Abstract
Snu114 is a U5 snRNP protein essential for pre-mRNA splicing. Based on its homology with the ribosomal translocase EF-G, it is thought that GTP hydrolysis by Snu114 induces conformational rearrangements in the spliceosome. We recently identified allele-specific genetic interactions between SNU114 and genes encoding three other U5 snRNP components, Prp8 and two RNA-dependent ATPases, Prp28 and Brr2, required for destabilization of U1 and U4 snRNPs prior to catalysis. To shed more light onto the function of Snu114, we have now directly analyzed snRNP and spliceosome assembly in SNU114 mutant extracts. The Snu114–60 C-terminal truncation mutant, which is synthetically lethal with the ATPase mutants prp28–1 and brr2–1, assembles spliceosomes but subsequently blocks U4 snRNP release. Conversely, mutants in the GTPase domain fail to assemble U5 snRNPs. These mutations prevent the interaction of Snu114 with Prp8 as well as with U5 snRNA. Since Prp8 is thought to regulate the activity of the DEAD-box ATPases, this strategy of snRNP assembly could ensure that Prp8 activity is itself regulated by a GTP-dependent mechanism.
Keywords: U5 snRNP, pre-mRNA splicing, U5-116kD, Snu114, GTPase, Prp8
INTRODUCTION
The spliceosome, the macromolecular complex that excises introns from pre-mRNA transcripts, is assembled from five snRNPs (small nuclear ribonucleoprotein particles), as well as additional accessory proteins. The U5 snRNP is an integral component of the spliceosome: Both the U5 snRNA and the large, conserved U5 snRNP protein Prp8 can be cross-linked to all sites of chemistry on the pre-mRNA transcript (for review, see Grainger and Beggs 2005). Prp8 interacts biochemically and genetically with the GTPase Snu114, which is an essential protein in Saccharomyces cerevisiae (Fabrizio et al. 1997; Achsel et al. 1998; Dix et al. 1998; Grainger and Beggs 2005). As a homolog of the ribosomal translocase elongation factor G, Snu114 is predicted to mediate rearrangements of the spliceosome (Fabrizio et al. 1997). In order to investigate the function of Snu114, we previously generated conditionally lethal alleles of the gene and tested for synthetic lethal interactions with other spliceosomal factors (Brenner and Guthrie 2005). We observed genetic interactions with two main classes of proteins: those that are involved in snRNP biogenesis, and those that are involved in spliceosome activation.
While the protein components of U5 snRNP have been identified (Stevens et al. 2001; for review, see Jurica and Moore 2003), the process by which the snRNP assembles is poorly understood. During snRNP biogenesis, a heptameric complex of Sm proteins binds to and stabilizes U5 snRNA, as well as most of the other snRNAs (Jones and Guthrie 1990; Will and Luhrmann 2001). Following the interaction of the Sm proteins with U5 snRNA, additional proteins assemble onto the snRNP. Snu114 and Prp8 appear to constitute the core of the U5 snRNP, as they both can be cross-linked directly to U5 snRNA (Dix et al. 1998). The strength of interactions among the snRNP protein components has been assessed by treating purified U5 snRNPs from HeLa extract with increasing concentrations of chaotropic salts. At high concentrations, Snu114 (U5–116 kDa in humans) remained bound only to Prp8 (U5–220 kDa) (Achsel et al. 1998). Under less stringent salt conditions, the Prp8/Snu114 dimer interacted with the ATPase Brr2 (U5–200 kDa) and a 40 kDa protein that does not appear to have a yeast ortholog (Achsel et al. 1998). U5 snRNP can also be found in complexes with other snRNPs; the U5•U4/U6 tri-snRNP includes the base-paired U4/U6 di-snRNP, and the penta-snRNP contains all five snRNPs (Cheng and Abelson 1987; Stevens et al. 2002).
In vitro studies have shown that during spliceosome assembly, the tri-snRNP binds to the pre-spliceosome, which already contains U1 and U2 snRNP bound to the 5′ splice site and branch point sequence, respectively (Staley and Guthrie 1998; for review, see Burge et al. 1999). However, isolation of a penta-snRNP suggests that a preassembled snRNP complex might bind to the pre-mRNA (Stevens et al. 2002). In either case, a substantial rearrangement of the spliceosome must occur in order for splicing to proceed (Brow 2002; Turner et al. 2004). During catalytic activation, the interaction between U1 snRNA and the 5′ splice site and the extensive base-pairings between U4 and U6 snRNAs are destabilized, releasing U1 and U4 snRNPs from tight association with the spliceosome. This allows U6 snRNA to interact with U2 snRNA and the 5′ splice site, which is necessary for the chemical steps of splicing. U5 snRNP proteins play an important role during these activation steps (for review, see Turner et al. 2004). Two DExD/H-box ATPases that are components of U5 snRNP—Prp28 and Brr2—have been implicated in release of U1 and U4, and Prp8 has been posited to play a role in regulating the activity of these ATPases (Laggerbauer et al. 1998; Raghunathan and Guthrie 1998; Kuhn et al. 1999, 2002; Staley and Guthrie 1999; Kuhn and Brow 2000). Additionally, several mutations in Snu114 block activation of the spliceosome (Bartels et al. 2002, 2003).
Here, we analyze our novel set of snu114 alleles (Brenner and Guthrie 2005) for in vitro defects during snRNP assembly and spliceosome activation. We find that several alleles, including those with mutations in the GTPase domain, inhibit formation of U5 snRNP. We also analyze the allele snu114–60, which truncates the last 70 amino acids; this allele exhibits genetic interactions with factors involved in spliceosome activation, including prp28–1, brr2–1, and alleles of prp8 (Brenner and Guthrie 2005). In this case we find that snu114–60 blocks spliceosome activation.
