Abstract
Nucleo-cytoplasmic shuttling is an important feature of proteins involved in nuclear export/import of RNAs, proteins, and also large ribonucleoprotein complexes such as ribosomes. The vast amount of proteomic data available shows that many of these processes are highly dynamic. Therefore, methods are needed to reliably assess whether a protein shuttles between nucleus and cytoplasm, and the kinetics with which it exchanges. Here we describe a combination of the classical heterokaryon assay with fluorescence recovery after photobleaching (FRAP) and fluorescence loss in photobleaching (FLIP) techniques, which allows an assessment of the kinetics of protein shuttling in the yeast Saccharomyces cerevisiae.
Keywords: nucleo-cytoplasmic shuttling, Saccharomyces cerevisiae
INTRODUCTION
Many analyses in yeast and higher eukaryotes have addressed the transport of factors between the nucleus and cytoplasm. However, previous assays for nucleo-cytoplasmic shuttling in yeast have either used a conventional heterokaryon assay (Flach et al. 1994) or changes in protein distribution in mutants defective in either import or export. Both of these approaches rely on assessments of the steady-state distribution of the protein, and therefore, do not allow the kinetics to be addressed. Moreover, yeast heterokaryon formation requires at least 1–2 h, so de novo protein synthesis must be inhibited, either by placing the gene of interest under the control of regulated promoter (Flach et al. 1994; Peng and Hopper 2000) or by the use of protein synthesis inhibitors (Oeffinger et al. 2004). This can introduce artifacts due to either altered levels of protein expression or the general inhibition of translation. In addition, inhibition of translation severely affects heterokaryon formation (K. Belaya, D. Tollervey, and M. Koš, unpubl.). The use of FLIP or FRAP to assess nucleo-cytoplasmic shuttling has been reported in higher eukaryotes (for review, see Koster et al. 2005). However, the very small size of yeast cells has prevented the application of these techniques. We therefore sought to develop a new approach, combining a heterokaryon assay with FRAP/FLIP to assess the shuttling properties of proteins in yeast.
RESULTS
To demonstrate the feasibility of the proposed approach, we decided to assess the shuttling properties of the ribosome synthesis factor Arx1p. Previous analyses identified Arx1p as a component of a late pre-60S ribosomes that were associated with putative export factors Nmd3p, Nug1p, and Nug2p (Bassler et al. 2001; Nissan et al. 2002). Arx1p was enriched in nucleus, but was also detected in cytoplasm. Together, these observations suggested, but did not formally demonstrate that Arx1p is a shuttling protein that accompanies the pre-60S particles during nuclear export (Nissan et al. 2002).
A strain expressing GFP-tagged versions of Arx1p was created. Arx1p is nonessential for viability, but arx1Δ strains are mildly impaired in growth (data not shown). No growth impairment was seen in the ARX1::GFP strain, indicting that the fusion construct is functional. The strain expressing Arx1-GFP was mated with a strain carrying the kar1-1 mutation, which prevents nuclear fusion following cell fusion, leading to formation of a heterokaryon with two nuclei. To reduce background fluorescence, mating was performed in complete synthetic medium.
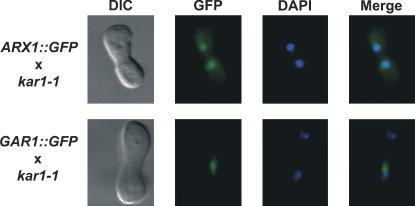
We initially assessed the shuttling of Arxp1 in a conventional heterokaryon assay (Fig. 1). Arx1p–GFP was detected in both nuclei of heterokaryons 3 h after mating. In contrast, a known nonshuttling nucleolar protein Gar1p–GFP (Girard et al. 1992) was detected in only one nucleus of the heterokaryon. This would be consistent with nuclear-cytoplasmic shuttling of Arx1–GFP, but might simply result from uptake of the cytoplasmic pool of Arx1p, slow leakage of Arx1p from the “donor” (i.e., Arx1–GFP expressing) nucleus over the 3-h time course of the mating, or even a high level of de novo synthesis.
