Abstract
TGF-β signaling involves a wide array of signaling molecules and multiple controlling events. Scaffold proteins create a functional proximity of signaling molecules and control the specificity of signal transduction. While many components involved in the TGF-β pathway have been elucidated, little is known about how those components are coordinated by scaffold proteins. Here, we show that Axin activates TGF-β signaling by forming a multimeric complex consisting of Smad7 and ubiquitin E3 ligase Arkadia. Axin depends on Arkadia to facilitate TGF-β signaling, as their small interfering RNAs reciprocally abolished the stimulatory effect on TGF-β signaling. Specific knockdown of Axin or Arkadia revealed that Axin and Arkadia cooperate with each other in promoting Smad7 ubiquitination. Pulse-chase experiments further illustrated that Axin significantly decreased the half-life of Smad7. Axin also induces nuclear export of Smad7. Interestingly, Axin associates with Arkadia and Smad7 independently of TGF-β signal, in contrast to its transient association with inactive Smad3. However, coexpression of Wnt-1 reduced Smad7 ubiquitination by downregulating Axin levels, underscoring the importance of Axin as an intrinsic regulator in TGF-β signaling.
Keywords: Arkadia, Axin, Smad7, TGF-β, ubiquitination
Introduction
TGF-β signaling plays a wide spectrum of roles from early embryonic development to mature tissues in controlling biological processes, including cell proliferation, differentiation, apoptosis, and cell fate determination (Massague, 2000). TGF-β superfamily members elicit biological responses through a complex cascade of signaling molecules that include receptors, Smads, and importantly the regulatory factors that positively or negatively regulate the signaling system (Shi and Massague, 2003).
The TGF-β superfamily can be divided into TGF-β, bone morphogenetic proteins (BMP), and Nodal. Schematically, much has been known about the molecular components involved in TGF-β signaling. TGF-β ligands bind to their receptors that are associated with a family of signal transducers called Smads (R-Smads) (Massague, 1998). TGF-β, BMP, and Nodal receptors make use of different sets of Smads. Upon ligand stimulation, different R-Smads bind to common mediator Smad4 (Co-Smad) to form activated Smad complex that is then translocated into the nucleus to regulate transcription of target genes (Massague and Chen, 2000; Massague and Wotton, 2000). The third subclass of Smads is comprised of inhibitory Smads (I-Smads), consisting of Smad6 and Smad7 in vertebrates. I-Smads inhibit TGF-β signaling through binding to activated type I receptors and competing with R-Smads for receptor interaction, and by recruiting the receptor for degradation (Hayashi et al, 1997; Imamura et al, 1997; Nakao et al, 1997; Hata et al, 1998).
Many ubiquitin ligases have been implicated in fine-tuning the levels of TGF-β signaling components including receptors, R-Smads and I-Smads. Smurf-1, an HECT type E3 ligase, was shown to bind to and cause the ubiquitin-mediated degradation of Smad1 and Smad5 (Zhu et al, 1999; Datto and Wang, 2005; Yamashita et al, 2005). In addition, Smurfs are recruited by inhibitory Smad6 and Smad7 to TGF-β and BMP receptors, resulting in the degradation of these receptors (Kavsak et al, 2000; Ebisawa et al, 2001; Suzuki et al, 2002; Murakami et al, 2003). However, a recent report shows that genetic disruption of the Smurf-1 gene does not alter the canonical Smad-mediated TGF-β or BMP signaling, but instead enhances the JNK MAPK cascade (Yamashita et al, 2005). Detailed analysis has shown that ubiquitination and degradation of MEKK2, an upstream kinase of JNK, was impaired in the Smurf1-deficient osteoblasts. Together with the finding that Smurf1 physically interacts with MEKK2, those observations suggest that Smurf is an E3 ligase for MEKK2 and that in Smurf−/− mice accumulated MEKK2 elevates JNK activity, at least in osteoblasts, leading to an age-dependent increase of bone mass seen in the mutant mice. Most recently, Ectodermin, another E3 ligase that possesses a RING finger on its N-terminal region, has been shown to play a crucial role in specification of the ectoderm by limiting the mesoderm-inducing activity of TGF-β by targeting Smad4 to ubiquitination and degradation (Dupont et al, 2005).
Arkadia was originally identified through an insertional mutagenesis in mice. Mice with Arkadia disrupted exhibit abnormal formation of the mammalian organizer during early embryogenesis (Episkopou et al, 2001; Niederlander et al, 2001). In Arkadia mutant embryos, anterior structures such as midbrain and forebrains are lost by mid-neurula stages of development. Structurally, it contains a RING finger domain in its C-terminal region that is responsible for its E3 ligase activity. Based on a genetic crossmating between heterozygous Arkadia and Nodal mice, it was revealed that Arkadia plays a role in Nodal signaling, and that Arkadia depends on Nodal in the induction of nodes. It was later shown that Arkadia enhances TGF-β signaling by physically interacting with, and inducing polyubiquitination and subsequent degradation of, the inhibitory Smad7 (Koinuma et al, 2003).
A remarkable feature in signaling transduction is that individual pathways rely on a group of proteins referred to as scaffolds. Major scaffolds include Axin in Wnt/β-catenin signaling (Salahshor and Woodgett, 2005), and JIP-1 in JNK MAP kinase signaling (Yasuda et al, 1999). These proteins are able to bind simultaneously to several components in the same signaling route, facilitating and augmenting specificity during signal transduction, presumably by changing conformation and providing molecular proximity towards one another. In the case of Axin in the Wnt pathway, in the absence of Wnt signal Axin interacts with APC, GSK3β, and casein kinase Iα to promote β-catenin phosphorylation by GSK3β, which leads to the degradation of β-catenin (Zeng et al, 1997; Hart et al, 1998; Peifer and Polakis, 2000; Liu et al, 2002). Whether such a scaffold exists in the TGF-β signaling pathway is unclear. SARA (Smad anchor for receptor activation) that binds to TGF-β receptors as well as Smad2/3 has been suggested to work as a scaffold protein to bring Smad substrates to the receptors and thus facilitate Smad activation (Tsukazaki et al, 1998). However, SARA is a membrane-bound protein mainly located in early endosomes, and is most likely confined to the perimembrane action (Hayes et al, 2002; Di Guglielmo et al, 2003). Here, we describe our finding that Axin is a major scaffold for TGF-β signaling. Remarkably, Axin interacts with not only Smad7 but also Arkadia. We also show that Axin2, which has been shown to be functionally equivalent to Axin (Chia and Costantini, 2005), also interacts with Arkadia and Smad7. Two-step co-immunoprecipitation experiment reveals that Arkadia, Axin, and Smad7 form a ternary complex. Axin enhances TGF-β signaling in an Arkadia-dependent manner. Axin sequesters Smad7 in the cytoplasm, where Arkadia facilitates Smad7 polyubiquitination and degradation. These data all suggest the possibility that Axin may well be a major scaffold in the TGF-β pathway, serving to promote Smad3 phosphorylation in response to TGF-β ligands (Furuhashi et al, 2001), and to downregulate negative factors such as Smad7.
Results
Identification of Arkadia and Smad7 as novel Axin-interacting proteins
In the course to identify new Axin-interacting proteins that may cooperate or antagonize the scaffolding roles of Axin in multiple pathways, we had previously employed a yeast two-hybrid screen using the C-terminus of Axin as a bait, and identified a variety of important factors (Rui et al, 2002). One of the clones such identified (designated as AIP7 for Axin-Interacting Protein 7) encodes an amino-acid sequence corresponding to aa (amino acids) 83–433 of Arkadia (Figure 1A). To test for the interaction in mammalian cells between Axin and Arkadia in vivo, we first raised and affinity-purified the antibody against Arkadia (for characterization of the Arkadia antibody, see Supplementary Figure 1). We then carried out immunoprecipitation using the lysates from HEK293T cells, with the newly raised anti-Arkadia and the anti-Axin C2b antibody as described (Rui et al, 2004). As shown in Figure 1B, Axin was readily detected in the precipitate by anti-Arkadia, and Arkadia detected in the immunoprecipitate of Axin, indicating that Axin indeed interacts with Arkadia at their endogenous levels.
