Abstract
A number of environmental factors can affect the development and severity of allergy and asthma; however, it can be argued that the most significant inhaled agents that modulate the development of these conditions are biologics. Sensitization to environmental allergens is an important risk factor for the development of asthma. Innate immune responses are often mediated by receptors on mononuclear cells whose primary ligands arise from microorganisms. Many pathogens, especially viruses, target epithelial cells and affect the host immune response to those pathogens. The acquired immune response to an allergen is influenced by the nature of the innate immune system. Products of innate immune responses to microbes promote TH1-acquired responses. In the absence of TH1 responses, TH2 responses can dominate. Central to TH1/TH2 balance is the composition of contaminants that derive from microbes. In this review we examine the biology of the response to allergens, viruses, and bacterial products in the context of the development of allergy and asthma.
Keywords: asthma, allergy, allergens, endotoxin, respiratory virus, immunoglobulins, tolerance, leukotrienes, neurotrophins
Allergy is a TH2-mediated immunologic phenomenon that is the most significant risk factor for development of childhood asthma. In the airway, the innate immune response to environmental agents gives rise to inflammation, enhancement of antigen presentation, and development of the primary (acquired) immune response. The inflammatory response results from the coordinated action of monocytes and macrophages, but it also involves responses of other cell types such as epithelial cells and neurons. Thus, while the immune response is central to the development of allergy, nonimmune structures also participate in this complex process.
A number of environmental factors have been reported to affect the development and severity of asthma, including outdoor air pollutants (e.g., particulates, ozone), indoor irritants, and agents such as environmental tobacco smoke. However, it can be argued that the most significant inhaled agents that modulate the development of respiratory allergy and asthma are biologics. Indeed, one important aspect of innate immunity involves the response of monocytes and macrophages, which is mediated by receptors whose primary ligands arise from various microorganisms. Furthermore, many pathogens, especially viruses, target epithelial cells, and the resulting responses of epithelial cells and surrounding monocytes greatly affect the host response to those pathogens.
It has been suggested that the primary acquired immune response to a given antigen is influenced by the nature of the innate immune system (and its associated cytokine response). Thus, products of innate responses to microbes that are more effectively cleared by IgG and TH1 inflammation might be expected to promote TH1-acquired responses. In the absence of such inflammation, TH2 responses can dominate, especially if inhaled bioaerosols contain agents that derive from multicellular organisms (which may mimic parasites). Ultimately, it is the total exposure and immune experience of an individual, coupled with genetic factors that control their innate and acquired immune responses, that determine if allergy develops in the airway. Central to TH1/TH2 balance is the composition of contaminants that derive from microbes. Hence, in this review we examine the biology of response to allergens, viruses, and bacterial products (primarily endotoxin) in the context of development of allergy and asthma.
Cockroach, Dust Mite, Mold, Rodent, and Pet Allergens and the Induction of Asthma
The question of asthma induction usually brings to mind infants who experience asthma for the first time; however, at least two other examples illustrate the importance of allergen exposure to asthma incidence in adults. The first example is occupational asthma, especially that caused by laboratory animal allergy, where 25–30% of workers who are sensitized to laboratory animal allergens develop symptoms within 1 year of beginning work (Bush et al. 1998). About 25% of symptomatic workers have asthma symptoms, thereby making laboratory animal allergens a relatively common cause of incident asthma associated with a new allergen exposure in adults (Bush et al. 1998). A second example is the report of markedly increased rates of asthma in primitive villagers from the New Guinea highlands. In the 1980s adult men in these villages developed severe asthma, and 91% were sensitive to many allergens, including house dust mites (Dowse et al. 1985). Cotton blankets that had been donated by Western charities were found to be heavily contaminated with dust mites, thus suggesting that they had been presented with a new, unique exposure that led to sensitization and incident asthma (Dowse et al. 1985).
Children who develop asthma typically have symptoms by the age of 4–5 years, and a significant portion of them develop persistent asthma (Stein and Martinez 2004). Data from birth cohort studies suggest that atopy (defined by family history, other allergic manifestations such as eczema, elevated IgE, or sensitization) is a major risk factor for the development of childhood asthma (Lowe et al. 2002; Martinez et al. 1995; Platts-Mills et al. 1997; Wahn et al. 1997). In asthmatic children age 6 years and older, sensitization to airborne environmental allergens is very common (80–90% of cases), and the combination of sensitization and exposure is strongly associated with more severe disease (Rosenstreich et al. 1997).
Allergens and their sources.
A number of allergen sources have been identified in the indoor environment (Table 1). House dust mites thrive in humid environments and live on human skin scales. Fecal particles, which contain the allergens, do not remain airborne for more than a few minutes after disturbance. Thus, the source has limited mobility, and exposure is limited primarily to bedding, carpeting, and upholstered furniture (Arlian and Platts-Mills 2001). Cockroaches cluster in narrow hiding places, coming out only to forage for food and water. The particles that contain the allergen are generally large, but the source is mobile so it is widespread in settled dust and, in many cases, accumulates in places inaccessible to cleaning (Eggleston and Arruda 2001). Rodents hide within walls and crevices, and leave high concentrations of allergen in inaccessible places. The allergens are found in urine and bodily secretions and are carried on small particles that remain airborne for extended periods of time. House dust is heavily contaminated, but removal is difficult because of the inaccessible reservoirs (Chapman and Wood 2001; Phipatanakul et al. 2004). Pets with fur produce allergens in their saliva and sebaceous secretions. Air sampling studies have shown that approximately 20–30% of airborne animal allergens are present on small particles of 1–5 μm diameter, in contrast to mite and cockroach allergens, which are carried on large particles of 10–40 μm diameter (Custovic et al. 1997; Luczynska et al. 1990). The animal allergens remain airborne for extended periods of time and are passively carried throughout the home as well as into public buildings and homes that have never housed a pet. After removal of a pet, household settled dust allergen levels decline over a period of 4–8 months (Wood et al. 1989). Air cleaners have been reported to reduce airborne pet allergen levels, but they have minimal effect on settled-dust allergen levels (Wood et al. 1998). The ecology of fungal allergen exposure is perhaps the least understood of all indoor allergens. Atopic persons are frequently sensitized, and fungi can easily be cultured from indoor dust and air. Fungal spores originate in the soil and are ubiquitous in the outdoor environment. The various fungal species and the levels of these spores fluctuate dramatically throughout the various seasons. These mold spores infiltrate the indoors via openings such as doors, windows, cracks and crevices. They are also transported inside by people and pets. Allergenic proteins have been isolated from fungi, but these allergens are not typically present in indoor environments. Recent data suggest that the allergens are only found in association with germinating fungal spores (Mitakakis et al. 2001).
Table 1.
Indoor allergen sources.
| Allergen | Animal source | Household source | Particle size (μm) | Distribution |
|---|---|---|---|---|
| Cockroach | Secretions | Mobile, hiding places | 5–35 | Dust, fabrics |
| Dust mite | Feces | Immobile, fastidious | 5–35 | Fabrics, beds |
| Rodent | Secretions, urine | Mobile, hiding places | 1–15 | Air, surfaces, fabrics |
| Pet | Secretions | Mobile, furniture | 1–5 | Air, widespread |
| Mold | NA | Moist surfaces, materials | 5–10 | Unknown |
NA, not applicable.
Exposure estimates.
In general, an exposure dose is determined by two factors: the exposure concentration (in the case of asthma, the airway or nasal concentration), and the exposure time. For allergens, the exposure concentration is uncertain. For simple sources, such as the house dust mite, allergen particles contaminate infested fabrics and then become airborne with disturbance (Platts-Mills and Chapman 1987). Particles are cleared by settling, but some are also absorbed onto walls, furniture, and other reservoirs (Platts-Mills and Chapman 1987). Reservoirs are in equilibrium with the air, regenerating airborne particles by physical disturbance or by air currents. Air concentrations are also influenced by ventilation and dilution by outside air. Finally, particles can be brought into the indoor environment by foot traffic or on clothing, generally adding to the reservoir dust and potentially adding to airborne particles that might be inhaled and contribute to an exposure dose.