RESULTS
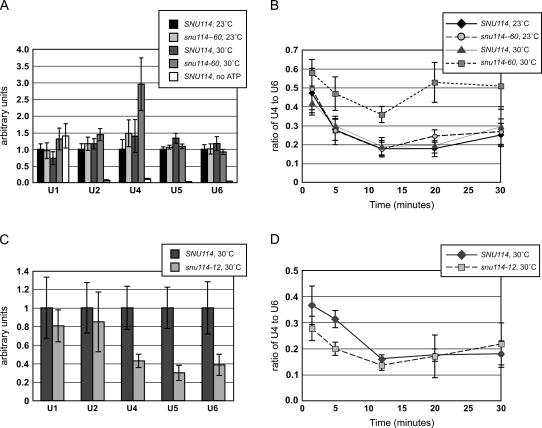
Our previous genetic data suggested that snu114–60, which causes weak growth defects at both high (37°C) and low (16°C) temperatures, inhibits spliceosome activation (Brenner and Guthrie 2005). We tested this hypothesis by monitoring spliceosome assembly and activation in vitro. Heat pretreatment blocks splicing activity in snu114–60 extract (Brenner and Guthrie 2005); we also found that incubating splicing reactions at 30°C instead of 23°C consistently inhibited splicing activity in snu114–60 extract, but not in wild-type extract (data not shown). In order to affinity-purify spliceosomes, mutant and wild-type extracts were incubated with biotinylated pre-mRNA transcript under splicing conditions at 23°C and 30°C. snRNAs that copurified with the transcript were quantified by real-time PCR. As a control for the specificity of snRNA binding, extracts were incubated with transcript in the absence of ATP. Only U1 snRNA was coprecipitated under these conditions (Fig. 1A); ATP is required for the other snRNA to associate with pre-mRNA (Bindereif and Green 1987; Legrain et al. 1988; Ruby and Abelson 1988). While snu114–60 extract incubated at 23°C behaved similarly to wild-type extract at either temperature, the mutant extract incubated at 30°C showed a different profile of snRNA binding (Fig. 1A). Similar amounts of U5 and U6 snRNAs were bound to transcript in all of the extracts, but an increased amount of U4 snRNA was consistently bound in snu114–60 extract incubated at 30°C. Because U4 and U6 assemble onto the spliceosome together in a base-paired form, and U4 is released during activation while U6 remains bound to transcript throughout splicing, the ratio of transcript-bound U4 to U6 is commonly monitored as a measure of catalytic activation. Over time, the ratio of U4 to U6 bound to transcript was consistently twofold higher for snu114–60 (Fig. 1B), indicating that snu114–60 extract is defective for releasing U4 from the spliceosome at elevated temperatures. Although snu114–60 cells also exhibit a slow-growth phenotype in the cold, incubating snu114–60 extract at 16°C did not block U1 or U4 release (data not shown).
FIGURE 1.
Spliceosome assembly and activation in snu114–60 and snu114–12 extracts. (A) SNU114 and snu114–60 extracts were incubated at 23°C and 30°C with biotinylated pre-mRNA and spliceosomes were affinity-purified. Levels of snRNAs associated with transcript after 20 min of incubation under splicing conditions are shown, as quantified by real-time PCR. Each coprecipitating snRNA was normalized to 1.0 for SNU114 at 23°C. As a control, SNU114 extract was incubated at 23°C with pre-mRNA in the absence of ATP. (B) Ratio of transcript-bound U4 snRNA vs. U6 snRNA over time for SNU114 and snu114–60 extracts. (C) snRNAs bound to biotinylated transcript after 20 min of incubation under splicing conditions in SNU114 and snu114–12 extract at 30°C. Each snRNA was normalized to 1.0 for SNU114. (D) Ratio of transcript-bound U4 snRNA vs. U6 snRNA over time for SNU114 and snu114–12 extracts. Data represent the average of at least five experiments; in A and B, n = 7 for the 30°C data and n = 5 for the 25°C data, and in C and D, n = 5. Error bars indicate standard error.
We then used this assay to gain insight into other snu114 alleles. The thermal-sensitive allele snu114–12 results from a point mutation within the GTPase domain and causes a splicing defect in vivo and in vitro, even when cells are grown at 25°C (Brenner and Guthrie 2005). The association of snRNAs with biotinylated transcript in snu114–12 extract was the same whether the incubation was at 23°C or 30°C; here we show only data from 30°C incubations (Fig. 1C). After 20 min of incubation, three- to fourfold less U4, U5, and U6 snRNAs bound to transcript in snu114–12 extract compared to wild-type. However, the ratio of U4 to U6 snRNAs bound to transcript over time was similar between wild-type and snu114–12 extracts (Fig. 1D). Thus, the main defect in snu114–12 appears to be spliceosome assembly rather than activation.
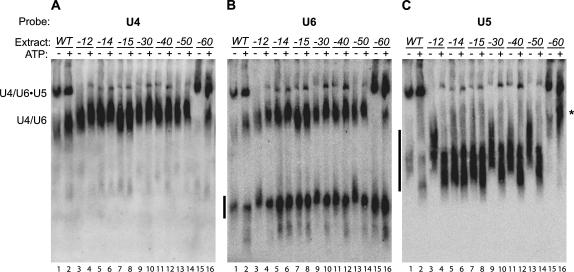
In order to determine whether the decreased tri-snRNP binding in snu114–12 extract resulted from a failure of tri-snRNP to interact with the pre-spliceosome or from decreased tri-snRNP levels, we assayed the snRNP profile of snu114–12 by native gel analysis (Raghunathan and Guthrie 1998). We also tested snu114–60 and five other conditionally lethal snu114 alleles that we previously identified (Brenner and Guthrie 2005). All extracts were made from cells grown at 30°C, except for the stronger temperature-sensitive allele snu114–15, which was grown at 25°C. With the exception of snu114–60, all of the mutants showed dramatically lower levels of tri-snRNP (Fig. 2). However, these mutants had high levels of U4/U6 di-snRNP and a free U5-species (Fig. 2), and the total levels of the snRNAs were not strongly perturbed (Fig. 3D; data not shown). Some of the snRNPs from mutant extracts exhibited slightly altered mobility in the native gels, which may reflect an altered conformation or composition. As noted previously, addition of ATP to wild-type extract causes an increase in U4/U6 di-snRNP levels (Fig. 2A,B, lanes 1,2), presumably due to ATP-dependent disassembly of U4/U6•U5 tri-snRNP and possibly of larger snRNP complexes (Raghunathan and Guthrie 1998; Stevens et al. 2001). A similar increase in di-snRNP was observed in snu114–60 extract, the only other extract with abundant tri-snRNP levels (Fig. 2A,B, lanes 15,16). Addition of ATP also led to the appearance of a slow-migrating U5 species in snu114–60 extract (Fig. 2C, lane 16, asterisk) with slightly slower mobility than U4/U6 di-snRNP. The composition of this species is unclear. In some cases, the abundance of tri-snRNP also increased when the extracts were incubated with ATP; we believe that ATP led to the dissociation of larger snRNP complexes (i.e., tetra-snRNP and penta-snRNP) that were otherwise unable to migrate into the gel. Overall, the native gel analysis demonstrated that tri-snRNP was abundant in snu114–60 extract, consistent with ability of tri-snRNP to assemble onto transcript. In contrast, tri-snRNP levels were low in many snu114 mutants, which could explain the defect of tri-snRNP addition in snu114–12 extract.