Figure 1.
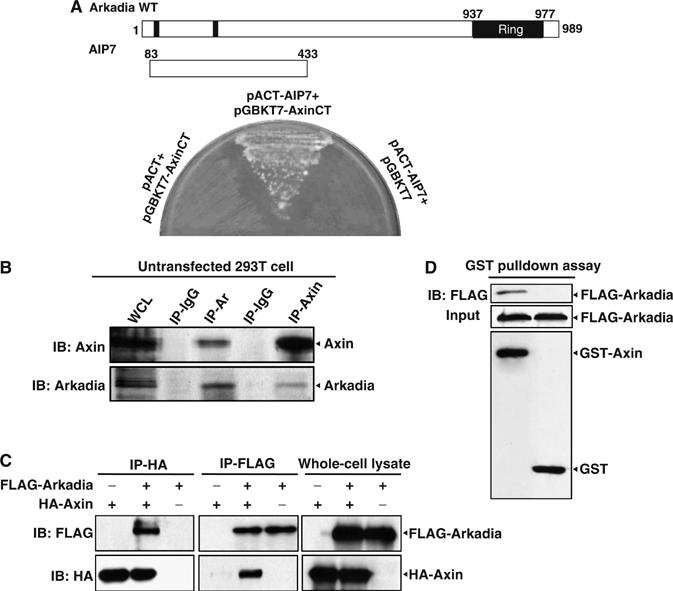
Identification of Arkadia as a novel Axin-interacting protein. (A) Yeast two-hybrid screening using Axin C-terminal as a bait was described previously (Rui et al, 2002). AIP7, one of the identified clones, contains a cDNA insert corresponding to aa 83–433 of Arkadia, as diagrammed on the top. AH109 cells cotransformed with pACT2-AIP7 and pGBKT7-AxinCT, but not the others, could grow on Ade−/Leu−/His−/Trp− medium plates. (B) Arkadia interacts with Axin at its endogenous levels. The 293T cells were treated with MG132 (10 μM for 4 h) before harvest. Cell lysates were immunoprecipitated with rabbit anti-Arkadia, rabbit anti-Axin, and control rabbit IgG, respectively, followed by immunoblotting with their respective antibodies as indicated. (C) Axin and Arkadia form complex in 293T cells. FLAG-tagged Arkadia and HA-tagged Axin were transfected either alone or together into HEK293T cells. At 32 h post-transfection, cells were treated for 4 h with 10 μM MG132, and were then subjected to immunoprecipitation, followed by Western blotting analysis with anti-HA or anti-FLAG as indicated. (D) GST pulldown assay. GST-Axin-fusion protein was expressed in E. coli cells and was purified as described previously (Rui et al, 2004); it was added to the lysate of MG132-treated 293T cells ectopically expressing FLAG-Arkadia. Note that only GST-Axin but not GST could pull down Arkadia. Experiments were repeated with essentially the same results.
We also carried out a reciprocal co-immunoprecipitation experiment using 293T cell lysates that contained ectopically expressed FLAG-tagged Arkadia and HA-tagged Axin (Figure 1C), and GST pulldown experiment (Figure 1D). The results also demonstrate that Axin and Arkadia strongly interact with each other.
Since it has been reported that the inhibitory Smad, Smad7, is a substrate of ubiquitin ligase Arkadia (Koinuma et al, 2003), we wondered if Axin might serve as a scaffold to bring I-Smad to the proximity of the E3 ligase for ubiquitination, which in turn facilitates TGF-β signaling. In particular, Smad3 has been shown to interact with Axin (Furuhashi et al, 2001). We cotransfected Axin separately with Smad3, Smad7 as well as other different Smads (indicated in Figure 2A), and carried out co-immunoprecipitation using anti-FLAG and anti-HA, respectively, for Smads and Axin (Figure 2A, left and right panels). Indeed, Axin interacted with Smad7, as strongly as with Smad3 (Figure 2A). In addition, Axin also bound to Smad6, albeit to a lesser extent. Immunoprecipitation using endogenous proteins in 293T cells also indicated that Axin interacts with Smad7 (Figure 2B).
Figure 2.
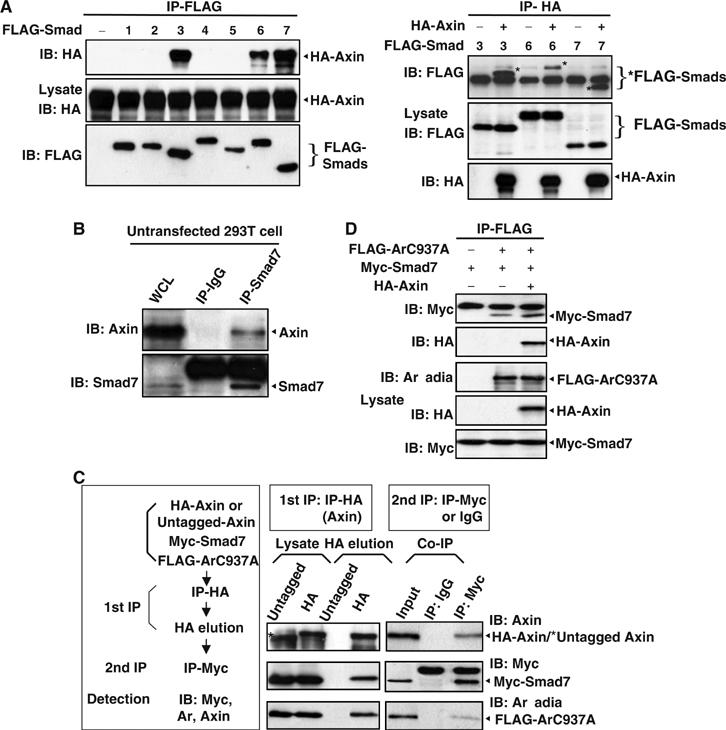
Axin interacts with Smad7, and Axin, Arkadia, and Smad7 form a ternary complex. (A) Identification of Smad7 as another novel Axin-interacting protein. FLAG-tagged Smads 1–7 were separately cotransfected with HA-Axin into 293T cells. Reciprocal immunoprecipitation with anti-FLAG and anti-HA was carried out, followed by Western blotting analysis. Smad3, 6, and 7 (marked by asterisks) were co-precipitated with Axin. (B) Endogenous Axin and Smad7 interact with each other in 293T cells. The 293T cell lysate was incubated with goat Smad7 polyclonal antibody (Santa Cruz Biotech.), followed by Western blotting with rabbit AxinC2b polyclonal antibody. Three separate experiments were carried out and similar results were obtained. (C) Two-step co-immunoprecipitation to test for ternary complex formation of Axin, Arkadia, and Smad7. The procedures of two-step co-immunoprecipitation are outlined in the left box. HEK293T cells were transfected with Myc-Smad7, FLAG-ArC937A, and HA-Axin (or untagged Axin as control, marked by asterisk). The first immunoprecipitation was performed with anti-HA antibody. The complex was eluted by using HA peptide, followed by the second step of immunoprecipitation with anti-Myc or control mouse IgG. Protein samples from each step were then subjected to Western blotting analysis separately by using anti-Axin, anti-Myc, and anti-Arkadia antibodies. The experiment was repeated with essentially the same result. (D) Axin increases the interaction affinity of Arkadia for Smad7. FLAG-ArC937A and Myc-Smad7 were cotransfected with or without Axin into 293T cells. Immunoprecipitation was carried out with anti-FLAG, followed by immunoblotting with anti-Myc to detect Smad7, and anti-HA to detect Axin. Increased amount of Myc-Smad7 was co-immunoprecipitated with Arkadia from cells coexpressing Axin.
