Abstract
Many human neuroimaging studies reported activity in the precentral gyrus (PcG) ipsilateral to the side of hand movements. This activity has been interpreted as the part of the primary motor cortex (M1) that controls bilateral or ipsilateral hand movements. For the better understanding of hand ipsilateral-PcG activity, we performed a functional MRI experiment in 8 healthy right-handed adults. Behavioral tasks involved hand or lower face movements on each side, or motor imagery of the same movements. Consistent with the known M1 organization, the hand contralateral-PcG activity was centered at the “hand-knob” portion of the PcG and the face contralateral-PcG activity was localized ventrolateral to it. The hand ipsilateral-PcG activity was identified in most subjects. However, converging results indicated that this ipsilateral PcG activity was situated in Brodmann’s area 6 in both hemispheres. The hand ipsilateral-PcG zones were active during not only hand movement but also face movements. Moreover, the hand ipsilateral-PcG zones revealed substantial imagery-related activity, which also failed to differentiate the hand and face. Statistical analyses confirmed poor effector-selectivity of the hand ipsilateral PcG activity during both the movement and imagery tasks. From these results, we conclude that the hand ipsilateral-PcG activity in healthy adults probably corresponds to a part of the ventral premotor cortex. By contrast, available evidence suggests that M1 does contribute to controlling the ipsilateral hand in children and patients after stroke recovery. It appears that, within the human PcG, there exist two parallel systems potentially capable of controlling ipsilateral hand movements: ventral premotor cortex and M1. These two systems may be differentially influenced by developmental or pathologic changes.
List of Abbreviation: ANOVA, analysis of variance; BA, Brodmann’s area; contra-PcG, activity in the precentral gyrus contralateral to the movement side; fMRI, functional magnetic resonance imaging; FOV, field of view; FWHM, full-width at half-maximum; ipsi-PcG, activity in the precentral gyrus ipsilateral to the movement side; M1, primary motor cortex; MANOVA, multivariate analysis of variance; PcG, precentral gyrus; TMS, transcranial magnetic stimulation; TE, echo time; TR, repetition time
Motor representations ipsilateral to the movement side may play an important role in motor recovery after stroke and in the control of complex movements. In fact, activity in the precentral gyrus ipsilateral to the side of hand movement (hand ipsi-PcG) was documented in many previous studies. Motor-related cortical potentials or fields were recorded from the ipsilateral PcG in healthy adults (Salmelin et al. 1995; Shibasaki and Kato 1975; Toma et al. 2002) or epilepsy patients by means of chronically implanted electrodes (Neshige et al. 1988). Neuroimaging studies revealed hand ipsi-PcG activity, especially during complex finger movement, in healthy adults (Cramer et al. 1999; Kim et al. 1993; Rao et al. 1993; Shibasaki et al. 1993; Toma et al. 2002) and patients after stroke recovery (Cao et al. 1998; Chollet et al. 1991; Cramer et al. 1999; Cramer et al. 1997; Weiller et al. 1992). Furthermore, transcranial magnetic stimulation (TMS) can induce hand movement ipsilateral to the stimulated hemisphere (Wassermann et al. 1994). In this study, the stimulation sites were close to those that elicited lower face movement. The hand ipsi-PcG zones have been interpreted as the part of primary motor areas (M1) or Brodmann’s area 4 (BA 4). This interpretation was based on the nonhuman primate studies that clearly demonstrated ipsilateral or bilateral hand motor representations within M1 (Aizawa et al. 1990; Gentilucci et al. 1988).
In humans, however, the exact location and functional characteristics of the hand ipsi-PcG zones have not been fully clarified. For example, M1 was often defined as the whole PcG in those previous studies. It is now obvious that the human PcG contains not only M1 but also the lateral premotor cortex, or BA6 (Rademacher et al. 2001). The hand representation of M1 is typically located at, or in the vicinity of, the “precentral hand-knob” (Yousry et al. 1997). This landmark has widely been used to identify the hand M1 activity in the contralateral PcG (hand contra-PcG). Curiously, the location of the hand ipsi-PcG activity does not coincide with the precentral hand-knob, at least in healthy adults (Cramer et al. 1999). This discrepancy may be explained by the hypothesis that the hand ipsi-PcG activity is located within the premotor cortex occupying the rostral part of the PcG. This possibility was briefly mentioned in a previous paper (Cramer et al. 1999), but has never been fully examined until now.
An objective of the present functional MRI experiment was to investigate precise localization of the ipsi-PcG activity during unilateral hand movement. The location of the hand ipsi-PcG activity was then compared with that of the M1 strip. The M1 strip was operationally defined by control motor tasks involving the hand and face. Another aim was to characterize hand ipsi-PcG activity in terms of somatotopic organization and specificity for motor execution. To clarify these issues, we employed motor imagery tasks involving the hand or lower face. Motor imagery induces activity in the motor cortices with a medial-lateral gradient for motor somatotopy (Buccino et al. 2001; Ehrsson et al. 2003) and also with a rostral-caudal gradient for movement specificity (Hanakawa et al. 2003b).
Materials and Methods
Subjects
Eight healthy adults (5 men and 3 women; age, 22–50 years old) participated in the experiment after giving informed consent approved by the institutional review board. All subjects were right-handed as judged by the Edinburgh Handedness Inventory (Oldfield 1971).
Behavioral tasks
Subjects lay down on a scanner bed, with wearing a headset and a button response unit for each hand. Each response unit was fixed at the wrist with a strap so that subjects would only move the fingers to press the buttons. Visual and auditory stimuli were delivered to the subjects for instruction (Fig. 1). Visual stimuli were projected into the scanner’s bore from a liquid crystal display projector. To inform the mode of performance, a uniform background was changed for 300 ms from default black to green for movement, or to red for imagery. Auditory stimuli were digitally recorded male voices lasting about 1 s. The auditory stimulus let the subjects know which body part (right hand, right face, left hand, or left face) to be targeted. The subjects were instructed to keep visual fixation on a cross-hair throughout the experiment. The visual fixation condition served as an implicit baseline in this event-related fMRI experiment.
Fig. 1. Experimental paradigm.