FIGURE 2.
snu114 mutants have low levels of tri-snRNP. Extracts from snu114 mutant strains grown at permissive temperature were incubated under splicing conditions, with or without ATP. snRNPs were resolved by native gel electrophoresis and transferred to nitrocellulose. Northern blots were probed for U4 (A), U6 (B), and U5 (C). U4/U6•U5 tri-snRNP and U4/U6 di-snRNP are labeled; free snRNPs are indicated by black bars to the left of each panel. The asterisk indicates the U5-snRNP species found in snu114–60.
FIGURE 3.
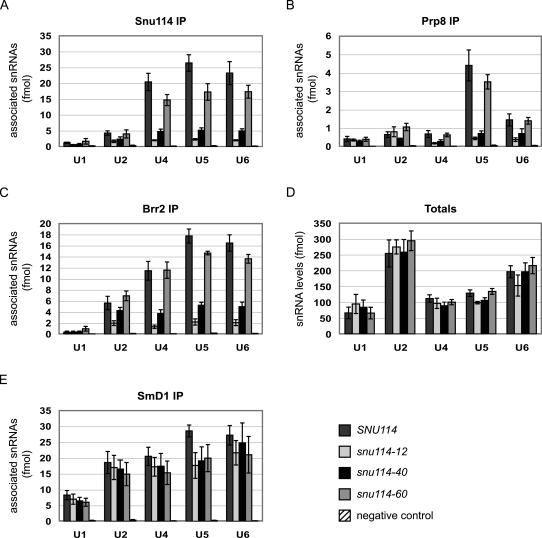
U5 snRNP proteins bind low levels of snRNAs in snu114–12 and snu114–40. Bars indicate levels of snRNAs copurified with snRNP proteins. (A) Immunoprecipitation of Snu114. (B) Immunoprecipitation of Prp8. (C) Affinity purification of TAP-tagged Brr2. (D) Total snRNA levels in the untagged extracts. (E) Immunoprecipitation of SmD1. We note that although SmD1 does not bind directly to U6 snRNA, U6 snRNA was likely coprecipitated with Sm-bound U4 snRNA. Copurifying snRNAs were reverse transcribed and quantified using real-time PCR. For A, B, and E, SNU114 extract was incubated with nonimmune sera as a negative control. For C, untagged SNU114 extract was used as negative control. See Materials and Methods for a description of how fmol were determined. Data represent the average of at least three experiments. In A, n = 6; B, n = 5; C, n = 3; D, n = 5; E, n = 5. Error bars indicate standard error.
The low levels of tri-snRNP in the snu114 mutants could arise from a failure of U5 snRNP to interact with U4/U6 di-snRNP, or from a defect in U5 snRNP formation or stability. To differentiate between these possibilities, we tested whether three core U5 snRNP proteins—Snu114, Prp8, and Brr2—were associated with U5 snRNA. We focused this analysis on snu114–12, snu114–60, and the strong thermal-sensitive allele snu114–40. Extracts were made from cells grown at 25°C. Snu114 and Prp8 were immunoprecipitated with polyclonal antibodies, and TAP-tagged Brr2 was precipitated with IgG resin; coprecipitating snRNAs were reverse-transcribed and quantified by real-time PCR. In wild-type extract, Snu114 and Brr2 immunoprecipitations pulled down similar amounts of U4, U5, and U6 snRNAs, while the Prp8 antibody pulled down predominantly U5 snRNA (Fig. 3A–C). This difference may suggest that Prp8 that has assembled into tri-snRNP is less accessible to the antibody. As observed previously, Brr2 also immunoprecipitated U2 snRNA (Raghunathan and Guthrie 1998). Consistent with the diminished tri-snRNP levels visualized by native gel, Snu114, Brr2, and Prp8 were each associated with five- to 10-fold less U4 and U6 snRNAs in snu114–12 and snu114–40 extracts (Fig. 3A–C). Furthermore, these U5 snRNP proteins were bound to similarly low amounts of U5 snRNA in snu114–12 and snu114–40 extracts, demonstrating that U5 snRNP itself either fails to form or is unstable in these mutants. In contrast, snu114–60 exhibited only a modest decrease in association with snRNAs, consistent with the robust tri-snRNP levels visualized by native gel electrophoretic analysis.
To rule out the possibility that decreased association of snRNAs with U5 snRNP proteins resulted from low snRNA levels, we measured the total snRNA levels in the extracts used for the immunoprecipitations. The mutant extracts contained similar amounts of each snRNA as found in wild-type extract (Fig. 3D), so this cannot account for the defects in snRNP formation. The stability of the snRNAs also depends on binding of the Sm proteins (Jones and Guthrie 1990). Therefore, as a further measure of functional snRNAs, we tested the interaction of SmD1 with snRNAs in the snu114 extracts (Fig. 3E). Although SmD1 was associated with slightly lower levels of snRNAs in the mutant extracts, this decrease was never more than twofold, which contrasts with the much larger changes in association of the other U5 snRNP proteins.
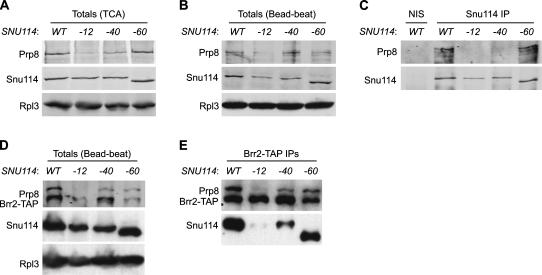
Because of the finding that Snu114, Prp8, and Brr2 associated with lower amounts of snRNAs in snu114–12 and snu114–40, we tested whether these core U5 proteins could interact with each other in the mutants. First, we assayed the total levels of the proteins by Western blot. While the protein levels of Snu114–12 and Snu114–40 were similar to wild-type levels in some cases (Fig. 4A), they commonly were two- to threefold lower (Fig. 4B,D). Quantitation of Prp8 levels was more challenging, as we found that Prp8 was particularly susceptible to degradation (Fig. 4B). In extracts made in the presence of TCA, in which proteolysis of Prp8 was minimal, Prp8 levels were reduced ∼10- and fivefold in snu114–12 and snu114–40 backgrounds, respectively (Fig. 4A). In the bead-beat extracts used for immunoprecipitations, where overall degradation of Prp8 occurred, the amount of full-length Prp8 was consistently reduced fivefold in snu114–12 strains (Fig. 4B). When Snu114 was immunoprecipitated with polyclonal antibodies, Prp8 was coprecipitated in wild-type and snu114–60 extracts (Fig. 4C). In contrast, the coprecipitation of Prp8 was diminished to almost background levels in snu114–12 and snu114–40 extracts (Fig. 4C).
