Abstract
Ubiquitin (Ub)-protein ligases (E3s) frequently modify their substrates with multiple Ub molecules in the form of a polyubiquitin (poly-Ub) chain. Although structurally distinct poly-Ub chains (linked through different Ub lysine (Lys) residues) can confer different fates on target proteins, little is known about how E3s select the Lys residue to be used in chain synthesis. Here, we used a combination of mutagenesis, biochemistry, and mass spectrometry to map determinants of linkage choice in chain assembly catalyzed by KIAA10, an HECT (Homologous to E6AP C-Terminus) domain E3 that synthesizes K29- and K48-linked chains. Focusing on the Ub molecule that contributes the Lys residue for chain formation, we found that specific surface residues adjacent to K48 and K29 are critical for the usage of the respective Lys residues in chain synthesis. This direct mechanism of linkage choice bears similarities to the mechanism of substrate site selection in sumoylation catalyzed by Ubc9, but is distinct from the mechanism of chain linkage selection used by the Mms2/Ubc13 (Ub E2 variant (UEV)/E2) complex.
Keywords: chain linkage, HECT domain, polyubiquitin chains, ubiquitin, ubiquitin ligase
Introduction
The conserved protein ubiquitin (Ub) regulates numerous biological processes through a mechanism involving covalent conjugation. Ub is attached to cellular proteins through the formation of an isopeptide bond between the C-terminus of Ub (G76) and a lysine (Lys) side chain of the target protein (Pickart and Eddins, 2004). Ub signals take two basic structural forms, comprising a single Ub moiety or polymeric chains linked through Lys residues of Ub itself. The corresponding conjugation processes are termed mono- and polyubiquitylation, respectively (Pickart and Fushman, 2004; Volk et al, 2005). The structures of polyubiquitin (poly-Ub) chains are further diversified through the use of different Ub Lys residues in chain assembly. In budding yeast, Ub is conjugated to other Ub molecules through each of its seven Lys residues (Peng et al, 2003; Xu et al, 2006).
Structurally distinct poly-Ub chains can be functionally distinct signals (Pickart and Fushman, 2004). For example, K48-linked chains constitute a well-established proteasome-targeting signal (Chau et al, 1989; Finley et al, 1994), whereas K63-linked chains are involved in nonproteolytic pathways including DNA damage tolerance, kinase activation, and protein trafficking and synthesis (Galan and Haguenauer-Tsapis, 1997; Spence et al, 2000; Sun and Chen, 2004; Ulrich, 2004). K6-linked chains have been implicated in DNA repair, while K29-linked chains seem to function in proteasome proteolysis (Pickart and Fushman, 2004).
Ubiquitylation is accomplished through the sequential actions of enzymes called E1, E2, and E3 (Pickart and Eddins, 2004; Petroski and Deshaies, 2005). E1 activates Ub by forming a thiol ester with the carboxyl group of Ub-G76. Ub is then transferred to a Cys residue of the E2; finally, Ub is transferred to a Lys residue of the substrate (or another Ub). This final reaction is usually E3 dependent. Most known E3s belong to either the HECT (Homologous to E6AP C-Terminus) or RING (Really Interesting New Gene) protein families. HECT E3s share a conserved ∼350-residue HECT domain that harbors an essential Cys residue; they form a thiol ester with Ub prior to attack of the substrate Lys residue (Huibregtse et al, 1995; Scheffner et al, 1995). RING E3s, in contrast, function as scaffolds that recruit the charged E2 and the substrate while facilitating direct attack of the substrate Lys residue on the E2-linked Ub (Zheng et al, 2000; Petroski and Deshaies, 2005).
E3 enzymes are the key determining factors in substrate protein selection. They also play a major role in specifying the linkage of poly-Ub chains. For example, yeast Rsp5, an HECT domain E3, collaborates with Ubc5 to modify specific substrates with mono-Ub, K48-linked, or K63-linked chains (Kee et al, 2005). Certain E3s show a dual specificity in chain assembly. The HECT E3 KIAA10 and its yeast ortholog Hul5 synthesize chains through K29 and K48 (Mastrandrea et al, 1999; You and Pickart, 2001), while the RING E3 parkin catalyzes K48- and K63-linked polyubiquitylation (Lim et al, 2005).
Little is known about how E3 enzymes determine target Lys specificity during chain assembly. The only available model for poly-Ub chain linkage selection derives from structural studies of the Ub E2 variant (UEV)/E2 complex Mms2/Ubc13, which synthesizes K63-linked chains (Hofmann and Pickart, 1999; Ulrich, 2004). The surface of the Mms2/Ubc13 heterodimer possesses two Ub interaction sites, one on Ubc13 that positions the donor Ub (contributing G76), and the other on Mms2 that binds the acceptor Ub (contributing K63) (VanDemark et al, 2001; McKenna et al, 2003; Tsui et al, 2005). The latter site docks Ub such that only K63 can access the Ubc13 active site. In this ‘indirect' mechanism of linkage selection, interactions between Mms2 and regions of the acceptor Ub that are distant from the targeted Lys are critical for site selectivity (Tsui et al, 2005).
In the present study, we exploited the dual linkage specificity of KIAA10 to address the molecular basis of linkage selectivity in HECT E3-catalyzed chain synthesis. In contrast to the case of Mms2/Ubc13, results with KIAA10 indicate that interactions with regions of the acceptor Ub surface that are proximal to the targeted Lys residues play a major role in linkage determination.
Results
Identification of acceptor Ub residues involved in linkage selectivity: experimental design and proof of concept
KIAA10 employs a simple mechanism of chain synthesis in which Ub monomers are added sequentially to the distal end of an unanchored poly-Ub chain (You and Pickart, 2001; Wang and Pickart, 2005). The Ub molecule that contributes the Lys residue in isopeptide bond synthesis, termed the acceptor Ub, binds noncovalently to KIAA10. We first sought to identify molecular determinants of linkage selectivity that reside in this Ub by searching for acceptor Ub mutations that affect target Lys selection.
To identify such mutations, we needed a biochemical assay that rigorously reported the loss of one Lys linkage. To this end, we developed the specialized Ub3 synthesis assay outlined in Figure 1A. The method relies on the individual inability of Lys-less Ub (K0-Ub) or C-terminally truncated Ub (Ub74) to be substrates for chain synthesis. However, these two variants can be linked to each other to produce Ub2, with Ub74 acting as the acceptor (provides Lys) and K0-Ub acting as the donor (provides G76) (Mastrandrea et al, 1999; You and Pickart, 2001). Based on the known ability of KIAA10 to target K29 in a K48-linked chain (Mastrandrea et al, 1999), we speculated that KIAA10 might conjugate K0-Ub molecules to K29 and K48 of one Ub74 molecule, producing a ‘forked' Ub3 chain (Figure 1A, top). However, if Ub74 carries a mutation that blocks utilization of K29 or K48, the formation of Ub3, but not Ub2, would be selectively impaired (Figure 1A, bottom). This assay has the advantage of simplicity: although conjugation is a multistep process, the mutant Ub is used solely in the final step of isopeptide bond formation.
Figure 1.

Design and proof of concept of acceptor Ub mutant screen. (A) Screen design. If KIAA10 is capable of generating forked Ub3 (top panel), then the loss of one Lys linkage can be monitored as loss of this forked Ub3 product (bottom panel). (B) Proof of concept (Coomassie-stained gel). Ub3 synthesis assays with KIAA10-CD were conducted as described in Materials and methods, using wt Ub74, Ub74-K48R, or Ub74-K29R as acceptors for K0-Ub.