Most studies of exposure have measured allergen levels in settled dust; only rarely have airborne concentrations been assessed. Settled dust and airborne dust mite allergen concentrations are highly variable, with reported coefficients of variation of 30% or more (Platts-Mills and Chapman 1987). Airborne concentrations of cat and other animal allergens are even more variable. Indeed, recent studies have shown that allergen concentrations in samples collected from the same home can vary by more than 3 orders of magnitude (Bollinger et al. 1996). This degree of uncertainty makes it difficult to determine the exposure dose that might be related to incident asthma. In general, airborne allergen concentrations do not correlate well with settled dust allergen concentrations (Swanson et al. 1989).
Birth cohort studies of incident asthma.
Several birth cohort studies have reported a relationship between exposure and incident asthma. The Multicentre Allergy Study, a prospective study of 1,318 infants born in five German cities, was the first to describe the “allergic march” whereby children became sensitized first to food allergens (especially egg), then to inhalant allergens (such as dust mite and cat) up to 3 years later (Lau et al. 2000). Those who became allergic to foods were at greater risk for development of later sensitization to inhalant allergens. Incident sensitization was related in a dose-response fashion to dust mite and cat allergen exposure. Children who were sensitized to indoor allergens were at risk for incident asthma, but settled dust exposure doses were not directly related to incident asthma (Lau et al. 2000). In another prospective birth cohort study of 505 children in Boston, Massachusetts, exposure to cockroach allergen was found to be a risk factor for wheezing respiratory illness but not diagnosed asthma (Gold et al. 1999). This group also found that settled dust endotoxin concentrations were related to incident asthma (Park et al. 2001). In contrast, the Dutch PIAMA (Prevention and Incidence of Asthma and Mite Allergy) study found no relationship between settled dust exposures and incident asthma (Brunekreef et al. 2002).
Preventing incident asthma.
To date, the results of two primary prevention trials have been reported. Arshad and Hide randomized a birth cohort of 124 mothers and their high risk infants to receive active or control environmental intervention. The active intervention included food avoidance measures during pregnancy and continued avoidance during breast-feeding. In addition, the child’s mattress was fitted with an allergen impervious cover. Asthma and sensitization were decreased in the first year of life in the active group, but the asthma effect was no longer statistically significant at 2, 4 and 8 years; however, a trend toward protection was consistent and was associated with p-values ranging from 0.10–0.06 (Arshad et al. 1992, 2003; Hide et al. 1994, 1996). A second intervention study was carried out in Manchester, United Kingdom, with 251 mothers and their newborn infants. The intervention included fitted mattress and pillow covers to the parent’s and child’s bed, laundry of bedding, and acaricide treatment of rugs and upholstered furniture. The intervention was successful in reducing mite allergen in the child’s bed and carpets by over 90% (Custovic et al. 2000). A recent article from this group reported significantly reduced airway resistance and a trend toward improved asthma symptoms in infants in the intervention group at 3 years of age (Woodcock et al. 2004).
What Makes an Allergen an Allergen?
A number of epidemiologic studies carried out over the past 25 years have shown that IgE-mediated sensitization to indoor allergens (including those that derive from house dust mites, cats, dogs, rodents, cockroaches, and fungi) is a risk factor for the subsequent development of asthma (Platts-Mills et al. 1997). These studies include case–control studies, prospective studies, and allergen avoidance trials. Indeed, a recent longitudinal general population survey that followed over 600 children from the onset of asthma to age 26 years showed that sensitization to house dust mite was one of the strongest risk factors for persistence of asthma [odds ratio (OR) 2.41; 95% confidence interval (CI), 1.42–4.09] and also for predicting asthma relapses (OR 2.18; 95% CI, 1.18–4.00] (Sears et al. 2003).
Inhaled allergens are the most common cause of IgE responses worldwide. Allergens belong to distinct protein families with a diverse array of biologic functions. They include enzymes, ligand binding proteins (e.g., lipocalins), enzyme inhibitors, structural proteins, and regulatory proteins (Chapman et al. 2000). These proteins have been cloned, sequenced, and produced in high-level expression vectors. Purified recombinant allergens have immunoreactivity that is comparable to their natural counterparts, and they are being used to develop improved allergy diagnostics and vaccines. High-resolution crystal structures for the most important allergens are now available, including house dust mite (Der p 2), cat (Fel d 1), and cockroach (Bla g 2) allergens (Derewenda et al. 2002; Kaiser et al. 2003b; Pomes et al. 2002). More than 20 allergen structures have been resolved, and these molecules constitute the most well-defined groups of biomedically important proteins. Several databases have been developed for comparing the structure, biological function, and immunologic properties of allergens. A partial listing of available online databases is shown in Table 2.
Table 2.
Online allergen databases.
| Database | Reference |
|---|---|
| WHO/IUIS Allergen Nomenclature | IUIS 2004 |
| Structural Database of Allergenic Proteins | UTMB 2004 |
| Food Allergy Research and Resource Program | FARRP 2004 |
| Protall | Protall 2004 |
| ALLERbase | ALLERbase 2004 |
| Allergome | Allergome 2004 |
| Central Science Laboratory | CSL 2004 |
Abbreviations: IUIS, International Union of Immunological Societies; UTMB, University of Texas Medical Branch; WHO, World Health Organization.
Why do allergens induce IgE responses?
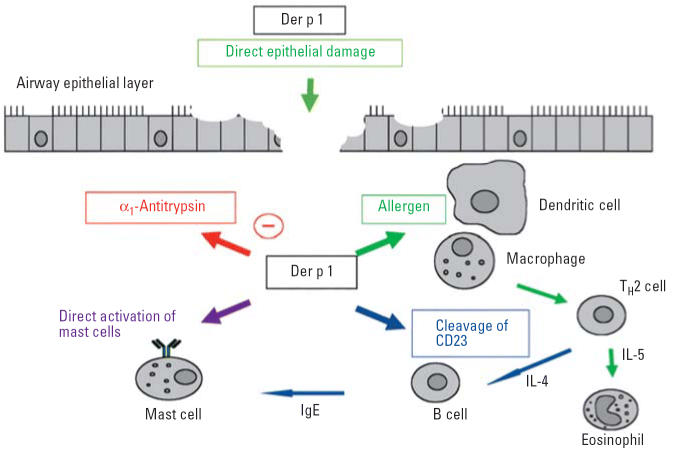
Two theories have been proposed to explain why allergens induce IgE responses (“allergenicity”). The “enzyme hypothesis” was originally developed as an explanation for why most dust mite allergens were proteolytic enzymes (principally cysteine and serine proteases, and chymotrypsin). Several lines of experimental evidence support this hypothesis (Figure 1) (Pomes et al. 2001; Sharma et al. 2003).
Figure 1.
Biological effects of Der p 1. Reproduced with permission of Blackwell Publishing Ltd. (Sharma et al. 2003).
Enzymatic activity directly promotes IgE synthesis through cleavage of the low-affinity IgE receptor (CD23) from activated B cells and by cleavage of the α subunit of the IL-2 receptor (CD25) on T cells (Hewitt et al. 1995; Shakib et al. 1998).
Mite proteinases (Der p 1, Der p 3, Der p 6, and Der p 9) damage lung epithelium and increase bronchial permeability by inducing pulmonary epithelial cell detachment and disruption of intercellular tight junctions (Wan et al. 1999).
Der p 1 induces production of proinflammatory cytokines in vitro [interleukin (IL)-8, IL-6, granulocyte-macrophage colony-stimulating factor] and induces IgE-independent mast cell and basophil degranulation (King et al. 1998).