Recently, it has been shown that Axin2/Conductin is functionally equivalent to Axin, at least as far as development is concerned (Chia and Costantini, 2005). This raised a critical question as to if Conductin can also facilitate TGF-β signaling. We generated the expression plasmid and carried out co-immunoprecipitation assay to address, first of all, whether Conductin also interacts with Smad7 and Arkadia. Indeed, Conductin was co-immunoprecipitated with Smad7 and Arkadia, underscoring the importance of Axin/Conductin functional linkage to the TGF-β pathway (see Supplementary Figure 2).
Axin, Arkadia, and Smad7 form a ternary complex
To further examine whether Axin, Arkadia, and Smad7 could form a ternary complex, we performed a two-step co-immunoprecipitation assay (Figure 2C) (Rui et al, 2004). As Arkadia is an E3 ubiquitin ligase, to prevent Arkadia-mediated protein degradation, an E3-defective mutant, ArC937A, was used in protein–protein interaction assay. HEK293T cells were transfected with HA–Axin, Myc-Smad7, and FLAG-ArC937A. As a control, Axin with no tag was transfected. In the first step of immunoprecipitation, anti-HA was used to pull down Axin, and HA peptide (Santa Cruz Biotech.) was used to elute the complex. The eluate was then immunoprecipitated with anti-Myc or control IgG, followed by Western blotting to detect Arkadia. As shown in Figure 2C, Arkadia was present in the final immunoprecipitate but not in the control sample, indicating that Axin, Smad7, and Arkadia are in a ternary complex. The observation that in the presence of overexpressed Axin higher levels of Myc-Smad7 were co-precipitated with FLAG-Arkadia is also consistent with a formation of the ternary complex, and indicates that Axin enhances the interaction of Arkadia with Smad7 (Figure 2D).
Determination of interaction regions of Axin with Arkadia and Smad7
The above data indicated that Arkadia and Smad7 each physically interact with Axin. We then determined the amino-acid regions of the three proteins that are required for their mutual interactions. Expression vectors containing wild-type as well as their different truncation mutants are indicated schematically (on the top of each panel, Figure 3). When HA–Axin-M1 alone was transfected, it was not present in the anti-FLAG (ArC937A) immunoco-precipitate (Figure 3A, first lane); however, when cotransfected with FLAG-ArC937A, full-length Axin was strongly co-immunoprecipitated with Arkadia. Axin deletion mutants C1, N2, and M1 retained their ability to form a complex with Arkadia, albeit with lower affinity compared to full-length Axin. However, Axin deletion mutants N1, C2, and ΔAr lost their ability to interact with Arkadia, indicating that the region around aa 507–757 in Axin is critical for Arkadia interaction.
Figure 3.
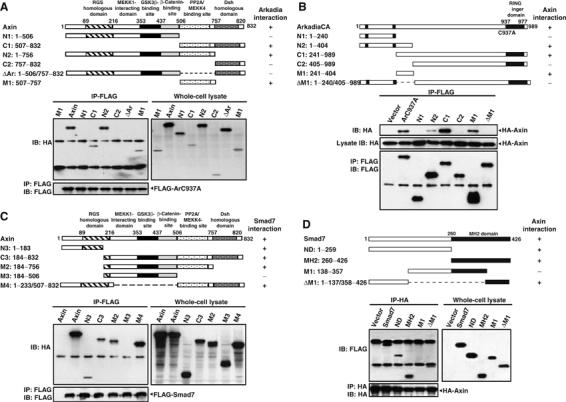
Determination of domains for interactions between Axin and Arkadia, Smad7. Structures of deletion mutants of Axin, Arkadia, and Smad7 are shown on the top of each panel. Functional domains of each protein are indicated above the schema and relative positions of the remaining fragment(s) in each deletion mutant are numbered on the left. HEK293T cells were cotransfected with different plasmid constructs as indicated. At 36 h post-transfection, cells were lysed and immunoprecipited with respective antibodies, followed by Western blotting. Of note, none of tagged deletion mutants when expressed alone was immunoprecipitated by the antibody against the other tag epitope. (A) Mapping of the domain in Axin for interaction with Arkadia. Shown on the top are schematic diagrams of the different Axin constructs used. The region around aa 507–757 in Axin is critical for Arkadia interaction. (B) Identification of Axin-binding sites on Arkadia. The RING domain of Arkadia (aa 937–977) is indicated. The ligase-defective mutant of Arkadia, ArC937A, was created by altering cysteine 937 to alanine. The region spanning aa 241–404 of Arkadia is responsible for Axin interaction. (C) Determination of Smad7-binding sites in Axin. Axin-N3 containing the N-terminal 183 aa showed partial Smad7-binding activity as compared to wild-type Axin; Axin-C3 and Axin-M2 that contain different C-terminal regions each also possessed partial activity to bind Smad7. Axin-M4 containing both the N- and C-terminal region retained a Smad7-binding affinity comparable to that of the wild-type Axin. (D) The MH2 domain (aa 260–426) is indicated. Note that both the N- and C-terminal regions of Smad7 are involved in the interaction with Axin.
We then cotransfected different deletion mutants of Arkadia with full-length Axin to 293T cells to determine the domain of Arkadia for Axin interaction. As shown in Figure 3B, the N1, C2, and ΔM1 deletion mutants could not interact with Axin, indicating that the region spanning aa 241–404 of Arkadia is critical for Axin interaction.
To determine the mutual interaction domains between Axin and Smad7, different constructs of Axin as indicated (on the top of Figure 3C) were cotransfected with Smad7 into 293T cells. The N-terminal region of aa 1–183 weakly interacted with Smad7; C-terminal region of aa 506–757 alone also showed partial binding ability. Of note, as C3 and M2 exhibited similar binding affinity for Smad7, the DIX domain of Axin (aa 757–832) is dispensable for Smad7 interaction. These results demonstrate that Axin requires both its N-terminal region (aa 1–183) and C-terminal region of aa 507–757 for maximal interaction with Smad7.
As shown in Figure 3D, among all the Smad7 mutants, only M1 that lacks both the N- and C-terminal regions failed to interact with Axin. Smad7 therefore appears to possess two domains for interaction with Axin, with either N- or MH2 domains alone capable of forming complex with Axin. It is interesting to note that both Axin and Smad7 require two regions for their interaction.
Axin colocalizes with Arkadia and Smad7
Next, we asked if Axin is colocalized in the cell with Arkadia and Smad7. We transfected FLAG-tagged Arkadia into COS-7 cells alone or together with Axin. When expressed alone, Arkadia was mainly localized in the nucleus in the absence of TGF-β (Figure 4A), in agreement with a previous report (Koinuma et al, 2003); when the cells were treated with TGF-β, Arkadia appeared to translocate into the cytoplasm and was distributed in the whole cell. In contrast, when coexpressed with Axin, even in the absence of TGF-β, Arkadia was translocated into the cytoplasm and was colocalized with Axin (Figure 4A, lower two panels).
Figure 4.