As proof of concept, we carried out this assay using Ub74 that contained both K29 and K48, or only one of these residues. The reaction cocktail also contained ATP, E1, UbcH5A (a cognate E2), KIAA10-CD, and K0-Ub. (KIAA10-CD, comprising the last 420 residues of KIAA10, is fully active in the synthesis of K29- and K48-linked chains (You and Pickart, 2001).) As seen in Figure 1B, wild-type (wt) Ub74 yielded Ub2 and Ub3 during chain assembly, while Ub74-K48R (no K48) and Ub74-K29R (no K29) produced Ub2 as virtually the sole product. (Trace amounts of Ub3 seen in the latter reactions probably reflect trace amounts of full-length Ub in the Ub74 due to incomplete trypsin cleavage during production of Ub74.) To verify further the structure of Ub3 produced from wt Ub74 and K0-Ub, the corresponding SDS–PAGE gel band was excised and analyzed by mass spectrometry for isopeptide linkages (Materials and methods). The results showed that (1) the K29 and K48 linkages were both detected and (2) no other linkage was detected (data not shown), although all seven possible linkages are observable by this method (Peng et al, 2003; Xu et al, 2006). Therefore, loss of the forked Ub3 product faithfully reports loss of one Lys linkage.
Ala scanning mutagenesis to identify acceptor Ub residues involved in linkage selectivity
Ala scanning mutagenesis has been widely used to map protein functional epitopes (e.g., Blaber et al, 1995; Morrison and Weiss, 2001; Sloper-Mould et al, 2001). All side-chain functional groups past the β-carbon are removed by Ala substitution, without the introduction of unusual dihedral angle preferences. The properties of Ala mutants therefore permit inferences to be drawn about the roles of specific side chains without complications due to unnatural backbone flexibility (Morrison and Weiss, 2001). Moreover, the small, neutral Ala side chain is unlikely to cause steric or electrostatic destabilization.
Hicke and co-workers previously performed a comprehensive Ala scan to identify surface residues required for Ub's essential functions in budding yeast (Sloper-Mould et al, 2001). Their collection of 54 yeast plasmids covers Ub's 63 surface residues; each mutant gene carries one to three mutations. Most mutations are to Ala; Ala residues and one Ser are mutated to Gly (Figure 2B below). We subcloned the 54 mutant genes to a bacterial vector and expressed the mutant proteins in Escherichia coli, then purified them and treated with trypsin under native conditions to release Ub's C-terminal GlyGly moiety (Cox et al, 1986). (To avoid anticipated inhibition of trypsin cleavage in Ub-R74A, we constructed a synthetic Ub74 gene for this mutant.) With this collection of mutant Ub74 molecules, pilot studies showed that high yields of Ub2 and Ub3 were obtained in assays of short duration with K0-Ub in excess over Ub74 (e.g., Figures 1B and 2A). Accordingly, in screening assays, we used 58 μM Ub74, 117 μM K0-Ub, and incubated for ⩽10 min. This concentration of Ub74 approximates the Km value for the acceptor Ub in Ub2 synthesis catalyzed by KIAA10 (You and Pickart, 2001).
Figure 2.
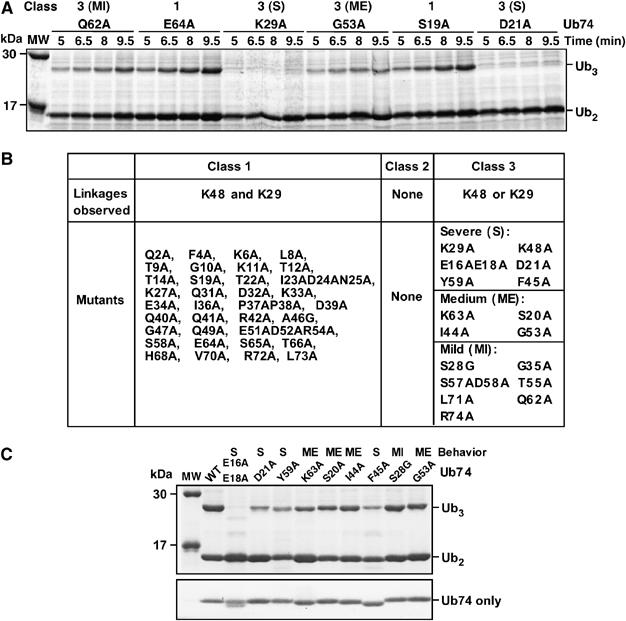
Data from acceptor Ub mutant screen. (A) Representative primary screening data (Coomassie-stained gel). The indicated Ub74 Ala mutants were tested in the Ub3 synthesis assay (incubation times as indicated). The mutants were classified as wt-like (class 1) or impaired in Ub3 synthesis in a severe (S), medium (ME), or mild (MI) manner (all class 3). (B) Summary of primary screening data. (C) Ub3 synthesis assays were conducted with the indicated Ub74 Ala mutants (Coomassie-stained gel). The bottom panel, also Coomassie stained, is from a separate gel where each Ub74 mutant was run alone to confirm equal loading.
We expected to identify at least three classes of Ub mutants. Class 1 mutants would produce Ub2 and Ub3 at similar levels to those seen with wt Ub74. Class 2 mutants would yield neither Ub2 nor Ub3, and thus would be altogether inactive as acceptors. Class 3 mutants would yield approximately normal amounts of Ub2, but reduced levels of Ub3. Such class 3 mutants are the targets of this study.
Representative primary screening data are shown in Figure 2A and Supplementary Figure 1A. Among the six Ub74 mutants assayed in this experiment, E64A and S19A produced a robust amount of Ub3; Q62A produced a slightly decreased amount of Ub3; G53A yielded still less Ub3; and K29A and D21A produced almost no Ub3. All six mutants retained normal Ub2 synthesis. These mutants were classified, respectively, into wt-like (class 1; Supplementary Figure 1), and three subtypes of class 3 mutants: mild (MI), medium (ME), and severe (S) (Figure 2A, top). No totally inactive (class 2) mutants were found.
All of the Ub74 mutants were assayed blind during the screening, so recovery of the K29A and K48A mutants (e.g., Figure 2A) represents a meaningful control. Wt-like (class 1) mutants were assayed once or twice; all other mutants were assayed at least three times to confirm their behaviors. Because the screening assay is qualitative, the absolute yields of Ub3 obtained from a given mutant varied between experiments; however, the relative yields of Ub3 from different class 3 mutants, which is the key parameter for our screen, was quite reproducible (e.g., Figure 2A and C).
Screening results for all of the mutants are summarized in Figure 2B. Besides K29A and K48A, a total of 14 mutants produced a decreased amount of Ub3, including four severe (E16AE18A, D21A, Y59A, F45A), four medium (K63A, S20A, I44A, G53A), and six mild mutants. All severe and medium, plus one mild, mutants were simultaneously assayed in Figure 2C (top panel), with the result for each mutant conforming to expectation from its behavior in the primary screen. Since the concentration of Ub74 affects the rate of Ub3 production (above), each Ub74 mutant was separately loaded to confirm that equal concentrations were used in the assays (Figure 2C, bottom panel). Aberrant electrophoretic migration of certain Ub Ala mutants, evident here for Ub-E16AE18A and F45A, has been observed previously (Tsui et al, 2005). Although Figure 2C shows some variation in the yield of Ub2, which could be indicative of effects on both linkages, it will be shown below that the medium and severe class 3 mutations affected just one linkage.