FIGURE 1.
Heterokaryon assay. Cells expressing either Arx1p–GFP or Gar1p–GFP were mated with kar1-1 strain and placed on YPD plate containing cycloheximide to inhibit de novo protein synthesis. After a 2-h incubation, heterokaryons were analyzed by fluorescence microscopy for presence of GFP signal in both nuclei.
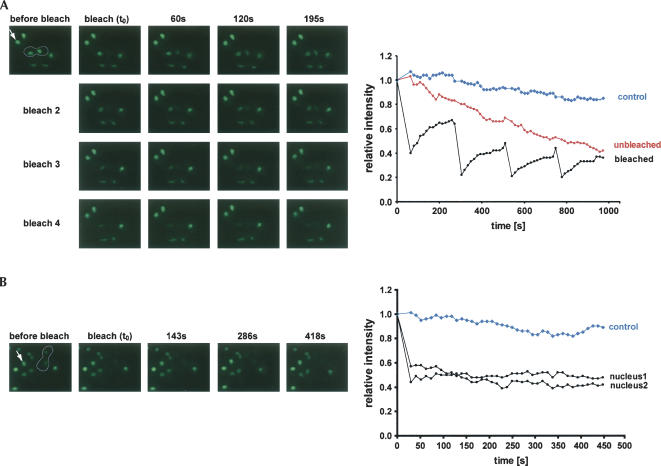
To allow a FRAP/FLIP analyses, one nucleus of the heterokaryon was completely bleached. Recovery of fluorescence in the target nucleus (FRAP) and loss of fluorescence in the unbleached nucleus (FLIP) were then followed in real time. To increase the resolution of the analysis, we repeated the bleaching of the target nucleus up to four times, after which the fluorescent signal in the unbleached nucleus was largely lost (Fig. 2A). The recovery of fluorescence in the bleached nucleus was approximately reciprocal to the loss of fluorescence in the unbleached nucleus, demonstrating rapid exchange of Arx1–GFP between both nuclei. The fluorescence of unbleached control nuclei in nearby cells was only mildly decreased by general photobleaching of the sample during viewing.
FIGURE 2.
Arx1p shuttles rapidly between nucleus and cytoplasm. (A) One nucleus of a heterokaryon (outlined) expressing Arx1p–GFP was bleached four times every 225 sec. The fluorescence intensity in both nuclei was recorded at 15-sec time points (graph on the right). A representative set of frames is shown. An arrow indicates the control nonbleached nucleus in a neighboring cell. (B) To control for cytoplasmic pool of Arx1p–GFP and de novo protein synthesis, both nuclei of heterokaryon were bleached and fluorescence intensity in both nuclei was followed by an 11-sec interval.
To control for de novo protein synthesis and recovery of nuclear signal from the cytoplasmic pool of Arx1p, we bleached both nuclei of a heterokaryon and followed the recovery of nuclear fluorescence (Fig. 2B). Little photo-recovery was seen in either nucleus, showing that neither the cytoplasmic pool nor de novo synthesis of Arx1p contributed significantly to the fluorescence recovery in cells with a single bleached nucleus.
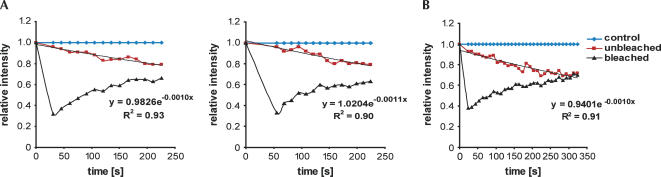
These observations demonstrate that Arx1p is a bona fide nucleo-cytoplasmic shuttling protein. Moreover, the kinetics of recovery/loss of fluorescence can be also used to estimate the flux of Arx1p. Figure 3A shows recovery and loss of fluorescence after the first bleach in two independent “multibleach” experiments. Figure 3B shows fluorescence recovery and loss in a heterokaryon after single bleach, which was followed for 5 min to allow complete equilibration of the signals in unbleached and bleached nucleus. Exponential curves were fitted to the obtained data. The shuttling half life can be calculated from the fitted exponential function (y = a.e−bx) as t1/2 = ln2/b. The average value, calculated from eight independent experiments, was 11.2 ± 1.3 min. If the cytoplasmic protein has an equal chance of reimport into either nucleus, the flux of export is double this value. The half life of nuclear Arx1p is therefore ∼5.6 min.