FIGURE 4.
Decreased association of Snu114 with Prp8 and Brr2 in snu114–12 and snu114–40 extracts. (A) Western blot of TCA extracts showing the levels of Prp8 and Snu114 in the indicated strains. (B) Western blot of bead-beat extracts showing total levels of Prp8 and Snu114. (C) Snu114 was immunoprecipitated using polyclonal antibodies. A Western blot of the precipitated proteins was probed for Snu114 and Prp8. As a control for the IP, SNU114 extract was incubated with nonimmune serum (NIS) conjugated to Protein A Sepharose. (D) Levels of Prp8, Brr2-TAP, and Snu114 in TAP-tagged Brr2 strains in bead-beat extract. (E) Brr2-TAP was affinity-purified, and a Western blot of the precipitated proteins was probed for Prp8, Brr2-TAP, and Snu114. Note that Snu114–60 migrates faster than Snu114 because the protein contains 70 fewer amino acids. In A, B, and D, Rpl3 was used as a loading control.
We also monitored the copurification of Snu114 and Prp8 in Brr2-TAP pull-downs (Fig. 4E). Affinity-purified Brr2 from wild-type extract was bound to both Prp8 and Snu114. In snu114–12 extract, Brr2 levels and Prp8 levels were reduced (Fig. 4D), and Brr2 bound to low amounts of both Prp8 and Snu114 (Fig. 4E). Brr2 also associated with less Snu114 and Prp8 in snu114–40 extract (Fig. 4C). TAP-tagged Brr2 enhanced the sickness of snu114–60 (data not shown); perhaps as a result, Brr2 levels were low in this strain (Fig. 4D,E). Overall, the data show that Prp8 levels are diminished in snu114–12 and snu114–40 extracts, and the association of Snu114 with both Prp8 and Brr2 is decreased in these mutants.
DISCUSSION
After the snRNPs have assembled onto the pre-mRNA transcript, dramatic rearrangements of the spliceosome must occur, including the unwinding of several RNA/RNA helices, the formation of new RNA helices, and a corresponding remodeling of protein interactions. The C-terminal truncation allele snu114–60 is synthetically lethal with mutations in PRP28 and BRR2 (Brenner and Guthrie 2005), ATPases that are believed to unwind and release U1 and U4 snRNAs during spliceosome activation (Laggerbauer et al. 1998; Raghunathan and Guthrie 1998; Staley and Guthrie 1999). Here we show that snu114–60 inhibits activation: Release of U4 from spliceosomes was blocked when extract was incubated at elevated temperature (Fig. 1A,B). Although the unwinding of U1 is generally believed to be highly coupled with that of U4 (Kuhn et al. 1999, 2002; Staley and Guthrie 1999), U1 and U4 unwinding are separable in some in vitro assays (Xie et al. 1998). While the snu114–60 allele predominantly affects the release of U4, likely through the activity of Brr2, genetic interactions suggest that the activities of Prp28, Brr2, and Snu114 in vivo are highly interrelated.
Previous studies have implicated additional regions of Snu114 in spliceosome activation. Truncation of the N-terminal 120 amino acids of Snu114, which corresponds to a domain that is not found in EF-G, inhibits release of U4, as does the allele D271N, which converts the protein from a GTPase to an XTPase (Bartels et al. 2002, 2003). GTP hydrolysis was directly implicated, since addition of XTP, but not nonhydrolyzable analogs, could relieve the block in D271N (Bartels et al. 2003). Altogether, this suggests that catalytic activation requires GTP hydrolysis by Snu114, and that both the N- and C-terminal domains are required to transmit a resulting rearrangement of the protein. A reasonable hypothesis is that upon GTP hydrolysis, Snu114 alters its interactions with Prp8 (Brenner and Guthrie 2005). This would relieve the inhibition by Prp8 on the ATPases Prp28 and Brr2, leading to U1 and U4 unwinding (Kuhn et al. 1999, 2002; Kuhn and Brow 2000).
It has long been known that Prp8 is essential for the formation of U5 snRNP and tri-snRNP, as illustrated by genetic depletion of Prp8 or heat inactivation of the prp8–1 allele (Brown and Beggs 1992). We previously found that prp8–1 is synthetically sick or lethal with many snu114 alleles, suggesting that these snu114 alleles may also affect snRNP formation (Brenner and Guthrie 2005). Here we demonstrate that the mutations snu114–12 and snu114–40 cause a defect in the assembly of U5 snRNP. These alleles disrupt the interaction between Snu114 and Prp8 (Fig. 4C) and cause lower Prp8 protein levels (Fig. 4A,B,D). Our data indicate that the stability of Prp8 depends on its ability to interact with Snu114. Additionally, the binding of Prp8 and Snu114 to U5 snRNA is decreased five- to 10-fold in snu114–12 and snu114–40 extracts (Fig. 3A,B). Because the levels of mutant Snu114 protein are similar to the wild-type protein and because the interaction of mutant Snu114 with Prp8 is reduced (Fig. 4A–C), we conclude that Snu114 does not bind to U5 snRNA without Prp8. It is unclear if Prp8 is unable to bind U5 snRNA alone or if the decreased association of Prp8 with U5 snRNA in the snu114 mutants results from instability of Prp8.
We also found that a decreased interaction between Snu114 and Prp8 correlates with diminished copurification of Snu114 with Brr2 (Fig. 4E). This corroborates yeast two-hybrid and far-Western analyses indicating Prp8 interacts directly with Brr2 and Snu114, while Brr2 and Snu114 do not directly interact (Achsel et al. 1998; Dix et al. 1998; van Nues and Beggs 2001; Grainger and Beggs 2005). Furthermore, our finding that the interaction of Brr2 with U5 snRNA is reduced when the interaction between Snu114 and Prp8 is low (Fig. 3C) suggests that Brr2 does not bind to the snRNA alone.
