148–6154. [PubMed] [Google Scholar]
- 29.Heimburger M., Palmblad J. E. Clin. Exp. Immunol. 1996;103:454–460. doi: 10.1111/j.1365-2249.1996.tb08302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjöström M., Jakobsson P.-J., Heimburger M., Palmblad J., Haeggström J. Z. Eur. J. Biochem. 2001;268:2578–2586. doi: 10.1046/j.1432-1327.2001.02142.x. [DOI] [PubMed] [Google Scholar]
- 31.Romppanen E.-L., Savolainen K., Mononen I. Anal. Biochem. 2000;279:111–114. doi: 10.1006/abio.1999.4457. [DOI] [PubMed] [Google Scholar]
- 32.Schröder O., Sjöström M., Qiu H., Jakobsson P. J., Haeggström J. Z. Cell. Mol. Life Sci. 2005;62:87–94. doi: 10.1007/s00018-004-4366-7. [DOI] [PubMed] [Google Scholar]
- 33.Sjöström M., Johansson A. S., Schröder O., Qiu H., Palmblad J., Haeggström J. Z. Arterioscler. Thromb. Vasc. Biol. 2003;23:e37–41. doi: 10.1161/01.ATV.0000082689.46538.DF. [DOI] [PubMed] [Google Scholar]
- 34.Miranda K. M., Espey M. G., Wink D. A. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 35.Beda N., Nedospasov A. Nitric Oxide. 2005;13:93–97. doi: 10.1016/j.niox.2005.05.002. [DOI] [PubMed] [Google Scholar]