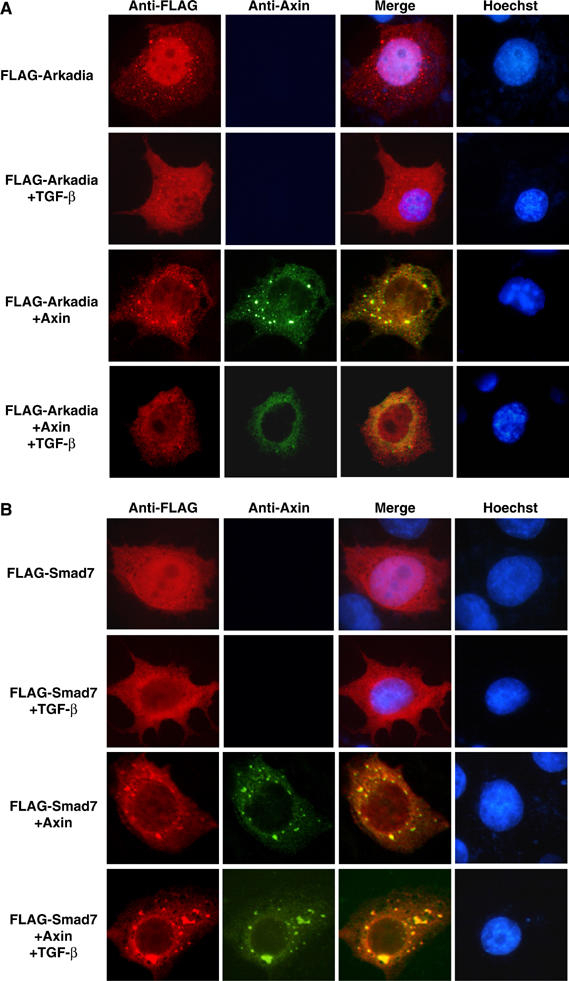
Axin is constitutively colocalized with Arkadia or Smad7 and induces cytoplasmic translocation of Smad7. (A) Axin colocalized with Arkadia. COS7 cells were transfected with FLAG-Arkadia with or without Axin. Cells treated with or without TGF-β then fixed and stained as described in Materials and methods. Anti-FLAG staining for Arkadia (red), anti-Axin for Axin (green), and nuclear staining by Hoechst were performed. (B) Axin induces cytoplasmic translocation of Smad7 and colocalized with Smad7 in the cytoplasm. Compared to expression of Smad7 alone, in Axin-coexpressing cells, great majority of Smad7 was seen in the cytoplasm, indicating that Axin caused translocation of Smad7 into the cytoplasm. Most representative results from three rounds of staining are shown.
Next, we performed similar experiments to see if Axin and Smad7 are also colocalized in the absence or presence of TGF-β. In the cells that were transfected with Smad7 alone, Smad7 was localized mainly in the nucleus when untreated (Figure 4B) (Itoh et al, 1998). In cells treated with TGF-β, Smad7 underwent nucleocytoplasmic shuttling to become mostly cytoplasmically localized, in accordance with the previous report (Itoh et al, 1998). When coexpressed with Axin, Smad7 was almost exclusively distributed in the cytoplasm and colocalized with Axin regardless of TGF-β treatment. These data suggest that Axin sequesters Smad7 in the cytoplasm.
Axin enhances TGF-β signaling through Arkadia
It was previously shown that Axin plays a positive role in TGF-β signaling by promoting Smad3 phosphorylation (Furuhashi et al, 2001). Our current finding suggested that Axin might regulate TGF-β signaling by an additional mean through complex formation with Arkadia and Smad7. To test this, we transfected the TGF-β-responsive luciferase reporter (12 × CAGA-Lux) in different combinations with Axin, AxinΔAr (incapable of interacting with Arkadia), Arkadia, and ArkadiaΔC (without the RING domain) into HepG2 and Mv1Lu/L17 cells. As expected, Axin and Arkadia each enhanced both basal and TGF-β-stimulated reporter activity in HepG2 and Mv1Lu/L17 cells (Figure 5A and B) (Furuhashi et al, 2001; Koinuma et al, 2003). When Arkadia and Axin were both expressed, a further increase of the reporter activity was observed in both of the cell lines. However, the Axin mutant AxinΔAr that lacks Arkadia-binding domain (and is hence also unable to bind Smad3 and is weak for Smad7 interaction) failed to enhance TGF-β signaling. Interestingly, ArkadiaΔC had a strong dominant-negative effect on Axin-induced TGF-β signaling. Of note, because Mv1Lu/L17 cells are null for TGF-β type I receptor (TβRI), a constitutively active TGF-β type I receptor (caTβRI) was cotransfected into Mv1Lu/L17 cells instead of treating cells with TGF-β ligand (Figure 5B).
Figure 5.
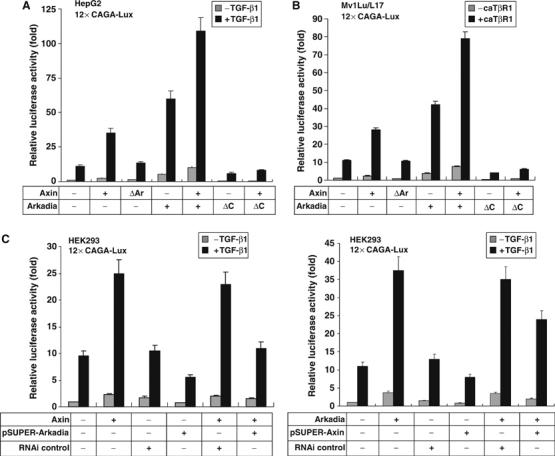
Axin enhances TGF-β-dependent transcriptional activity through Arkadia. 12 × CAGA-Lux reporter was cotransfected with different Axin or Arkadia constructs into HepG2, Mv1Lu/L17, and HEK293 cells as indicated. Cells were either treated with TGF-β (A, C) or cotransfected with caTβRI (B). Variation in transfection efficiency among samples was less than 10% based on activities of cotransfected β-galactosidase. All transfections were performed in duplicate and the data are presented as means±s.d. of at least three separate experiments after normalizing luciferase activity from vector control to 1. (A) Axin, but not AxinΔAr, can activate TGF-β-dependent gene transcription of 12 × CAGA-Lux reporter; the dominant-negative mutant of Arkadia, ArΔC, diminished Axin-mediated activation of the reporter gene in HepG2 cells. (B) Axin depends on Arkadia for stimulation of reporter transcriptional activity in Mv1Lu/L17 cells. (C) The siRNA against Arkadia, but not control siRNA, attenuated Axin-stimulated TGF-β signaling in 293 cells (left panel). Conversely, Axin siRNA diminished the Arkadia-induced activation of TGF-β signaling (right panel).
Functional dependence between Axin and Arkadia was further demonstrated by introducing vector-based small interfering RNA (siRNA) of Arkadia (for knockdown efficiency and specificity, see Supplementary Figure 3). The Arkadia siRNA could attenuate Axin-stimulated TGF-β signaling (Figure 5C). Conversely, siRNA against Axin (Rui et al, 2004) diminished Arkadia-induced TGF-β signaling, indicating that Axin and Arkadia depend on each other to facilitate TGF-β signaling.
Axin enhances Arkadia-dependent Smad7 ubiquitination
It is reported that Arkadia can strongly induce polyubiquitination of Smad7 (Koinuma et al, 2003). We then examined whether Axin facilitates the function of Arkadia in this aspect. As expected, wild-type Arkadia, but not ArkadiaΔC, could induce the polyubiquitination of Smad7 in transfected 293T cells (Figure 6A). Ubiquitination of Smad7 was enhanced in cells overexpressing increasing amounts of Axin (Figure 6A and B). Axin-induced Smad7 polyubiquitination was blocked by ArkadiaΔC in a dose-dependent manner (Figure 6C), suggesting that the latter exerts a dominant-negative effect on the Axin-induced polyubiquitination of Smad7. To further test the cooperation between Axin and Arkadia, vector-based siRNA of Arkadia was introduced into Axin- and Smad7-transfected cells (Figure 6D). Consistent with the above data, siRNA against Arkadia could attenuate Axin-induced polyubiquitination of Smad7. Similarly, when siRNA of Axin was expressed in Arkadia-transfected cells, polyubiquitinated Smad7 was reduced (Figure 6E). These findings suggest that Axin and Arkadia depend on each other to promote the ubiquitination of Smad7.