In snu114–12 and snu114–40 extracts, Snu114, Prp8, and Brr2 proteins were associated with 10- and fivefold less U5 snRNA, respectively (Fig. 3A–C). However, the association of SmD1 with U5 snRNA was reduced less than twofold (Fig. 3E), and the snRNA itself was stable (Fig. 3D). Thus, we suggest that the U5 species detected by native gel electrophoresis (Fig. 2), which is present in most of the snu114 mutant extracts, represents U5 snRNA bound to the Sm proteins, but not to the other U5 snRNP proteins.
Previously, the stability of various U5 deletion mutants, many of which do not support yeast viability, was assayed in the presence of wild-type U5 snRNA (Frank et al. 1994). Deletion of Internal loop 1 (IL2 in human), a domain that can be cross-linked to Snu114 and Prp8 (Dix et al. 1998), abolishes binding of both proteins to the RNA in yeast and mammalian systems (Hinz et al. 1996; Dix et al. 1998; Segault et al. 1999). However, this deletion did not affect U5 RNA stability (Frank et al. 1994). Only mutations that affect the binding of the Sm proteins caused degradation of the RNA (Frank et al. 1994). Thus, the binding of the Sm proteins in the snu114 alleles assayed here appears to maintain the stability of the snRNAs, despite decreased Prp8 and Snu114 binding. While Brown and Beggs (1992) found that depletion of Prp8 caused a drop in U4, U5, and U6 snRNA levels, it is likely that the degree of Prp8 depletion was much greater under their conditions than in the snu114 mutants, thus giving rise to a more severe phenotype than we observed.
Reduced levels of U5- and tri-snRNP have been observed in two other snu114 mutants: R487E, which disrupts a predicted interdomain salt bridge, and the XTPase allele D271N (Bartels et al. 2003). This phenotype of snu114-D271N may arise from defects in nucleotide binding, due to low cellular levels of XTP (Bartels et al. 2003). Many of the snu114 alleles that we characterized here may affect a conformational rearrangement of the protein that arises from changes in nucleotide binding. We observed decreases in tri-snRNP levels, which we suspect correspond with decreases in U5 snRNP levels, in six snu114 alleles containing single-point mutations in domains outside of the extreme N and C termini. Two of these mutations, snu114–12 and snu114–15, are located within conserved motifs in the GTPase domain. While the mutations snu114–30, snu114–40, and snu114–50 are outside of the GTPase domain, they may affect transmission of a signal from the GTPase domain, which is caused by a change in nucleotide binding, to the C-terminal domains of Snu114 and to factors that interact with the protein (Brenner and Guthrie 2005). Overall, each of these mutations may stabilize a protein conformation that is unfavorable for binding to Prp8. It is possible that Snu114 must hydrolyze GTP in order to stabilize the interaction with Prp8. However, since GTP hydrolysis is likely needed at the time of spliceosome activation, we favor the model that GTP binding is required for stable interaction with Prp8. Formation of the Prp8/Snu114 heterodimer would allow productive association with U5 snRNA and subsequent formation of tri-snRNP. This mechanism would ensure that U5 snRNP cannot join the spliceosome without the presence of GTP-bound Snu114. Following addition of tri-snRNP to the spliceosome, GTP hydrolysis by Snu114 would be stimulated, allowing spliceosome activation to occur.
MATERIALS AND METHODS
Strains
Strains and plasmids were described in Brenner and Guthrie (2005), except as noted. For affinity purification of spliceosomes, strains derived from yTB23 by replacing pTB1 with pTB106 (SNU114), pTB107 (snu114–12), or pTB113 (snu114–60) were grown at 30°C. The strains for the native gel analysis were derived from yTB13 by replacing pTB1 with plasmids pTB92 through pTB102; cells were grown at 30°C, with the exception of snu114–15, which was grown at 25°C.
For immunoprecipitations, the following MATa and MATα strains were used: SNU114 (yTB127 and yTB128), snu114–12 (yTB175 and yTB171), snu114–40 (yTB162 and yTB163), and snu114–60 (yTB164 and yTB165). The MATa strains were constructed from yTB127 (MATa his3Δ leu2Δ ura3Δ met15Δ) in an identical manner to the MATα strains, as described in Brenner and Guthrie (2005). The TAP-tagged Brr2 strain (MATa his3Δ leu2Δ ura3Δ met15Δ BRR2-TAP::HIS3), a gift from the O'Shea lab (Ghaemmaghami et al. 2003), was crossed to yTB171 (snu114–12), yTB163 (snu114–40), and yTB165 (snu114–60) and sporulated to obtain yTB176, yTB177, and yTB178, respectively, which contain both BRR2-TAP::HIS3 and chromosomal snu114 mutations. Because the integrated snu114 alleles exhibited a more severe thermal-sensitive growth phenotype than strains carrying the mutant alleles on plasmids, cells were grown at 25°C. At 25°C, the doubling time of snu114–12 was 1.3 times slower than wild-type, snu114–40 was 1.1 times slower, and snu114–60 grew similarly to wild type.
Affinity purification of spliceosomes
Splicing extracts were prepared as described (Umen and Guthrie 1995). The following protocol was adapted from Staley and Guthrie (1999): Actin pre-mRNA was transcribed in the presence of 5% biotin-11-UTP (Sigma). Standard splicing reactions (Lin et al. 1985) included 4 nM biotinylated actin and were performed at 23°C or 30°C. At 1.5, 5, 12, 20, and 30 min, 20-μL aliquots were removed into siliconized tubes containing 60-μL ice-cold splicing buffer/buffer D (40%/60%) with 20 mM EDTA and 25-μL streptavidin Sepharose beads (Amersham), prepared according to Staley and Guthrie (1999). As a control, ATP was depleted from extracts by incubating splicing reactions in the presence of 2 mM glucose for 20 min at room temperature prior to adding pre-mRNA. Following addition of biotinylated transcript, glucose-treated reactions were incubated for 12 min at 23°C or 30°C and quenched as above. Spliceosomes and beads were incubated with rotation for 90 min at 4°C. Beads were washed four times with 1 mL of Net2–50 (50 mM Tris at pH 8.0, 50 mM NaCl, 0.5 mM DTT, 0.05% NP40). To elute the RNA, 100 μL Buffer G (0.3 M NaOAc, 10 mM EDTA, 1% SDS) with 0.0125 mg/mL proteinase K was added, and tubes were incubated at 37°C for 20 min. RNA was phenol-extracted and ethanol-precipitated in the presence of glycogen. The five snRNAs and the actin transcript were reverse-transcribed and quantified by real-time PCR. The levels of copurifying snRNAs were normalized to the amount of precipitated actin in each reaction.