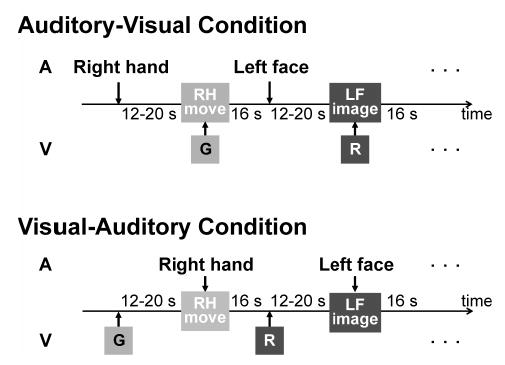
In the auditory (A)-visual (V) condition, an auditory stimulus specifying the effector (right hand, left hand, right face or left face) was presented first. After a variable delay period (12~20 s), subjects moved the specified body part or imagined it moving in response to a green (G) or red (R) visual stimulus, respectively. In the visual-auditory condition, a green or red color stimulus was presented first. After a delay period, an auditory stimulus prompted subjects to move the instructed body part or imagine it moving. For example, right hand movement (RH move) should occur in response to the visual stimulus (G) in the auditory-visual condition and to the auditory stimulus (right hand) in the visual-auditory condition.
Two different experimental conditions were employed to assess the consistency of brain activity and to extract components responsible more for motor execution than for sensory processing. In the ‘auditory-visual’ condition, an auditory stimulus was presented first to specify the body part to be involved. After a semi-randomized delay period (12~20 s), a visual stimulus (green or red) was presented to instruct the subject on the performance mode (move or imagine). In this condition, motor performance was visually triggered when subjects were informed about “how” to execute the task. In the ‘visual-auditory’ condition, a visual stimulus was presented first. After a delay period, subjects moved or imagined the body part specified by the following auditory stimulus. In this condition, performance was cued by an auditory stimulus that provided the information about “which” body part to be involved.
The hand movement task was sequential tapping with the unilateral index, middle, and ring fingers of either the right or left hand as brisk and large as possible. The tapping sequence (e.g., middle-index-ring) was fixed within an experimental run and varied across different runs. Before each run, an experimenter told the subject about which tapping sequence should be employed in the coming run. For behavioral reports, subjects sequentially pressed the buttons as instructed. The responses were recorded with Presentation software (http://nbs.neuro-bs.com) on a personal computer, which also controlled the presentations of the sensory stimuli. For the face movement task, repetitive contraction of the unilateral risorius muscle was requested. More specifically, subjects were asked to pull their unilateral oral angle to the specified side three times in a row. This task was chosen because TMS to the cerebral hemisphere can evoke exclusively contralateral risorius muscle activation (Cohen and Hallett 1988). Unilateral activation of the perioral muscles was visually checked while subjects were briefly trained on the face movement task before the experiment. During the fMRI experiment, a custom-made mirror was attached onto a head coil to allow the visualization of the mouth and bilateral cheek areas. This allowed us to monitor face movement through a digital video camera with a telephoto lens. The video was digitally recorded on a Power Macintosh computer (Apple Computer, Inc., Cupertino, CA). In addition, one of the authors continuously monitored face movements, so that compliance with the required task was immediately recognized on-line. Unfortunately, however, parts of the behavioral data were lost and subsequent analyses were not possible.
For the motor imagery tasks, subjects were instructed to imagine the specified movement without accompanying any overt movement. Subjects were asked to imagine movements performed by the subjects themselves (1st person perspective) as opposed to movements performed by someone else (Ruby and Decety 2001). They were also encouraged to imagine the movement kinesthetically rather than visually, although the clear distinction between the two strategies was not always easy. Prior to the experiment, subjects were briefly trained on the imagery tasks. Absence of overt movement was confirmed through visual inspection.
fMRI experiment
The fMRI experiment was conducted on a General Electric 1.5-T scanner with a standard head coil. Gradient-echo, echo planar images were obtained by using the GE epiVP sequence (TR = 2 s, TE = 43 ms, flip angle = 90 degree, 64 × 64 matrix, 21 slices, FOV = 22 cm, thickness = 5 mm with 1.5-mm gap). This sequence permitted on-line reconstruction and display of the image time-series, so that image quality could be checked during the experiment. Trigger pulses from the scanner were used to synchronize the timing between stimulus presentation and slice acquisition. Each experimental run lasted for 6 min (180 images). The experimental run was repeated three times for each of the ‘visual-auditory’ and ‘auditory-visual’ condition in semi-randomized order. In total, 6 scanning runs were performed for each subject. A T1-weighted axial image was acquired for each subject, by using three-dimensional volume acquisition for precise anatomic localization.
Image analysis
Image preprocessing and statistical analyses of the fMRI data were performed with the SPM99 software package (http://www.fil.ion.ucl.ac.uk/spm). The image preprocessing included realignment in time and space, and spatial normalization to fit to a standard stereotaxic space (Montreal Neurological Institute template). During the normalization process, original images were re-sampled into 2 × 2 × 2-mm voxel size. Spatial smoothing was applied with a Gaussian kernel of 6-mm full width at half maximum (FWHM). The T1-weighted anatomic images were coregistered onto the functional images and spatially normalized.
Statistical analyses were based on a fixed-effects model, multi-regression analysis for each individual. Movement and imagery events were modeled separately for each effector. Brain responses related to the instruction cues were also taken into account. In total, therefore, 9 event types of interest were defined for each run: one for instruction events, 4 for movement events (right hand, left hand, right face or left face) and 4 for imagery events (right hand, left hand, right face or left face). The brain response for each event type was modeled with a canonical hemodynamic response function and its temporal derivative. These functions were convolved with a train of delta functions representing each event type to create covariates in the multi-regression analysis. A constant term for each run was modeled as a nuisance variable. A single design matrix including those multiple covariates was thus built for each subject. Parameter estimates for each covariate were calculated from the least-mean square fit with the fMRI time series. This process was performed with the aid of session-specific scaling to deal with mean intensity differences across different runs (no within-session scaling). Also, 4-s high-cut and 120-s low-cut temporal filters were applied to compensate for the autocorrelation of the data and to increase a signal-to-noise ratio, respectively.
In this particular report, we concentrate on demonstrating activities in the bilateral PcG. Movements were visual triggered in the ‘auditory-visual’ condition and auditory triggered in the ‘visual-auditory’ condition. First, we explored brain activity common to visual-cued movement and auditory-cued movement, by employing a conjunction analysis (Price and Friston 1997). Planned linear contrasts were applied to the parameter estimates of covariates, and this operation yielded statistical parametric maps of t-statistics. To detect possibly small “ipsi-PcG” activity, the statistical threshold was set at P < .001 without correction for multiple comparisons. Second, the ipsi-PcG activity detectable during the visual-cued movement only or during auditory-cued movement only was also explored at the same threshold. For consistency, we used the same threshold to detect contra-PcG activities, although contra-PcG activities were typically much greater than ipsi-PcG activities. We thereby defined movement-related activity for each effector in the contra-PcG and ipsi-PcG for both hemispheres.