Figure 6.
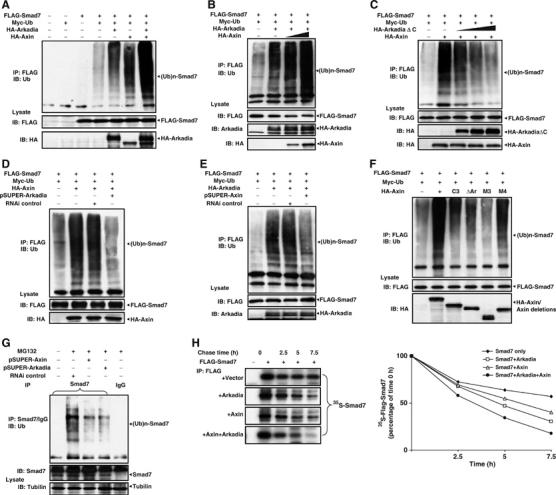
Axin promotes Arkadia-induced ubiquitin-dependent degradation of Smad7. (A) Axin induces ubiquitination of Smad7. The 293T cells were transfected as indicated, and treated with 10 μM of MG132 for 4 h before cell lysis. Lysates from cells were subjected to anti-FLAG immunoprecipitation followed by anti-ubiquitin immunoblotting. Polyubiquitination species of Smad7, (Myc-ubiquitin)n–FLAG-Smad7, are indicated in the top panel. (B) Axin promotes Arkadia-induced polyubiquitination of Smad7 in a dose-dependent manner. Increasing amounts (0, 0.5, and 2 μg) of HA-Axin were cotransfected with 2 μg of Arkadia into 293T cells. Smad7 ubiquitination was determined as described above. (C) Axin-induced polyubiquitination of Smad7 is negatively regulated by ArkadiaΔC in a dose-dependent manner. (D) Arkadia siRNA diminished polyubiquitination of Smad7 induced by Axin. The 293T cells were transfected with Axin with or without pSUPER-Arkadia as indicated to assess the effect of siRNA against Arkadia on Smad7 ubiquitination. (E) Axin siRNA attenuated Arkadia-induced polyubiquitination of Smad7. pSUPER-Axin (4 μg) was transfected into 293T cells. (F) Axin scaffolds Arkadia and Smad7 to enhance the polyubiquitination of Smad7. The 293T cells were transfected with FLAG-Smad7, Myc-ubiquitin, and wild-type or different Axin deletion mutants as indicated. (G) Specific knockdown of Axin or Arkadia reduced polyubiquitinated Smad7 in 293T cells. HEK293T cells were transfected with pSUPER vectors expressing control siRNA, Axin siRNA, or Arkadia siRNA as indicated. MG132 (10 μM) was added to the cells at 32 h post-transfection, and were incubated for additional 4 h. Cell lysates were then subjected to immunoprecipitation with anti-Smad7 or control IgG, followed by Western blotting with anti-ubiquitin. (H) Axin facilitates Arkadia to accelerate ubiquitin-dependent degradation of Smad7. The 293T cells were transfected with FLAG-Smad7 with or without wild-type Arkadia or wild-type Axin, or both of them. [35S]methionine- and cysteine-labeled cell lysates were immunoprecipitated by anti-FLAG antibody followed by autoradiography after separation on SDS–PAGE. The autoradiographic signals were quantified and the values plotted relative to 0-h values. All of the above experiments were repeated with essentially the same results.
Since Axin, Arkadia, and Smad7 form a ternary complex, to assess the functional significance of the complex formation, we transfected into 293T cells with Axin constructs lacking the Arkadia-binding site (AxinΔAr) or both of Arkadia- and Smad7-binding sites (Axin-M3), and determined their contribution to the polyubiquitination of Smad7 (Figure 6F). The Axin-M4 deletion mutant that retains both Smad7- and Arkadia-binding sites was also included for comparison. Deletion of both Arkadia- and Smad7-binding sites (Axin-M3) rendered Axin completely incapable of enhancing the ubiquitination of Smad7. Axin mutants lacking Arkadia-binding site (AxinΔAr that also lacks the C-terminal portion of Smad7 interaction site) or the N-terminal part of the Smad7-binding site (Axin-C3) drastically lost the function, whereas Axin-M4 induced polyubiquitination of Smad7 as effectively as did the wild-type Axin (Figure 6F). Specific depletion of endogenous Axin or Arkadia by their respective siRNA significantly reduced the levels of polyubiquitinated Smad7 in 293T cells (Figure 6G). These observations indicate that Axin scaffolds Arkadia and Smad7 to facilitate Arkadia-induced polyubiquitination of Smad7.
To further ascertain the augmentative effect of Axin on Arkadia-induced proteasome-dependent degradation of Smad7, we analyzed turnover of Smad7 by pulse-chase experiments (Figure 6H). As expected, Smad7 was rapidly degraded in the presence of Arkadia or Axin alone. Moreover, it is found that Axin could significantly enhance Arkadia-induced degradation of Smad7. The results thus demonstrate that Axin accelerates turnover of Smad7 by recruiting an E3 ubiquitin ligase, Arkadia.
Axin associates with Arkadia and Smad7 independently of TGF-β signaling
In the case of Smad3 interaction with Axin, it has been shown that Smad3 becomes disassociated upon stimulation by TGF-β signal. Our results obtained from the cell staining experiments, however, raised the possibility that Axin may be colocalized with Smad7 and Arkadia before and after TGF-β treatment. To verify this, we carried out interaction assays by co-immunoprecipitation between Axin and separately Arkadia, Smad7, along with Smad3, Smad3D407E, and Smurf1, after cotransfection with or without caTβRI as indicated (Figure 7A). Indeed, Arkadia and Smad7 bind to Axin with the same affinities regardless of coexpression of caTβRI. Smurf1 did not interact with Axin. In the absence of TGF-β signaling, Smad3 strongly interacted with Axin; however, when coexpressed with caTβRI, Smad3 dissociated from the Axin complex. In contrast, the Smad3 mutant D407E that is not phosphorylated upon TGF-β stimulation (Goto et al, 1998) remained associated with Axin in the presence of TGF-β signaling, in agreement with the previous report that the mutant D407E is constitutively associated with Axin. These data have thus demonstrated that whereas only unstimulated Smad3 but not stimulated Smad3 interacts with Axin, Arkadia and Smad7 are constitutively associated with Axin. Consistently, caTβRI did not have any effect on Axin and Arkadia-induced polyubiquitination of Smad7, as determined by both ubiquitination and pulse-chase labeling experiments (Supplementary Figure 4A and B).
Figure 7.