This evidence also suggests that proteolytic allergens could contribute to lung damage and inflammation in asthma.
An alternative hypothesis is that the route of administration, dose of allergen inhaled (or ingested), and genetic predisposition are the principal factors that affect allergen recognition and development of allergen-specific TH2 responses that ultimately lead to IgE production. These factors apply to potent allergens, regardless of whether they are proteolytic enzymes. Recent structural studies have shown that several potent allergens are not enzymes. The group 2 mite allergens elicit IgE responses in 90% of mite allergic patients (Smith et al. 2001). The crystal structure of Der p 2 revealed a hydrophobic pocket within the molecule (Derewenda et al. 2002). Recent studies show that Der p 2 has structural homology to MD-2, a lipopolysaccharide (LPS) binding protein, and to a cholesterol binding protein C2 associated with Niemann-Pick disease (Gruber et al. 2004). The crystal structure of Fel d 1 revealed that the allergen was homologous to uteroglobin and contained an internal, asymmetric, amphipathic ligand binding pocket (Kaiser et al. 2003a, 2003b). Cockroach allergens are strongly associated with asthma among lower socioeconomic groups in innercity, rural, and suburban areas, yet none of the cockroach allergens identified to date has proteolytic activity. The most important allergen associated with IgE responses, Bla g 2, belongs to a subgroup of the aspartic proteinase family of enzymes that is enzymatically inactive (Arruda et al. 2001; Pomes et al. 2002). Attempts to render the Bla g 2 enzymatically active by selected site-directed mutagenesis of the active site catalytic triads have been largely unsuccessful. The high-resolution crystal structure of recombinant Bla g 2 defined the structural features that explain why the allergen is not an active enzyme and also showed that the allergen is a zinc binding protein (Pomes et al. 2002; Gustchina et al. 2005).
Modified TH2 responses to allergens and immunological tolerance.
Dose-related effects of allergen exposure on IgE responses have been studied most extensively using cat allergen (Fel d 1). Several recent studies have reported that the prevalence of sensitization to cat is reduced when children live with one or more cats (Hesselmar et al. 1999). Moreover, exposure to high levels of Fel d 1 (> 20 μg/g dust) has been associated with a reduced prevalence of IgE antibody responses to Fel d 1 and an increase in IgG4 antibody responses (Custovic et al. 2001; Platts-Mills et al. 2001). At lower exposure levels (1–10 μg/g dust), the prevalence of IgE responses was increased. These studies have further demonstrated a “modified” TH2 response among a subset of individuals who develop IgG1 and IgG4 responses to Fel d 1, without an IgE response. These individuals appear to have a form of immunological tolerance to Fel d 1. In keeping with this, recent studies have identified tolerogenic T-cell peptides on Fel d 1 that are associated with the production of IL-10 in vitro and that stimulate increased IL-10 production in patients receiving allergen immunotherapy (Reefer et al. 2004). T-cell mapping experiments have identified peptides on Fel d 1 chain 1 that are associated with IL-5 production in allergic individuals and peptides associated with immune tolerance in modified TH2 responders (Platts-Mills et al. 2004; Reefer et al. 2004).
The induction of a form of immune tolerance following high-dose allergen exposure has obvious implications for the development of new vaccines to treat allergic diseases. New approaches to immunotherapy are being developed that rely on increasing the dose of allergen administered while reducing the potential for adverse reactions (Chapman et al. 2000). This effect has been achieved by generating genetically engineered “hypoallergens” that retain their ability to stimulate T cells but that have reduced IgE antibody binding capacity. Another approach has been the use of deoxycytidyl–deoxyguanosine dinucleotide (CpG)–coupled allergens, which demonstrate reduced allergenicity and promote the development of modified TH2 responses. An alternative strategy has been to use peptide-based vaccines to induce T-cell anergy or tolerance. Clinical trials are currently under way using hypoallergens, CpG-coupled allergens, and allergen peptides for immunotherapeutic purposes. Successful clinical outcomes have been reported in some of the initial trials using hypoallergens and CpG vaccines to treat pollen allergy (Chapman et al. 2000; Creticos et al. 2004; Niederberger et al. 2004). A recent study has also reported significant improvement in allergic symptoms using a vaccine containing several purified recombinant timothy pollen allergens (Jutel et al. 2005). It remains to be established whether any of these approaches will be effective for patients with asthma, who tend to be more difficult to treat with allergen immunotherapy. Nonetheless, these approaches offer the possibility of designing rational, safe, and more effective immunologic treatments for allergic disease.
Viruses and Asthma
A number of studies have implicated viral lower respiratory tract infections early in life as a risk factor for the subsequent development of asthma (Piedimonte and Simoes 2002). In particular, it has been suggested that respiratory syncytial virus (RSV) infection may enhance the development of “allergic” inflammatory responses when the host is exposed to allergens after an episode of bronchiolitis.
Although RSV infection is usually self-limited and the virus is cleared from the respiratory tract of immune-competent children within several weeks, there is growing evidence to suggest that RSV infection may have long-term sequelae in the developing respiratory system (Piedimonte 2002). In fact, epidemiologic evidence from several retrospective studies as well as from more recent well-controlled prospective studies supports the association between early life RSV lower respiratory tract illness and recurrent episodes of wheezing and the development of asthma during the first decade of life (Sigurs et al. 2000; Stein et al. 1999). Indeed, RSV bronchiolitis and asthma share several clinical features (wheezing, increased work of breathing, tachypnea, and reversible changes in pulmonary function), but they also differ substantially in terms of response to bronchodilator and anti-inflammatory therapies. Despite extensive research, the precise molecular mechanisms and pathways by which RSV infection causes airway inflammation and affects long-term control of airway function subsequent to the initial insult remain unclear.
Viral infection and neuroimmune interactions.
Compromised epithelial integrity, the elaboration of local proinflammatory mediators, and dysfunction of neural pathways may influence airway responses to environmental stimuli. Some investigators postulate that infection with RSV or other viral pathogens can precipitate an imbalance in local cell-mediated immune responses (Lemanske 1998). Others hypothesize that infant bronchiolitis may result in alterations to neuronal pathways that influence airway smooth muscle tone and airway patency via the release of neurotransmitters (Larsen and Colasurdo 1999). Piedimonte has proposed that combined neuroimmune interactions primed by the virus can initiate and propagate a cascade of events leading to recurrent cycles of airway inflammation and obstruction (Figure 2) (Piedimonte 2001).
Figure 2.
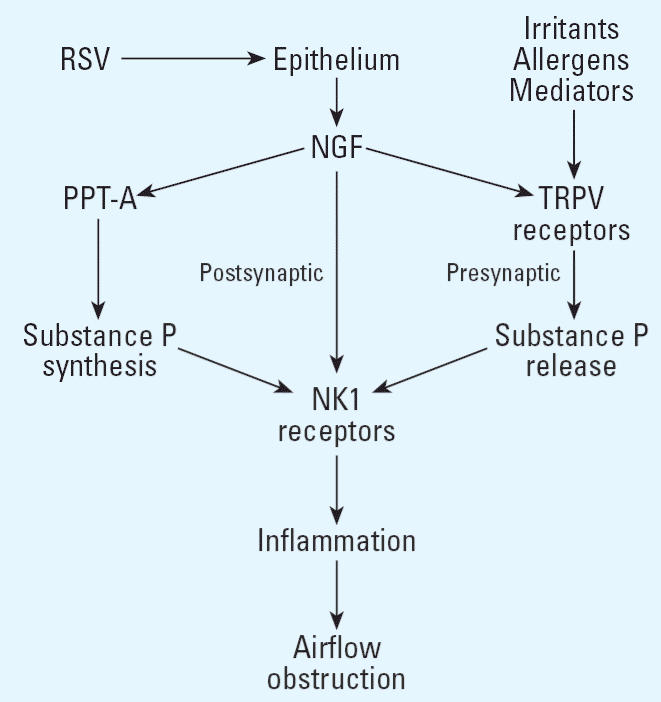
Viral infection and neuroimmune interactions. Abbreviations: RSV, respiratory syncytial virus; NGF, nerve growth factor; PPT-A, pre-protachykinin A; NK1, neurokinin 1; TRPV, transient receptor potential vanilloid.