Recent anatomical evidence shows that the precentral sulcus and the central sulcus form the rostral border and caudal border of the human M1, respectively (Rademacher et al. 2001). Our search volume was therefore limited from the caudal bank of the precentral sulcus (rostral border) to the rostral bank of the central sulcus (caudal border). However, the perfect distinction of whether activity exclusively involved the rostral bank or the caudal bank of the sulci was not always easy. This was due to limited spatial resolution of the present fMRI method. We excluded activity in the depth of the precentral sulcus because this zone often shows bilateral activity during not only complex motor tasks but also cognitive tasks (Hanakawa et al. 2003a; Hanakawa et al. 2002; Picard and Strick 2001). Mean stereotaxic coordinates were calculated from the most significantly activated voxel (i.e., statistical peak) within each cluster. The Montreal Neurological Institute coordinates were converted into Talairach and Tournoux’s coordinates (Talairach and Tournoux 1988), by using a non-linear transformation (http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml).
We intended to reproduce a previous result indicating the asymmetry of hand contra- and ipsi-PcG zones (Cramer et al. 1999). For this purpose, multivariate analysis of variance (MANOVA) was applied to the 3 stereotaxic coordinates of the PcG activities. We thereby compared the location of the contra-PcG and ipsi-PcG in the same hemisphere separately for the hand and face movements. Second, a 3-dimensional vector representing the spatial relationship among the three PcG activities was computed for each subject (vector analysis). This analysis took into consideration subtle differences in each individual’s anatomy and potentially imperfect spatial normalization. The hand contra-PcG activity was assigned to the origin of this computation because the correspondence between the hand contra-PcG activity and the precentral hand-knob was quite tight. More specifically, assigned to the origin was the most significantly activated voxel in the hand contra-PcG activity at, or in the proximity of, the precentral hand-knob. The following vectors were calculated by using the statistical peak from each activity cluster. Our operational definition was that the vector from the hand contra-PcG to the face contra-PcG should represent the direction of the M1 strip in the stereotaxic coordinate system (M1 vector). A vector from the hand contra-PcG to the hand ipsi-PcG (defined by the task with the other hand) was also computed (hand ipsi-PcG vector). Then, the orientation of the hand ipsi-PcG vectors was compared with that of the M1 vector, by putting the three stereotaxic coordinates into MANOVA as variables. Finally, the mean coordinates of each PcG activity was evaluated in reference to a probability map of BA 4 in humans. A statistical analysis was performed with a Student t-test to compare the hand ipsi-PcG location with the rostral border of BA 4 described for 11 brains (Rademacher et al. 2001).
The magnitude of movement- and imagery-related responses was estimated at the most significantly activated voxel in each PcG zone. Mean percent changes of task-related MRI signals were calculated in reference to the fixation baseline. We analyzed movement-related activities and coexisting imagery-related activities by ANOVA, with taking laterality (right or left), effector (face or hand), and stimulus (auditory-cued or visual-cued) as within-subject variables.
Results
Location of the contralateral and ipsilateral PcG activities during movement tasks
Task performance was judged to be satisfactory for all subjects through the behavioral monitoring during the fMRI experiment. All movement tasks induced activities in the bilateral PcG, but the ipsi-PcG activity was less consistently observed during the right hand movement task as compared with the other movement tasks (Table 1). The conjunction analysis (auditory-cued & visual-cued) detected hand contra-PcG activity and face contra- and ipsi-PcG activities in almost all subjects. The conjunction analysis revealed hand ipsi-PcG activity in 4 out of 8 subjects for each hand movement. Separate analyses for the visual-cued movement only and the auditory-cued movement only gave consistent findings with the conjunction analysis. In the analysis only on the auditory-cued movement, ipsi-PcG activity was found in 2 more subjects for the right hand movement and 4 more subjects for the left hand movement. However, the analysis only on the visual-cued movement failed to show hand ipsi-PcG activity in subjects with negative findings by the conjunction analysis. Cortical activity ipsilateral to the side of hand movement was also observed in the parietal cortex including the postcentral gyrus. The very edge of such ‘ipsilateral parietal’ activity sometimes extended into the central sulcus (See parietal activity in white in Fig 2E). Yet, the ipsilateral parietal activity never had statistical peaks in the central sulcus, and thus was dissociable from the ipsi-PcG activity.
Table 1.
Location of movement-related activities in the precentral gyrus (PcG)
| Coordinates (mean±S.D.)
|
|||||
|---|---|---|---|---|---|
| Activity | x | y | z | Anatomical location | N of subjects |
| R hand movement task | |||||
| L PcG (contra-hand) | −37±5 | −19±4 | 54±3 | CS = 8 | A&V = 8 |
| R PcG (ipsi-hand)* | 54±7 | 5±10 | 42±5 | PcS = 4; Conv. = 1; CS = 1 | A&V = 4; A = 2 |
| L hand movement task | |||||
| L PcG (ipsi-hand) | −49±7 | 2±6 | 46±6 | Conv. = 5; PcS = 3 | A&V = 4; A = 4 |
| R PcG (contra-hand) | 38±5 | −17±4 | 54±5 | CS = 8 | A&V = 8 |
| R face movement task | |||||
| L PcG (contra-face) | −53±3 | −6±4 | 38±4 | Conv. = 4; CS = 4 | A&V = 8 |
| R PcG (ipsi-face) | 56±2 | −1±4 | 37±4 | Conv. = 4; CS = 4 | A&V = 8 |
| L face movement task | |||||
| L PcG (ipsi-face) | −54±3 | −6±3 | 38±7 | Conv. = 5; CS = 3 | A&V = 7; A = 1 |
| R PcG (contra-face) | 55±4 | −2±3 | 36±5 | Conv. = 4; CS = 4 | A&V = 7; A = 1 |
The coordinates were obtained from the most significantly activated voxel from the conjunction analysis or the separate analysis only on the auditory-cued movement for each individual. All PcG zones, except for the hand ipsi-PcG in the right hemisphere, yielded data from all subjects. Listed are Talairach and Tournoux’s coordinates converted from the Montreal Neurological Institute coordinates. R, right; L, left; Conv., convexity part of PcG; PcS, caudal bank of precentral sulcus; CS, rostral bank of PcG; A&V, conjunction analysis for auditory-cued and visual-cued movements; A, analysis on the auditory-cued movement only.
Fig. 2.