Identification of affected Lys linkages
We next conducted secondary mutagenesis to determine which linkage was affected by selected Ala mutations. Only the medium and severe mutants were subjected to this analysis. First, the K29R mutation was introduced into the E16AE18A, D21A, Y59A, K63A, S20A, I44A, F45A and G53A, mutants. Because the resulting Ub74 double/triple mutants have only K48 available for Ub2 synthesis, their relative yields of Ub2, compared to a Ub74-K29R control, reports the effects of these Ala mutations on the K48 linkage. As shown in Figure 3A, mutants K29RY59A, K29RI44A, and K29RF45A displayed decreased Ub2 formation, indicating that Ub-Y59, I44, and F45 are important for K48 usage. The E16AE18A, D21A, K63A, and S20A mutations (Figure 3A), as well as the G53A mutation (data not shown), did not affect K48 usage.
Figure 3.

Secondary mutagenesis data for acceptor Ub Ala mutants (Coomassie-stained gels). (A) Effects of Ala mutations on use of K48. The K29R mutation was introduced into the indicated Ub74 Ala mutants and Ub2 synthesis assays were conducted. (B) Effects of Ala mutations on use of K29. Reactions were carried out as in panel A, except that the K48R mutation was introduced into the indicated Ub74 Ala mutants. The underlined mutations affected use of the indicated Lys sites.
To determine the effects of specific Ala mutations on the usage of K29, the K48R mutation was introduced into the E16AE18A, D21A, Y59A, K63A, S20A, and G53A mutants. The results showed that Ub-E16AE18AK48R, D21AK48R, and K63AK48R displayed decreased Ub2 formation, while Ub-S20AK48R reproducibly displayed increased Ub2 formation (Figure 3B). Thus, E16 and/or E18, D21 and K63 are needed for optimal K29 usage, while the S20A mutation selectively favors use of K29. The Y59A and G53A mutations had no effect on K29 usage (Figure 3B and data not shown).
To examine whether E16, or E18, or both affect K29 usage, Ub74 mutants E16A, E16AK29R, E16AK48R, E18A, E18AK29R, and E18AK48R were generated and analyzed as described above. The results indicated that the E16A and E18A mutations each inhibit K29 usage, while neither mutation affects K48 usage (data not shown).
Consistent with the primary screening data, all but one of the Ala mutations examined in the secondary screen affected only one Lys linkage. The outlier was G53A, which had no effect on either Lys linkage in the secondary screen despite its effect on Ub3 synthesis in the primary screen. The basis of this discrepancy with Ub-G53A remains to be determined; one possibility is that the G53A mutation inhibits chain elongation (above n=2) regardless of chain structure.
Mass spectrometry to identify residues involved in K29 versus K48 linkage choice
To analyze the linkage properties of selected mutants by an independent method, we applied semiquantitative mass spectrometric (MS) analysis to selected class 3 Ala mutants as outlined in Figure 4A and B. The Ub2 produced by wt, S20A, D21A, I44A, Y59A, and K63A Ub74 in the Ub3 synthesis assay was excised from a gel (two time points for each mutant), trypsinized, and the peptides were eluted. Eluted peptides were ionized and signature peptides for each linkage were detected in an MS survey scan followed by one data-dependent MS/MS scan. The acquired MS/MS spectra were matched and manually verified. As expected, only K29 and K48 were detected as linkage sites in all of the Ub2 samples. Chain linkages were quantified as described in Materials and methods.
Figure 4.
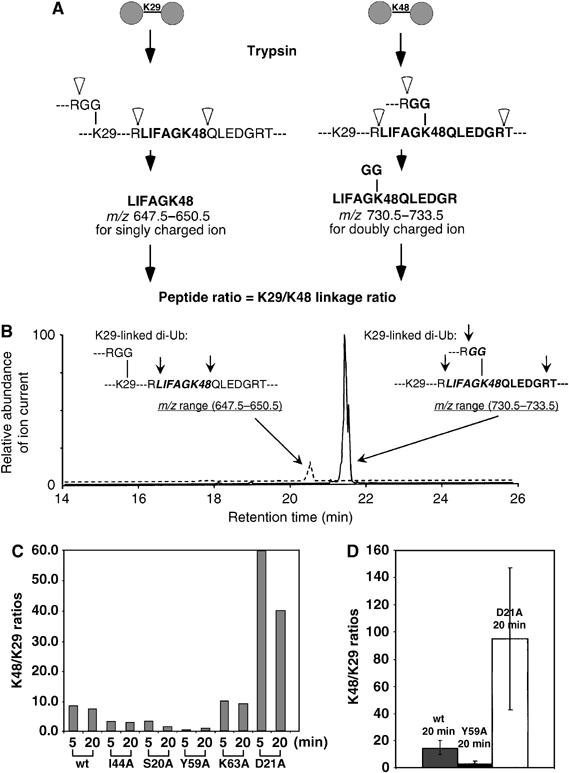
MS analysis of chain synthesis involving acceptor Ub Ala mutants. (A) Design of MS assay (see Materials and methods). (B) Representative MS data from one of the Ub2 molecules analyzed in panel C. (C) K48/K29 linkage ratios observed in Ub2 synthesized from the indicated acceptor Ub Ala mutants. Products were analyzed at two different time points in the respective reactions. (D) Data for replicate determinations (±s.d.) are shown for reactions with wt Ub, Ub-Y59A, and Ub-D21A as acceptors.
The molar ratios of K48/K29 linkages measured in selected mutant Ub2 molecules are shown in Figure 4C. Notably, Ub2 synthesized from wt Ub74 has a K48/K29 linkage ratio of ∼8, indicating that KIAA10 prefers K48 over K29 when both sites are available. Ub2 produced from mutants S20A, I44A, and Y59A had a decreased K48/K29 ratio (Figure 4C). This is consistent with the secondary mutagenesis results, which showed that the S20A mutation increased K29 usage, while the I44A and Y59A mutations decreased K48 usage (Figure 3); in all cases, the effect is to decrease the K48/K29 ratio. Mutants D21A and K63A had an increased K48/K29 ratio (Figure 4C), consistent with the decreased K29 usage revealed by secondary mutagenesis (Figure 3). Among the five mutants examined by MS, the K48/K29 ratio changed more markedly for severe mutants (D21A and Y59A) than for medium mutants (S20A, I44A, and K63A) (Figure 4C and D), also in agreement with the primary screening results. Importantly, the MS data directly show that the mutations altered linkage selectivity—even though the yield of Ub2 product was essentially unchanged by severe mutations (see Figure 2A), the distribution between the two linkages was significantly altered (Figure 4C and D).