Oligos
U2, U4, U5, and U6 oligos were described by Inada and Guthrie (2004). Oligos for U1 were oTB148 (5′-TGACTACTTTTCTCTAGCGTGCC-3′) and oTB149 (5′-CATAACGGGAACGAGCAAAGTTG-3′). Actin was amplified using oTB141 (5′-CGGTTCTGGTATGTGTAAAGC-3′) and oTB142 (5′-CAGGTCGACTCTAGAGGATC-3′). When in vitro transcribed snRNAs were used as a standard, the original U2 oligos could not be used, as they amplify a region of the fungal domain that is not included in the in vitro transcript. Instead, oTB166 (5′-GGCTTAGATCAAGTGTAGTATCTGT-3′) and oTB167 (5′-TTTTATTATTTTGGGTGCCAAAAAA-3′) were used.
Quantitation of RNAs
In order to reverse-transcribe the RNAs, a mix of the primers used for reverse PCR amplification (250 nM final concentration) was incubated with RT buffer (50 mM HEPES at pH 8.3, 75 mM KCl) and RNA at 65°C for 5 min and then on ice for 5 min. Reverse transcription reactions, containing 3 mM MgCl2, 10 mM DTT, 500 μM each dNTP, 50 mM HEPES at pH 8.3, 75 mM KCl, and MMLV reverse transcriptase, were then incubated at 42°C for 2 h. Quantitative real-time PCR was performed as described (Inada and Guthrie 2004). PCR conditions were as follows: 95°C for 3 min; 32 cycles of 95°C for 15 sec, 57°C for 30 sec, 72°C for 60 sec, followed by measuring; 72°C for 5 min; and determination of melting curves.
In vitro transcribed actin was reverse-transcribed and used for normalization of affinity-purified biotinylated actin. Standard curves of each snRNA were initially generated from cDNA of reverse-transcribed total RNA isolated from wild-type splicing extract. We then decided to compare absolute amounts of snRNAs, as opposed to proportions of total levels in extract. Therefore, the five snRNAs were in vitro transcribed using T7 polymerase using previously described constructs (Fabrizio et al. 1989; McPheeters et al. 1989; Ghetti et al. 1995). The concentrations of gel-purified RNAs were determined by A260. To validate our quantitations, we in vitro transcribed the snRNAs in the presence of γ32P GTP, which labels only the 5′ end of each snRNA. Gel-purified snRNAs were quantified both by A260 and by scintillation counting; there was virtually a 1:1 correspondence between the measurements. Equal molar amounts of each snRNA were then reverse-transcribed and used for standard curves. According to this method, the relative levels of snRNAs in splicing extract are approximately as follows: U2 and U6 are at similar levels, U4 and U5 are approximately threefold less abundant, and U1 is threefold less abundant yet. In a standard splicing extract, the concentrations of U2 and U6 are ∼2 nM.
snRNAs affinity-purified by binding to biotinylated pre-mRNA in SNU114 versus snu114–60 extract were normalized based on total WT RNA. In all other experiments, the in vitro transcribed snRNAs were used for normalization. Because we found that U4 was threefold less abundant than U6 in total RNA, the initial values for the ratios of U4 to U6 in snu114–60 extracts were divided by three to obtain the values shown in Figure 1B. Figure 3D shows the amount of each snRNA present in the total volume of extract that was immunoprecipitated, and the remaining panels in Figure 3 show the calculated number of fmol of snRNAs that were coprecipitated from the entire IP.
Native gels
Native gel analysis was performed as described by Raghunathan and Guthrie (1998), with the following changes: Reactions were incubated with or without 2mM ATP for 30 min at 25°C. Spermidine was not included in the reactions. Gels were transferred to Hybond-N membrane in 0.5X TAE. Northern blots were sequentially reprobed after being stripped with 0.1% SDS in boiling water.
Immunoprecipitations
Between 50 and 200 mL of culture was grown at 25°C to OD 0.8–1.0. Pellets were resuspended in 1 mL IPP150 (10 mM Tris at pH 8.0, 150 mM NaCl, 0.1% NP40) in the presence of 1 mM PMSF, 1 mM benzamidine, 1 μg/mL leupeptin, 1 μg/mL aprotinin, and 0.5 μg/mL pepstatin A. Cells were disrupted in the presence of 0.5 mm zirconia/silica beads (Biospec Products, Inc.) by four 1.5-min pulses in a Mini-beadbeater (Biospec Products, Inc.). Extracts were normalized to the same concentration by Bradford assay (BioRad). For each immunoprecipitation, 10 μL Protein A Sepharose (Amersham) were preincubated for 2 h at 23°C with 2 μL Snu114 antisera, 5 μL Prp8 antisera, 2 μL SmD1 antisera, or 2 μL nonimmune sera in the presence of IPP150. Resin was washed three times with IPP150. For TAP purifications, 10-μL bed volume IgG sepharose (Amersham) were used. For the affinity purification shown in Figure 4D, the equivalent of 100 mL of culture at OD 0.9 was used. In all other cases, 500 μg extract plus IPP150 were added to a total volume of 300 μL. Reactions were incubated at 4°C for 2 h and washed four times with 1 mL IPP150. Proteins were eluted by adding 80 μL elution buffer (50 mM Tris at pH 8.0, 150 mM NaCl, 10 mM EDTA, 1% SDS) and rotating at room temperature for 5 min. Half of the supernatant was removed into 6× SDS-PAGE loading dye. Proteinase K was added to the remaining liquid and resin, and tubes were incubated at 37°C for 20 min. RNA was phenol-extracted and ethanol-precipitated. In parallel, RNA was also isolated from 20 μL of total extract to determine total snRNA levels.
Antibodies and Western blot analysis
Polyclonal Snu114, Prp8, and SmD1 antibodies were gifts from P. Fabrizio (Max-Planck Institute of Biophysical Chemistry, Goettingen, Germany), J. Beggs (University of Edinburgh, Scotland), and S.-C. Cheng (Academia Sinica, Taipei, Taiwan), respectively. The Prp8.4 antibody was used for immunoprecipitations, while the Prp8.1 antibody was used for Western blotting (Lossky et al. 1987; Jackson et al. 1988). Monoclonal Rpl3 was a gift from J. Warner (Albert Einstein College of Medicine, Bronx, NY) (Vilardell and Warner 1997). As a negative control, we used antisera from rabbits that failed to interact with any of the snRNAs above the level of background of Protein A Sepharose alone; the antisera was a gift from A. Kutach.