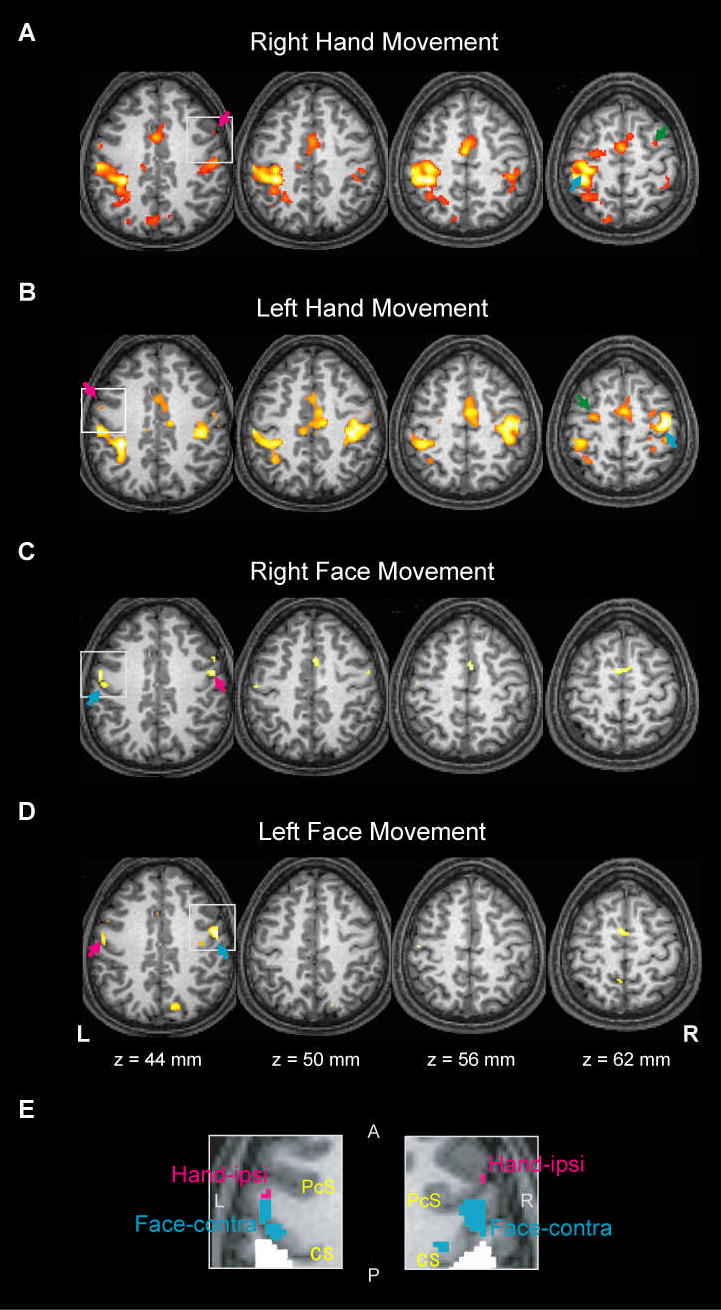
Brain activity during right hand movement (A), left hand movement (B), right face movement (C), and left face movement (D) from a conjunction analysis in a representative subject. The contralateral PcG activity and ipsilateral PcG activity are indicated by the cyan and magenta arrows, respectively. Ipsilateral activities anterior to the precentral hand-knob at z = 62 mm (green arrows in A and B) are located in the depth of the precentral sulcus and should beregarded as the dorsal premotor cortex (See Methods for clarification). (E) The areas encompassed by the white squares at z = 44 mm in (A)–(D) are magnified and overlaid for each hemisphere. The hand ipsi-PcG activity (magenta) and face contra-PcG activity (cyan) are shown. The areas in white indicate the ‘ipsilateral parietal’ activity. The coordinates are based on the Montreal Neurological Institute template. The right side of the brain (R) is shown in the right side of the images. L, left; A, anterior (= rostral); P, posterior (= caudal); CS, central sulcus; PcS, precentral sulcus
The hand contra-PcG activity often formed an activity cluster involving both precentral and postcentral gyri (e.g., Fig 2A). However, the statistical peak of the hand contra-PcG activity was consistently found at, or in the vicinity of, the precentral hand-knob in both hemispheres. The hand ipsi-PcG activity was smaller in extent than the hand contra-PcG activity (e.g., Fig 2A and B). The statistical peaks of the ipsi-PcG could be found within the limited range from the precentral sulcus (e.g., Fig 2A) through the PcG convexity (e.g., Fig 2B) to the rostral bank of the central sulcus. The hand ipsi-PcG activity was always situated rostral and lateral to the precentral hand-knob. In the ventral-dorsal axis, the hand ipsi-PcG activity was typically situated ventral to the precentral hand-knob. These findings held true for both hemispheres.
The spatial relationship between the contra- and ipsi-PcG activities was tested by MANOVA, using x, y and z coordinates as variables. It turned out that the hand ipsi-PcG activity for one movement side differed in location from the hand contra-PcG activity for the other movement side (P = .001 for both hemispheres). This indicated that the hand ipsi-PcG was a distinct zone from the hand contra-PcG/precentral hand knob. By contrast, face ipsi-PcG activity for one movement side was indistinguishable from face contra-PcG activity for the other movement side (P = .885 for the left hemisphere and P = .183 for the right hemisphere, by MANOVA). Consistent with a previous TMS study (Wassermann et al. 1994), the face PcG activity and hand ipsi-PcG activity were sometimes located very close with each other (See left panel of Fig 2E for example).
The vector analysis was performed to confirm that hand ipsi-PcG activity was distinct from face PcG activity. The mean hand ipsi-PcG vector was different than the mean M1 vector for both hemispheres (F [3, 10] = 9.3, P = .003 on the left; F [3, 12] = 7.3, P = .005 on the right, both by MANOVA). Fig 3 illustrates that the mean hand ipsi-PcG vector directs rostral and dorsal to the mean M1 vector in both hemispheres. In the left hemisphere, the hand ipsi-PcG vector was significantly shifted rostral to the M1 vector in the caudal-rostral (y) direction (P = .030) and dorsal in the ventral-dorsal (z) direction (P = .012). There was no difference in the medial-lateral (x) direction (P = .183). In the right hemisphere, the hand ipsi-PcG vector was significantly shifted dorsal to the M1 vector (P = .040). There was no significant difference in the caudal-rostral (P = .160) and medial-lateral (P = .599) directions. This analysis confirmed that the hand ipsi-PcG activity was outside of the functionally determined M1 strip.
Fig. 3.
