Abstract
Processing bodies (P-bodies) are cellular structures that have critical roles in mRNA degradation and post-transcriptional gene silencing. Some patients with autoimmune disease have high titer antibodies directed against P-bodies, and certain sera have been used as markers for the GW182 component of these structures. This study shows that available reference sera are unreliable markers for GW182 because the sera contain antibodies directed against Ge-1, a second P-body autoantigen.
Keywords: mRNA processing body, mRNA decay, RISC complex, autoantigen
Some of the enzymes involved in mRNA degradation are concentrated in discrete cytoplasmic foci known as mRNA processing bodies (P-bodies, also known as “GW182 bodies”) (Eystathioy et al. 2003b; Sheth and Parker 2003; Cougot et al. 2004). Components of the RNA-induced silencing complex (RISC) also localize to P-bodies, implicating P-bodies in the process of post-transcriptional gene silencing (Liu et al. 2005b; Sen and Blau 2005). Liu et al. (2005a) and Jakymiw et al. (2005) reported that P-body autoantigen GW182 interacts with RISC component Argonaute 2 (Ago2). These studies relied, in part, on human GW182 reference sera 18033 and IC-6. In previous studies, we used reference serum Ge to identify a second P-body autoantigen (Ge-1 [Bloch et al. 1994; Yu et al. 2005], also known as Hedls [Fenger-Gron et al. 2005]). Fenger-Gron et al. (2005) reported that Ge-1 interacts with P-body decapping components DCP1a and DCP2 and enhances mRNA decapping activity. Because GW182 and Ge-1 have similar predicted molecular masses, we tested the reference sera for the ability to recognize one or both P-body components.
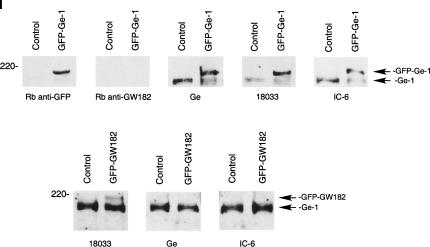
Green fluorescent protein (GFP)-GW182 and GFP-Ge-1 fusion proteins were expressed in Hep-2 cells, and cells were stained with anti-GFP antibodies and the human reference sera. The Hep-2 cells used in this study were previously shown to lack detectable endogenous GW182 (Bloch et al. 2005; Yu et al. 2005; see Fig. 2, panel I, below). In GFP-Ge-1-transfected cells, Ge-1 reference serum (patient Ge) and GW182 reference sera 18033 and IC-6 stained P-bodies intensely, suggesting that all three sera contain anti-Ge-1 antibodies (Fig. 1, panel I). In GFP-GW182-expressing Hep-2 cells, serum 18033 stained P-bodies but sera Ge and IC-6 stained P-bodies weakly, or not at all (Fig. 1, panel II). These results suggest that the latter two sera contain little, if any, anti-GW182 antibodies. In addition, it appears that overexpression of GW182 displaces Ge-1 from P-bodies, obscures Ge-1 epitopes, or decreases the cellular level of Ge-1. The observation that GW182 reference serum 18033 contains anti-GW182 and anti-Ge-1 antibodies while reference sera Ge and IC-6 contain anti-Ge-1, but not anti-GW182 antibodies, was confirmed by immunoblot prepared with extracts of GFP-Ge-1- and GFP-GW182-transfected cells (Fig. 2, panel I).
FIGURE 2.
(Continued on next page)
FIGURE 2.
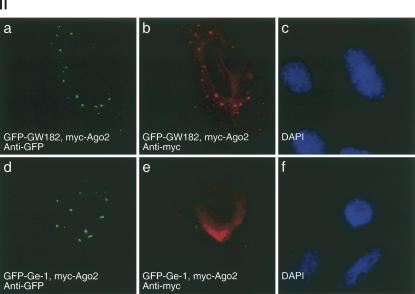
(Panel I) Detection of anti-Ge-1 and anti-GW182 antibodies in human reference sera by immunoblot. Extracts were prepared from Hep-2 cells transfected with GFP-Ge-1, GFP-GW182, or GFP (control) as indicated. Reference sera Ge, 18,033, and IC-6 reacted with GFP-Ge-1, as well as endogenous Ge-1 in Hep-2 cells. Note that rabbit anti-GW182 did not react with a ∼180-kD band, consistent with the absence of detectable, endogenous GW182 in these cells. Serum 18033, but not Ge or IC-6, reacted with GFP-GW182. (Panel II) GW182, but not Ge-1, recruits Ago2 to P-bodies. Hep-2 cells were transfected with either myc-Ago2 and GFP-GW182 (a–c) or myc-Ago2 and GFP-Ge-1 (d–f) and cells were stained with anti-myc and anti-GFP antiserum. DAPI staining in c and f indicates the location of cell nuclei.
FIGURE 1.