Ub mutations affecting K48 usage are specific to KIAA10
To test if the effects of selected mutations are specific to KIAA10, E2-25K and the E6AP-HECT domains were utilized in chain assembly assays with several of the Ub Ala mutants that impede chain synthesis catalyzed by KIAA10. E2-25K and E6AP specifically synthesize unanchored K48-linked chains (Chen and Pickart, 1990; Wang and Pickart, 2005). With KIAA10, the E16AE18A and D21A mutations inhibit K29 usage, while I44A, F45A, and Y59A reduce K48 usage; Ub-R74A is a mild mutant (Figure 2B). As shown in Figure 5A, only a subset of these mutations affected E6AP-HECT-catalyzed Ub2 synthesis: Ub-F45A and R74A showed slightly decreased activity and Ub-I44A showed modest inhibition. The same conclusion can be drawn based on the yields of autoubiquitylated E6AP-HECT; this reaction also produces K48-linked chains (Glockzin et al, 2003). Importantly, the Y59A mutation, which significantly reduces Ub-K48 usage by KIAA10 (Figure 3A), has no effect on K48 usage by E6AP-HECT (Figure 5A). With E2-25K, all of the mutants except Y59A and R74A supported robust chain synthesis (Figure 5B). The importance of Ub-Y59 for chain synthesis by E2-25K was known previously (Pickart et al, 1992).
Figure 5.

Specificity of Ala mutant effects. (A, B) The indicated full-length Ubs (wt or mutant) were assayed in steady-state chain synthesis (Coomassie-stained gels). (A) E6AP-HECT-catalyzed synthesis of unanchored K48-linked chains with UbcH5A as cognate E2. (B) E2-25K-catalyzed synthesis of unanchored K48-linked chains. Ub4 doublets in some lanes reflect the presence of linear and cyclized chains (Yao and Cohen, 2000). (C) Summary of mutant effects on three (K48) conjugation systems; +++ indicates activity indistinguishable from wt Ub. (D) Thialysine at position 29 stimulates Ub's acceptor activity with KIAA10-CD (autoradiograph). Ub-K29CK48R was treated with ethyleneimine to introduce thialysine at position 29. After conversion to Ub74 with trypsin, this acceptor was used in Ub2 synthesis assays with 125I-Ub as the donor (Wang and Pickart, 2005). Control reactions used Ub74-K48R (which retains K29) as the acceptor. All lanes are from the same gel, but several intervening lanes were excised. Ub2 produced in the absence of Ub74 derives from the conjugation of 125I-Ub to itself. Bottom, quantification of Ub2 by γ-counting (corrected for signal in absence of Ub74).
Although E6AP is an HECT E3, it uses a different mechanism of chain synthesis from KIAA10—the acceptor Ub is covalently bound to the E6AP-HECT Cys residue and the chain is built up at this site prior to release by hydrolysis (Wang and Pickart, 2005). The fact that certain Ala mutations differentially affect KIAA10- and E6AP-catalyzed synthesis is therefore not surprising. However, because of this mechanistic distinction, it is possible that Ub mutants found to be defective in the E6AP reaction are actually impaired at an upstream step. We can exclude this possibility in most cases. Each mutant Ub is functional in the E1 step (under the conditions of our assay) because each of them shows moderate or greater activity in at least one conjugation system (Figure 5A and B). Moreover, the majority of these Ala mutants support yeast viability (Sloper-Mould et al, 2001), indicating that they are active in essential conjugation systems in vivo. However, the Ub-R74A mutation appears to reduce the activity of the UbcH5A∼Ub thiol ester (see below). This effect could explain the slight reduction in E6AP-HECT-catalyzed chain synthesis seen in Figure 5A.
The key point for the present study is that the spectrum of mutational effects seen with KIAA10-CD is unique to this enzyme (Figure 5C), indicating that these effects cannot be explained by changes in local structure around Ub-K48. Rather, they are most simply explained if the mutations disrupt specific, functionally important interactions between KIAA10 and the acceptor Ub.
Because no known enzyme besides KIAA10 (and its yeast ortholog Hul5) synthesizes homopolymeric K29-linked chains, we could not conduct a similar functional analysis of mutants that affected K29 usage by KIAA10. The major concern is that mutations in E16, E18, or D21, which are spatially close to K29 (see Figure 7 below), could affect the physical or electronic properties of the K29 side chain. However, previous NMR studies suggest that while there is strong electrostatic interaction between D21 and K29, such interactions are absent for E16 and E18 (Sundd et al, 2002). Therefore, the similar effects of the three acidic side-chain mutations cannot be attributed to a general disruption of electrostatic interactions between these side chains and K29.
Figure 7.
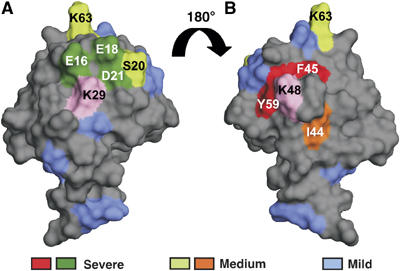
Locations of acceptor Ub mutations that affect linkage selection by KIAA10. Blue denotes mild mutations (effects on linkage selection were not determined); dark green denotes severe mutations that reduce use of K29; and light green (K63A, S20A) denotes medium mutations that affect use of K29. Red denotes severe mutations that reduce use of K48; orange (I44A), medium mutation that reduces use of K48. K29 and K48 are shown in pink. Panel B was obtained by rotating panel A 180°.
Because D21 and K29 interact, the D21A mutation is expected to lower the side-chain pKa of K29 due to loss of the stabilizing influence of the negatively changed carboxylate. To test if such an effect would be intrinsically detrimental for K29 usage by KIAA10, we substituted K29 with thialysine (S-aminoethylcysteine), whose pKa is 1.3 U less than that of Lys (Gloss and Kirsch, 1995). This was accomplished by alkylating Ub-K29CK48R with ethyleneimine. Compared to Lys at this position, the presence of thialyine at position 29 stimulated Ub2 synthesis (∼4- to 15-fold; Figure 5D). This result can be explained if deprotonation of K29 normally limits KIAA10-catalyzed chain synthesis; consistent with this idea, KIAA10-CD-catalyzed synthesis of K29- and K48-linked chains is strongly stimulated at elevated pH (pH 8.0 versus 7.6; CM Pickart, unpublished data). Thus, a lowering of the side chain pKa of K29 cannot explain the inhibition of K29 usage by the Ub-D21A mutation. Although we cannot exclude that alterations in the orientation of K29 contribute to the effects of the D21A mutation, this explanation is unlikely to apply with the E16A and E18A mutations (above). Overall, it is most likely that these mutations impair functionally important interactions between the acceptor Ub and KIAA10.
Screen for donor Ub determinants of linkage selectivity in KIAA10-catalyzed chain synthesis
In the above-described screen (Figure 2), the mutations were present in the acceptor (Ub74) molecule. To address whether the donor Ub also plays a role in determining linkage specificity, we conducted a donor Ub screen. Here, all of the Lys residues on each (full-length) Ub Ala mutant were blocked by reductive N-methylation to produce K0-Ala-Ub mutants, whose properties were then analyzed in Ub3 synthesis with wt Ub74 as the acceptor. The results of these assays are summarized in Figure 6A: K0-Q40A-Ub, K0-R73A-Ub, and K0-R74A-Ub were defective in both Ub2 and Ub3 synthesis, with Q40A showing a slightly weaker defect. Seven other Ala mutants were selectively defective in Ub3 formation (Figure 6A and B).
Figure 6.