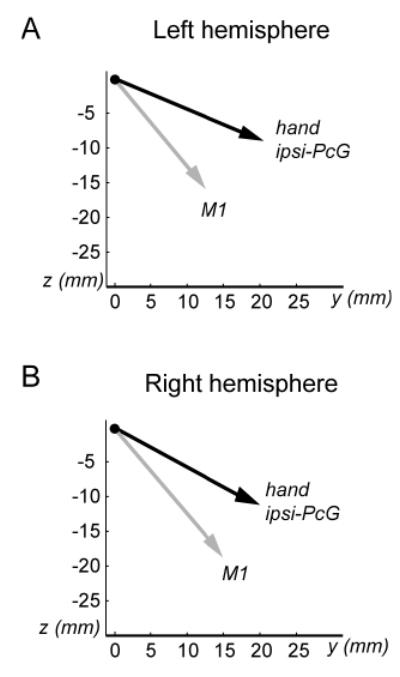
A vectorial presentation of the hand ipsi-PcG location in the left (A) and right (B) hemispheres. The hand ipsi-PcG vector (black) and the M1 vector (gray) indicate the direction of the hand ipsi-PcG activity and the face contra-PcG activity, respectively. These vectors are computed in reference to the hand contra-PcG activity as the origin (closed circle). The vectors are projected onto the caudal-rostral (y) and ventral-dorsal (z) plane for presentation. The data are not presented for the medial-lateral axis (x). The scale is in millimeters.
The stereotaxic coordinates of the PcG activities (See Table 1) were referred to the probability map of BA 4 in humans (Rademacher et al. 2001). The hand contra-PcG and the face contra- and ipsi-PcG were located within the 20% boundary of BA 4. However, the hand ipsi-PcG activity was located rostral to the BA 4 rostral border in the left hemisphere (mean distance in y coordinate = 6.6 mm; t [17] = 2.6; P = .018). The hand ipsi-PcG activity was also located rostral to the BA 4 rostral border in the right hemisphere (mean distance in y coordinate = 4.5 mm), although this difference did not reach statistical significance (t [15] = 1.3; P = .21). Note, however, that the hand ipsi-PcG was situated dorsal to the face PcG defining the BA 4 rostral border. The PcG/BA 4 runs diagonal in the stereotaxic coordinate system and directs ventrally as it goes rostrally. Taken together, the hand ipsi-PcG activities were likely to be within BA 6, rather than BA 4, in both hemispheres.
Motor somatotopy of the contralateral and ipsilateral PcG zones
Motor somatotopy expressed by movement-related activities was evaluated for each PcG zone. Although the hand ipsi-PcG activity was more frequently found during the auditory-cued movements, none of the PcG zones exhibited statistically significant effects of the stimulus. In fact, the somatotopy findings were virtually the same between the auditory-cued and the visual-cued activities. Thus, only the results from the auditory-cued activities were illustrated in Fig 4 for simplicity. The hand contra-PcG zone showed activity almost exclusively for the contralateral hand movements, indicating clear-cut somatotopic organization. This interpretation was underscored by the significant main effects of the laterality (P < .001 by ANOVA) and effector (P < .001), and by the laterality-by-effector interactions (P < .001) for both hemispheres. By contrast, the hand ipsi-PcG activity reflected not only bilateral hand movements but also bilateral lower face movements (no significant effects of laterality, effector, or laterality-by-effector interactions).
Fig. 4.
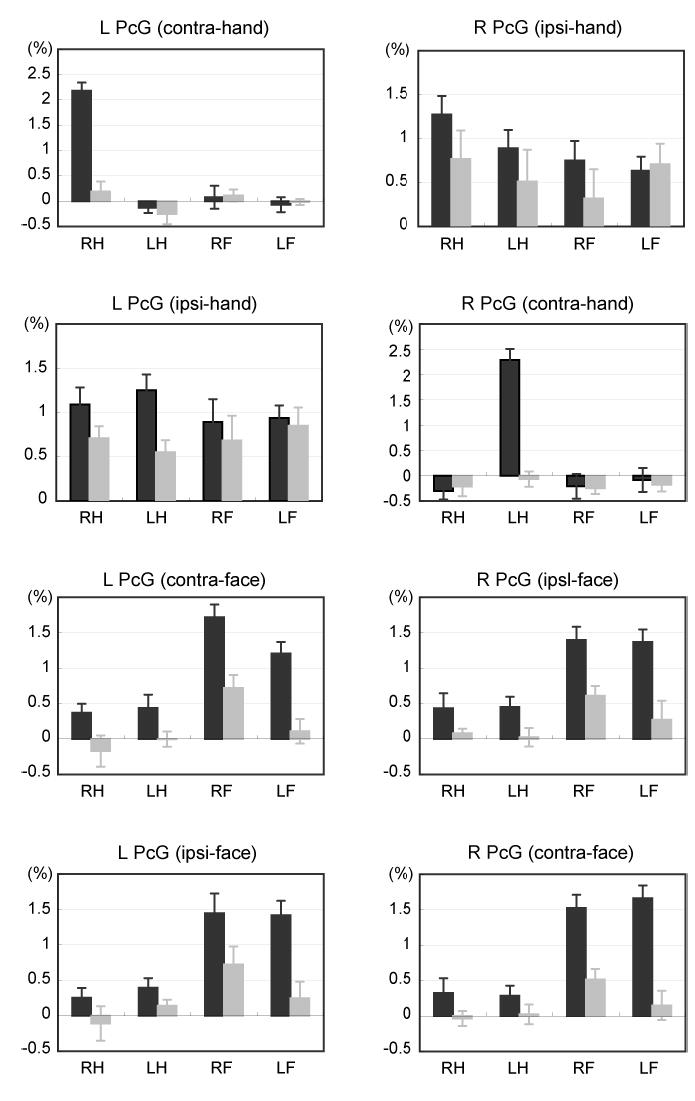
Somatotopy represented in the PcG zones during the auditory-cued movement (dark gray) and auditory-cued motor imagery (light gray). The contra- and ipsi-PcG zones (See Table 1 for the information) were determined for each effector in each individual. Task-related percent signal changes were averaged across subjects. Error bars represent standard errors of mean. RH, right hand; LH, left hand ; RF, right face; LF, left face
The contra- and ipsi-face PcG zones showed significant effects of the effector (P < .001) but not the laterality, indicating that each face PcG zone primarily represents bilateral lower face movements. Nonetheless, the significant effector-by-laterality interactions (P = .02) in the face contra-PcG in the left hemisphere signified a predominant role of this zone for contralateral face movements.
Imagery-related activity in the contralateral and ipsilateral PcG zones
The hand contra-PcG zones showed almost no imagery-related activity for either the hand or the face (Fig. 4). Statistically, the hand contra-PcG zones showed neither main effects nor interactions, except for moderate laterality effects (P = .02) in the hand contra-PcG zone of the left hemisphere. In the hand ipsi-PcG zones, hand motor imagery-related activity was half as much as hand movement-related activity. Moreover, imagery-related activity was also found during face motor imagery in the hand ipsi-PcG zones. Statistical analyses failed to show the effects of the imagined effector in both hemispheres (i.e., no somatotopy). As for the face PcG zones, face imagery-related activity was a quarter as much as actual face movement-related activity on both sides. The face PcG activity during motor imagery was selective for the face. There were significant main effects of effector in the face contra-PcG zone in the right hemisphere (P = .02) and in the face ipsi-PcG zone in the left (P = .03) and the right (P = .01) hemispheres. The face contra-PcG zone in the left hemisphere was selective for contralateral face motor imagery as indicated by significant effector-by-laterality interactions (P = .04).