In the airway, a dense network of sensory nerve fibers is strategically placed just below the epithelial surface, so that any change in the bronchial environment may stimulate the release of the proinflammatory neuropeptide substance P (Piedimonte 1995). During RSV infection, stimulation of these nerves causes a marked increase in airway vascular permeability and results in an increase in overall inflammatory status (Piedimonte et al. 1999). Our work has revealed that these changes are mediated by the high affinity receptor for substance P (NK1 receptor), the expression of which is greatly increased by RSV (King et al. 2001; Piedimonte et al. 1999). This up-regulation presumably occurs at the pretranslational level because NK1 receptor mRNA levels increase substantially during RSV infection. We have also shown that T-lymphocyte subpopulations, predominantly CD4+ cells, within the bronchial-associated lymphoid tissue (BALT) of RSV-infected lungs express high levels of the NK1 receptor (Auais et al. 2003). As a consequence, stimulation of the sensory nerves by airborne irritants has the potential to cause a new inflammatory cycle that is mediated by the attraction of NK1 receptor–expressing T-helper lymphocytes and monocytes into the airway and activated by substance P. This mechanism may establish important neuroimmune interactions that undergo long-term dysregulation following RSV infection and predispose to airway inflammation and hyperreactivity.
Viral infection, mast cells, and leukotrienes.
RSV also dramatically affects the distribution and function of mast cells in the airway mucosa (Wedde-Beer et al. 2002). Histopathological analysis with an antibody against tryptase identified numerous mast cells in sections from RSV-infected lungs, with an approximately 7-fold increase compared with the lungs of non-infected controls. In addition, most of these mast cells were in close spatial association with nerve fibers, suggesting functional mast cell–nerve interactions similar to those previously reported in other organ systems, particularly the skin, central nervous system, and gastrointestinal tract (Bauer and Razin 2000). Among the inflammatory mediators released from mast cells, cysteinyl leukotrienes (cysLTs) have been shown to cause airway inflammation and airway smooth muscle contraction during RSV infection, accounting for the wheezing observed in bronchiolitis. Increased leukotriene C4 (LTC4) levels were observed in nasopharyngeal secretions of children during the acute phase of RSV infection, and their concentration correlated with clinical severity, being higher in patients with lower respiratory tract involvement than in children with upper respiratory illness alone (van Schaik et al. 1999; Volovitz et al. 1988). Furthermore, cysLTs play critical roles in the pathophysiology of asthma and could represent an important component in the link between RSV and asthma.
Time course analysis of infected lung tissues indicated that the effect of RSV on 5-lipoxy-genase (5-LO) gene expression is transient; levels are maximal by 3 days postinoculation, already reduced by 5 days, and resolved by 30 days (Wedde-Beer et al. 2002). A similar profile was observed for the concentration of cysLTs in the same tissues, with almost complete return to pathogen-free levels by 5 days postinoculation. These findings suggest that the exaggerated neurogenic inflammation in the intrapulmonary airways infected by RSV in early life involves the concomitant release of cysLTs and activation of the cysLT1 receptor, as manifested by the potent inhibitory effect of the receptor antagonist montelukast on neurogenic-mediated vascular leakage.
On the basis of these studies, we speculate that following the early phase of the viral respiratory infection, leukotriene production and release rapidly return to baseline levels, but they can be reactivated by stimulation of the numerous mast cells still present in the lung tissues, for example, by substance P released upon stimulation of sensory nerve terminals. Another implication of these data is that the increased susceptibility of RSV-infected intra-pulmonary airways to the inflammatory effects of sensory nerves may be dependent, at least in part, on increased neurostimulation of mucosal mast cells, with consequent release of cysLTs. This effect, in turn, can amplify the release of tachykinins from sensory nerves, thereby forming a local neuron-mast cell feedback loop.
Viral infection, nerve growth factor, and neurotrophins.
Recent studies show that RSV infection promotes a large increase in the expression of nerve growth factor (NGF) and neurotrophin receptors (Hu et al. 2002). NGF was the first discovered component of the neurotrophin family (Levi-Montalcini 1987), which includes the brain-derived neurotrophic factor (BDNF) and the neurotrophins 3 (NT-3) and 4/5 (NT-4/5). Neurotrophins modulate survival, differentiation and apoptosis of peripheral afferent and efferent neurons, and specifically control the expression of genes that encode the precursors of substance P and other peptide neurotransmitters. These effects are mediated by binding to high-affinity tyrosine kinase (trk) receptors (generally promoting neuron survival and differentiation) or to the low-affinity panneurotrophin receptor p75 (generally mediating apoptosis and death). The high-affinity receptor for NGF is the trkA subtype (Kernie and Parada 2000). Neurotrophins exert changes in the functional activity of peripheral neurons in a number of ways that collectively define “neuronal plasticity” (Renz 2001). Examples from studies in vitro and in vivo include increased production of neuro-transmitters, increased number of nerves that produce specific neuropeptides, and increased neurotransmitter release from nerve terminals mediated by increased expression and function of the vanilloid receptor TRPV1 (the capsaicin receptor). NGF is also synthesized in several nonneuronal cell types including epithelial and inflammatory cells (e.g., mast cells and CD4+ T cells) that also express trk receptors (Ehrhard et al. 1993; Leon et al. 1994; Nilsson et al. 1997). This function may target the innervation of specific tissues, but there is growing evidence that NGF functions as a potent and eclectic neuroimmunomodulator that releases and is released by a variety of inflammatory mediators. In particular, patients with bronchial asthma and allergic rhinoconjunctivitis display high serum levels of NGF, thereby suggesting an important pathogenetic role of neurotrophins in allergic disorders (Braun et al. 1999).
Because NGF is released from airway epithelial cells, increases the production and release of substance P and other tachykinins from adult sensory neurons, and induces sensory hyperinnervation in the airways of transgenic mice, it represents an ideal link between virus-infected respiratory epithelium and the dense subepithelial network of unmyelinated sensory fibers. RSV-induced release of NGF may lead to short- and long-term changes in the distribution and reactivity of sensory nerves across the respiratory tract, thus participating in exaggerated inflammatory reactions during and after the infection. NGF and its receptors may also amplify other immunologic and neuronal pathways contributing to airway inflammation and hyperreactivity. On the basis of these observations, we postulate that changes of neurotrophin expression in the respiratory tract may coordinate a variety of interactions between sensory afferent nerves and multiple components of the immune system and inflammatory pathways, thereby generating a pathophysiological link between early-life viral infections and childhood asthma.
The Role of Endotoxin in Asthma
Allergens—such as those that derive from pollens, pets, rodents, cockroaches, house dust mites, or foods—might be considered harmless environmental antigens. Such antigens are recognized by the immune system, and the “normal” immune response is the development of clinical tolerance. In allergy and asthma, such antigens are recognized as “dangerous,” and the immune systems mounts an inflammatory response characterized by proliferation and activation of TH2 cells. Two key questions arise from this concept. First, how is the development of clinical tolerance regulated? Second, why is the immune system of atopic individuals not able to develop in this fashion?
Role of early-life exposures and the hygiene hypothesis.