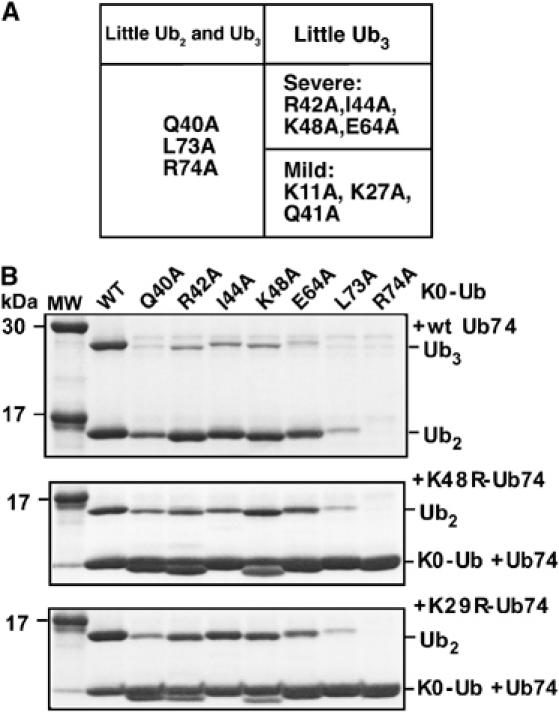
Donor Ub Ala mutant screen. The screen was carried out as described in the text using reductively N-methylated Ub (wt or mutant) as the donor in Ub3 synthesis. (A) Summary of primary screening data. (B) Search for linkage-specific effects of donor Ub mutations (Coomassie-stained gels). Inactive and severe mutants from panel A were retested with wt Ub74 (upper panel), Ub74-K48R (middle panel), and Ub74-K29R (lower panel) as the acceptor.
Each defective mutant was assayed with wt Ub74, Ub74-K48R, or Ub74-K29R to examine whether the donor Ub Ala mutations affected K29 or K48 usage (Figure 6B). Surprisingly, all of the mild and severe mutants identified in the primary donor screen were impaired in the use of both conjugation sites. These results argue against any role for donor Ub determinants in linkage determination. Note that the design of the donor Ub screen requires the mutant Ubs to function in several steps prior to the reaction of interest. In fact, while all of the mutants are functional with E1 under our assay conditions (above), the results of additional assays, summarized below, suggest that most of the donor Ub mutations impede the E2-catalyzed step rather than E3-dependent isopeptide bond synthesis.
Assays of UbcH5A∼Ub thiol ester formation showed that K0-K48A-Ub and K0-E64A-Ub produce the E2–Ub thiol ester intermediate with a significantly reduced efficiency relative to wt Ub (data not shown). This effect can explain the severe defects of these two mutants in the donor Ala mutant screen (Figure 6A and B)—although these assays were programmed to have excess E2 activity with unmutated K0-Ub, we did not check if this condition was met with each individual Ala mutant.
In pulse-chase assays, the Q40A, I44A, L73A, and R74A K0-Ub mutants retained some or all of the E2–Ub intermediate even after chasing with KIAA10-CD, indicating that these mutants are inefficiently transferred from UbcH5A to KIAA10-CD (data not shown). The requirement of these Ub side chains for E2-to-E3 transfer explains the low activity seen in the donor Ub screen (Figure 6). Interestingly, all of these residues reside at the E2/Ub interface in an NMR-derived model of the Ubc1–Ub thiol ester intermediate (Hamilton et al, 2001). The current results are among the first to suggest that these contacts, which are likely to be present in many E2–Ub adducts, could be functionally significant. Given the upstream defects of these Ub mutants (Figure 6A), we cannot exclude that they are also defective in isopeptide bond synthesis. However, the low number of mutants recovered in the donor screen suggests that, in general, acceptor Ub determinants play more important roles than donor Ub determinants in Lys site selection by KIAA10.
Discussion
Mechanism of target Lys selection by KIAA10
We used a combination of biochemistry, mutagenesis, and mass spectrometry to identify surface residues of the acceptor Ub that are important for linkage selectivity in chain synthesis catalyzed by the HECT domain E3 KIAA10. To our knowledge, this is the first such analysis to be conducted for E3-catalyzed chain synthesis. Positions of these functionally important residues on the Ub surface are shown in Figures 7A and B. Mutations colored in blue elicit mild effects; the effects of these mutations on linkage selection were not determined. Mutations colored in dark/light green affect the use of K29 in the acceptor Ub (Figure 7A); mutations colored in red/orange inhibit the use of K48 (Figure 7B). We note that while mutating A46 (to Gly) and G47 (to Ala) did not affect the use of K48, more drastic mutations at these positions might well have elicited inhibitory effects. Strikingly, most of the residues affecting a given linkage are close both to one another, and to the affected linkage site. They form two patches surrounding K29 and K48, with the K29-proximal residues important for K29 linkage selectivity and the K48-proximal residues important for K48 linkage selectivity. These two functionally important patches are on opposite faces of the Ub molecule (Figure 7).
How E3s select Ub conjugation sites is poorly understood. In fact, it is unclear if target proteins are usually modified at one specific Lys residue (see Pickart and Eddins, 2004). Target Lys specificity during poly-Ub chain assembly seems to be more marked, although the number of E3s characterized is small. Two general models of Lys site selection have been developed based on conjugation catalyzed by UEV/E2 complexes and E2 enzymes. In the first case, the surface of the acceptor Ub surrounding I44 contacts a specific surface of Mms2 in the Mms2/Ubc13 (UEV/E2) complex (VanDemark et al, 2001; McKenna et al, 2003; Tsui et al, 2005). The contact surface on Ub is distant from the target Lys (Ub-K63), but when this contact is made, other Ub Lys residues are excluded from the Ubc13 active site. In contrast, Ub residues that are important for the use of K48 or K29 by KIAA10 are clustered in the immediate vicinity of these Lys residues. It is therefore unlikely that an indirect, Mms2-like mechanism is relevant in the case of KIAA10.
A different, direct mechanism of Lys site selection is seen in many conjugation events involving the Ub-like protein SUMO (Small Ub-like MOdifier). Ubc9, the SUMO E2, can recognize and modify its substrate RanGAP1 in the absence of an E3. The consensus sequence for sumoylation comprises a four-residue motif φ-K-X-D/E; the structure of the Ubc9/RanGAP1 complex reveals multiple interactions between this consensus peptide and an active site proximal surface of Ubc9 (Bernier-Villamor et al, 2002). Here, residues immediately adjacent to the targeted Lys residue interact with the E2 to specify the substrate conjugation site. Recent results show that these Ubc9/RanGAP1 contacts are unaltered in the presence of a SUMO E3 (Reverter and Lima, 2005).
Nearly all of the acceptor Ub residues important for Lys selection in chain synthesis catalyzed by KIAA10 are proximal to the Lys residues themselves, suggesting a direct mechanism analogous to that used by Ubc9 in targeting consensus SUMO sites. In the case of KIAA10, however, the functionally important residues of the ‘substrate' (Ub) contact the E3 rather than the E2. We propose that the Lys and its surrounding residues interact with specific residues near the KIAA10 active site, thus facilitating isopeptide bond formation at the selective Lys residue. Given the identities of the relevant side chains, it is likely that these contacts, as with Ubc9, are used for binding rather than chemical catalysis. Independent of whether the KIAA10 active site harbors chemical catalytic groups (e.g., a general base), such noncovalent binding interactions can facilitate the chemical step by immobilizing and orienting the targeted Lys residue in a favorable manner for reaction. Similar interactions could facilitate the ubiquitylation of non-Ub target proteins by KIAA10 or other E3s, provided that these reactions target substrate Lys residues that occur in specific surface environments. As noted above, the generality of site-specific substrate ubiquitylation remains unclear.