To find somatotopically organized hand motor imagery-related activity was originally outside of the scope of this experiment. Nevertheless, such a region was found within the left superior precentral sulcus (mean coordinate; x, y, z = −32, −2, 63) consistently across the subjects (n = 7). The imagery-related activity of this region was significantly greater during hand motor imagery than face motor imagery (P < .05). This activity did not show the effect of laterality (P = .302) or effector-by-laterality interaction (P = .130). Notably, the same region exhibited somatotopically organized movement-related activity selective for the right hand movement (effector, P < .05; laterality, P = .281; effector-by-laterality interaction, P < .05). Only a small number of subjects (n = 4) showed similar activity in the right superior precentral sulcus during the left hand motor imagery task. No further analysis was performed because of the inconsistency.
Discussion
Technically, we avoided employing a voxel-based group analysis from multiple subjects. Larger smoothing kernels (10~12 mm FWHM) typically used for such an analysis may obscure the exact localization and extent of brain activity. This seemed important since we occasionally found obviously 2 separate activities in the hemisphere ipsilateral to hand movements. One was in the precentral sulcus or PcG convexity and the other was in the parietal cortex (i.e., ‘ipsilateral parietal’ activity). A group-averaged analysis may merge these two separate activities together, which may result in a false finding of PcG activation situated in between. We were still able to confirm previous reports showing that unilateral independent finger movement induced activity in the PcG not only contralateral but also ipsilateral to the movement side (Cramer et al. 1999; Kim et al. 1993; Rao et al. 1993; Shibasaki et al. 1993). The ipsilateral PcG activity was less consistently observed during the right hand movement task than the other tasks. This observation supports a previous report in which the right PcG was activated mostly during contralateral hand movements whereas the left PcG was activated by both ipsilateral and contralateral hand movements (Kim et al. 1993). Also, the location of the hand ipsi-PcG activity is congruent with the one reported previously (Cramer et al. 1999).
The mean coordinates of the hand and face contra-PcG activities were consistent with those of the M1 activations in a previous report (Lotze et al. 2000). The hand contra-PcG activity tuned finely to the contralateral hand movement fits the conventional notion for M1 functions. By contrast, the hand ipsi-PcG zone showed several properties atypical as M1. First, the hand ipsi-PcG zones were located rostral to the BA4 rostral border, most likely within BA 6. Moreover, notwithstanding the definition by hand movement, the hand ipsi-PcG zones demonstrated almost comparable activity for lower face movement. This means that the hand ipsi-PcG zones lack motor somatotopy, a hallmark of M1. M1 has sequential and clearly separated motor representations of the head, upper extremity and lower extremity, although the intra-limb sequential somatotopy is questioned (Schieber 2001). The peaks of the face contra-PcG and ipsi-PcG zones were localized within BA 4, although these activities might extend rostrally into BA 6. The present results demonstrated that the face M1 might have control over bilateral lower face muscles to some extent. Still, the significant effector-by-laterality interactions in the face contra-PcG in the left hemisphere indicates that this zone may relate more to contralateral than ipsilateral movements. This finding agrees with moderate lower face paresis predominantly on the contralateral side to the lesion involving the cerebral hemisphere.
The hand ipsi-PcG zones showed considerable imagery-related activity. Such imagery-related activity was scarcely observed in the hand contra-PcG zones in the present study. M1 may reveal slight motor imagery-related activity (Porro et al. 1996; Roth et al. 1996), or none at least at a group level (Gerardin et al. 2000; Hanakawa et al. 2003b). Actually, the face PcG zones consistent with the face M1 disclosed mild imagery-related activity. Hence, the presence or absence of motor imagery-related activity per se may not adequately characterize M1. It should be noted, however, that the face PcG zones preserved effector-selectivity in both movement- and imagery-related activities whereas the hand ipsi-PcG zones did not. There is accumulating evidence indicating that motor imagery evokes somatotopically organized activity in the premotor cortex and possibly M1 (Buccino et al. 2001; Ehrsson et al. 2003). Therefore, imagery-related activity without following somatotopy argues against that the hand ipsi-PcG zones belong to M1. Instead, we found hand imagery-selective activity in the superior precentral sulcus, which probably corresponds to the caudal part of the dorsal premotor cortex (Hanakawa et al. 2002; Hanakawa et al. 2003b; Picard and Strick 2001).
Information available from nonhuman primate studies is invaluable for interpreting the present data. At least, there are three candidate regions as the counterpart of the hand ipsi-PcG zones shown here in humans. First, a classic study localized ipsilateral limb motor representation adjacent to the superior precentral dimple mainly in BA 6 (Bucy and Fulton 1933). This dorsal premotor area predominantly manifests hindlimb movement, and thus is unlikely the correlate of the hand ipsi-PcG zone. Second, several studies described a small distinct subdivision of M1 that encodes ipsilateral or bilateral distal forelimb movements (Aizawa et al. 1990; Gentilucci et al. 1988). Such a region is situated between the face and distal forelimb representations of M1. In the present study, however, the hand ipsi-PcG zones were found rostral to the M1 strip. Additionally, the percentage of neurons related to ipsilateral or bilateral distal forelimb movement is rather small (8 %) in M1 (Tanji et al. 1988). The chance to detect activity from such a small portion of neurons would not be very high with the present method. Third and last, distal forelimb movements are also represented in F5 and rostral F4 sectors of the ventral premotor cortex (Gentilucci et al. 1988). In the ventral premotor cortex, most of the distal forelimb neurons are active during movement on either side (Rizzolatti et al. 1988; Tanji et al. 1988). Notably, the distal forelimb and face representations are partially overlapped in the ventral premotor cortex in contrast to the clear segregation between them in M1 (Gentilucci et al. 1988). This somatotopic overlap would account for the activity during face movement in the hand ipsi-PcG. Also, a part of the ventral premotor cortex contains visuomotor neurons that are active during active movement as well as visual stimulus presentation relevant to motor behavior (Murata et al. 1997). Such visuomotor properties could explain activity during motor imagery in the hand ipsi-PcG. Among the three candidate regions, therefore, the properties of the ventral premotor cortex can best explain the hand ipsi-PcG activity in the present experiment. The ventral premotor cortex contains a particular class of neurons that are active while an organism makes a movement (typically grasping movement) and also while the organism observes someone else executing the same movement (Gallese et al. 1996; Rizzolatti et al. 1996). In agreement with our interpretation, the hand ipsi-PcG activity coincides in location with the ventral premotor cortex activity during passive observation of hand movements (Buccino et al. 2001).