FIGURE 3.
Kinetics of Arx1p shuttling. (A) Graphs representing the fluorescence loss and recovery in the heterokaryon nuclei during the first bleach from two independent experiments. The fluorescence intensity was normalized to the intensity in the control nucleus. An exponential curve was fitted to the nonbleached nucleus and the calculated exponential function and correlation coefficient are shown. (B) As in A, but the heterokaryon was bleached only once and the fluorescence loss and recovery were followed until complete equilibration of the intensity in both nuclei.
DISCUSSION
This study shows that FLIP/FRAP photobleaching techniques can be applied to yeast heterokaryons to measure kinetics of a protein shuttling. The method described here overcomes the limitation of the small size of yeast cells and allows characterization of proteins without interfering with their expression levels. Moreover, it does not require additional positive or negative controls, and thus circumvents problems with differing strain backgrounds and reduces the number of samples. The recently described photo-activated fluorescent proteins (Lukyanov et al. 2005) may provide useful variations of the technique described here. The kinetic data can provide valuable information about protein function.
From the data obtained we estimate that the nuclear pool of Arx1p is exchanged with the cytoplasmic pool with a half life of ∼5.5 min. The abundance of Arx1p was estimated from large-scale affinity purification studies at 45,000 molecules per cell (Ghaemmaghami et al. 2003). As shown in Figure 1, Arx1p is predominantly nuclear, and thus the shuttling rate of Arx1p is ∼4000 molecules per minute. This figure is in excess over the estimated ribosome synthesis rate of ∼2000 ribosomes per minute (Warner 1999). It is possible that more than one molecule of Arx1p is exported per ribosome. Alternatively, Arx1p might participate in other export pathways. We have observed an apparent defect in pre-tRNA maturation in strains lacking Arx1p (data not shown), but it is currently unclear whether this reflects impaired pre-tRNA export.
The technique we describe overcomes many limitations of the conventional heterokaryon assay in yeast and should be of use in analyses of many other nuclear-cytoplasmic shuttling factors.
MATERIALS AND METHODS
Yeast strains and plasmids
Strains and plasmids used in this study are listed in Table 1. All strains were handled as described (Guthrie and Fink 1991).
TABLE 1.
Yeast strains and plasmids used in this study
Heterokaryon assay
The classical assay was done as described in Flach et al. (1994) and Peng and Hopper (2000) with modifications. Briefly, cells were grown in synthetic complete medium until OD600 ∼1. The mating was initiated by mixing 1 mL of ARX1::GFP (D912) or Gar1-GFP expressing cells (YKB2) with kar1-1 strain (D660). Cells were concentrated by filtration onto 25-mm, 0.45-mm nitrocellulose filters and then placed onto YPD plate. Cells were monitored visually and after ∼40 min, when first heterokaryons started to appear, the filters were placed on YPD plate containing 50 μg/mL cycloheximide and incubated for another 2 h. Cells were washed off the membrane and fixed in 4% (v/v) formaldehyde and analyzed by microscopy (Leica HC).
For FLIP/FRAP experiments, the cells were grown in SD complete medium, mixed for mating with kar1-1 strain, and deposited on a filter, which was placed on a plate for 3 h. Then, cells were washed and spotted on a thin layer of 2% agarose in SD medium (growth chamber) on a slide. Fluorescence time-lapse imaging was performed on Zeiss Axiovert 200M microscope at room temperature. For bleaching, a 377-nm Nitrogen pulse laser (Photonics Instruments) was used. A nucleus of a heterokaryon was photobleached by a 10-msec pulse and a single focal plane with 400 msec exposure time was recorded every 15 sec (11 sec for the experiment with both nuclei of heterokaryon bleached). Fluorescence of the regions of interest was quantified at each time point using SlideBook 4.0 software. Mean fluorescence was calculated as intensity per area and normalized to the level before photobleaching.