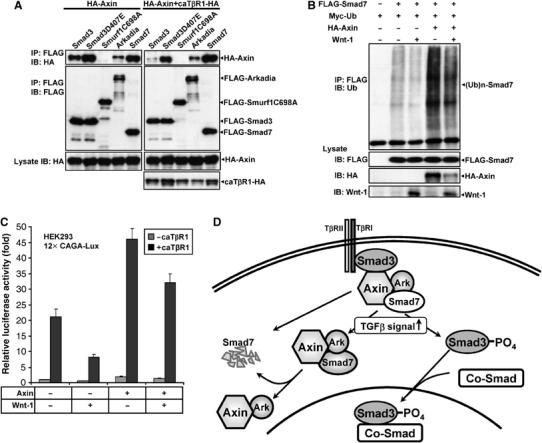
Axin constitutively associates with Arkadia and Smad7. (A) HA-Axin together with the various plasmids indicated were cotransfected with or without caTβRI into 293T cells. After 4 h treatment with MG132 (10 μM), cells were lysed and lysates were immunoprecipitated by anti-FLAG, followed by Western blotting using anti-HA and rabbit anti-FLAG. (B) Wnt-1 attenuated both basal and Axin-induced Smad7 ubiquitination. HEK293T cells were transfected with 0.5 μg of FLAG-Smad7, Myc-ubiquitin with or without 1.5 μg of HA-Axin or/and Wnt-1 as indicated. Cells were treated with MG132 (10 μM) for 4 h before lysed. Cell lysates were subjected to immunoprecipitation with anti-FLAG antibody and polyubiquitination of Smad7 was detected with anti-ubiquitin antibody. Wnt-1 in total cell lysates was probed with anti-Wnt-1. Similar results were obtained in three separate experiments. (C) Effects of Wnt-1 on the TGF-β-dependent transcriptional activity. 12 × CAGA-Lux was transfected into HEK293 cells with or without Axin, caTβRI, or/and Wnt-1 as indicated. Wnt-1 could reduce the reporter gene transcription in the presence or absence of transfected Axin. (D) Model of scaffolding roles of Axin in TGF-β signaling. Axin forms a complex with Smad3, Arkadia, and Smad7, of which Smad3 has physical contact with the type I receptor. Upon ligand stimulation, Axin promotes Smad3 phosphorylation; phosphorylated Smad3 dissociates from the Axin complex, and then combines with Smad4 to activate transcription in the nucleus. In addition, Axin acts as a scaffold to facilitate Arkadia-mediated polyubiquitination of Smad7 regardless of TGF-β signaling, leading to Smad7 degradation. Implied here is also that Axin can sequester Smad7 in the cytoplasm.
However, it is possible that Wnt proteins may exert an effect on Axin-mediated Smad7 ubiquitination as the stability and function of Axin have been shown to be regulated by the Wnt proteins. We therefore coexpressed mouse Wnt-1 with Smad7 in the presence or absence of overexpressed Axin and determined the levels of Smad7 ubiquitination in 293T cells. As shown in Figure 7B, Wnt-1 significantly reduced Smad7 ubiquitination in the cell. In addition, Wnt-1 also attenuated Axin-induced Smad7 ubiquitination, in agreement with its ability to downregulate the levels of Axin (Yamamoto et al, 1999), further underscoring the importance of Axin in the induction of Smad7 degradation. In parallel, we assayed for the effect of Wnt-1 on the transcriptional activity of the 12 × CAGA-Lux as shown in Figure 7C. Wnt-1 could reduce the TGF-β-dependent transcriptional activity of the reporter gene in the presence or absence of overexpressed Axin. The results were reproducible in three separate experiments.
Discussion
TGF-β signaling has been an area of intensive studies, owing to its plethoric biological roles in development and cellular homeostasis. Although many signaling molecules have been elucidated, how these intracellular signaling components are coordinated remains largely obscure. Here, we have uncovered a novel aspect of TGF-β signaling regulation in that promotion of R-Smad activation and I-Smad downregulation are coordinated by the same scaffolding molecule Axin that has been demonstrated to play a central and scaffolding role in the Wnt and JNK MAP kinase pathways (Luo and Lin, 2004; Salahshor and Woodgett, 2005). We present a simplified summary of the roles of Axin in the regulation of TGF-β signaling in Figure 7D. Despite the observation that Axin forms a complex with Arkadia and Smad7 independently of TGF-β signaling, Wnt-1, a Wnt ligand, appears to play an inhibitory role in Axin-stimulated TGF-β signaling by downregulating Axin levels in the cell. We have thus provided new mechanistic insights into the notion that Axin is an integrator for the Wnt and TGF-β signaling pathways (Attisano and Labbe, 2004).
Axin acts as a scaffold for multiple components in the TGF-β superfamily signaling pathway
Abundant evidence exists to suggest that Wnt signaling interacts or crosstalks with TGF-β signaling (Labbe et al, 2000; Nishita et al, 2000; Edlund et al, 2005). In this study, we have demonstrated that Axin, a central scaffold in the Wnt pathway, interacts with the E3 ubiquitin ligase Arkadia that has been linked to TGF-β signaling. The interaction has been confirmed by multiple means, a yeast two-hybrid interaction assay, co-immunoprecipitation of endogenous proteins, and subcellular colocalization by immunofluorescent staining. This unexpected finding prompted us to check whether Axin would also bind to Smads as they have been shown to be Arkadia substrates. Indeed, Axin also interacts with Smad7; moreover, ternary complex assay by two-step co-immunoprecipitation has revealed that Axin simultaneously interacts with Arkadia and Smad7. Interestingly, the C-terminal region of Axin participates in the interaction with both Arkadia and Smad7, suggesting that Axin can provide proximity of Smad7 to its E3 ligase Arkadia. As for the structural requirements of Smad7 for Axin interaction, both the N-terminus region and the carboxyl region of MH2 are both needed. This may imply that Axin may also modulate Smad7 functions in pathways other than TGF-β signaling, as the MH2 domain alone is responsible for receptor interaction, formation of homomeric and heteromeric Smad complexes.
Axin as an activator of TGF-β by downregulating I-Smads through sequestration and polyubiquitination-mediated degradation
Smad3 in its inactive state binds to Axin. When phosphorylated in response to TGF-β treatment, it dissociates from the Axin complex (Furuhashi et al, 2001), suggesting that Axin may not be directly involved in Smad3-dependent transcriptional activation of target genes. In contrast, Axin seems to be constitutively associated with Arkadia, and Smad7 unless degraded. Even in the absence of TGF-β treatment, coexpression of Axin itself can translocate Smad7 from the nucleus to the cytoplasm as revealed by immunostaining. Co-immunoprecipitation experiment also showed that complex formation between Axin and Arkadia or Smad7 did not change after stimulation by the constitutively active type I receptor. Moreover, TGF-β signal did not seem to further increase Axin-induced Smad7 polyubiquitination and degradation (Supplementary Figure 4). All these observations indicate that Axin is closely associated with Arkadia and Smad7, and acts to constantly decrease cellular levels of the I-Smad(s) in the presence or absence of TGF-β.
The nucleocytoplasmic translocation of I-Smad induced by Axin may be achieved by two possible means. One is that Axin serves as a carrier to mediate nuclear export, and the other is that Axin simply traps I-Smad in the cytoplasm to prevent nuclear entry of Smad7. The first possibility is supported by the fact that Axin is also a nuclear protein, albeit mostly cytoplasmic (Cong and Varmus, 2004; Wiechens et al, 2004), and by our previous observation that it is able to undergo nucleocytoplasmic shuttling upon stimulation by signals such as UV (Rui et al, 2004). However, Smad7 was still present in the cytoplasm when coexpressed with an Axin mutant that did not exhibit any discernible nuclear localization after its NLS sequences were deleted (data not shown). Therefore, it remains unclear how Axin induces Smad7 nuclear export.
It is demonstrated here that Axin facilitates TGF-β signaling in an Arkadia-dependent manner. First of all, AxinΔAr that is deleted of the Arkadia-interacting domain fails to activate TGF-β signaling. Second, when Arkadia expression is knocked down by siRNA, the Axin-induced activation of TGF-β signaling is drastically diminished. Moreover, E3 ligase-deficient mutant ArkadiaΔC also greatly attenuated Axin activation of TGF-β signaling. All these observations point to a critical role of Arkadia in the biological function of Axin. Furthermore, the observation that Arkadia always stimulates TGF-β signaling to a greater extent than Axin is also consistent with the above notion. To test if the stimulatory role of Arkadia is indeed exerted by its E3 ligase activity, a series of ubiquitination assays as well as half-life determination of Smad7 were carried. Indeed, Axin strongly promotes polyubiquitination of Smad7 and cooperates with Arkadia to reduce stability of Smad7.