Bilateral hand movements can be easily elicited by stimulation to M1 in healthy children, but this phenomenon disappears after age 10 (Muller et al. 1997). In adults with congenital mirror movement, bilateral hand movements can be evoked by M1 stimulation (Cohen et al. 1991). Hemiparetic patients due to stroke may present with typical precentral hand-knob activity ipsilateral to the affected hand (Cao et al. 1998; Carey et al. 2002), although such activity could be, in part, ascribed to association movements of the intact hand (Weiller et al. 1993). Some hemiparetic patients experience motor disturbance of the hand ipsilateral to a lesion (Colebatch and Gandevia 1989; Jones et al. 1989). These lines of evidence suggest that M1 does have a potential to represent ipsilateral or bilateral hand movements in humans. To reconcile the discrepancy between these findings and the present data, we propose a possible scheme of age- or disease-associated changes of the two parallel motor systems originating from the ipsilateral PcG (Fig. 6). It appears that both premotor cortex and M1 control the ipsilateral hand in childhood. Presumably, the M1 function controlling the ipsilateral hand is gradually suppressed in the course of the development of inhibitory systems. Such inhibitory systems would enhance the independent and agile use of each hand. The inhibitory systems may include the transcallosal inhibition from the contralateral M1. Inter-hemispheric interactions between the bilateral M1 seem effective and mostly inhibitory in human adults (Ferbert et al. 1992), although the callosal connections between them are relatively sparse (Rouiller et al. 1994). Because of the suppression of ipsilateral M1, only the ventral premotor cortex may concern ipsilateral or bilateral hand movements in healthy adults. Maldevelopment of the inhibitory systems possibly results in the congenital mirror movement, and the withdrawal of the inhibition due to acquired lesions might lead to reemergence of the ipsilateral M1 function for compensation. Consistent with this scheme, it has been nicely shown that the size of congenital lesions affects the development or withdrawal of the two ipsilateral motor systems on the lateral convexity of the hemisphere (Staudt et al. 2002).
In summary, ipsilateral PcG activity often observed during independent finger movements likely corresponds to a portion of the ventral premotor cortex rather than M1. We need further studies to test if this notion applies to other types of movement, such as a forceful grip. Also, movement of the proximal arm may well involve ipsilateral M1. It is possible that primates have developed a specialized system to subserve the dexterity of independent finger movements. Evidence from split-brain primates indicates that the ipsilateral motor system effectively controls the arm but not the hand or fingers (Brinkman and Kuypers 1973). Recent studies in stroke patients support this notion by showing that the intact parts of the affected hemisphere might be more important for the paretic hand control (Brinkman and Kuypers 1973; Cramer et al. 2002; Fridman et al. 2004; Werhahn et al. 2003). These new findings cast doubt on the predominant role of the ipsilateral motor system during stroke recovery. Nonetheless, rehabilitation strategies to enhance effective use of the ipsilateral system may still be plausible, if we can develop a way to utilize the properties of the ipsilateral system, such as the visuomotor/imagery functions.
Fig. 5.
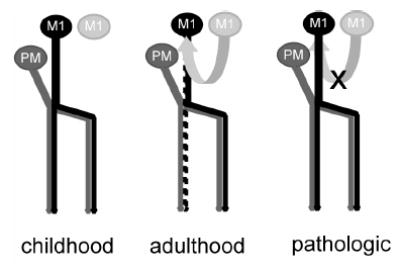
A possible scheme for the ipsilateral motor systems originating from the PcG. Both premotor cortex and M1 may control the ipsilateral hand below age 10 (childhood). The ipsilateral M1 function is gradually suppressed in the course of the normal development of inhibitory systems such as the transcallosal inhibition from the contralateral M1. In normal adults, only the premotor cortex usually controls ipsilateral or bilateral distal limb movements (adulthood). Withdrawal of the inhibitory inputs due to acquired lesions might lead to the reemergence of ipsilateral M1 function (pathologic).
Acknowledgments
T.H. was supported by an NINDS Intramural Competitive Fellowship Award and also at a manuscript writing stage by a Grant-in-Aid for Young Researchers (B) 15700257 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- Aizawa H, Mushiake H, Inase M, Tanji J. An output zone of the monkey primary motor cortex specialized for bilateral hand movement. Exp Brain Res. 1990;82:219–221. doi: 10.1007/BF00230856. [DOI] [PubMed] [Google Scholar]
- Brinkman J, Kuypers GJM. Cerebral control of contalateral and ipsilateral arm, hand and finger movements in split-brain rhesus monkey. Brain. 1973;96:653–674. doi: 10.1093/brain/96.4.653. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- Bucy P, Fulton J. Ipsilateral representation in the motor and premotor cortex of monkeys. Brain. 1933;56:318–342. [Google Scholar]
- Cao Y, D’Olhaberriague L, Vikingstad EM, Levine SR, Welch KM. Pilot study of functional MRI to assess cerebral activation of motor function after poststroke hemiparesis. Stroke. 1998;29:112–122. doi: 10.1161/01.str.29.1.112. [DOI] [PubMed] [Google Scholar]
- Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, Ugurbil K. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol. 1991;29:63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Hallett M. Noninvasive mapping of human motor cortex. Neurology. 1988;38:904–909. doi: 10.1212/wnl.38.6.904. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Meer J, Tarkka I, Bierner S, Leiderman DB, Dubinsky RM, Sanes JN, Jabbari B, Branscum B, Hallett M. Congenital mirror movements. Abnormal organization of motor pathways in two patients. Brain. 1991;114 ( Pt 1B):381–403. doi: 10.1093/brain/114.1.381. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain. 1989;112 ( Pt 3):749–763. doi: 10.1093/brain/112.3.749. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Finklestein SP, Schaechter JD, Bush G, Rosen BR. Activation of distinct motor cortex regions during ipsilateral and contralateral finger movements. J Neurophysiol. 1999;81:383–387. doi: 10.1152/jn.1999.81.1.383. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Mark A, Barquist K, Nhan H, Stegbauer KC, Price R, Bell K, Odderson IR, Esselman P, Maravilla KR. Motor cortex activation is preserved in patients with chronic hemiplegic stroke. Ann Neurol. 2002;52:607–616. doi: 10.1002/ana.10351. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, Kennedy DN, Finklestein SP, Rosen BR. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Geyer S, Naito E. Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. J Neurophysiol. 2003;90:3304–3316. doi: 10.1152/jn.01113.2002. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119 ( Pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G. Functional organization of inferior area 6 in the macaque monkey. I. Somatotopy and the control of proximal movements. Exp Brain Res. 1988;71:475–490. doi: 10.1007/BF00248741. [DOI] [PubMed] [Google Scholar]