TCA extracts of proteins were prepared as described (Preker et al. 2002). Extracts were separated by electrophoresis on 7.5% SDS-polyacrylamide gels (with the exception of Prp8 in Fig. 4B, which used a 10% gel) and blotted to nitrocellulose membrane. Membranes were cut and separate portions were probed with antibodies against Prp8 (1:1000 dilution), Snu114 (1:5000 dilution), and Rpl3 (1:5000 dilution). Protein A within the TAP-tag of Brr2-TAP was detected with Prp8 antibodies. Proteins were detected with enhanced chemiluminescence (Amersham) using goat anti-rabbit or goat anti-mouse antibodies conjugated to horseradish peroxidase (BioRad) at a dilution of 1:3000. Alternatively, proteins were detected and quantified using the Odyssey System (Li-Cor Biosciences) with fluorescent Alexa Fluor 680 goat anti-rabbit (Molecular Probes) and IR dye 800 donkey anti-mouse (Rockland) antibodies at a dilution of 1:20,000.
ACKNOWLEDGMENTS
We thank Jean Beggs, Soo-Chen Cheng, Patrizia Fabrizio, and John Warner for gifts of antibodies, and Jon Staley for sharing protocols. Thank you to John Abelson, Hiten Madhani, Mike Springer, and members of the Guthrie lab for discussions and comments on the manuscript. T.J.B. was a Howard Hughes Medical Institute predoctoral fellow. C.G. is an American Cancer Society Research Professor of Molecular Genetics. This work was supported by NIH grant GM21119.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2319806.
REFERENCES
- Achsel T., Ahrens K., Brahms H., Teigelkamp S., Luhrmann R. The human U5-220kD protein (hPrp8) forms a stable RNA-free complex with several U5-specific proteins, including an RNA unwindase, a homologue of ribosomal elongation factor EF-2, and a novel WD-40 protein. Mol. Cell. Biol. 1998;18:6756–6766. doi: 10.1128/mcb.18.11.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels C., Klatt C., Luhrmann R., Fabrizio P. The ribosomal translocase homologue Snu114p is involved in unwinding U4/U6 RNA during activation of the spliceosome. EMBO Rep. 2002;3:875–880. doi: 10.1093/embo-reports/kvf172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels C., Urlaub H., Luhrmann R., Fabrizio P. Mutagenesis suggests several roles of Snu114p in pre-mRNA splicing. J. Biol. Chem. 2003;278:28324–28334. doi: 10.1074/jbc.M303043200. [DOI] [PubMed] [Google Scholar]
- Bindereif A., Green M.R. An ordered pathway of snRNP binding during mammalian pre-mRNA splicing complex assembly. EMBO J. 1987;6:2415–2424. doi: 10.1002/j.1460-2075.1987.tb02520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner T.J., Guthrie C. Genetic analysis reveals a role for the C terminus of the Saccharomyces cerevisiae GTPase Snu114 during spliceosome activation. Genetics. 2005;170:1063–1080. doi: 10.1534/genetics.105.042044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brow D.A. Allosteric cascade of spliceosome activation. Annu. Rev. Genet. 2002;36:333–360. doi: 10.1146/annurev.genet.36.043002.091635. [DOI] [PubMed] [Google Scholar]
- Brown J.D., Beggs J.D. Roles of PRP8 protein in the assembly of splicing complexes. EMBO J. 1992;11:3721–3729. doi: 10.1002/j.1460-2075.1992.tb05457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge C.B., Tuschl T.H., Sharp P.A. Splicing of precursors to mRNAs by the spliceosomes. In: Gesteland R.F., et al., editors. The RNA world. 2d ed., Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1999. pp. 525–560. [Google Scholar]
- Cheng S.C., Abelson J. Spliceosome assembly in yeast. Genes & Dev. 1987;1:1014–1027. doi: 10.1101/gad.1.9.1014. [DOI] [PubMed] [Google Scholar]
- Dix I., Russell C.S., O'Keefe R.T., Newman A.J., Beggs J.D. Protein–RNA interactions in the U5 snRNP of Saccharomyces cerevisiae . RNA. 1998;4:1239–1250. doi: 10.1017/s1355838298981109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P., McPheeters D.S., Abelson J. In vitro assembly of yeast U6 snRNP: A functional assay. Genes & Dev. 1989;3:2137–2150. doi: 10.1101/gad.3.12b.2137. [DOI] [PubMed] [Google Scholar]
- Fabrizio P., Laggerbauer B., Lauber J., Lane W.S., Luhrmann R. An evolutionarily conserved U5 snRNP-specific protein is a GTP-binding factor closely related to the ribosomal translocase EF-2. EMBO J. 1997;16:4092–4106. doi: 10.1093/emboj/16.13.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D.N., Roiha H., Guthrie C. Architecture of the U5 small nuclear RNA. Mol. Cell. Biol. 1994;14:2180–2190. doi: 10.1128/mcb.14.3.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami S., Huh W.K., Bower K., Howson R.W., Belle A., Dephoure N., O'Shea E.K., Weissman J.S. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- Ghetti A., Company M., Abelson J. Specificity of Prp24 binding to RNA: A role for Prp24 in the dynamic interaction of U4 and U6 snRNAs. RNA. 1995;1:132–145. [PMC free article] [PubMed] [Google Scholar]
- Grainger R.J., Beggs J.D. Prp8 protein: At the heart of the spliceosome. RNA. 2005;11:533–557. doi: 10.1261/rna.2220705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz M., Moore M.J., Bindereif A. Domain analysis of human U5 RNA. Cap trimethylation, protein binding, and spliceosome assembly. J. Biol. Chem. 1996;271:19001–19007. doi: 10.1074/jbc.271.31.19001. [DOI] [PubMed] [Google Scholar]