Increasing evidence suggests that prenatal and early postnatal environmental determinants play an important role in the development of allergy and asthma. Tolerance programming starts in early life, even before birth. Indeed, the presence of allergen-specific T cells has been demonstrated in humans at the time of birth, thus suggesting that specific immune responses can develop in utero (Prescott et al. 1999; Szepfalusi et al. 1997). Moreover, transplacental allergen transfer has been demonstrated in animals and humans (Holloway et al. 2000). Maturation of the fetal immune system occurs primarily during the first two trimesters of pregnancy. The development of clinical tolerance continues after birth and the first 2 years of life seems to be particularly important (Prescott et al. 1998; Prescott et al. 1999).
It is now well recognized that natural exposure to microbes through mucosal surfaces in the gastrointestinal tract, respiratory tract, and skin are critical for the development of clinical tolerance. These observations are directly linked to the “hygiene hypothesis,” which states that exposure to microbial antigens plays an important role in immunoprotection and is required for the development of clinical tolerance (Renz and Herz 2002). In fact, microbes are now viewed as important immunoregulators in addition to their role as pathogens. How are these facts linked to the development of allergy and asthma? Recent longitudinal and cross-sectional cohort studies have found that the traditional farming environment in the European Alps protects against the development of allergy and asthma (Braun-Fahrlander et al. 2002; von Mutius et al. 2000). Two factors were identified that presumably transmit this protection during the early postnatal period (the first year of life): consumption of raw (nonpasteurized) milk and daily exposure to farm animals (Braun-Fahrlander et al. 2002; von Mutius et al. 2000). To identify further the microbial components involved in this protection, investigators collected dust samples from over 800 families, and endotoxin (bacterial lipopolysaccharide or LPS) measurements were made. The results indicate a strong inverse association between natural, chronic exposure to endotoxin and the risk of allergic sensitization and clinical manifestations of respiratory tract allergy and asthma (Braun-Fahrlander et al. 2002).
Endotoxin and the immune system.
The system of LPS recognition is highly complex and involves multiple components of the innate immune system. Recently, several molecules have been identified that play critical roles in this context. The LPS binding protein (LBP) acts as a carrier of LPS. This complex assembles with soluble or membrane bound CD14 molecules and allows recognition by the toll-like receptor 4 (TLR4) on the surface of immune cells such as macrophages. A schematic of this complex recognition system is illustrated in Figure 3.
Figure 3.
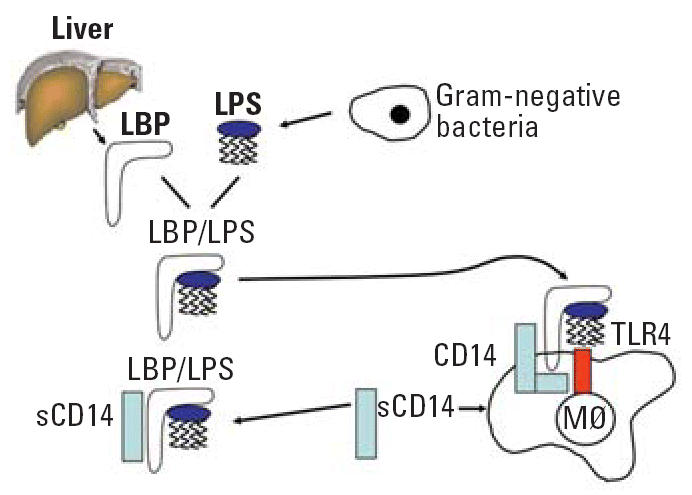
LPS recognition system. Abbreviation: LPS, lipopolysaccharide; LBP, lipopolysaccharide binding protein; CD, cluster of differentiation; sCD14, soluble CD14; TLR4, toll-like receptor 4; Mφ, macrophage.
To test further the concept that LPS exposure is linked to protection against the development of respiratory allergies, animal studies were conducted. Exposure of adult mice to LPS suppressed IgE production, airway inflammation, and development of bronchial hyperresponsiveness (Gerhold et al. 2003). LPS acted in a dose-dependent manner; high-dose exposure (equivalent to 100 μg LPS intranasally) promoted TH1 immune responses, and low-dose exposure (0.1 μg LPS intra-nasally) had a proallergic effect (Eisenbarth et al. 2002). To explore further the role of LPS in this process, a murine model of prenatal allergen exposure has been used. In this model LPS was administered intranasally to pregnant mice. Offspring were then sensitized to a conventional allergen (ovalbumin, OVA) followed by OVA aerosol challenges to induce experimental asthma. At birth, mice from LPS-exposed mothers had an elevated neonatal IFN-γ response. When these mice were sensitized to OVA, the development of anti-OVA IgE and IgG1 antibodies was markedly suppressed, whereas the levels of anti-OVA-IgG2a antibodies remained unchanged (Blumer et al. 2005). Furthermore, splenic mononuclear cells re-exposed in vitro to OVA produced significantly less IL-5 and IL-13 but not IFN-γ, thus indicating a selective suppression of the TH2 arm of the immune system. This effect was also reflected in the analysis of bronchoalveolar lavage fluid following OVA aerosol challenges. The influx of eosinophils, macrophages, and lymphocytes into the airways was also markedly suppressed; however, these mice remained hyperresponsive to metacholine. Together, these data provide experimental evidence that prenatal exposure to a microbial component such as LPS can modify the immune response to allergen exposure later in life. Further experiments are now under way to delineate the precise molecular mechanisms responsible for this effect.
Other microbial components as immunomodulators.
Bacterial LPS is not the only microbial component that can act as an immunomodulator. In the studies cited above of European farmers, a polymorphism in the TLR-2 promoter has been associated with reduced allergic sensitization, asthma and hay fever (Eder et al. 2004). TLR-2 recognizes, among other things, peptidoglycans primarily produced by gram-positive bacteria, lipoprotein and zymosan, which is a component of yeast. Furthermore, the level of muramic acid, a major component of peptidoglycan that can be considered a marker for exposure to gram-positive bacteria, was inversely correlated with wheezing and asthma regardless of farming and endotoxin exposure (van Strien et al. 2004).
An updated hygiene hypothesis.
Although it is clear that the prenatal and early postnatal environment influences the development of allergy and asthma, the exact nature of this influence is not completely understood. The updated “hygiene hypothesis” states that microbial load and chronic exposure to microbial compounds play an important role in the development of clinical tolerance and subsequently confer protection against allergic diseases. Future studies will be necessary to define precisely the components of this protective microbial load. Timing and duration of exposure seem to be critical. In terms of the duration, it is necessary to distinguish acute and chronic events. Dosing also seems to be critical, as experimental studies clearly indicate a differential effect of low- and high-dose exposures. Furthermore, the route of exposure must be considered. Nonmucosal LPS exposure is clearly an unwanted phenomenon that triggers an inflammatory response, whereas mucosal LPS exposure seems to be of particular benefit. Delineation of these and other aspects of the biology of microbes as immunomodulators might lead to the development of new avenues of allergy prevention and treatment in near future.
Conclusion
In this article we have reviewed the role of allergens, viruses, and endotoxin in the development of allergy and asthma. While these agents may appear to be ubiquitous, there are variations in exposure to them that may affect the host. It seems likely that increasing endotoxin exposure and decreasing allergen and viral exposures would decrease development of allergic airway responses. The importance of these exposures cannot be overestimated, as they are sources of stimulatory ligands for lymphocytes and antigen-presenting cells. However, the complex immune and inflammatory interactions that result from exposure to these ligands are still not completely understood. As our understanding of the influence of these interactions on the development of allergy improves, novel interventions designed to modulate the host response to these asthmagenic exposures can be developed and implemented.
Footnotes
This article is part of the mini-monograph “Environmental Influences on the Induction and Incidence of Asthma.”
The authors thank S. London, S. Kleeberger, and M.J. Selgrade for helpful suggestions during preparation of this article.