Importantly, we could exclude several trivial explanations for the inhibitory effects of the Lys-proximal acceptor Ub mutations. Results obtained with other K48-specific chain-synthesizing enzymes indicate that Ala mutations at Ub residues 44, 45, and 59 do not impair the local structure around the K48 side chain. Although we could not conduct a similar test for K29-specific mutations, this explanation is very unlikely for the E16A and E18A mutations (Sundd et al, 2002). We could also exclude a reduced pKa of the K29 ɛ-amino group as the basis for inhibition by the D21A mutation (Figure 5C).
Implications for acceptor Ub interaction(s) with KIAA10
How are the presumptive Ub contact surfaces mapped in this study (Figure 7) brought into play? The precise orientation in which the acceptor Ub binds to KIAA10 remains unknown. One explanation for our data is that KIAA10 possesses two independent acceptor Ub binding sites, with the K48-selective site having a higher affinity or reactivity (leading to preferential use of K48, discussed below). Alternatively, the acceptor Ub could bind in a single orientation, perhaps in a conformationally flexible region of KIAA10, which allows subsequent reaction of the E3-bound donor Ub with opposite faces of the bound acceptor Ub. In the latter case, we might have expected to find many acceptor Ub mutants that failed to bind at all, leading to defects in usage of the K29 and K48 sites. Instead, all but one of the medium and severe class 3 mutants proved to be defective in the use of just one Lys site (the G53A mutant was not obviously defective for either linkage). However, if a single binding interface on Ub encompasses multiple side chains, each of which makes a modest contribution to affinity, the relevant mutants might not have shown strong defects in our primary screen. (For example, we cannot exclude that the mild mutations inhibit the use of both target Lys residues.)
Although a more thorough knowledge of binding properties would be informative, so far we have been unable to quantify the affinities of the acceptor Ub mutants for KIAA10. The chain synthesis activities of the severe/medium mutants were too low for reliable determination of Km values. These experiments, and attempts to use these mutants as competitive inhibitors (generating Ki values), were further complicated by the use of new target Lys residues at high concentrations of the mutants. (At high acceptor Ub concentrations, KIAA10-CD ubiquitylates Ub-K6 and K33 with a low efficiency (Volk et al, 2005).) We also undertook mutagenesis of KIAA10-CD in an effort to identify cognate E3 regions involved in the determination of linkage selectivity. However, although we focused on predicted surface loops and used a chimera-based approach that was expected to be protective of proper folding, all the mutants were devoid of any activity.
Intrinsic preference of KIAA10 for K48
Although several E3s are known to synthesize poly-Ub chains through more than one Lys residue (see Introduction), the relative susceptibilities of different Lys residues to targeting by a single E3 have not previously been established. We report here that with wt Ub, KIAA10 strongly prefers to ubiquitylate K48 over K29 (eightfold difference; Figure 4C). This outcome was initially surprising since previous studies yielded similar kinetic parameters for the KIAA10-catalyzed ubiquitylation of K48 and K29 (Mastrandrea et al, 1999). In retrospect, these earlier results probably reflected Lys misincorporation at AGA (Arg) codons of Ub (You et al, 1999) or the use of additional target Lys residues at high acceptor Ub concentrations, because recent kinetic data suggest that K48 is preferred over K29 by ⩾3-fold (CM Pickart, unpublished data). The K48 preference uncovered in the present work can be explained by a more efficient E3 interaction of the K48-proximal (hydrophobic) residues compared to the K29-proximal (acidic) residues, but direct measurements of Ub binding are needed to confirm this model.
We do not know if KIAA10 continues to use the same linkage throughout a chain when both Lys residues are available. We speculate that if the chain product has a defined conformation, Ub/Ub interactions could selectively impede access of the E3 to one Ub binding surface, leading to altered linkage selectivity in downstream isopeptide synthesis steps. Interestingly, some of the residues important for K48 usage by KIAA10, for example, I44, occur at or near a Ub/Ub interface in K48-linked chains (Varadan et al, 2002), suggesting that K29 usage could be favored in longer chain acceptors. This possibility remains to be addressed experimentally. Our results also suggest how the susceptibilities of individual Lys residues might be regulated through the participation of trans-acting factors such as E4 enzymes (Hoppe, 2005). Some E4s, such as Ufd2, bind directly to Ub and/or poly-Ub chains (Koegl et al, 1999). Our findings suggest that such E4s could block access to one Ub binding surface or create a new binding interface, thus disfavoring or favoring, respectively, the use of a specific Ub Lys residue.
In this study, we made use of a forked (heteropolymeric) chain for diagnostic purposes. Although at least one forked chain has been detected in vivo (Peng et al, 2003), the signaling properties of such chains have not been yet been examined. Our ability to generate forked chains in high yield using KIAA10 (and perhaps, other linkage-specific conjugating systems) opens the door to functional studies of such chains in the in vitro setting.
Materials and methods
Plasmids and cloning
Plasmids specifying the Ub Ala mutants (in a yeast vector) were generously provided by L Hicke, Northwestern University (Sloper-Mould et al, 2001). These plasmids were used as templates for amplifying the mutant Ub genes with flanking sites for ligation into the BamHI and NdeI sites of the pET3a E. coli vector. All Ub double or triple mutants (and the E16A and E18A single mutants) were cloned by overlap extension PCR. All inserts were verified by DNA sequencing.
Protein expression and purification
All Ub mutant proteins were expressed in E. coli strain BL21(DE3)pJY2 (supplemented with the AGA-decoding tRNA) and purified as described (Haldeman et al, 1997; You et al, 1999). K0-Ub, in which all Lys residues are mutated to arginines, was purified avoiding strong acid precipitation (You et al, 1999; Volk et al, 2005). To make Ub74, wt or mutant Ubs (∼5 mg/ml) were treated with 0.1 mg/ml trypsin (Sigma) in the presence of 20 mM NH4HCO3 at 37°C for 90 min, followed by the addition of 0.2 mg/ml soybean trypsin inhibitor (Sigma). Ub74-R74A was expressed from a synthetic gene. To prepare K0-Ala-Ub mutants, the Lys residues of each Ub Ala mutant (∼0.3 mg in 0.3 ml) were blocked by reductive N-methylation in 6 M urea (Volk et al, 2005). Ub-K29CK48R was alkylated with ethyleneimine (Chemservice) as described (Piotrowski et al, 1997).
H6-tagged murine E1 (Raasi and Pickart, 2003), untagged E2-25K (carrying the benign C170S mutation) (Haldeman et al, 1997), GST-UbcH5A, and GST-E6AP-HECT (Wang and Pickart, 2005) were expressed and purified as described; UbcH5A and E6AP-HECT were released from the respective fusion proteins by thrombin cleavage (Wang and Pickart, 2005). KIAA10-CD was produced from refolded GST-KIAA10-CD (You and Pickart, 2001).
Chain synthesis assays
Ub3 synthesis assays (37°C) employed 0.1 μM E1, 0.5 μM UbcH5A, 1 μM KIAA10-CD, 58 μM Ub74 (wt or mutants), and 117 μM K0-Ub (wt or mutants). Assays were initiated by adding a reaction cocktail contributing (final concentrations): 50 mM Tris–HCl (pH 7.6), 5 mM MgCl2, 2 mM ATP (plus regenerating system), 0.3 U/ml inorganic pyrophosphatase, and 0.5 mM DTT. Reactions were incubated for 10 min (or as indicated), quenched with sample buffer (SB), and resolved by SDS–PAGE. Products were detected by Coomassie staining.