- Inada M., Guthrie C. Identification of Lhp1p-associated RNAs by microarray analysis in Saccharomyces cerevisiae reveals association with coding and noncoding RNAs. Proc. Natl. Acad. Sci. 2004;101:434–439. doi: 10.1073/pnas.0307425100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S.P., Lossky M., Beggs J.D. Cloning of the RNA8 gene of Saccharomyces cerevisiae, detection of the RNA8 protein, and demonstration that it is essential for nuclear pre-mRNA splicing. Mol. Cell. Biol. 1988;8:1067–1075. doi: 10.1128/mcb.8.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.H., Guthrie C. Unexpected flexibility in an evolutionarily conserved protein-RNA interaction: Genetic analysis of the Sm binding site. EMBO J. 1990;9:2555–2561. doi: 10.1002/j.1460-2075.1990.tb07436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurica M.S., Moore M.J. Pre-mRNA splicing: Awash in a sea of proteins. Mol. Cell. 2003;12:5–14. doi: 10.1016/s1097-2765(03)00270-3. [DOI] [PubMed] [Google Scholar]
- Kuhn A.N., Brow D.A. Suppressors of a cold-sensitive mutation in yeast U4 RNA define five domains in the splicing factor Prp8 that influence spliceosome activation. Genetics. 2000;155:1667–1682. doi: 10.1093/genetics/155.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn A.N., Li Z., Brow D.A. Splicing factor Prp8 governs U4/U6 RNA unwinding during activation of the spliceosome. Mol. Cell. 1999;3:65–75. doi: 10.1016/s1097-2765(00)80175-6. [DOI] [PubMed] [Google Scholar]
- Kuhn A.N., Reichl E.M., Brow D.A. Distinct domains of splicing factor Prp8 mediate different aspects of spliceosome activation. Proc. Natl. Acad. Sci. 2002;99:9145–9149. doi: 10.1073/pnas.102304299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laggerbauer B., Achsel T., Luhrmann R. The human U5-200kD DEXH-box protein unwinds U4/U6 RNA duplices in vitro. Proc. Natl. Acad. Sci. 1998;95:4188–4192. doi: 10.1073/pnas.95.8.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain P., Seraphin B., Rosbash M. Early commitment of yeast pre-mRNA to the spliceosome pathway. Mol. Cell. Biol. 1988;8:3755–3760. doi: 10.1128/mcb.8.9.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R.J., Newman A.J., Cheng S.C., Abelson J. Yeast mRNA splicing in vitro. J. Biol. Chem. 1985;260:14780–14792. [PubMed] [Google Scholar]
- Lossky M., Anderson G.J., Jackson S.P., Beggs J. Identification of a yeast snRNP protein and detection of snRNP-snRNP interactions. Cell. 1987;51:1019–1026. doi: 10.1016/0092-8674(87)90588-5. [DOI] [PubMed] [Google Scholar]
- McPheeters D.S., Fabrizio P., Abelson J. In vitro reconstitution of functional yeast U2 snRNPs. Genes & Dev. 1989;3:2124–2136. doi: 10.1101/gad.3.12b.2124. [DOI] [PubMed] [Google Scholar]
- Preker P.J., Kim K.S., Guthrie C. Expression of the essential mRNA export factor Yra1p is autoregulated by a splicing-dependent mechanism. RNA. 2002;8:969–980. doi: 10.1017/s1355838202020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan P.L., Guthrie C. RNA unwinding in U4/U6 snRNPs requires ATP hydrolysis and the DEIH-box splicing factor Brr2. Curr. Biol. 1998;8:847–855. doi: 10.1016/s0960-9822(07)00345-4. [DOI] [PubMed] [Google Scholar]
- Ruby S.W., Abelson J. An early hierarchic role of U1 small nuclear ribonucleoprotein in spliceosome assembly. Science. 1988;242:1028–1035. doi: 10.1126/science.2973660. [DOI] [PubMed] [Google Scholar]
- Segault V., Will C.L., Polycarpou-Schwarz M., Mattaj I.W., Branlant C., Luhrmann R. Conserved loop I of U5 small nuclear RNA is dispensable for both catalytic steps of pre-mRNA splicing in HeLa nuclear extracts. Mol. Cell. Biol. 1999;19:2782–2790. doi: 10.1128/mcb.19.4.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley J.P., Guthrie C. Mechanical devices of the spliceosome: Motors, clocks, springs, and things. Cell. 1998;92:315–326. doi: 10.1016/s0092-8674(00)80925-3. [DOI] [PubMed] [Google Scholar]
- Staley J.P., Guthrie C. An RNA switch at the 5′ splice site requires ATP and the DEAD box protein Prp28p. Mol. Cell. 1999;3:55–64. doi: 10.1016/s1097-2765(00)80174-4. [DOI] [PubMed] [Google Scholar]
- Stevens S.W., Barta I., Ge H.Y., Moore R.E., Young M.K., Lee T.D., Abelson J. Biochemical and genetic analyses of the U5, U6, and U4/U6 × U5 small nuclear ribonucleoproteins from Saccharomyces cerevisiae . RNA. 2001;7:1543–1553. [PMC free article] [PubMed] [Google Scholar]
- Stevens S.W., Ryan D.E., Ge H.Y., Moore R.E., Young M.K., Lee T.D., Abelson J. Composition and functional characterization of the yeast spliceosomal penta-snRNP. Mol. Cell. 2002;9:31–44. doi: 10.1016/s1097-2765(02)00436-7. [DOI] [PubMed] [Google Scholar]
- Turner I.A., Norman C.M., Churcher M.J., Newman A.J. Roles of the U5 snRNP in spliceosome dynamics and catalysis. Biochem. Soc. Trans. 2004;32:928–931. doi: 10.1042/BST0320928. [DOI] [PubMed] [Google Scholar]
- Umen J.G., Guthrie C. A novel role for a U5 snRNP protein in 3′ splice site selection. Genes & Dev. 1995;9:855–868. doi: 10.1101/gad.9.7.855. [DOI] [PubMed] [Google Scholar]
- van Nues R.W., Beggs J.D. Functional contacts with a range of splicing proteins suggest a central role for Brr2p in the dynamic control of the order of events in spliceosomes of Saccharomyces cerevisiae . Genetics. 2001;157:1451–1467. doi: 10.1093/genetics/157.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardell J., Warner J.R. Ribosomal protein L32 of Saccharomyces cerevisiae influences both the splicing of its own transcript and the processing of rRNA. Mol. Cell. Biol. 1997;17:1959–1965. doi: 10.1128/mcb.17.4.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will C.L., Luhrmann R. Spliceosomal UsnRNP biogenesis, structure and function. Curr. Opin. Cell Biol. 2001;13:290–301. doi: 10.1016/s0955-0674(00)00211-8. [DOI] [PubMed] [Google Scholar]
- Xie J., Beickman K., Otte E., Rymond B.C. Progression through the spliceosome cycle requires Prp38p function for U4/U6 snRNA dissociation. EMBO J. 1998;17:2938–2946. doi: 10.1093/emboj/17.10.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]