This research was supported by the Intramural Research Program of the NIH, NIEHS (D.C.Z), NIH P01 ES09606 (P.E.), NIH R44 ES011920 (M.C.), NIH R01 HL61007 (G.P.), EU-QLK4-CT-2001-00250 (H.R.), and NIH R01 ES012706 (D.P.).
References
- ALLERbase 2004. Eat Right 4 Your Type (D’Adamo PJ, ed). Available: http://www/dadamo.com/allerbase/allerbase.cgi [accessed 1 October 2004].
- Allergome 2004. Allergome: A Database of Allergenic Molecules. Latina, Italy:Allergy Data Laboratories. Available: http://www.allergome.org [accessed 1 October 2004].
- Arlian LG, Platts-Mills TA. The biology of dust mites and the remediation of mite allergens in allergic disease. J Allergy Clin Immunol. 2001;107(suppl 3):S406–413. doi: 10.1067/mai.2001.113670. [DOI] [PubMed] [Google Scholar]
- Arruda LK, Vailes LD, Ferriani VP, Santos AB, Pomes A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol. 2001;107(3):419–428. doi: 10.1067/mai.2001.112854. [DOI] [PubMed] [Google Scholar]
- Arshad SH, Bateman B, Matthews SM. Primary prevention of asthma and atopy during childhood by allergen avoidance in infancy: a randomised controlled study. Thorax. 2003;58(6):489–493. doi: 10.1136/thorax.58.6.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad SH, Matthews S, Gant C, Hide DW. Effect of allergen avoidance on development of allergic disorders in infancy. Lancet. 1992;339(8808):1493–1497. doi: 10.1016/0140-6736(92)91260-f. [DOI] [PubMed] [Google Scholar]
- Auais A, Adkins B, Napchan G, Piedimonte G. Immunomodulatory effects of sensory nerves during respiratory syncytial virus infection in rats. Am J Physiol Lung Cell Mol Physiol. 2003;285:L105–L113. doi: 10.1152/ajplung.00004.2003. [DOI] [PubMed] [Google Scholar]
- Bauer O, Razin E. Mast cell-nerve interactions. News Physiol Sci. 2000;15:213–218. doi: 10.1152/physiologyonline.2000.15.5.213. [DOI] [PubMed] [Google Scholar]
- Blumer N, Herz U, Wegmann M, Renz H. Prenatal lipopolysaccharide exposure prevents allergic sensitization and airway inflammation, but not airway responsiveness, in a murine model of experimental asthma. Clin Exp Allergy. 2005;35:397–402. doi: 10.1111/j.1365-2222.2005.02184.x. [DOI] [PubMed] [Google Scholar]
- Bollinger ME, Eggleston PA, Flanagan E, Wood RA. Cat antigen in homes with and without cats may induce allergic symptoms. J Allergy Clin Immunol. 1996;97(4):907–914. doi: 10.1016/s0091-6749(96)80064-9. [DOI] [PubMed] [Google Scholar]
- Braun A, Lommatzsch M, Lewin GR, Virchow JC, Renz H. Neurotrophins: a link between airway inflammation and airway smooth muscle contractility in asthma. Int Arch Allergy Immunol. 1999;118:163–165. doi: 10.1159/000024056. [DOI] [PubMed] [Google Scholar]
- Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347(12):869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Smit J, de Jongste J, Neijens H, Gerritsen J, Postma D, et al. The prevention and incidence of asthma and mite allergy (PIAMA) birth cohort study: design and first results. Pediatr Allergy Immunol. 2002;13 (suppl 15):55–60. doi: 10.1034/j.1399-3038.13.s.15.1.x. [DOI] [PubMed] [Google Scholar]
- Bush R, Wood R, Eggleston P. Laboratory animal allergy. J Allergy Clin Immunol. 1998;102:99–112. doi: 10.1016/s0091-6749(98)70060-0. [DOI] [PubMed] [Google Scholar]
- Chapman MD, Smith AM, Vailes LD, Arruda LK, Dhanaraj V, Pomes A. Recombinant allergens for diagnosis and therapy of allergic disease. J Allergy Clin Immunol. 2000;106(3):409–418. doi: 10.1067/mai.2000.109832. [DOI] [PubMed] [Google Scholar]
- Chapman MD, Wood RA. The role and remediation of animal allergens in allergic diseases. J Allergy Clin Immunol. 2001;107(suppl 3):S414–421. doi: 10.1067/mai.2001.113672. [DOI] [PubMed] [Google Scholar]
- Creticos PS, Chen YH, Schroeder JT. New approaches in immunotherapy: allergen vaccination with immunostimulatory DNA. Immunol Allergy Clin North Am. 2004;24(4):569–581. doi: 10.1016/j.iac.2004.06.012. [DOI] [PubMed] [Google Scholar]
- CSL 2004. The Allergen Database. Sand Hutton, York, UK:Central Science Laboratory. Available: http://allergen.csl.gov.uk/ 1 October [accessed 2004].
- Custovic A, Green R, Fletcher A, Smith A, Pickering CA, Chapman MD, et al. Aerodynamic properties of the major dog allergen Can f 1: distribution in homes, concentration, and particle size of allergen in the air. Am J Respir Crit Care Med. 1997;155(1):94–98. doi: 10.1164/ajrccm.155.1.9001295. [DOI] [PubMed] [Google Scholar]
- Custovic A, Hallam CL, Simpson BM, Craven M, Simpson A, Woodcock A. Decreased prevalence of sensitization to cats with high exposure to cat allergen. J Allergy Clin Immunol. 2001;108(4):537–539. doi: 10.1067/mai.2001.118599. [DOI] [PubMed] [Google Scholar]
- Custovic A, Simpson BM, Simpson A, Hallam C, Craven M, Brutsche M, et al. Manchester Asthma and Allergy Study: low-allergen environment can be achieved and maintained during pregnancy and in early life. J Allergy Clin Immunol. 2000;105(2 pt 1):252–258. doi: 10.1016/s0091-6749(00)90073-3. [DOI] [PubMed] [Google Scholar]
- Derewenda U, Li J, Derewenda Z, Dauter Z, Mueller GA, Rule GS, et al. The crystal structure of a major dust mite allergen, Der p 2, and its biological implications. J Mol Biol. 2002;318(1):189–197. doi: 10.1016/S0022-2836(02)00027-X. [DOI] [PubMed] [Google Scholar]
- Dowse GK, Turner KJ, Stewart GA, Alpers MP, Woolcock AJ. The association between Dermatophagoides mites and the increasing prevalence of asthma in village communities within the Papua New Guinea highlands. J Allergy Clin Immunol. 1985;75(1 pt 1):75–83. doi: 10.1016/0091-6749(85)90016-8. [DOI] [PubMed] [Google Scholar]
- Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrlander C, et al. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol. 2004;113(3):482–488. doi: 10.1016/j.jaci.2003.12.374. [DOI] [PubMed] [Google Scholar]
- Eggleston PA, Arruda LK. Ecology and elimination of cockroaches and allergens in the home. J Allergy Clin Immunol. 2001;107(suppl 3):S422–429. doi: 10.1067/mai.2001.113671. [DOI] [PubMed] [Google Scholar]
- Ehrhard P, Erb P, Graumann U, Otten U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc Nat Acad Sci USA. 1993;90:10984–10988. doi: 10.1073/pnas.90.23.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196(12):1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARRP 2004. FARRP Allergen Database. Lincoln, NE:Food Allergy Research and Resource Program. Available: http://www.allergenonline.com [accessed 1 October 2004].