ACKNOWLEDGMENTS
We thank Kevin Hardwick and Karen May for their help with photobleaching techniques. K.B. was supported by the Darwin Trust, D.T. was supported by the Wellcome Trust, and M.K. was supported by an EU Marie Curie Fellowship MEIF-CT-2003-501083.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2301806.
REFERENCES
- Bassler J., Grandi P., Gadal O., Lessmann T., Petfalski E., Tollervey D., Lechner J., Hurt E. Identification of a 60S preribosomal particle that is closely linked to nuclear export. Mol. Cell. 2001;8:517–529. doi: 10.1016/s1097-2765(01)00342-2. [DOI] [PubMed] [Google Scholar]
- Flach J., Bossie M., Vogel J., Corbett A., Jinks T., Willins D.A., Silver P.A. A yeast RNA-binding protein shuttles between the nucleus and the cytoplasm. Mol. Cell. Biol. 1994;14:8399–8407. doi: 10.1128/mcb.14.12.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami S., Huh W.K., Bower K., Howson R.W., Belle A., Dephoure N., O'Shea E.K., Weissman J.S. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- Girard J.P., Lehtonen H., Caizergues-Ferrer M., Amalric F., Tollervey D., Lapeyre B. GAR1 is an essential small nucleolar RNP protein required for pre-rRNA processing in yeast. EMBO J. 1992;11:673–682. doi: 10.1002/j.1460-2075.1992.tb05099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C., Fink G.R. Methods in enzymology: Guide to yeast genetics and molecular biology. Vol. 194. Academic Press, NY; 1991. [PubMed] [Google Scholar]
- Koster M., Frahm T., Hauser H. Nucleocytoplasmic shuttling revealed by FRAP and FLIP technologies. Curr. Opin. Biotechnol. 2005;16:28–34. doi: 10.1016/j.copbio.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Lukyanov K.A., Chudakov D.M., Lukyanov S., Verkhusha V.V. Innovation: Photoactivatable fluorescent proteins. Nat. Rev. Mol. Cell Biol. 2005;6:885–891. doi: 10.1038/nrm1741. [DOI] [PubMed] [Google Scholar]
- Nissan T.A., Bassler J., Petfalski E., Tollervey D., Hurt E. 60S pre-ribosome formation viewed from assembly in the nucleolus until export to the cytoplasm. EMBO J. 2002;21:5539–5547. doi: 10.1093/emboj/cdf547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeffinger M., Dlakic M., Tollervey D. A pre-ribosome-associated HEAT-repeat protein is required for export of both ribosomal subunits. Genes & Dev. 2004;18:196–209. doi: 10.1101/gad.285604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G., Hopper J.E. Evidence for Gal3p's cytoplasmic location and Gal80p's dual cytoplasmic-nuclear location implicates new mechanisms for controlling Gal4p activity in Saccharomyces cerevisiae . Mol. Cell. Biol. 2000;20:5140–5148. doi: 10.1128/mcb.20.14.5140-5148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumtel S., Leger-Silvestre I., Gleizes P.E., Teulieres F., Gas N. Assembly and functional organization of the nucleolus: Ultrastructural analysis of Saccharomyces cerevisiae mutants. Mol. Biol. Cell. 2000;11:2175–2189. doi: 10.1091/mbc.11.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallen E.A., Hiller M.A., Scherson T.Y., Rose M.D. Separate domains of KAR1 mediate distinct functions in mitosis and nuclear fusion. J. Cell Biol. 1992;117:1277–1287. doi: 10.1083/jcb.117.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J.R. The economics of ribosome biosynthesis in yeast. Trends Biochem. Sci. 1999;24:437–440. doi: 10.1016/s0968-0004(99)01460-7. [DOI] [PubMed] [Google Scholar]