Detection of anti-Ge-1 and anti-GW182 antibodies in human reference sera by indirect immunofluorescence. (Panel I) GFP-Ge-1 was expressed in Hep-2 cells and cells were stained with anti-GFP antiserum (a,d,g) and human serum Ge (b), 18033 (e), or IC-6 (h). The human sera stained larger and more numerous P-bodies in cells expressing GFP-Ge-1 compared with nontransfected cells, indicating the presence of anti-Ge-1 antibodies in all three sera. (Panel II) GFP-GW182 was expressed in Hep-2 cells and cells were stained with anti-GFP antiserum (a,d,g) and human reference sera. The number of P-bodies recognized by GW182 reference serum 18033 (b) was increased in transfected, compared with nontransfected cells, indicating the presence of anti-GW182 antibodies. Reference sera Ge (e) and IC-6 (h) stained P-bodies (indicated by arrows) in transfected cells weakly (in the case of Ge) or not at all (IC-6), indicating that these sera contained little or no anti-GW182 antibodies. DAPI staining (c,f,i, in Panels I and II) indicates the location of cell nuclei.
Because GW182 reference sera contain antibodies to both GW182 and Ge-1 or Ge-1 alone, and because these sera were used to show an interaction between Ago2 and GW182, we re-examined the relationship between Ago2 and P-bodies. Hep-2 cells were transfected with Ago2 and either GW182 or Ge-1. Ago2 localized to P-bodies in cells expressing GFP-GW182, but not in cells expressing GFP-Ge-1 (Fig. 2, panel II), suggesting that GW182 is required to recruit Ago2 to P-bodies.
In summary, GW182 reference sera are unreliable markers for the GW182 component of P-bodies because they contain antibodies directed against a second P-body autoantigen (Ge-1). Previous studies (Eystathioy et al. 2002, 2003a; Yang et al. 2004) performed with these sera may need to be reconsidered. However, the observation that GW182, but not Ge-1, recruits Ago2 to P-bodies supports the findings of previous investigators that GW182 interacts with components of the RISC complex (Jakymiw et al. 2005; Liu et al. 2005a).
ACKNOWLEDGMENTS
We thank M. Fritzler for providing rabbit anti-GW182 antiserum and human reference serum.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.17406.
REFERENCES
- Bloch D.B., Rabkina D., Quertermous T., Bloch K.D. The immunoreactive region in a novel autoantigen contains a nuclear localization sequence. Clin. Immunol. Immunopathol. 1994;72:380–389. doi: 10.1006/clin.1994.1156. [DOI] [PubMed] [Google Scholar]
- Bloch D.B., Yu J.H., Yang W.H., Graeme-Cook F., Lindor K.D., Viswanathan A., Bloch K.D., Nakajima A. The cytoplasmic dot staining pattern is detected in a subgroup of patients with primary biliary cirrhosis. J. Rheumatol. 2005;32:477–483. [PubMed] [Google Scholar]
- Cougot N., Babajko S., Seraphin B. Cytoplasmic foci are sites of mRNA decay in human cells. J. Cell Biol. 2004;165:31–40. doi: 10.1083/jcb.200309008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eystathioy T., Chan E.K., Tenenbaum S.A., Keene J.D., Griffith K., Fritzler M.J. A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol. Biol. Cell. 2002;13:1338–1351. doi: 10.1091/mbc.01-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eystathioy T., Chan E.K., Takeuchi K., Mahler M., Luft L.M., Zochodne D.W., Fritzler M.J. Clinical and serological associations of autoantibodies to GW bodies and a novel cytoplasmic autoantigen GW182. J. Mol. Med. 2003a;81:811–818. doi: 10.1007/s00109-003-0495-y. [DOI] [PubMed] [Google Scholar]
- Eystathioy T., Jakymiw A., Chan E.K., Seraphin B., Cougot N., Fritzler M.J. The GW182 protein colocalizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies. RNA. 2003b;9:1171–1173. doi: 10.1261/rna.5810203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenger-Gron M., Fillman C., Norrild B., Lykke-Andersen J. Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping. Mol. Cell. 2005;20:905–915. doi: 10.1016/j.molcel.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Jakymiw A., Lian S., Eystathioy T., Li S., Satoh M., Hamel J.C., Fritzler M.J., Chan E.K. Disruption of GW bodies impairs mammalian RNA interference. Nat. Cell Biol. 2005;7:1167–1174. doi: 10.1038/ncb1334. [DOI] [PubMed] [Google Scholar]
- Liu J., Rivas F.V., Wohlschlegel J., Yates J.R., Parker R., Hannon G.J. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 2005a;7:1161–1166. doi: 10.1038/ncb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Valencia-Sanchez M.A., Hannon G.J., Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat. Cell Biol. 2005b;7:719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G.L., Blau H.M. Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat. Cell Biol. 2005;7:633–636. doi: 10.1038/ncb1265. [DOI] [PubMed] [Google Scholar]
- Sheth U, Parker R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science. 2003;300:805–808. doi: 10.1126/science.1082320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Jakymiw A., Wood M.R., Eystathioy T., Rubin R.L., Fritzler M.J., Chan E.K. GW182 is critical for the stability of GW bodies expressed during the cell cycle and cell proliferation. J. Cell Sci. 2004;117:5567–5578. doi: 10.1242/jcs.01477. [DOI] [PubMed] [Google Scholar]
- Yu J.H., Yang W.H., Gulick T., Bloch K.D., Bloch D.B. Ge-1 is a central component of the mammalian cytoplasmic mRNA processing body. RNA. 2005;11:1795–1802. doi: 10.1261/rna.2142405. [DOI] [PMC free article] [PubMed] [Google Scholar]