- Gerhold K, Bluemchen K, Franke A, Stock P, Hamelmann E. Exposure to endotoxin and allergen in early life and its effect on allergen sensitization in mice. J Allergy Clin Immunol. 2003;112(2):389–396. doi: 10.1067/mai.2003.1646. [DOI] [PubMed] [Google Scholar]
- Gold DR, Burge HA, Carey V, Milton DK, Platts-Mills T, Weiss ST. Predictors of repeated wheeze in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med. 1999;160(1):227–236. doi: 10.1164/ajrccm.160.1.9807104. [DOI] [PubMed] [Google Scholar]
- Gruber A, Mancek M, Wagner H, Kirschning CJ, Jerala R. Structural model of MD-2 and functional role of its basic amino acid clusters involved in cellular lipopolysaccharide recognition. J Biol Chem. 2004;279(27):28475–28482. doi: 10.1074/jbc.M400993200. [DOI] [PubMed] [Google Scholar]
- Gustchina A, Li M, Wunschmann S, Chapman MD, Pomes A, Wlodawer A. Crystal structure of cockroach allergen Bla g 2, an unusual zinc binding aspartic protease with a novel mode of self-inhibition. J Mol Biol. 2005;348:433–444. doi: 10.1016/j.jmb.2005.02.062. [DOI] [PubMed] [Google Scholar]
- Hesselmar B, Aberg N, Aberg B, Eriksson B, Bjorksten B. Does early exposure to cat or dog protect against later allergy development? Clin Exp Allergy. 1999;29(5):611–617. doi: 10.1046/j.1365-2222.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- Hewitt CR, Brown AP, Hart BJ, Pritchard DI. A major house dust mite allergen disrupts the immunoglobulin E network by selectively cleaving CD23: innate protection by antiproteases. J Exp Med. 1995;182(5):1537–1544. doi: 10.1084/jem.182.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hide DW, Matthews S, Matthews L, Stevens M, Ridout S, Twiselton R, et al. Effect of allergen avoidance in infancy on allergic manifestations at age two years. J Allergy Clin Immunol. 1994;93(5):842–846. doi: 10.1016/0091-6749(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Hide DW, Matthews S, Tariq S, Arshad SH. Allergen avoidance in infancy and allergy at 4 years of age. Allergy. 1996;51(2):89–93. [PubMed] [Google Scholar]
- Holloway JA, Warner JO, Vance GH, Diaper ND, Warner JA, Jones CA. Detection of house-dust-mite allergen in amniotic fluid and umbilical-cord blood. Lancet. 2000;356(9245):1900–1902. doi: 10.1016/S0140-6736(00)03265-7. [DOI] [PubMed] [Google Scholar]
- Hu C, Wedde-Beer K, Auais A, Rodriguez MM, Piedimonte G. Nerve growth factor and nerve growth factor receptors in respiratory syncytial virus-infected lungs. Am J Physiol Lung Cell Mol Physiol. 2002;283:L494–L502. doi: 10.1152/ajplung.00414.2001. [DOI] [PubMed] [Google Scholar]
- IUIS 2005. List of Allergens. Vienna, Austria:International Union of Immunological Societies. Available: www.iuisonline.org [accessed 1 October 2004].
- Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–613. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Kaiser L, Gronlund H, Sandalova T, Ljunggren HG, Achour A, Schneider G, et al. Three-dimensional structure of Fel d 1, the major allergen in cat. Int Arch Allergy Immunol. 2003a;132(1):25–26. doi: 10.1159/000073261. [DOI] [PubMed] [Google Scholar]
- Kaiser L, Gronlund H, Sandalova T, Ljunggren HG, van Hage-Hamsten M, Achour A, et al. The crystal structure of the major cat allergen Fel d 1, a member of the secretoglobin family. J Biol Chem. 2003b;278(39):37730–37735. doi: 10.1074/jbc.M304740200. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Parada LF. The molecular basis for understanding neurotrophins and their relevance to neurologic disease. Arch Neurol. 2000;57:654–657. doi: 10.1001/archneur.57.5.654. [DOI] [PubMed] [Google Scholar]
- King C, Brennan S, Thompson PJ, Stewart GA. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. J Immunol. 1998;161(7):3645–3651. [PubMed] [Google Scholar]
- King KA, Hu C, Rodriguez MM, Romaguera R, Jiang X, Piedimonte G. Exaggerated neurogenic inflammation and substance P receptor upregulation in RSV-infected weanling rats. Am J Respir Cell Mol Biol. 2001;24:101–107. doi: 10.1165/ajrcmb.24.2.4264. [DOI] [PubMed] [Google Scholar]
- Larsen GL, Colasurdo GN. Neural control mechanisms within airways: disruption by respiratory syncytial virus. J Pediatr. 1999;135:S21–S27. [PubMed] [Google Scholar]
- Lau S, Illi S, Sommerfeld C, Niggemann B, Bergmann R, von Mutius E, et al. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Multicentre Allergy Study Group. Lancet. 2000;356(9239):1392–1397. doi: 10.1016/s0140-6736(00)02842-7. [DOI] [PubMed] [Google Scholar]
- Lemanske JR. 1998. Immunologic mechanisms in RSV-related allergy and asthma. In: RSV and Asthma: Is There a Link? (Hiatt PW, ed). New York:American Thoracic Society, 11–16.
- Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, et al. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci USA. 1994;91:3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Lowe L, Murray CS, Custovic A, Simpson BM, Kissen PM, Woodcock A. Specific airway resistance in 3-year-old children: a prospective cohort study. Lancet. 2002;359(9321):1904–1908. doi: 10.1016/S0140-6736(02)08781-0. [DOI] [PubMed] [Google Scholar]
- Luczynska CM, Li Y, Chapman MD, Platts-Mills TA. Airborne concentrations and particle size distribution of allergen derived from domestic cats (Felis domesticus). measurements using cascade impactor, liquid impinger, and a two-site monoclonal antibody assay for Fel d I. Am Rev Respir Dis. 1990;141(2):361–367. doi: 10.1164/ajrccm/141.2.361. [DOI] [PubMed] [Google Scholar]
- Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332(3):133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- Mitakakis TZ, Barnes C, Tovey ER. Spore germination increases allergen release from Alternaria. J Allergy Clin Immunol. 2001;107(2):388–390. doi: 10.1067/mai.2001.112602. [DOI] [PubMed] [Google Scholar]
- Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci USA. 2004;101(suppl 2):14677–14682. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G, Forsberg-Nilsson K, Xiang Z, Hallbook F, Nilsson K, Metcalfe D. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur J Immunol. 1997;27:2295–2301. doi: 10.1002/eji.1830270925. [DOI] [PubMed] [Google Scholar]
- Park JH, Gold DR, Spiegelman DL, Burge HA, Milton DK. House dust endotoxin and wheeze in the first year of life. Am J Respir Crit Care Med. 2001;163(2):322–328. doi: 10.1164/ajrccm.163.2.2002088. [DOI] [PubMed] [Google Scholar]
- Phipatanakul W, Cronin B, Wood RA, Eggleston PA, Shih MC, Song L, et al. Effect of environmental intervention on mouse allergen levels in homes of inner-city Boston children with asthma. Ann Allergy Asthma Immunol. 2004;92(4):420–425. doi: 10.1016/S1081-1206(10)61777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedimonte G. Tachykinin peptides, receptors, and peptidases in airway disease. Exp Lung Res. 1995;21:809–834. doi: 10.3109/01902149509031765. [DOI] [PubMed] [Google Scholar]
- Piedimonte G. Neural mechanisms of respiratory syncytial virus-induced inflammation and prevention of respiratory syncytial virus sequelae. Am J Respir Crit Care Med. 2001;163:S18–S21. doi: 10.1164/ajrccm.163.supplement_1.2011113. [DOI] [PubMed] [Google Scholar]
- Piedimonte G. The association between respiratory syncytial virus infection and reactive airway disease. Respir Med. 2002;96(suppl B):S26–S30. [PubMed] [Google Scholar]
- Piedimonte G, Rodriguez MM, King KA, McLean S, Jiang X. Respiratory syncytial virus upregulates expression of the substance P receptor in rat lungs. Am J Physiol Lung Cell Mol Physiol. 1999;277:L831–L840. doi: 10.1152/ajplung.1999.277.4.L831. [DOI] [PubMed] [Google Scholar]
- Piedimonte G, Simoes EA. Respiratory syncytial virus and subsequent asthma: one step closer to unraveling the Gordian knot? Eur Respir J. 2002;20:515–517. doi: 10.1183/09031936.02.00404602. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357(9258):752–756. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TA, Chapman MD. Dust mites: immunology, allergic disease, and environmental control. J Allergy Clin Immunol. 1987;80(6):755–775. doi: 10.1016/s0091-6749(87)80261-0. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100(6 pt 1):S2–S24. doi: 10.1016/s0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TA, Woodfolk JA, Erwin EA, Aalberse R. Mechanisms of tolerance to inhalant allergens: the relevance of a modified Th2 response to allergens from domestic animals. Springer Semin Immunopathol. 2004;25(3–4):271–279. doi: 10.1007/s00281-003-0149-8. [DOI] [PubMed] [Google Scholar]
- Pomes A, Chapman MD, Vailes LD, Blundell TL, Dhanaraj V. Cockroach allergen Bla g 2: structure, function, and implications for allergic sensitization. Am J Respir Crit Care Med. 2002;165(3):391–397. doi: 10.1164/ajrccm.165.3.2104027. [DOI] [PubMed] [Google Scholar]
- Pomes A, Smith AM, Gregoire C, Vailes LD, Arruda LK, Chapman MD. Functional properties of cloned allergens from dust mite, cockroach and cat. Allergy Clin Immunol Inter. 2001;13:162–169. [Google Scholar]
- Prescott SL, Macaubas C, Holt BJ, Smallacombe TB, Loh R, Sly PD, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol. 1998;160(10):4730–4737. [PubMed] [Google Scholar]
- Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353(9148):196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- Protall 2004. Food Allergens of Plant Origin. Colney, Norwich, UK:Institute of Food Research. Available: http://www.ifr.bbsrc.ac.uk/protall/ [accessed 1 October 2004].