- Gerardin E, Sirigu A, Lehericy S, Poline JB, Gaymard B, Marsault C, Agid Y, Le Bihan D. Partially overlapping neural networks for real and imagined hand movements. Cereb Cortex. 2000;10:1093–1104. doi: 10.1093/cercor/10.11.1093. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Honda M, Okada T, Fukuyama H, Shibasaki H. Differential activity in the premotor cortex subdivisions in humans during mental calculation and verbal rehearsal tasks: a functional magnetic resonance imaging study. Neurosci Lett. 2003a;347:199–201. doi: 10.1016/s0304-3940(03)00692-x. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Honda M, Sawamoto N, Okada T, Yonekura Y, Fukuyama H, Shibasaki H. The role of rostral Brodmann area 6 in mental-operation tasks: an integrative neuroimaging approach. Cereb Cortex. 2002;12:1157–1170. doi: 10.1093/cercor/12.11.1157. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Immisch I, Toma K, Dimyan MA, van Gelderen P, Hallett M. Functional properties of brain areas associated with motor execution and imagery. J Neurophysiol. 2003b;89:989–1002. doi: 10.1152/jn.00132.2002. [DOI] [PubMed] [Google Scholar]
- Jones RD, Donaldson IM, Parkin PJ. Impairment and recovery of ipsilateral sensory-motor function following unilateral cerebral infarction. Brain. 1989;112 ( Pt 1):113–132. doi: 10.1093/brain/112.1.113. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ashe J, Hendrich K, Ellermann JM, Merkle H, Ugurbil K, Georgopoulos AP. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science. 1993;261:615–617. doi: 10.1126/science.8342027. [DOI] [PubMed] [Google Scholar]
- Lotze M, Erb M, Flor H, Huelsmann E, Godde B, Grodd W. fMRI evaluation of somatotopic representation in human primary motor cortex. Neuroimage. 2000;11:473–481. doi: 10.1006/nimg.2000.0556. [DOI] [PubMed] [Google Scholar]
- Muller K, Kass-Iliyya F, Reitz M. Ontogeny of ipsilateral corticospinal projections: a developmental study with transcranial magnetic stimulation. Ann Neurol. 1997;42:705–711. doi: 10.1002/ana.410420506. [DOI] [PubMed] [Google Scholar]
- Murata A, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G. Object representation in the ventral premotor cortex (area F5) of the monkey. J Neurophysiol. 1997;78:2226–2230. doi: 10.1152/jn.1997.78.4.2226. [DOI] [PubMed] [Google Scholar]
- Neshige R, Luders H, Shibasaki H. Recording of movement-related potentials from scalp and cortex in man. Brain. 1988;111 ( Pt 3):719–736. [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Porro CA, Francescato MP, Cettolo V, Diamond ME, Baraldi P, Zuiani C, Bazzocchi M, di Prampero PE. Primary motor and sensory cortex activation during motor performance and motor imagery: a functional magnetic resonance imaging study. J Neurosci. 1996;16:7688–7698. doi: 10.1523/JNEUROSCI.16-23-07688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. NeuroImage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Burgel U, Geyer S, Schormann T, Schleicher A, Freund HJ, Zilles K. Variability and asymmetry in the human precentral motor system. A cytoarchitectonic and myeloarchitectonic brain mapping study. Brain. 2001;124:2232–2258. doi: 10.1093/brain/124.11.2232. [DOI] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, Lisk LM, Morris GL, Mueller WM, Estkowski LD, et al. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43:2311–2318. doi: 10.1212/wnl.43.11.2311. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M. Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Exp Brain Res. 1988;71:491–507. doi: 10.1007/BF00248742. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Roth M, Decety J, Raybaudi M, Massarelli R, Delon-Martin C, Segebarth C, Morand S, Gemignani A, Decorps M, Jeannerod M. Possible involvement of primary motor cortex in mentally simulated movement: a functional magnetic resonance imaging study. Neuroreport. 1996;7:1280–1284. doi: 10.1097/00001756-199605170-00012. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp Brain Res. 1994;102:227–243. doi: 10.1007/BF00227511. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nat Neurosci. 2001;4:546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Forss N, Knuutila J, Hari R. Bilateral activation of the human somatomotor cortex by distal hand movements. Electroencephalogr Clin Neurophysiol. 1995;95:444–452. doi: 10.1016/0013-4694(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Constraints on somatotopic organization in the primary motor cortex. J Neurophysiol. 2001;86:2125–2143. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Kato M. Movement-associated cortical potentials with unilateral and bilateral simultaneous hand movement. J Neurol. 1975;208:191–199. doi: 10.1007/BF00630632. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Sadato N, Lyshkow H, Yonekura Y, Honda M, Nagamine T, Suwazono S, Magata Y, Ikeda A, Miyazaki M, et al. Both primary motor cortex and supplementary motor area play an important role in complex finger movement. Brain. 1993;116:1387–1398. doi: 10.1093/brain/116.6.1387. [DOI] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krageloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain. 2002;125:2222–2237. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- Talairach J and Tournoux P. Co-planar stereotaxic atlas of the human brain New York: Thieme Medical Publishers, 1988.
- Tanji J, Okano K, Sato KC. Neuronal activity in cortical motor areas related to ipsilateral, contralateral, and bilateral digit movements of the monkey. J Neurophysiol. 1988;60:325–343. doi: 10.1152/jn.1988.60.1.325. [DOI] [PubMed] [Google Scholar]
- Toma K, Matsuoka T, Immisch I, Mima T, Waldvogel D, Koshy B, Hanakawa T, Shill H, Hallett M. Generators of movement-related cortical potentials: fMRI-constrained EEG dipole source analysis. Neuroimage. 2002;17:161–173. doi: 10.1006/nimg.2002.1165. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Pascual-Leone A, Hallett M. Cortical motor representation of the ipsilateral hand and arm. Exp Brain Res. 1994;100:121–132. doi: 10.1007/BF00227284. [DOI] [PubMed] [Google Scholar]
- Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. 1992;31:463–472. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol. 1993;33:181–189. doi: 10.1002/ana.410330208. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Conforto AB, Kadom N, Hallett M, Cohen LG. Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann Neurol. 2003;54:464–472. doi: 10.1002/ana.10686. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]