A general role of Axin in cytostasis?
TGF-β plays a crucial role in maintaining proliferative homeostasis (Massague, 1998). In light of all the existing evidence, it is fascinating to speculate that Axin, a known tumor suppressor (Polakis, 2000; Hsu et al, 2001), exerts a dual role in a constitutive manner to prevent escape of cells from cytostasis by acting to downregulate oncogenic β-catenin, in one way, and to enforce antiproliferative function induced by TGF-β. As far as Wnt signaling is concerned, Axin in unstimulated cells provides an architectural platform for molecular assembly containing APC, GSK-3β, casein kinases, and β-catenin to enable phosphorylation of β-catenin, leading to ubiquitination and degradation. In the case of TGF-β signaling, besides a role in facilitating Smad3 phosphorylation upon TGF-β treatment (Furuhashi et al, 2001), Axin promotes ubiquitination and degradation of Smads that play an inhibitory role in this pathway, again leading to enhancement of antiproliferative mode. Equally interesting is that Axin directly stimulates p53 function through augmenting its phosphorylation by HIPK-2, leading to cell apoptosis (Rui et al, 2004). Of note, Axin-mediated JNK MAP kinase activation seems to also play a role in the induction of apoptosis by Axin (Neo et al, 2000). These lines of evidence all point to a general role of Axin in downregulating cellular proliferation. This conjecture raises another interesting question as to whether Axin has evolved as a major tumor suppressor, in that when one aspect of antiproliferative function is lost, it can still manage to utilize another avenue to prevent cell from escape from cytostasis. Certainly, much awaits further investigation.
Materials and methods
Plasmid constructions
Full-length cDNA encoding mouse Arkadia was cloned through fusing the EST clone with HA- or FLAG-tag using a PCR-based approach. Arkadia point mutation C937A and Smurf1C698A, and various deletion mutants were generated as described previously (Rui et al, 2004). The constructs of caTβRI, Smad1–7, Smurf1, and Smad7 mutants were as described previously (Chen et al, 1998). The expression plasmid for mouse Wnt-1 was purchased from Upstate Biotech. (Lake Placid, NY).
Cell culture and transient transfection
HEK293T, HEK293, and HepG2 were obtained from ATCC (Manassas, VA). All of the three cell lines and Mv1Lu/L17 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and antibiotics, and maintained at 37°C, 5% CO2, in a humidified incubator. Transfections were performed in 60-mm dishes using calcium phosphate precipitation.
Preparation of antibodies
Mouse anti-HA (F-7), anti-Myc (9E10), anti-ubiquitin (P4D1), and goat anti-Smad7 (N-19) were purchased from Santa Cruz Biotech. Mouse anti-FLAG (M2) was purchased from Sigma. The polyclonal antibody against Axin was described previously (Rui et al, 2004). Arkadia GST-fusion protein-containing amino-acid sequence of aa 1–132 was produced in BL21 Escherichia coli cells and was injected into rabbits following purification using glutathione–agarose beads. The Arkadia antibody was purified and characterized as described previously (Rui et al, 2004). Rabbit polyclonal Wnt-1 antibody was purchased from Zymed (Cat. no.: 36-5800).
Co-immunoprecipitation and Western blotting
Cells were transfected with various plasmids and incubated for 36 h before analysis. For inhibition of proteasomal degradation, cells were treated with 10 μM MG132 (Sigma) for 4 h. Cell lysate preparation, immunoprecipitation, and Western blotting were performed as described previously (Zhang et al, 1999). Essentially the same results were obtained in at least three separate experiments for each interaction assay.
GST pulldown assay
The GST-Axin (full length) was prepared as described previously (Rui et al, 2004). Approximately 4 mg of GST-fusion protein bound to agarose beads were added to each total lysate from 293T cells, and incubated for 3 h with gentle rotation. The beads were washed three times with cell lysis buffer, and the proteins were eluted with 2 × SDS sample buffer. Protein samples were analyzed by Western blotting.
Immunofluorescent staining
COS7 cells grown on coverslips in six-well plates were transfected with expression plasmids as indicated. After 30 h, immunofluorescent staining was carried out as described previously (Rui et al, 2004). To determine the effects of TGF-β, cells were washed with serum-free medium and treated with TGF-β (10 ng/ml, in serum-free medium) for 8 h before fixation. Experiments were repeated for multiple times.
Transcription reporter assay
HEK293, HepG2, and Mv1Lu/L17 cells were transfected with 0.1 μg of 12 × CAGA-Lux, and 2 μg each of Axin and Arkadia constructs, with or without 0.5 μg of caTβRI or/and 0.5 μg Wnt-1 as indicated. At 36 h post-transfection, luciferase activity was measured and presented as described previously (Rui et al, 2004). HepG2 and HEK293 cells were washed with serum-free medium and incubated for additional 12 h in serum-free medium supplemented with TGF-β1 (5 ng/ml) before analysis. All transfections were performed in duplicate and the data are presented as means±s.d. of at least three separate experiments after normalizing luciferase activity from vector control to 1.
Two-step co-immunoprecipitation
Two-step co-immunoprecipitation was performed essentially according to the procedures described previously (Rui et al, 2004). Briefly, 293T cells were transfected with HA–Axin, Myc-Smad7 and FLAG-ArC937A. For the control of first immunoprecipitation, the Axin lacking the HA-tag was used. At 36 h after transfection, the cells were lysed with lysis buffer (Zhang et al, 1999), sonicated briefly, and centrifuged. The supernatant was incubated with anti-HA antibody bound to protein A/G–agarose beads for 3 h at 4°C. The beads were washed with lysis buffer three times, and the HA–Axin protein complex was eluted with 300 μl of lysis buffer containing 250 mM NaCl and HA peptide for 3 h at 4°C. Second immunoprecipitation was performed using 150 μl of eluate from the first immunoprecipitation and 350 μl of lysis buffer containing 464 mM NaCl and 10 μl of anti-Myc antibody or control IgG followed by the addition of protein A/G–agarose beads.
Metabolic labeling and pulse-chase analysis
HEK293T cells transfected with plasmids indicated were treated with 100 μCi/ml [35S]methionine and cysteine (Amersham) in methionine- and cysteine-free DMEM (Amersham), and were incubated for 1 h at 37°C, then washed with PBS twice and chased in DMEM supplemented with 10% FBS, 2 mM methionine, and 0.5 mM cysteine for the time periods indicated. Cells were lysed with RIPA buffer and subjected to immunoprecipitation followed by SDS–PAGE. The gels were fixed, dried, and examined by autoradiography.
Supplementary Material
Supplementary Materials and Methods
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Acknowledgments
We thank our colleagues for technical assistance and Drs Jiawei Wu and Zhenguo Wu for critical comments. This research was supported by grants from Research Grant Council of Hong Kong (HKUST6127/04M; HKUST6416/05M), National Outstanding Young Investigator Award (No.30125012; 30125021), and grants from the National Science Foundation of China (Nos. 90208015 and 30370306), as a grant from Fujian Council of Science and Technology (2002F002).