- Reefer AJ, Carneiro RM, Custis NJ, Platts-Mills TA, Sung SS, Hammer J, et al. A role for IL-10-mediated HLA-DR7-restricted T cell-dependent events in development of the modified Th2 response to cat allergen. J Immunol. 2004;172(5):2763–2772. doi: 10.4049/jimmunol.172.5.2763. [DOI] [PubMed] [Google Scholar]
- Renz H. Neurotrophins in bronchial asthma. Respir Res. 2001;2:265–268. doi: 10.1186/rr66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz H, Herz U. The bidirectional capacity of bacterial antigens to modulate allergy and asthma. Eur Respir J. 2002;19(1):158–171. doi: 10.1183/09031936.02.00254202. [DOI] [PubMed] [Google Scholar]
- Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336(19):1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349(15):1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- Shakib F, Schulz O, Sewell H. A mite subversive: cleavage of CD23 and CD25 by Der p 1 enhances allergenicity. Immunol Today. 1998;19(7):313–316. doi: 10.1016/s0167-5699(98)01284-5. [DOI] [PubMed] [Google Scholar]
- Sharma S, Lackie PM, Holgate ST. Uneasy breather: the implications of dust mite allergens. Clin Exp Allergy. 2003;33(2):163–165. doi: 10.1046/j.1365-2222.2003.01605.x. [DOI] [PubMed] [Google Scholar]
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Cell Mol Biol. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- Smith AM, Benjamin DC, Hozic N, Derewenda U, Smith WA, Thomas WR, et al. The molecular basis of antigenic cross-reactivity between the group 2 mite allergens. J Allergy Clin Immunol. 2001;107(6):977–984. doi: 10.1067/mai.2001.115629. [DOI] [PubMed] [Google Scholar]
- Stein RT, Martinez FD. Asthma phenotypes in childhood: lessons from an epidemiological approach. Paediatr Respir Rev. 2004;5(2):155–161. doi: 10.1016/j.prrv.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Stein RT, Sherril D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- Swanson MC, Campbell AR, Klauck MJ, Reed CE. Correlations between levels of mite and cat allergens in settled and airborne dust. J Allergy Clin Immunol. 1989;83(4):776–783. doi: 10.1016/0091-6749(89)90014-6. [DOI] [PubMed] [Google Scholar]
- Szepfalusi Z, Nentwich I, Gerstmayr M, Jost E, Todoran L, Gratzl R, et al. Prenatal allergen contact with milk proteins. Clin Exp Allergy. 1997;27(1):28–35. [PubMed] [Google Scholar]
- UTMB 2004. SDAP: Structural Database of Allergic Proteins. Galveston, TX:University of Texas Medical Branch. Available: http://fermi.utmb.edu/SDAP [accessed 1 October 2004].
- van Schaik SM, Tristram DA, Nagpal IS, Hintz KM, Welliver RCI, Welliver RC. Increased production of IFN-gamma and cysteinyl leukotrienes in virus-induced wheezing. J Allergy Clin Immunol. 1999;103:630–636. doi: 10.1016/s0091-6749(99)70235-6. [DOI] [PubMed] [Google Scholar]
- van Strien RT, Engel R, Holst O, Bufe A, Eder W, Waser M, et al. Microbial exposure of rural school children, as assessed by levels of N-acetyl-muramic acid in mattress dust, and its association with respiratory health. J Allergy Clin Immunol. 2004;113(5):860–867. doi: 10.1016/j.jaci.2004.01.783. [DOI] [PubMed] [Google Scholar]
- Volovitz B, Welliver RC, De Castro G, Krystofik D, Ogra PL. The release of leukotrienes in the respiratory tract during infection with respiratory syncytial virus: role in obstructive airway disease. Pediatr Res. 1988;24:504–507. doi: 10.1203/00006450-198810000-00018. [DOI] [PubMed] [Google Scholar]
- von Mutius E, Braun-Fahrlander C, Schierl R, Riedler J, Ehlermann S, Maisch S, et al. Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin Exp Allergy. 2000;30(9):1230–1234. doi: 10.1046/j.1365-2222.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- Wahn U, Bergmann R, Kulig M, Forster J, Bauer CP. The natural course of sensitisation and atopic disease in infancy and childhood. Pediatr Allergy Immunol. 1997;8(suppl 10):16–20. [PubMed] [Google Scholar]
- Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104(1):123–133. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedde-Beer K, Hu C, Rodriguez MM, Piedimonte G. Leukotrienes mediate neurogenic inflammation in lungs of young rats infected with respiratory syncytial virus. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1143–L1150. doi: 10.1152/ajplung.00323.2001. [DOI] [PubMed] [Google Scholar]
- Wood RA, Chapman MD, Adkinson NF, Jr, Eggleston PA. The effect of cat removal on allergen content in household-dust samples. J Allergy Clin Immunol. 1989;83(4):730–734. doi: 10.1016/0091-6749(89)90006-7. [DOI] [PubMed] [Google Scholar]
- Wood RA, Johnson EF, Van Natta ML, Chen PH, Eggleston PA. A placebo-controlled trial of a HEPA air cleaner in the treatment of cat allergy. Am J Respir Crit Care Med. 1998;158(1):115–120. doi: 10.1164/ajrccm.158.1.9712110. [DOI] [PubMed] [Google Scholar]
- Woodcock A, Lowe LA, Murray CS, Simpson BM, Pipis SD, Kissen P, et al. Early life environmental control: effect on symptoms, sensitization, and lung function at age 3 years. Am J Respir Crit Care Med. 2004;170(4):433–439. doi: 10.1164/rccm.200401-083OC. [DOI] [PubMed] [Google Scholar]