Ub2 synthesis assays (37°C) were carried out similarly, except that 117 μM Ub74 (wt or mutants) was used.
Steady-state chain synthesis assays (37°C) employed 0.1 μM E1, 5 μM E2-25K (or 2 μM UbcH5A plus ∼4 μM E6AP-HECT), and 117 μM wt or mutant Ub. Assays were initiated by adding reaction cocktail (above). Reactions were incubated for 3 h, quenched with SB, and resolved by SDS–PAGE. Products were detected by Coomassie staining.
Mass spectrometry
Excised gel slices containing ∼2 μg Ub2 or Ub3 were trypsinized by an in-gel digestion protocol (Shevchenko et al, 1996). Released peptides were dissolved in buffer A (0.4% acetic acid, 0.005% heptafluorobutyric acid, 5% acetonitrile). Approximately 2% of each sample was loaded onto a 100 μm i.d. × 12 cm self-packed, fused-silica C18 capillary column (Peng and Gygi, 2001), and then eluted with a 30-min gradient from 0 to 30% buffer B (0.4% acetic acid, 0.005% heptafluorobutyric acid, 95% acetonitrile; flow rate: ∼300 nl/min). Eluted peptides were ionized under high voltage (1.8 kV), and detected in an MS survey scan (m/z 400–1700 and 3 μscans) followed by one data-dependent MS/MS scan (3 μscans each, isolation width of 3m/z, 35% normalized collision energy and dynamic range of 1 min) in a completely automated manner on an LCQ-DECA XP-Plus ion trap mass spectrometer (Thermo Finnigan, San Jose, CA).
Quantification of Ub linkages was based on data from the MS survey scans of the ubiquitylated peptide (LIFAGK48QLEDGR containing GlyGly-K48, called the K48-GG peptide) and a second reference peptide (LIFAGK48) (Figure 4A and B). The extracted ion currents of these two peptides were used to derive the molar ratios of K48/K29 linkages after normalizing for the efficiencies of peptide extraction, ionization, and detection (see below). The LIFAGK48 peptide derives from Ub74 and is present in an identical molar amount to the K29-GG peptide because (1) the K48 residue is not recognized by trypsin after linkage to K0-Ub, and thus LIFAGK48 can only arise from the proximal Ub molecule in non-K48 linked Ub2 (Figure 4B); (2) the distal K0-Ub produces the equivalent LIFAGR peptide, but this peptide differs in mass from LIFAGK; and (3) all non-K48-linked Ub2 is conjugated through K29 since only K29 and K48 were detected as modified in all Ub2 samples (above). We did not use the K29-GG peptide for quantification because its early elution during chromatography caused its ion current signal to vary dramatically between experiments. The conditions used in trypsin cleavage afforded quantitative cleavage at R42, R54, K48, and R74 (Xu et al, 2006). No miscleavage products were detected in any of the analyses.
The acquired MS/MS spectra were matched against a database containing Ub mutants, trypsin and keratin, using the SEQUEST algorithm (Eng et al, 1994). The parameters were set to allow parent ion mass tolerance to be 3 and to consider only b and y ion series. Modifications were permitted to allow the detection of the following (mass shift shown in Daltons): oxidized methionine (+16) and ubiquitylated Lys (+114) (Peng et al, 2003). The spectra for all matched ubiquitylated peptides were manually verified.
The ion current ratio of the two quantified peptides (see Results) will not reflect their real molar ratio if the two peptides display different efficiencies in peptide extraction, electrospray ionization, and ion detection. To eliminate these variables, we determined a normalization factor through the following steps: (1) defining the ion current ratio of K48-GG peptide versus another Ub peptide (TLSDYNIQK63) to be 0.70 when they were present at the same molar amount in a digested forked Ub3 sample (in which wt Ub74 was conjugated to two molecules of K0-Ub at K29 and K48 simultaneously); (2) measuring the ion current ratio of the LIFAGK48 peptide versus the TLSDYNIQK63 peptide to be 0.75 when they were equimolar in a sample of digested wt Ub monomer; and (3) therefore deriving the ion current ratio of the K48-GG peptide and the equimolar LIFAGK48 peptide to be 0.93. Finally, we used this factor to normalize the measured ratio of the K48 and K29 linkages. The two quantified peptides were present in all Ub species except the Ub-I44A mutant. In this case, we measured the ion currents of the mutant K48-GG peptide and the LAFAGK48 peptide without normalization, but a significant variation was not anticipated.
Supplementary Material
Supplementary Figure 1
Acknowledgments
We are grateful to L Hicke for providing the Ala mutant template plasmids and to J Sims for help with Figure 7. We thank R Cohen and one of the anonymous reviewers for particularly helpful comments. This research was supported by grants from the US National Institutes of Health to CMP (DK46984) and JP (DK69580).
References
- Bernier-Villamor V, Sampson DA, Matunis MJ, Lima CD (2002) Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell 108: 345–356 [DOI] [PubMed] [Google Scholar]
- Blaber M, Baase WA, Gassner N, Matthews BW (1995) Alanine scanning mutagenesis of the alpha-helix 115–123 of phage T4 lysozyme: effects on structure, stability and the binding of solvent. J Mol Biol 246: 317–330 [DOI] [PubMed] [Google Scholar]
- Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK, Varshavsky A (1989) A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science 243: 1576–1583 [DOI] [PubMed] [Google Scholar]
- Chen Z, Pickart CM (1990) A 25-kiloDalton ubiquitin carrier protein (E2) catalyzes multi-ubiquitin chain synthesis via lysine-48 of ubiquitin. J Biol Chem 265: 21835–21842 [PubMed] [Google Scholar]
- Cox MJ, Shapira R, Wilkinson KD (1986) Tryptic peptide mapping of ubiquitin and derivatives using reverse-phase high performance liquid chromatography. Anal Biochem 154: 345–352 [DOI] [PubMed] [Google Scholar]
- Eng J, McCormack AL, Yates JR (1994) An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 5: 976. [DOI] [PubMed] [Google Scholar]
- Finley D, Sadis S, Monia BP, Boucher P, Ecker DJ, Crooke ST, Chau V (1994) Inhibition of proteolysis and cell cycle progression in a multiubiquitination-deficient yeast mutant. Mol Cell Biol 14: 5501–5509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JM, Haguenauer-Tsapis R (1997) Ubiquitin lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J 16: 5847–5854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glockzin S, Ogi F-X, Hengstermann A, Scheffner M, Blattner C (2003) Involvement of the DNA repair protein hHR23 in p53 degradation. Mol Cell Biol 23: 8960–8969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloss LM, Kirsch JF (1995) Decreasing the basicity of the active site base, Lys-258, of aspartate amino transferase by replacement with gamma-thialysine. Biochemistry 34: 3990–3998 [DOI] [PubMed] [Google Scholar]