References
- Attisano L, Labbe E (2004) TGFbeta and Wnt pathway cross-talk. Cancer Metast Rev 23: 53–61 [DOI] [PubMed] [Google Scholar]
- Chen YG, Hata A, Lo RS, Wotton D, Shi Y, Pavletich N, Massague J (1998) Determinants of specificity in TGF-beta signal transduction. Genes Dev 12: 2144–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia IV, Costantini F (2005) Mouse axin and axin2/conductin proteins are functionally equivalent in vivo. Mol Cell Biol 25: 4371–4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong F, Varmus H (2004) Nuclear-cytoplasmic shuttling of Axin regulates subcellular localization of beta-catenin. Proc Natl Acad Sci USA 101: 2882–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datto M, Wang XF (2005) Ubiquitin-mediated degradation a mechanism for fine-tuning TGF-beta signaling. Cell 121: 2–4 [DOI] [PubMed] [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL (2003) Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol 5: 410–421 [DOI] [PubMed] [Google Scholar]
- Dupont S, Zacchigna L, Cordenonsi M, Soligo S, Adorno M, Rugge M, Piccolo S (2005) Germ-layer specification and control of cell growth by Ectodermin, a Smad4 ubiquitin ligase. Cell 121: 87–99 [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Fukuchi M, Murakami G, Chiba T, Tanaka K, Imamura T, Miyazono K (2001) Smurf1 interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. J Biol Chem 276: 12477–12480 [DOI] [PubMed] [Google Scholar]
- Edlund S, Lee SY, Grimsby S, Zhang S, Aspenstrom P, Heldin CH, Landstrom M (2005) Interaction between Smad7 and beta-catenin: importance for transforming growth factor beta-induced apoptosis. Mol Cell Biol 25: 1475–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Episkopou V, Arkell R, Timmons PM, Walsh JJ, Andrew RL, Swan D (2001) Induction of the mammalian node requires Arkadia function in the extraembryonic lineages. Nature 410: 825–830 [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Yagi K, Yamamoto H, Furukawa Y, Shimada S, Nakamura Y, Kikuchi A, Miyazono K, Kato M (2001) Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway. Mol Cell Biol 21: 5132–5141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto D, Yagi K, Inoue H, Iwamoto I, Kawabata M, Miyazono K, Kato M (1998) A single missense mutant of Smad3 inhibits activation of both Smad2 and Smad3, and has a dominant negative effect on TGF-beta signals. FEBS Lett 430: 201–204 [DOI] [PubMed] [Google Scholar]
- Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P (1998) Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol 8: 573–581 [DOI] [PubMed] [Google Scholar]
- Hata A, Lagna G, Massague J, Hemmati-Brivanlou A (1998) Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev 12: 186–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA Jr, Wrana JL, Falb D (1997) The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell 89: 1165–1173 [DOI] [PubMed] [Google Scholar]
- Hayes S, Chawla A, Corvera S (2002) TGF beta receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J Cell Biol 158: 1239–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W, Shakya R, Costantini F (2001) Impaired mammary gland and lymphoid development caused by inducible expression of Axin in transgenic mice. J Cell Biol 155: 1055–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K (1997) Smad6 inhibits signalling by the TGF-beta superfamily. Nature 389: 622–626 [DOI] [PubMed] [Google Scholar]
- Itoh S, Landstrom M, Hermansson A, Itoh F, Heldin CH, Heldin NE, ten Dijke P (1998) Transforming growth factor beta1 induces nuclear export of inhibitory Smad7. J Biol Chem 273: 29195–29201 [DOI] [PubMed] [Google Scholar]
- Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL (2000) Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell 6: 1365–1375 [DOI] [PubMed] [Google Scholar]
- Koinuma D, Shinozaki M, Komuro A, Goto K, Saitoh M, Hanyu A, Ebina M, Nukiwa T, Miyazawa K, Imamura T, Miyazono K (2003) Arkadia amplifies TGF-beta superfamily signalling through degradation of Smad7. EMBO J 22: 6458–6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe E, Letamendia A, Attisano L (2000) Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc Natl Acad Sci USA 97: 8358–8363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X (2002) Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837–847 [DOI] [PubMed] [Google Scholar]
- Luo W, Lin SC (2004) Axin: a master scaffold for multiple signaling pathways. Neurosignals 13: 99–113 [DOI] [PubMed] [Google Scholar]
- Massague J (1998) TGF-beta signal transduction. Annu Rev Biochem 67: 753–791 [DOI] [PubMed] [Google Scholar]
- Massague J (2000) How cells read TGF-beta signals. Nat Rev Mol Cell Biol 1: 169–178 [DOI] [PubMed] [Google Scholar]
- Massague J, Chen YG (2000) Controlling TGF-beta signaling. Genes Dev 14: 627–644 [PubMed] [Google Scholar]
- Massague J, Wotton D (2000) Transcriptional control by the TGF-beta/Smad signaling system. EMBO J 19: 1745–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami G, Watabe T, Takaoka K, Miyazono K, Imamura T (2003) Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Mol Biol Cell 14: 2809–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P (1997) Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature 389: 631–635 [DOI] [PubMed] [Google Scholar]
- Neo SY, Zhang Y, Yaw LP, Li P, Lin SC (2000) Axin-induced apoptosis depends on the extent of its JNK activation and its ability to down-regulate beta-catenin levels. Biochem Biophys Res Commun 272: 144–150 [DOI] [PubMed] [Google Scholar]
- Niederlander C, Walsh JJ, Episkopou V, Jones CM (2001) Arkadia enhances nodal-related signalling to induce mesendoderm. Nature 410: 830–834 [DOI] [PubMed] [Google Scholar]
- Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, Cho KW (2000) Interaction between Wnt and TGF-beta signalling pathways during formation of Spemann's organizer. Nature 403: 781–785 [DOI] [PubMed] [Google Scholar]
- Peifer M, Polakis P (2000) Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 287: 1606–1609 [DOI] [PubMed] [Google Scholar]
- Polakis P (2000) Wnt signaling and cancer. Genes Dev 14: 1837–1851 [PubMed] [Google Scholar]
- Rui HL, Fan E, Zhou HM, Xu Z, Zhang Y, Lin SC (2002) SUMO-1 modification of the C-terminal KVEKVD of Axin is required for JNK activation but has no effect on Wnt signaling. J Biol Chem 277: 42981–42986 [DOI] [PubMed] [Google Scholar]
- Rui Y, Xu Z, Lin S, Li Q, Rui H, Luo W, Zhou HM, Cheung PY, Wu Z, Ye Z, Li P, Han J, Lin SC (2004) Axin stimulates p53 functions by activation of HIPK2 kinase through multimeric complex formation. EMBO J 23: 4583–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahshor S, Woodgett JR (2005) The links between axin and carcinogenesis. J Clin Pathol 58: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700 [DOI] [PubMed] [Google Scholar]
- Suzuki C, Murakami G, Fukuchi M, Shimanuki T, Shikauchi Y, Imamura T, Miyazono K (2002) Smurf1 regulates the inhibitory activity of Smad7 by targeting Smad7 to the plasma membrane. J Biol Chem 277: 39919–39925 [DOI] [PubMed] [Google Scholar]
- Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL (1998) SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell 95: 779–791 [DOI] [PubMed] [Google Scholar]
- Wiechens N, Heinle K, Englmeier L, Schohl A, Fagotto F (2004) Nucleo-cytoplasmic shuttling of Axin, a negative regulator of the Wnt-beta-catenin pathway. J Biol Chem 279: 5263–5267 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A (1999) Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem 274: 10681–10684 [DOI] [PubMed] [Google Scholar]
- Yamashita M, Ying SX, Zhang GM, Li C, Cheng SY, Deng CX, Zhang YE (2005) Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell 121: 101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda J, Whitmarsh AJ, Cavanagh J, Sharma M, Davis RJ (1999) The JIP group of mitogen-activated protein kinase scaffold proteins. Mol Cell Biol 19: 7245–7254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL III, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F (1997) The mouse fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90: 181–192 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Neo SY, Wang X, Han J, Lin SC (1999) Axin forms a complex with MEKK1 and activates c-Jun NH(2)-terminal kinase/stress-activated protein kinase through domains distinct from Wnt signaling. J Biol Chem 274: 35247–35254 [DOI] [PubMed] [Google Scholar]
- Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH (1999) A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 400: 687–693 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and Methods
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4