- Haldeman MT, Xia G, Kasperek EM, Pickart CM (1997) Structure and function of ubiquitin conjugating enzyme E2-25K: the tail is a core-dependent activity element. Biochemistry 36: 10526–10537 [DOI] [PubMed] [Google Scholar]
- Hamilton KS, Ellison MJ, Barber KR, Williams RS, Huzil JT, Mckenna S, Ptak C, Glover M, Shaw GS (2001) Structure of a conjugating enzyme–ubiquitin thiolester intermediate reveals a novel role for the ubiquitin tail. Structure 9: 897–904 [DOI] [PubMed] [Google Scholar]
- Hofmann RM, Pickart CM (1999) Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell 96: 645–653 [DOI] [PubMed] [Google Scholar]
- Hoppe T (2005) Multiubiquitylation by E4 enzymes: ‘one size' doesn't fit all. Trends Biochem Sci 30: 183–187 [DOI] [PubMed] [Google Scholar]
- Huibregtse JM, Scheffner M, Beaudenon S, Howley PM (1995) A family of proteins structurally and functionally related to the E6-AP ubiquitin-protein ligase. Proc Natl Acad Sci USA 92: 2563–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee Y, Lyon N, Huibregtse JM (2005) The Rsp5 ubiquitin ligase is coupled to and antagonized by the Ubp2 deubiquitinating enzyme. EMBO J 24: 2414–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S (1999) A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell 96: 635–644 [DOI] [PubMed] [Google Scholar]
- Lim KL, Chew KC, Tan JM, Wang C, Chung KK, Zhang Y, Tanaka Y, Smith W, Engelender S, Ross CA, Dawson VL, Dawson TM (2005) Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J Neurosci 25: 2002–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrandrea LD, You J, Niles EG, Pickart CM (1999) E2/E3-mediated assembly of lysine 29-linked polyubiquitin chains. J Biol Chem 274: 27299–27306 [DOI] [PubMed] [Google Scholar]
- McKenna S, Moraes T, Pastushok L, Ptak C, Xiao W, Spyracopoulos L, Ellison MJ (2003) An NMR-based model of the ubiquitin-bound human ubiquitin conjugation complex Mms2–Ubc13. The structural basis for lysine 63 chain synthesis. J Biol Chem 278: 13151–13158 [DOI] [PubMed] [Google Scholar]
- Morrison KL, Weiss GA (2001) Combinatorial alanine-scanning. Curr Opin Chem Biol 5: 302–307 [DOI] [PubMed] [Google Scholar]
- Peng J, Gygi SP (2001) Proteomics: the move to mixtures. J Mass Spectrom 36: 1083–1091 [DOI] [PubMed] [Google Scholar]
- Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP (2003) A proteomics approach to understanding protein ubiquitination. Nat Biotechnol 21: 921–926 [DOI] [PubMed] [Google Scholar]
- Petroski MD, Deshaies RJ (2005) Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol 6: 9–20 [DOI] [PubMed] [Google Scholar]
- Pickart CM, Eddins MJ (2004) Ubiquitin: structures, function, mechanisms. Biochem Biophys Acta 1695: 55–72 [DOI] [PubMed] [Google Scholar]
- Pickart CM, Fushman D (2004) Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol 8: 610–616 [DOI] [PubMed] [Google Scholar]
- Pickart CM, Haldeman MT, Kasperek EM, Chen Z (1992) Iodination of tyrosine-59 of ubiquitin selectively blocks ubiquitin's acceptor activity in di-ubiquitin synthesis catalyzed by E2-25K. J Biol Chem 267: 14418–14423 [PubMed] [Google Scholar]
- Piotrowski J, Beal R, Hoffman L, Wilkinson KD, Cohen RE, Pickart CM (1997) Inhibition of the 26S proteasome by polyubiquitin chains synthesized to have defined lengths. J Biol Chem 272: 23712–23721 [DOI] [PubMed] [Google Scholar]
- Raasi S, Pickart CM (2003) Rad23 ubiquitin-associated domains (UBA) inhibit 26S proteasome-catalyzed proteolysis by sequestering lysine 48-linked polyubiquitin chains. J Biol Chem 278: 8951–8959 [DOI] [PubMed] [Google Scholar]
- Reverter D, Lima CD (2005) Insights into E3 ligase activity revealed by a SUMO–RanGAP1–Ubc9–Nup358 complex. Nature 435: 687–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, Nuber U, Huibregtse JM (1995) Protein ubiquitination involving an E1–E2–E3 enzyme ubiquitin thioester cascade. Nature 373: 81–83 [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68: 850–858 [DOI] [PubMed] [Google Scholar]
- Sloper-Mould KE, Jemc J, Pickart CM, Hicke L (2001) Distinct functional surface regions on ubiquitin. J Biol Chem 276: 30483–30489 [DOI] [PubMed] [Google Scholar]
- Spence J, Gali RR, Dittmar G, Sherman F, Karin M, Finley D (2000) Cell cycle-regulated modification of the ribosome by a variant multiubiquitin chain. Cell 102: 67–76 [DOI] [PubMed] [Google Scholar]
- Sun L, Chen ZJ (2004) The novel functions of ubiquitination in signaling. Curr Opin Cell Biol 16: 119–126 [DOI] [PubMed] [Google Scholar]
- Sundd M, Iverson N, Ibarra-Molero B, Sanchez-Ruiz JM, Robertson AD (2002) Electrostatic interactions in ubiquitin: stabilization of carboxylates by lysine amino groups. Biochemistry 41: 7586–7596 [DOI] [PubMed] [Google Scholar]
- Tsui C, Raguraj A, Pickart CM (2005) Ubiquitin binding site of the ubiquitin E2 variant (UEV) protein Mms2 is required for DNA damage tolerance in the yeast RAD6 pathway. J Biol Chem 280: 19829–19835 [DOI] [PubMed] [Google Scholar]
- Ulrich HD (2004) How to activate a damage-tolerant polymerase: consequences of PCNA modifications by ubiquitin and SUMO. Cell Cycle 3: 15–18 [PubMed] [Google Scholar]
- VanDemark AP, Hofmann RM, Tsui C, Pickart CM, Wolberger C (2001) Molecular insights into polyubiquitin chain assembly: crystal structure of the Mms2/Ubc13 heterodimer. Cell 105: 711–720 [DOI] [PubMed] [Google Scholar]
- Varadan R, Walker O, Pickart CM, Fushman D (2002) Structural properties of polyubiquitin chains in solution. J Mol Biol 324: 637–647 [DOI] [PubMed] [Google Scholar]
- Volk S, Wang M, Pickart CM (2005) Chemical and genetic strategies for manipulating polyubiquitin chain structure. Methods Enzymol 399: 21–36 [DOI] [PubMed] [Google Scholar]
- Wang M, Pickart CM (2005) Different HECT domain ubiquitin ligases employ distinct mechanisms of polyubiquitin chain synthesis. EMBO J 24: 4324–4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Cheng D, Duong DM, Rush J, Roelofs J, Finley D, Peng J (2006) A proteomic strategy for quantifying polyubiquitin chain topologies. Israel J Chem (in press) [Google Scholar]
- Yao T, Cohen RE (2000) Cyclization of polyubiquitin by the E2-25K ubiquitin conjugating enzyme. J Biol Chem 275: 36862–36868 [DOI] [PubMed] [Google Scholar]
- You J, Pickart CM (2001) A HECT domain E3 enzyme assembles novel polyubiquitin chains. J Biol Chem 276: 19871–19878 [DOI] [PubMed] [Google Scholar]
- You J, Cohen RE, Pickart CM (1999) Construct for high-level expression and low misincorporation of lysine for arginine during expression of pET-encoded eukaryotic proteins in Escherichia coli. Biotechniques 27: 950–954 [DOI] [PubMed] [Google Scholar]
- Zheng N, Wang P, Jeffrey PD, Pavletich NP (2000) Structure of a c-Cbl–UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell 102: 533–539 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1


